Abstract
The LEW.1AR1-iddm rat is an animal model of human type 1 diabetes (T1D), which arose through a spontaneous mutation within the major histocompatibility complex (MHC)-congenic background strain LEW.1AR1. The LEW.1AR1-iddm rat is characterized by two phenotypes: diabetes development with a diabetes incidence of 60% and a variable T cell frequency in peripheral blood. In this study the immune cell repertoire of LEW.1AR1-iddm rats was analysed over time from days 30 to 90 of life and compared to the background strain LEW.1AR1 and the LEW rat strain as well as the LEW.1WR1 rat strain. The LEW.1AR1-iddm rats are characterized by a high variability of CD3+, CD4+ and CD8+ T cell frequencies in peripheral blood over time, and the frequency is unique for each animal. The variability within the frequencies resulted in changes of the CD4+ : CD8+ T cell ratio. The other three rat strains studied were characterized by a stable but nevertheless strain-specific T cell frequency resulting in a specific CD4+ : CD8+ T cell ratio. The frequency of natural killer (NK) cells and B cells in LEW.1AR1-iddm rats was increased, with a higher variability compared to the other strains. Only monocytes showed no differences in frequency and variability between all strains studied. These variabilities of immune cell frequencies in the LEW.1AR1-iddm rats might lead to imbalances between autoreactive and regulatory T cells in peripheral blood as a prerequisite for diabetes development.
Keywords: animal model, immune cells, LEW.1AR1-iddm rat, rat strains, type 1 diabetes
Introduction
The LEW.1AR1-iddm rat is a model for human type 1 diabetes (T1D), which arose through a spontaneous mutation in the LEW.1AR1 strain 1. This model shows apoptotic β cell death, induced by proinflammatory cytokines released from infiltrating immune cells in the pancreatic islets 2,3. The autoimmune nature of the diabetic syndrome has been proved by adoptive transfer experiments 4,5.
The diabetic syndrome of the LEW.1AR1-iddm rat follows an autosomal recessive mode of inheritance, with a diabetes incidence of about 60% within the colony 2. Three diabetes susceptibility regions have been discovered by linkage analysis using two back-cross populations with the Brown Norway (BN) and with the PAR (Paris) strain 6,7. Iddm8 and Iddm9 are mapped on RNO1 and Iddm1 is mapped on RNO20 containing the major histocompatibility complex (MHC) region. The MHC is generally accepted as the diabetes predisposing locus in humans and animals. In rats, the MHC class II RT1-B/Du haplotype is permitting autoimmune diabetes. The mutation of the LEW.1AR1-iddm rat leading to diabetes manifestation has been mapped within the Iddm8 region on RNO1 6,7.
In addition to autoimmune diabetes, the LEW.1AR1-iddm rats manifest a second phenotype, which has been described as a ‘variable CD3+ T cell frequency’ and could also be mapped within the Iddm8 region 8. Thus, the mutation in the Iddm8 region does not only confer susceptibility to diabetes but also to the variable CD3+ T cell frequency in peripheral blood. The background strain LEW.1AR1 is diabetes-resistant and shows a stable T cell frequency.
The MHC-II RT1-B/Du haplotype is indispensible for diabetes development in rats, as also documented for the other rat models of T1D, the BioBreeding diabetes-prone (BBdp) rat and the Komeda rat 9,10. The LEW.1AR1 (RT1-Aa, RT1-B/Du, RT1-Cu) strain and LEW.1WR1 (RT1-Au, RT1-B/Du, RT1-Ca) strain express also the MHC-II RT1-B/Du haplotype. Interestingly, only the LEW.1WR1 strain develops diabetes spontaneously, with a very low incidence of 2% 11. Conversely, the original LEW (RT1-Al, RT1-B/Dl, RT1-Cl) strain cannot develop diabetes because of the missing RT1-B/Du haplotype.
Therefore, these congenic LEW strains, LEW, LEW.1AR1 and LEW.1WR1 rats, were chosen to compare the frequencies of immune cell subpopulations in peripheral blood to those in LEW.1AR1-iddm rats. The identification of possible differences in the immune cell population in peripheral blood may indicate changes in the immune cell system leading to diabetes development.
The aim of this study was (i) to analyse the frequency of T cells and other immune cells in the peripheral blood of LEW.1AR1-iddm rats from days 30 to 90 of life and (ii) to compare the findings with the background strain LEW.1AR1 as well as the LEW.1WR1 and the LEW strains.
