Abstract
Kawasaki disease (KD) is an acute vasculitis syndrome of unknown aetiology in children. The administration of Candida cell wall antigens induced KD-like coronary vasculitis in mice. However, the responses of KD patients to Candida cell wall antigen are unknown. In this study, we examined the response of KD patients to β-glucan (BG), one of the major fungal cell wall antigens, by measuring the anti-BG titre. In KD patients, the anti-C. albicans cell wall BG titre was higher than that in normal children. The anti-BG titre was also higher in KD patients compared to children who served as control subjects. The efficacy of intravenous immunoglobulin (IVIG) therapy in KD is well established. We categorized the KD patients into three groups according to the therapeutic efficacy of intravenous immunoglobulin (IVIG) and compared the anti-BG titre among these groups. Anti-BG titres were similar in the control group and the non-responsive group. In the fully responsive group, the anti-BG titre showed higher values than those in the normal children. This study demonstrated clinically that KD patients have high antibody titres to Candida cell wall BG, and suggested the involvement of Candida cell wall BG in the pathogenesis of KD. The relationship between IVIG therapy and anti-BG titre was also shown. These results provide valuable insights into the therapy and diagnosis of KD.
Keywords: β-glucan, antibody, Candida albicans, IVIG, Kawasaki disease
Introduction
Kawasaki disease (KD) is an acute vasculitis syndrome of unknown aetiology that affects children 1,2. KD vasculitis primarily involves the coronary arteries. It is a leading cause of acquired heart disease in children in developed countries. The diagnosis of KD is usually based on clinical symptoms that include prolonged fever lasting longer than 5 days and cervical lymphadenopathy. However, some patients do not meet the diagnostic criteria of KD, and are wrongly diagnosed. To date, there are no specific diagnostic tests for KD. The first line of treatment for KD is the administration of a high dose of intravenous immunoglobulin (IVIG) plus aspirin. IVIG therapy is performed typically within 10 days of the onset of the illness, under controlled conditions.
Several epidemiological studies on KD have reported that genetic and environmental factors contribute to the disease pathogenesis 3–6. Some reports have suggested the involvement of microbial infections such as bacteria and fungi in KD 7. Murata et al. have established a mouse model of coronary arteritis, which is histopathologically similar to that of the human KD, by the administration of Candida albicans, a major pathogenic fungi-derived substance 8. We also induced similar coronary arteritis in mice by the administration of C. albicans water-soluble fraction (CAWS) obtained from C. albicans culture supernatant 9. The therapeutic effects of IVIG or anti-TNF-α were examined using this mouse model 10–12.
C. albicans colonizes the intestinal tract and causes invasive deep mycosis in an immunocompromised host. β-glucan (BG) is one of the main components of fungal cell wall and fungal pathogen-associated molecular patterns (PAMPs). BG stimulates the host immune system, and induces an inflammatory response leading to the production of inflammatory mediators 13. Several researchers have studied the host immune response to pathogenic fungi and fungal PAMPs. Dectin-1, complement receptor 3 and lactosylceramide have all been cited as candidates for BG receptors and are important for phagocytosis and other biological activities 14–16. We detected antibodies against BG in human sera as a BG recognition molecule in the acquired immune response 17. This antibody titre fluctuated in patients with deep mycosis whose sera were β-1,3-glucan-positive 18,19. These results suggested that anti-BG serve as an indicator of the human response to BG and could be used to further understand the immune responses to BG in humans.
The administration of Candida cell wall antigens induced a KD-like coronary vasculitis in the mouse. However, the response to Candida cell wall antigen in KD patients is unknown. In this study, we examined the specific response to BG, one of the major fungal cell wall antigens in KD patients by the measurement of anti-BG titre.
Materials and methods
Materials
C. albicans and Aspergillus niger-solubilized cell wall glucan (CSBG and ASBG) were prepared by the NaClO-oxidation method according to a procedure used previously 20,21. Polysaccharide fractions (AgHWE) from Agaricus brasiliensis (= A. blazei Murrill sensu Heinem) were also prepared as described 22.