Materials and methods
Animals
Rats were bred under specific pathogen-free (SPF) conditions and housed thereafter under standard conditions in the Central Animal Facility of Hannover Medical School 8. They were serologically negative for specific viruses 1,12. All strains were submitted regularly to genetic monitoring in order to verify the authenticity of the strain 13,14. All experiments were conducted in accordance with the principles of laboratory care approved by the local institutional Animal Care and Research Advisory Committee of Hannover Medical School.
Six to 20 animals were analysed from the different rat strains: LEW.1AR1-iddm, LEW.1AR1, LEW.1WR1 and LEW. Blood was taken from all animals every fifth day, starting at age 30 days until day 70, and thereafter every 10th day until day 90 (n = 11 in total). Blood glucose concentrations were measured three times a week until day 90 (Glucometer Elite™; Bayer, Leverkusen, Germany). The diabetes incidence (blood glucose >10 mmol/l) was 60% with a manifestation at day 62 ± 2 and an average blood glucose concentration of 22·7 ± 2·4 mmol/l. Diabetic LEW.1AR1-iddm rats were treated with Linplant™ insulin pellets (LinShin Canada, Ontario, Canada). All animals were killed at day 120 and pancreata were examined morphologically.
Morphology
Pancreas tissue specimens were fixed in 4% paraformaldehyde in 0·15 M phosphate-buffered saline, and paraffin-embedded for histological analyses. Serial pancreas sections were stained with the avidin–biotin complex (ABC) technique to identify the main infiltrating immune cells in the islet infiltrate 15. The following rat-specific primary antibodies were used: insulin (5330-3369G), infiltrating CD68+ macrophages (ED1), CD4+ T cells (OX-35) and CD8+ T cells (OX-8). Antibodies were from AbD Serotec (Düsseldorf, Germany) and used in dilutions of between 1:200 and 1:800.
Flow cytometry
In order to determine the different immune cell subpopulations in peripheral blood flow, cytometric analyses were performed using the following labelled rat-specific monoclonal antibodies: CD4 (OX-38) fluorescein isothiocyanate (FITC), CD3 (G4·18) phycoerythrin (PE) (Becton Dickinson, Heidelberg, Germany), CD8 (OX-8) FITC, CD8 (OX-8) PE, CD45RA (OX-33) FITC, NKR (10/78) FITC and CD68 (ED1) FITC (AbD Serotec). Blood was prepared 8 and immune cell subpopulations were analysed in a flow cytometer (Becton Dickinson). For the intracellular ED1 staining the immune cells were pretreated with Leukoperm® (AbD Serotec), according to the manufacturer's instructions. Data were analysed using Cellquest version 3·0·1 software.
Calculations of the analysed parameters
In a first analysis of the immune cell composition of peripheral blood, a mean value for each immune cell subpopulation was calculated for each animal from all measurements over time (at days 30, 35, 40, 45, 50, 55, 60, 65, 70, 80 and 90; n = 11) performed between days 30 and 90. From these single mean values for each animal, a ‘total mean value’ for each strain was calculated. The same procedure was adopted to obtain the ‘total mean value’ for the coefficient of variation (CV) for each strain.
In a second analysis, at each time-point (at days 30, 35, 40, 45, 50, 55, 60, 65, 70, 80 and 90) a mean value was calculated from all animals of each strain and for each immune cell subpopulation.
In a third analysis each animal was followed-up from days 30 to 90 to document possible individual variations over time.
Statistical analyses
Mean values ± standard error of the mean (s.e.m.), analysis of variance (anova) plus Tukey's post-test and CV were calculated using the GraphPad Prism version 5 software (La Jolla, CA, USA).
Results
Infiltration of pancreatic islets in LEW.1AR1-iddm rats compared with other MHC congenic LEW strains
Only animals of the LEW.1AR1-iddm strain developed diabetes at day 62 ± 2 (Table 1). In diabetic rats, 60 days after diabetes manifestation, only a few remaining islets with β cells showed a moderate immune cell infiltration (Fig. 1a–d). Immunohistochemistry documented an islet T cell infiltration with a predominance of CD8+ versus CD4+ T cells (Fig. 1a,b). The other main infiltrating immune cells were CD68+ macrophages (Fig. 1d). The pancreatic islets of all normoglycaemic animals from the LEW.1AR1-iddm strain (Fig. 1e), the LEW.1AR1 background strain (Fig. 1f), the LEW.1WR1 (Fig. 1g) and the LEW strain (Fig. 1h) showed well-preserved β cells (Fig. 1e–h) without immune cell infiltration.
Table 1.
Rat strains used in the studies.
| Rat strain | MHC | Number of animals (n) | Diabetes incidence (%) | Blood glucose (mmol/l) |
|---|---|---|---|---|
| LEW.1AR1 | RT1 (AaB/DuCu) | 10 | 0 | 5·6 ± 0·2 |
| LEW.1AR1-iddm | RT1 (AaB/DuCu) | 20 | 60 | (d) 22·7 ± 2·4 |
| Onset (day 62 ± 2) | (nd) 5·7 ± 0·2 | |||
| LEW | RT1 (AlB/DlCl) | 6 | 0 | 5·2 ± 0·1 |
| LEW.1WR1 | RT1 (AuB/DuCa) | 6 | 0 | 5·1 ± 0·1 |
(d): diabetic; (nd): non-diabetic; MHC = major histocompatibility complex.
Fig. 1.
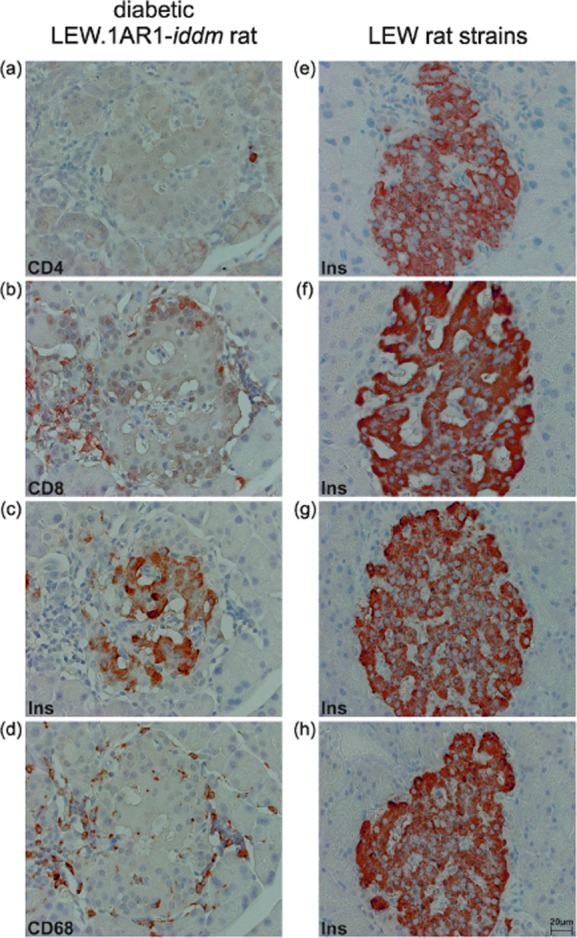
Pancreatic islets with β cells from diabetic animals with immune cell infiltration or from normoglycaemic animals without immune cell infiltration. Immunostaining for CD4+ (a), CD8+ T cells (b), insulin (c), and CD68+ infiltrating macrophages (d) from the diabetic LEW.1AR1-iddm rats (a-d) and immunostaining for insulin (e–h) for normoglycaemic LEW.1AR1-iddm rats (e), the LEW.1AR1 (f), LEW.1WR1 (g) and LEW rats (h). The immune cell composition comprised CD8+ T cells and CD68+ macrophages with a small number of CD4+ T cells after diabetes manifestation. The remaining β cells after diabetes manifestation as well as the β cells in the absence of diabetes showed a moderate to dense insulin immunostaining. Diaminobenzidene (DAB) immunohistochemistry with haematoxylin counterstaining.
Immune cell frequency in peripheral blood of LEW.1AR1-iddm rats compared with other MHC congenic LEW strains
In a first step, the immune cell composition in peripheral blood over time was compared between the inbred strains LEW.1AR1-iddm, LEW.1AR1, LEW.1WR1 and LEW. Therefore, a mean value for each immune cell subpopulation was calculated for each animal from all measurements (at days 30, 35, 40, 45, 50, 55, 60, 65, 70, 80 and 90) performed. From these single mean values for each animal, a total mean value for each strain was calculated. The same procedure was adopted to obtain the total mean value for the CV for each strain.