Subjects and specimens
Infants and children who met the diagnostic criteria for KD were enrolled into the study. This study included 18 KD patients, 21 children who served as child control subjects and nine adults who served as adult healthy control subjects. The demographic characteristics are shown in Table 1. All KD patients met the diagnostic criteria for KD as established by the Japanese Kawasaki Disease Research Committee. All KD patients were treated with IVIG (2 g/kg) and oral aspirin. Serum samples of KD patients were first collected on the first day of admission before the start of IVIG, the second after IVIG and a month after disease onset. The response to IVIG treatment in patients with Kawasaki disease was defined as follows: no response, high fever continued after IVIG; effective, high fever declined 24 h after IVIG termination followed by periodic rise in body temperature; complete response, body temperature returned to normal 24 h after IVIG termination. Fever was not observed after defervescence. All child control subjects had a fever. Serum samples were stocked at −30°C until the assay was performed. A peripheral venous blood sample was obtained from each participant. The study protocol was approved by the ethics committee of Nippon Medical School, and informed written consent was obtained from all study participants.
Table 1.
Demographic characteristics of patients with Kawasaki disease and controls.
| Kawasaki disease (KD) | Child control (CC) | |
|---|---|---|
| Age (months) | ||
| Mean ± s.d. | 32·4 ± 13·5 | 23·5 ± 30·8 |
| Range | 12–56 | 2–144 |
| Gender (male/female) | 10/8 | 9/12 |
s.d. = standard deviation.
Enzyme-linked immunosorbent assay (ELISA) of the anti-BG
A 96-well Nunc plate was coated overnight with the glucan preparation (25 μg/ml) in 0·1 M carbonate buffer (pH 9·6) by incubation at 4°C. The plate was washed with phosphate-buffered saline (PBS) containing 0·05% Tween 20 (Wako Pure Chemical Co., Osaka, Japan) (PBST) and blocked with 0·5% bovine serum albumin (BSA; Sigma, St Louis, MO, USA) at 37°C for 60 min. After additional washing, the plate was incubated with diluted human serum at 37°C for 60 min. For the measurement of IgG+M+A or immunoglobulin (Ig)G titre serum samples were diluted 2000-fold, and for IgM or IgA titres serum samples were diluted 200-fold. The plate was then washed with PBST and treated with an antibody for peroxidase-conjugated anti-human IgG+M+A, IgG, IgM or IgA (Sigma) in PBST containing 0·1% bovine serum albumin (BSA) (BPBST) and was developed with a 3,3′,5,5′-tetramethylbenzidine (TMB) substrate system (KPL Inc., Gaithersburg, MD, USA). Colour development was stopped with 1 N phosphoric acid and optical density was measured at 450 nm. To standardize the reactivity of each individual experiment, human pooled serum was used as standard sera. Each unit of anti-BG was calculated by the absorbance of the standard sera (Fig. S1, S3).
ELISA of the anti-ovalbumin (OVA)
A 96-well Nunc plate was coated with the ovalbumin (25 μg/ml) in 0·1 M carbonate buffer (pH 9·6) by incubation overnight at 4°C. The plate was washed with PBST and blocked with 0·5% bovine serum albumin (BSA; Sigma) at 37°C for 60 min. After additional washing, the plate was incubated with 500-fold diluted human serum at 37°C for 60 min. The plate was then washed with PBST and treated with an antibody for peroxidase-conjugated anti-human IgG+M+A (Sigma) in PBST containing 0·1% BSA (BPBST) and developed with a TMB substrate system (KPL Inc.). Colour development was stopped with 1 N phosphoric acid and optical density was measured at 450 nm.
Statistical analyses
The Mann–Whitney U-test was used for the comparisons between the different study groups. These statistical analyses were performed using MedCalc statistical software, version 11·6·1·0.