The percentage of CD3+ T cells in peripheral blood in diabetic and non-diabetic LEW.1AR1-iddm rats (diabetic: 51·9 ± 1·0%, non-diabetic: 51·8 ± 2·1%) was decreased significantly compared to the other three rat strains (P < 0·001; LEW.1AR1: 66·4 ± 0·7%; LEW.1WR1: 63·1 ± 1·0%; LEW: 67·4 ± 1·2%) [Fig. 2a(i)]. Additionally, the variability was significantly higher in the diabetic and non-diabetic LEW.1AR1-iddm rats (CV, diabetic: 15·4 ± 1·4%, non-diabetic: 15·7 ± 1·1%) compared to the other rat strains (P < 0·001; LEW.1AR1: 6·7 ± 0·8%; LEW.1WR1: 5·4 ± 0·5%; LEW: 5·7 ± 0·7%) [Fig. 2b(i)].
Fig. 2.
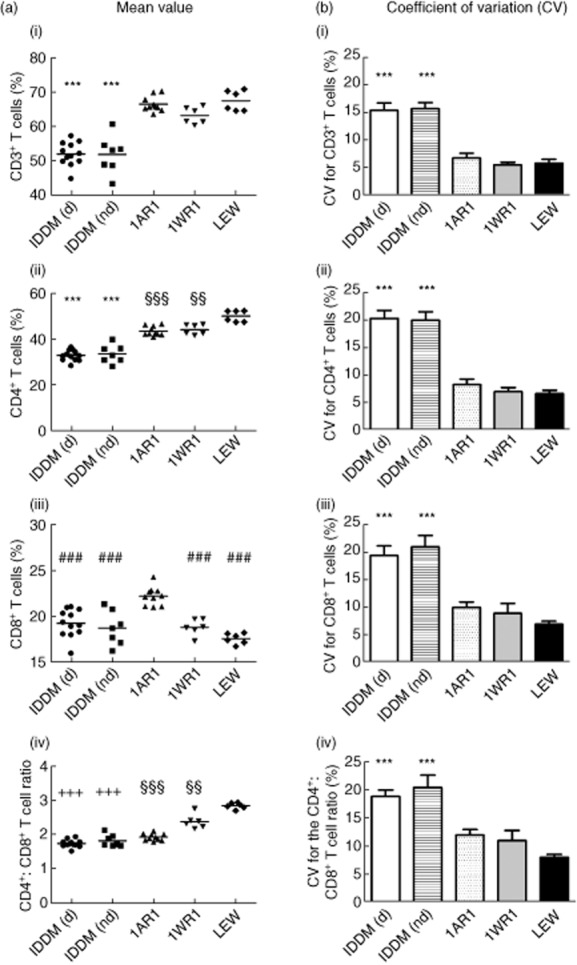
T cell frequency in different rat strains. A mean value for each T cell subpopulation was calculated for each single animal from all measurements (n = 11) performed. From these single mean values for each animal, a ‘total mean value’ for each strain was calculated (column a) for (i) CD3+ T cells, (ii) CD4+ T cells, (iii) CD8+ T cells and (iv) the CD4+ : CD8+ T cell ratio. The same procedure was adopted to obtain the ‘total mean value’ for the coefficient of variation (CV) for each strain (column b). ‘Total mean values’ were all expressed in % with exception of the mean value of the CD4+ : CD8+ T cell ratio [a(iv)]. IDDM (d) = LEW.1AR1-iddm diabetic, IDDM (nd) = LEW.1AR1-iddm non-diabetic, 1AR1 = LEW.1AR1, 1WR1 = LEW.1WR1. ***P < 0·001 IDDM (d) and IDDM (nd) versus 1AR1, 1WR1 and LEW; ###P < 0·001 IDDM (d), IDDM (nd), 1WR1 and LEW versus 1AR1; +++P < 0·001 IDDM (d) and IDDM (nd) versus 1WR1 and LEW; §§§P < 0·001 1AR1 versus LEW; §§P < 0·01 1WR1 versus LEW.