Results
Anti-BG titre in KD patients and control subjects
The antibody to CSBG was detected in the sera of KD patients and both adult and child control subjects (Fig. 1). The anti-BG titre in the normal children was lower than in normal adults. In KD patients, anti-BG titre was higher than that in the normal children. Conversely, the anti-OVA titre did not differ among the three groups (KD versus CC: P = 0·11; KD versus AC: P = 0·06, CC versus AC: P = 0·37).
Fig. 1.
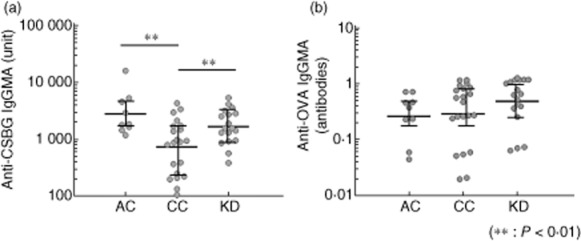
Comparison of anti-β-glucan titre in control subjects and Kawasaki disease patients. An enzyme-linked immunosorbent assay (ELISA) plate was coated with (a) Candida albicans solubilized cell wall glucan (CSBG) and (b) ovalbumin (OVA). The sera were added to the plate, and the plate-bound immunoglobulin (Ig) was determined with peroxidase-conjugated anti-human IgG+M+A. Enzyme activity was measured by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. AC = adult healthy control subjects (n = 9); CC = child control subjects (n = 21); KD = Kawasaki disease patients hospitalized (n = 18); **P < 0·01; Mann–Whitney U-test.
Next, we examined the relationship between anti-BG antibody and age in KD patients and normal children (Fig. 2). In both groups, anti-BG antibody titre increased proportionately with age and showed a positive correlation between them. When anti-BG titres per age in the two groups were compared, the anti-BG titre was higher in KD patients compared to normal children.
Fig. 2.
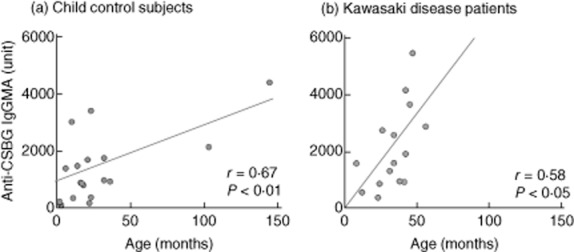
Relationship between age and anti-β-glucan titre in control subjects and patients with Kawasaki disease. The graph shows that the changes in anti-β-glucan titre positively correlates with the changes in age.
Candida cell wall glucan is composed of β-1,3-glucan and β-1,6-glucan 18. To examine whether anti-BG reacted with β-1,3-glucan or β-1,6-glucan chain, we analysed the reactivity of anti-BG to ASBG, composed mainly of β-1,3-glucan, and AgHWE, composed mainly of β-1,6-glucan, in KD patients (Fig. S2). The sera of KD patients indicated high reactivity to both antigens (Fig. 3).
Fig. 3.
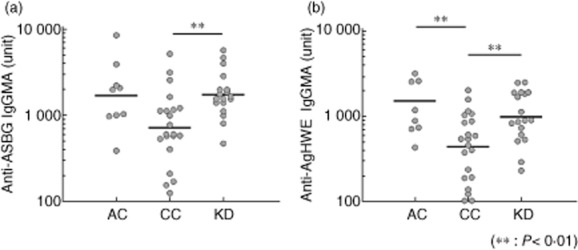
Comparison of anti-β-1,3-glucan [Aspergillus niger solubilized cell wall glucan (ASBG)] and anti-β-1,6-glucan [Agaricus blazei by repeated extraction with hot water (AgHWE)] titre in control subjects and Kawasaki disease patients. The enzyme-linked immunosorbent assay (ELISA) plate was coated with (a) ASBG and (b) AgHWE. The sera were added to the plate, and the plate-bound immunoglobulin (Ig) was determined with peroxidase-conjugated anti-human IgG+M+A. Enzyme activity was measured by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. AC = adult healthy control subjects (n = 9); CC = child control subjects (n = 21); KD = Kawasaki disease patients hospitalized (n = 18); **P < 0·01; Mann–Whitney U-test.