In parallel to the reduced CD3+ T cell frequency the percentage of CD4+ T cells was also decreased significantly in LEW.1AR1-iddm rats independent from the metabolic state (diabetic LEW.1AR1-iddm rats: 32·7 ± 0·7%; non-diabetic LEW.1AR1-iddm rats: 33·4 ± 1·5%) compared to the other three rat strains (P < 0·001; LEW.1AR1: 43·4 ± 0·7%; LEW.1WR1: 44·0 ± 0·9%; LEW: 49·9 ± 1·0%) [Fig. 2a(ii)]. Remarkably, the CD4+ T cell frequency in the LEW.1AR1 (P < 0·001) and the LEW.1WR1 strains (P < 0·01) was reduced significantly compared to the LEW strain. As shown for CD3+ T cells, the variation was significantly higher in the LEW.1AR1-iddm rats (CV, diabetic: 20·3 ± 1·5%, non-diabetic: 19·9 ± 1·5%) compared to the other rat strains (P < 0·001; LEW.1AR1: 8·3 ± 1·0%; LEW.1WR1: 7·0 ± 0·7%; LEW: 6·6 ± 0·6%) [Fig. 2b(ii)].
The frequency of CD8+ T cells was decreased significantly in LEW.1AR1-iddm rats (diabetic: 19·3 ± 0·4%, non-diabetic: 18·7 ± 0·7%) only in comparison to the LEW.1AR1 background strain (P < 0·001; 22·2 ± 0·3%), but not to the LEW.1WR1 and LEW strains [Fig. 2a(iii)]. The CD8+ T cells in the LEW.1WR1 and LEW strains (LEW.1WR1: 18·8 ± 0·4%; LEW: 17·6 ± 0·2%) were also lower compared to the LEW.1AR1 strain. Again, the variation was significantly higher for CD8+ T cells in LEW.1AR1-iddm rats (CV, diabetic: 19·4 ± 1·8%, non-diabetic: 21·0 ± 2·0%; P < 0·001 compared to LEW.1AR1: 10·0 ± 1·0%; LEW.1WR1: 8·9 ± 1·8%; LEW: 6·9 ± 0·6%) [Fig. 2b(iii)].
Due to the differences between the T cell subpopulations, the CD4+ : CD8+ T cell ratio is strain-specific. The ratio in the LEW.1AR1-iddm (diabetic: 1·7 ± 0·1, non-diabetic: 1·8 ± 0·1) and the LEW.1AR1 strain (2·0 ± 0·1) was significantly lower than in the LEW.1WR1 strain (P < 0·001; 2·4 ± 0·1) and the LEW strain (P < 0·001; 2·8 ± 0·1) [Fig. 2a(iv)]. The variation of the ratio was higher in LEW.1AR1-iddm rats in comparison to the other strains (CV, diabetic: 18·8 ± 1·1%, non-diabetic: 20·4 ± 2·2%, P < 0·001; LEW.1AR1: 12·0 ± 1·0%; LEW.1WR1: 11·0 ± 1·8%; LEW: 8·0 ± 0·5%) [Fig. 2b(iv)].
In contrast to the T cell subpopulations the frequency of NK cells in LEW.1AR1-iddm rats, independent from the diabetic state (diabetic: 5·9 ± 0·4%, P < 0·001; non-diabetic: 4·7 ± 0·2%, P < 0·01) was increased significantly compared to the other three rat strains (LEW.1AR1: 2·8 ± 0·1%; LEW.1WR1: 2·7 ± 0·1%; LEW: 2·7 ± 0·1%) [Fig. 3a(i)]. The variability was increased significantly in diabetic LEW.1AR1-iddm rats compared to all other groups (CV, diabetic LEW.1AR1-iddm: 70·3 ± 10·3%; P < 0·05 compared to non-diabetic LEW.1AR1-iddm: 38·1 ± 3·0%; P < 0·001 compared to LEW.1AR1: 28·8 ± 3·4%, LEW.1WR1: 28·6 ± 1·7%; LEW: 27·9 ± 5·6%) [Fig. 3b(i)].
Fig. 3.