The anti-BG in each isotype was detected in the serum samples of normal children (Fig. 4). In child control subjects, anti-BG IgG showed a lower titre compared to the normal adults. When compared with the normal children, the KD patients showed higher anti-BG titre in each isotype. In particular, the anti-BG IgM showed a higher titre compared to both groups of control subjects.
Fig. 4.
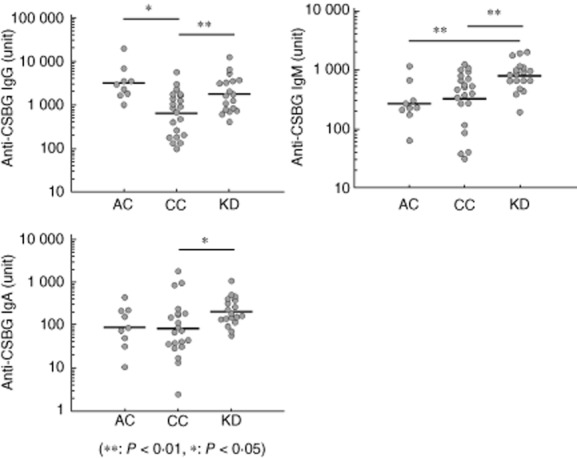
Comparison of anti-β-glucan isotype titre in control subjects and Kawasaki disease patients. The enzyme-linked immunosorbent assay (ELISA) plate was coated with Candida albicans solubilized cell wall glucan (CSBG). The sera were added to the plate, and the plate-bound immunoglobulin (Ig) was determined with peroxidase-conjugated anti-human IgG, M or A. Enzyme activity was measured by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. AC = adult healthy control subjects (n = 9); CC = child control subjects (n = 21); KD = Kawasaki disease patients hospitalized (n = 18); *P < 0·05; **P < 0·01; Mann–Whitney U-test.
Relationship between anti-BG titre and IVIG therapy in KD patients
The efficacy of IVIG therapy in KD is well established 2. We compared the anti-BG titre before and after IVIG therapy (Fig. 5). After IVIG therapy, the anti-BG titre was increased in all KD patients. Moreover, in some patients the high titre was sustained for up to 1 month after IVIG therapy. Next, we categorized the KD patients into three groups based on the therapeutic effect of IVIG and compared the anti-BG titre before the start of IVIG among these groups (Fig. 6). The anti-BG titre was similar in normal children and the group that did not respond to the treatment. However, in the fully responsive group, the anti-BG titre showed a higher reactivity compared to that of the normal children.
Fig. 5.
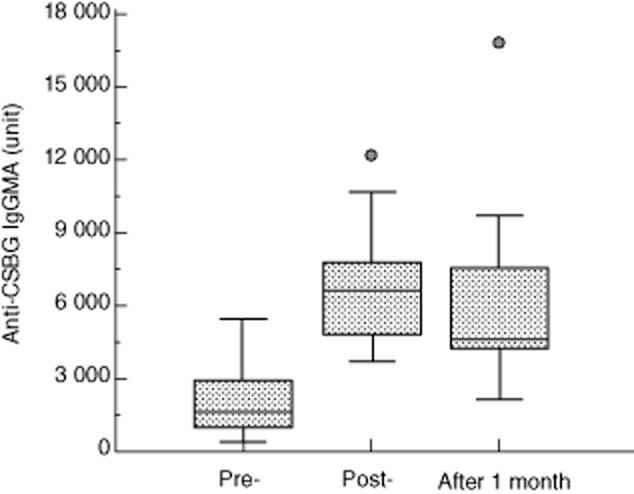
Effect of γ-globulin therapy on anti-Candida albicans solubilized cell wall glucan (CSBG) titre in Kawasaki disease patients. The enzyme-linked immunosorbent assay (ELISA) plate was coated with CSBG. The sera were added to the plate, and the plate-bound immunoglobulin (Ig) was determined with peroxidase-conjugated anti-human IgG+M+A. Enzyme activity was measured by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. Pre = Kawasaki disease patients hospitalized (n = 18); post = Kawasaki disease patients after γ-globulin therapy (n = 18); post 1 month = Kawasaki disease patients 1 month after γ-globulin therapy (n = 18).
Fig. 6.
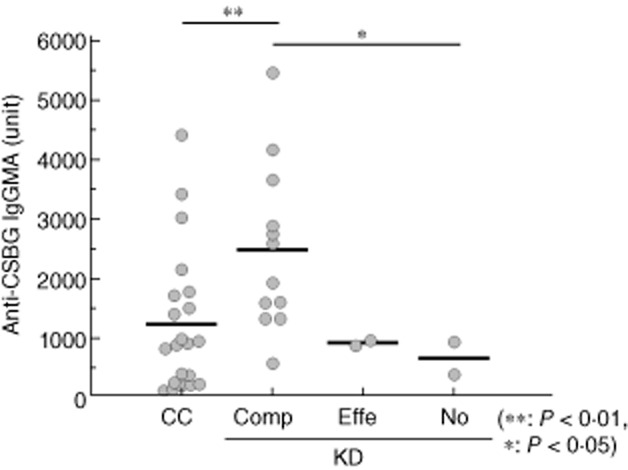
Comparison of the effect of γ-globulin therapy and anti-β-glucan titre in Kawasaki disease patients. An enzyme-linked immunosorbent assay (ELISA) plate was coated with Candida albicans solubilized cell wall glucan (CSBG). The sera were added to the plate, and the plate-bound immunoglobulin (Ig) was determined with peroxidase-conjugated anti-human IgG+M+A. Enzyme activity was measured by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. CC = child control subjects (n = 21); KD = Kawasaki disease patients hospitalized (n = 16); KD patients were divided into three groups based on the effect of intravenous Ig (IVIG) treatment. [Comp = complete response (n = 12); Effe = effective (n = 2); No = no effect (n = 2)].
Discussion
KD is an acute vasculitis syndrome of unknown aetiology in children 1. Nevertheless, the involvement of infection in the pathogenesis of KD has been suggested. We have reported the induction of vasculitis, similar to KD, by the administration of CAWS in mice 9. In this study we examined the antibody titre to BG, one of the major fungal PAMPs in KD patients. KD patients showed high anti-BG titre compared to the children in the control group. Conversely, anti-OVA titre did not increase in KD patients. In addition, we compared anti-lipopolysaccharide (LPS) as one of the major bacterial PAMPs between KD patients and control subjects (data not shown). Anti-LPS titres did not differ among the three groups (KD versus CC: P = 0·06; KD versus AC: P = 0·68; CC versus AC: P = 0·17). There was no significant correlation between anti-LPS and anti-BG titre (P = 0·98, r < 0·01). These results suggested the increasing specific immune response to Candida antigen in KD patients.
Candida colonizes the intestinal tract. The eukaryotic fungal community in the gut and skin was recently called ‘mycobiome’ 23,24. It was also reported that the mycobiome plays an important role in several diseases 25–27. We and other groups have reported that the anti-BG detected in adult human serum is one of the parameters which reflected the host response to BG 18,28. For the first time, we detected the anti-BG of individual isotypes in the serum of children. Anti-BG titre was lower in child serum compared to adult serum, especially the IgG class. We have shown that the IgG subclass of the anti-BG is IgG2. The placental transfer of IgG2 antibody is much lower when compared to other antibodies of the IgG subclass 29. Therefore, it was suggested that this antibody was produced after birth in response to BG.
A relationship between anti-BG titre and age indicated that the anti-BG titre increased with age. We have reported that anti-BG titre increased with age in bovine serum and was absent in fetal bovine serum (FBS) 30. These results suggested that anti-BG is induced depending on the exposure to fungal BG antigen and the immune responses to BG. We examined the anti-BG titre in patients with fungal infection or those with a higher risk of fungal infection, such as cancer or dialysis patients 31,32. Those patients with weak immune responses to fungi had a lower anti-BG titre. This suggested that the anti-BG is a potential indicator of the immune response to BG. In this study, the KD patients had a higher anti-BG titre. These results suggested that KD patients are increasingly exposed to the immune responses induced by Candida cell wall β-glucan.