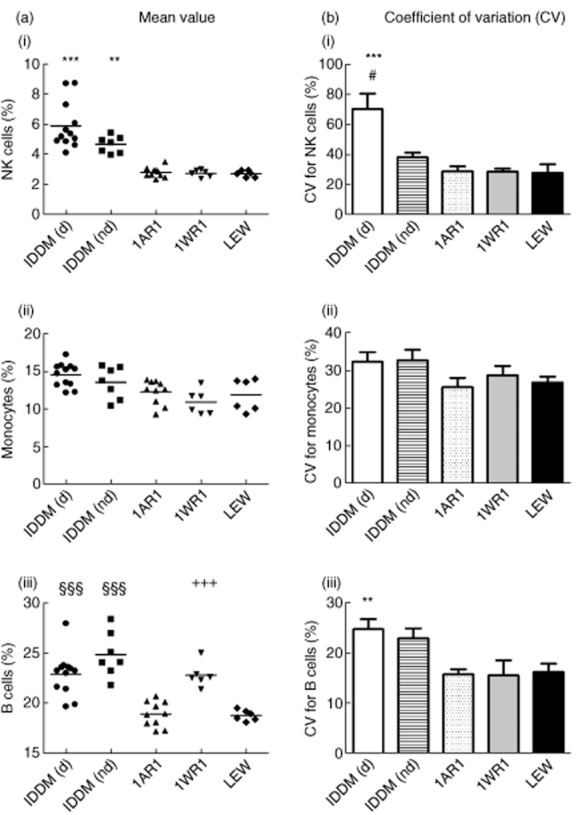
Immune cell frequency in different rat strains. As explained in Fig. 2, a mean value for each immune cell subpopulation was calculated (column a) for (i) natural killer (NK) cells, (ii) monocytes and (iii) B cells and coefficient of variation (CV) for each strain (column b). ‘Total mean values’ were all expressed in %. IDDM (d) = LEW.1AR1-iddm diabetic, IDDM (nd) = LEW.1AR1-iddm non-diabetic, 1AR1 = LEW.1AR1, 1WR1 = LEW.1WR1. ***P < 0·001; **P < 0·01 IDDM (d) and IDDM (nd) versus 1AR1, 1WR1 and LEW; §§§P < 0·001 IDDM (d) and IDDM (nd) versus 1AR1 and LEW; +++P < 0·001 1AR1 versus 1WR1 and LEW; #P < 0·001 IDDM (d) versus IDDM (nd).
A tendency towards an increase of monocytes was observed in the diabetic LEW.1AR1-iddm rats (diabetic LEW.1AR1-iddm: 14·6 ± 0·5%; non-diabetic LEW.1AR1-iddm: 13·6 ± 0·8%; LEW.1AR1: 12·3 ± 0·5%; LEW.1WR1: 10·9 ± 0·7%; LEW: 11·9 ± 0·9%) [Fig. 3a(ii)]. No differences in variability [Fig. 3b(ii)] were detectable, although the variability was relatively high in general.
The frequencies of B cells in LEW.1AR1-iddm rats, independent from the health status (diabetic: 22·9 ± 0·6%, non-diabetic: 24·9 ± 0·8%), and LEW.1WR1 rats (22·8 ± 0·5%), were significantly higher than in the background strain LEW.1AR1 (P < 0·001; 18·9 ± 0·4%) and the LEW strain (P < 0·001; 18·8 ± 0·2%) [Fig. 3a(iii)]. A significant increase in variability was detectable only within the diabetic LEW.1AR1-iddm group (CV: 24·8 ± 2·0%) in comparison to the LEW.1AR1 strain (P < 0·01; 15·8 ± 1·0%), the LEW.1WR1 strain (P < 0·01; 15·6 ± 3·0%) and the LEW strain (P < 0·01; 16·3 ± 1·6%) [Fig. 3b(iii)].
Time–course of the T cell frequency in peripheral blood of the LEW.1AR1-iddm rats compared with other MHC congenic LEW strains
Changes in the CD3+, CD4+ and CD8+ T cell frequencies over time were determined in LEW.1AR1-iddm rats and compared to all other rat strains in this study. Diabetic and non-diabetic LEW.1AR1-iddm rats showed the lowest and strongest non-linear course in all T cell frequencies over time (Fig. 4a,b). The T cell frequencies for the LEW.1AR1 strain were more stable and higher than in LEW.1AR1-iddm rats, with only a small decline of the CD3+ and CD4+ T cells at day 60 (Fig. 4c). The higher CD3+ and CD4+ frequencies showed a relatively stable course in the LEW.1WR1 and the LEW strains with the exception of a small increase at day 65 in the LEW.1WR1 strain (Fig. 4d,e). The percentage of CD8+ T cell frequency was around 20% in all rat strains.
Fig. 4.