We prepared the limulus factor G-activating substance, CAWS, from the culture supernatant of C. albicans 33. CAWS is composed of the mannan–glucan complex and induces vasculitis, similar to KD, in mice. The BG of CAWS influences its biological activities. BG is one of the major fungal PAMPs and activates complement and inflammatory cytokine production, such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-17. We have reported previously that the response to fungal BG was different among mouse stains 34,35. The DBA/2 mouse strain is highly responsive to BG and showed a high anti-BG titre, and induced severe coronary arteritis after CAWS administration. In addition, anti-BG titre in mouse serum and induction of coronary arteritis through the administration of CAWS were high compared to those observed in control mice. It was also reported that BG was involved in autoimmune diseases such as arthritis, inflammatory bowel disease and encephalomyelitis in mice 36,37. Anti-BG could not only be an indicator of the immune response to BG, but could also be an enhancer of inflammatory responses by forming an immune complex.
IVIG administration is a well-established therapeutic strategy to effectively treat KD 2. However, some KD patients showed resistance to IVIG therapy, and were required to receive other aggressive treatments. Therefore, early determination of the effect on IVIG is a critical factor in KD treatment. In KD patients, after IVIG therapy, the anti-BG titre was increased. We have reported that anti-BG was detected in human immunoglobulin preparations of IVIG therapy 17. The titre was very high and could be detected effectively in human serum even when diluted 20000-fold. It was suggested that this increase in anti-BG titre resulted from the anti-BG in human immunoglobulin preparations.
We also examined the relationship between anti-BG titre and the effect of IVIG therapy in KD patients. Interestingly, in the group that responds fully to IVIG therapy, the anti-BG showed a high titre compared to that of the other groups. These results suggested that, in KD patients with high reactivity to Candida β-glucan antigen, IVIG therapy was effective. The probable mechanism contributing to the efficacy of IVIG therapy is the exclusion and neutralization of Candida BG antigen. The antigen–antibody complexes induce their biological activity through Fc receptors. The Fc-γ receptors I and III transfer the activating signal. Conversely, the Fc-γ receptor IIB transfers the inhibitory signal. The host normally maintains an optimum activating/inhibitory ratio to facilitate the biological activity of the immune complex 38. Anti-BG in the IVIG could induce an inhibitory effect to the inflammatory responses mediated by Fc-γ IIB receptors.
This study demonstrated that the KD patients have a high antibody titre to CSBG and suggested the involvement of CSBG in the pathogenesis of KD. The relationship between IVIG therapy and anti-BG titre was also established. These results provided valuable insights towards the therapy and diagnosis of KD.
Disclosures
The authors have declared that no financial and commercial conflicts of interest exist.
Author contributions
K. I. carried out the studies and data analyses, and wrote the manuscript. R. F. collected and organized the samples, performed data analyses and helped to draft the manuscript. N. M. and Y. A. designed the study. S. O. and N. O. coordinated the study and helped draft the manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Standard curves of enzyme-linked immunosorbent assay (ELISA) of anti-β-glucan (BG).
Fig. S2. 13C-NMR spectra of Candida albicans solubilized cell wall glucan (CSBG), Aspergillus niger solubilized cell wall glucan (ASBG) and Agaricus blazei by repeated extraction with hot water (AgHWE) in dimethylsulphoxide (DMSO)-d6.
Fig. S3. The binding of immunoglobulin (Ig) to no-antigen enzyme-linked immunosorbent assay (ELISA) plate.
References
- 1.Gerding R. Kawasaki disease: a review. J Pediatr Health Care. 2011;25:379–387. doi: 10.1016/j.pedhc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Kuo HC, Yang KD, Chang WC, Ger LP, Hsieh KS. Kawasaki disease: an update on diagnosis and treatment. Pediatr Neonatol. 2012;53:4–11. doi: 10.1016/j.pedneo.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Rowley AH. Kawasaki disease: novel insights into etiology and genetic susceptibility. Annu Rev Med. 2011;62:69–77. doi: 10.1146/annurev-med-042409-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japan Kawasaki Disease Genome Consortium; US Kawasaki Disease Genetics Consortium. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012;44:517–521. doi: 10.1038/ng.2220. [DOI] [PubMed] [Google Scholar]
- 5.Burns JC, Cayan DR, Tong G, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–225. doi: 10.1097/01.ede.0000152901.06689.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohga K, Yamanaka R, Kinumaki H, Awa S, Kobayashi N. Kawasaki disease and rug shampoo. Lancet. 1983;1:930. doi: 10.1016/s0140-6736(83)91358-2. [DOI] [PubMed] [Google Scholar]
- 7.Mader R, Keystone EC. Infections that cause vasculitis. Curr Opin Rheumatol. 1992;4:35–38. [PubMed] [Google Scholar]
- 8.Murata H. Experimental candida-induced arteritis in mice. Relation to arteritis in the mucocutaneous lymph node syndrome. Microbiol Immunol. 1979;23:825–831. doi: 10.1111/j.1348-0421.1979.tb02815.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohno N. Chemistry and biology of angitis inducer, Candida albicans water-soluble mannoprotein–beta–glucan complex (CAWS) Microbiol Immunol. 2003;47:479–490. doi: 10.1111/j.1348-0421.2003.tb03409.x. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Oharaseki T, Yokouchi Y, et al. Administration of human immunoglobulin suppresses development of murine systemic vasculitis induced with Candida albicans water-soluble fraction: an animal model of Kawasaki disease. Mod Rheumatol. 2010;20:160–167. doi: 10.1007/s10165-009-0250-5. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi R, Fukazawa R, Watanabe M, et al. Etanercept suppresses arteritis in a murine model of Kawasaki disease: a comparative study involving different biological agents. Int J Vasc Med. 2013 doi: 10.1155/2013/543141. Article ID 543141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song MS, Lee SB, Sohn S, et al. Infliximab treatment for refractory Kawasaki disease in Korean children. Korean Circ J. 2010;40:334–338. doi: 10.4070/kcj.2010.40.7.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–315. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 14.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 15.Ross GD, Cain JA, Myones BL, Newman SL, Lachmann PJ. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman JW, Lindermuth J, Fish PA, Palace GP, Stevenson TT, DeMong DE. A novel carbohydrate–glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J Biol Chem. 1998;273:22014–22020. doi: 10.1074/jbc.273.34.22014. [DOI] [PubMed] [Google Scholar]
- 17.Masuzawa S, Yoshida M, Ishibsahi K, et al. Solubilized Candida cell wall β-glucan, CSBG, is epitope of natural human antibody. Drug Dev Res. 2003;58:179–189. [Google Scholar]
- 18.Ishibashi K, Yoshida M, Nakabayashi I, et al. Role of anti-beta-glucan antibody in host defense against fungi. FEMS Immunol Med Microbiol. 2005;44:99–109. doi: 10.1016/j.femsim.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M, Ishibashi K, Hida S, et al. Rapid decrease of anti-beta-glucan antibody as an indicator for early diagnosis of carinii pneumonitis and deep mycotic infections following immunosuppressive therapy in antineutrophil cytoplasmic antibody-associated vasculitis. Clin Rheumatol. 2009;28:565–571. doi: 10.1007/s10067-009-1096-0. [DOI] [PubMed] [Google Scholar]