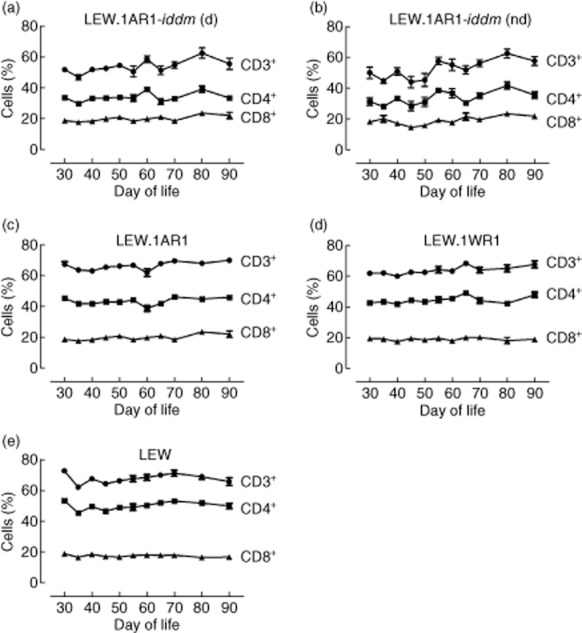
T cell frequency in different rat strains over time from days 30 to 90 of life. The CD3+ T cell frequency as well as the CD4+ and CD8+ T cell frequency was measured in the different rat strains. For each time-point a mean value ± standard error of the mean (s.e.m.) was calculated for each group. Diabetic and non-diabetic LEW.1AR1-iddm rats showed strong variations in the T cell frequency over the lifetime (a,b). The background strain LEW.1AR1 showed only little variation in the T cell frequency, with a strain-dependent decrease of CD3+ and CD4+ T cells at day 60 of life (c). The LEW and LEW.1WR1 strain showed only slight variations in T cell frequency over time (d,e). (d) = diabetic, (nd) = non-diabetic.
In LEW.1AR1-iddm rats the CD4+ : CD8+ T cell ratio changed over time due to the non-linear course of the CD4+ and CD8+ T cells, while the CD4+ : CD8+ T cell ratio in the other strains was more stable (Supporting information, Fig. S1).
Time–course of the NK cell, monocyte and B cell frequencies in peripheral blood of the LEW.1AR1-iddm rats compared to MHC congenic LEW strains
In addition to the T cell subpopulations the frequencies of NK cells, monocytes and B cells were determined over time in LEW.1AR1-iddm rats and compared to the other strains. The NK cells increased over time in LEW.1AR1-iddm rats (Fig. 5a,b), independent of the metabolic state, while the NK cell frequency in the LEW.1AR1, the LEW.1WR1 and the LEW strain was completely stable over time (Fig. 5c–e). Although the B cell frequency was variable in all strains, the highest variations occurred in diabetic and non-diabetic LEW.1AR1-iddm rats. The frequency of monocytes was also variable in all strains over time (Fig. 5a–e), but LEW.1AR1-iddm rats showed a higher frequency of monocytes in the early days of life (Fig. 5a,b).
Fig. 5.
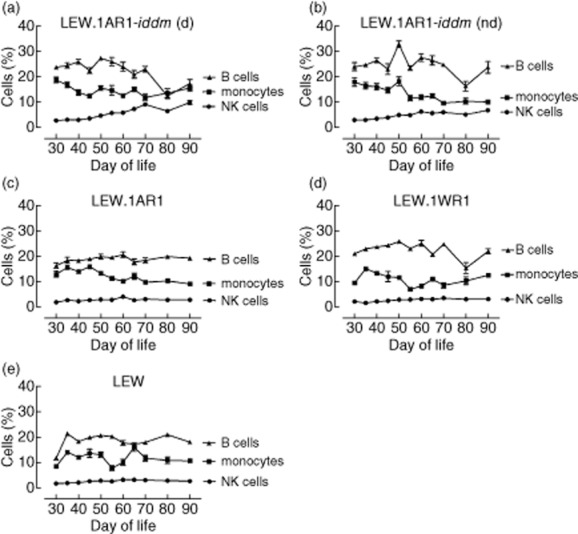
Immune cell frequency in different rat strains over time from days 30 until 90 of life. The frequency of natural killer (NK) cells as well as the frequency of monocytes and B cells was measured in the different rat strains. For each time-point a mean value ± standard error of the mean (s.e.m.) was calculated for each group. Diabetic and non-diabetic LEW.1AR1-iddm rats showed more changes in the immune cell frequency over time (a,b) than the other strains. (d) = diabetic, (nd) = non-diabetic.