- 20.Ohno N, Uchiyama M, Tsuzuki A, et al. Solubilization of yeast cell-wall beta-(1–>3)-D-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr Res. 1999;316:161–172. doi: 10.1016/s0008-6215(99)00049-x. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi K, Miura NN, Adachi Y, Tamura H, Tanaka S, Ohno N. The solubilization and biological activities of Aspergillus beta-(1–> 3)-D-glucan. FEMS Immunol Med Microbiol. 2004;42:155–166. doi: 10.1016/j.femsim.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T. Antitumor beta glucan from the cultured fruit body of Agaricus blazei. Biol Pharm Bull. 2001;24:820–828. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 23.Ghannoum MA, Jurevic RJ, Mukherjee PK, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLOS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21:334–341. doi: 10.1016/j.tim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliev ID, Funari VA, Taylor KD, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Velden WJ, Netea MG, de Haan AF, Huls GA, Donnelly JP, Blijlevens NM. Role of the mycobiome in human acute graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:329–332. doi: 10.1016/j.bbmt.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Park HK, Ha MH, Park SG, Kim MN, Kim BJ, Kim W. Characterization of the fungal microbiota (mycobiome) in healthy and dandruff-afflicted human scalps. PLOS ONE. 2012;7:e32847. doi: 10.1371/journal.pone.0032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiani P, Bromuro C, Cassone A, Torosantucci A. Anti-beta-glucan antibodies in healthy human subjects. Vaccine. 2009;27:513–519. doi: 10.1016/j.vaccine.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishibashi K, Dogasaki C, Iriki T, et al. Anti-β-glucan antibody in bovine sera. Int J Med Mushrooms. 2005;7:533–545. [Google Scholar]
- 31.Ishibashi K, Yoshida M, Nakabayashi I, et al. Characterization of blood beta-1,3-glucan and anti-beta-glucan antibody in hemodialysis patients using culinary–medicinal Royal Sun AgaricusAgaricus brasiliensis S. Wasser et al. (Agaricomycetideae) Int J Med Mushrooms. 2011;13:101–107. doi: 10.1615/intjmedmushr.v13.i2.10. [DOI] [PubMed] [Google Scholar]
- 32.Motoi M, Ishibashi K, Mizukami O, Miura N, Adachi Y, Ohno N. Anti-β-glucan antibody in cancer patients. Int J Med Mushrooms. 2011;13:101–107. doi: 10.1615/intjmedmushr.v13.i2.10. [DOI] [PubMed] [Google Scholar]
- 33.Uchiyama M, Ohno N, Miura NN, et al. Chemical and immunochemical characterization of limulus factor G-activating substance of Candida spp. FEMS Immunol Med Microbiol. 1999;24:411–420. doi: 10.1111/j.1574-695X.1999.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 34.Harada T, Miura NN, Adachi Y, Nakajima M, Yadomae T, Ohno N. IFN-gamma induction by SCG, 1,3-beta-D-glucan from Sparassis crispa, in DBA/2 mice in vitro. J Interferon Cytokine Res. 2002;22:1227–1239. doi: 10.1089/10799900260475759. [DOI] [PubMed] [Google Scholar]
- 35.Harada T, Nagi Miura N, Adachi Y, Nakajima M, Yadomae T, Ohno N. Antibody to soluble 1,3/1,6-beta-D-glucan, SCG in sera of naive DBA/2 mice. Biol Pharm Bull. 2003;26:1225–1228. doi: 10.1248/bpb.26.1225. [DOI] [PubMed] [Google Scholar]
- 36.Ruutu M, Thomas G, Steck R, et al. β-glucan triggers spondylarthritis and Crohn's disease-like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–2222. doi: 10.1002/art.34423. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Gonnella P, Safavi F, et al. Low dose zymosan ameliorates both chronic and relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2013;254:28–38. doi: 10.1016/j.jneuroim.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karsten CM, Köhl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Standard curves of enzyme-linked immunosorbent assay (ELISA) of anti-β-glucan (BG).
Fig. S2. 13C-NMR spectra of Candida albicans solubilized cell wall glucan (CSBG), Aspergillus niger solubilized cell wall glucan (ASBG) and Agaricus blazei by repeated extraction with hot water (AgHWE) in dimethylsulphoxide (DMSO)-d6.
Fig. S3. The binding of immunoglobulin (Ig) to no-antigen enzyme-linked immunosorbent assay (ELISA) plate.