Time–course of the individual T cell frequency in single animals representative for each strain
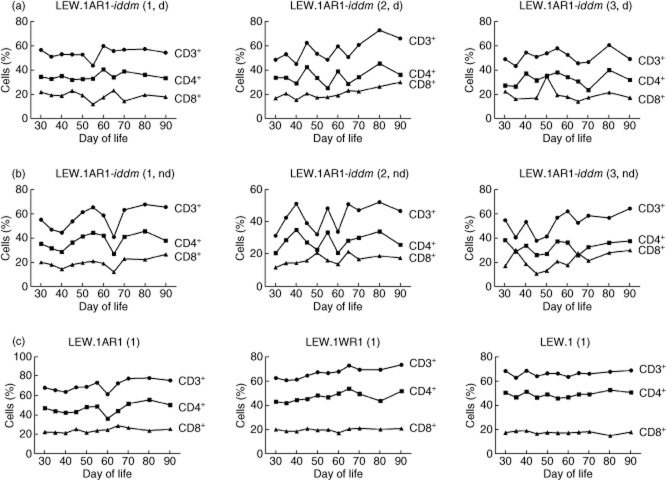
The highest variations were found in the T cell subpopulations. Therefore individual time courses for each strain were studied. The frequencies of CD3+, CD4+ and CD8+ T cells in the LEW.1AR1-iddm rats showed a unique time–course from days 30 to 90 (Fig. 6a,b), in contrast to a uniform time–course for each single animal of all other strains (Fig. 6c).
Fig. 6.
T cell frequency (CD3+, CD4+ and CD8+ T cells) was studied from days 30 to 90 of life for each single animal. In diabetic (a) and non-diabetic (b) LEW.1AR1-iddm rats the T cell frequency was unique for each single rat of the LEW.1AR1-iddm strain independent of diabetes development. In the LEW.1AR1 strain, the LEW.1WR1 strain and the LEW strain the T cell frequency was more stable over time compared to the LEW.1AR1-iddm rats (c). In the background strain LEW.1AR1 all animals showed a strain-specific decrease of CD3+ and CD4+ T cells at day 60 of life while the LEW.1WR1 and LEW rats showed only small changes in the T cell frequency over the lifetime (c). (d) = diabetic, (nd) = non-diabetic.
Besides heterogeneity, LEW.1AR1-iddm rats exhibited two subtypes. In the first subtype, analogous time–courses were recorded for all subsets of CD3+, CD4+ and CD8+ T cells both in the diabetic and non-diabetic LEW.1AR1-iddm rats (graphs for a representative animal in the first column of Fig. 6a,b). In the other subtype, CD3+ and CD4+ T cell frequencies followed an analogous time–course, whereas the changes of the CD8+ T cells followed an independent time–course (graphs for a representative animal each in the second and third columns of Fig. 6a,b, respectively).
The T cell frequency in the LEW.1AR1 background strain was more stable in comparison with the LEW.1AR1-iddm strain during the observation period, with a drop of CD3+ and CD4+ T cells only at day 60 (graph for a representative animal in the first column of Fig. 6c).
The stable course in the LEW.1WR1 strain exhibited only a small peak at day 65, whereas in the LEW strain the time–course in the T cell frequency was constant (graphs for a representative animal each in the second and third column of Fig. 6c).
Discussion
The present study presents for the first time, to our knowledge, a longitudinal analysis of the immune cell frequencies involved in T1D development. Our findings show clearly that the course of the T cell frequency from days 30 to 90 of life follows a strain-specific pattern, while a variable T cell frequency is unique for the LEW.1AR1-iddm rat compared to all other rat strains of this study. In addition, the frequency of NK cells and B cells was variable only in the LEW.1AR1-iddm rat. Differences in the monocyte frequency could not be observed between all the strains due to a generally high variability.
The average frequency over time of the CD3+ T cells, especially CD4+ T cells, was decreased significantly within the LEW.1AR1-iddm strain compared with the LEW.1AR1, LEW.1WR1 and LEW strains. These findings are in agreement with our recently published results 8. The variability of the frequency of the CD3+, CD4+ and CD8+ T cells in peripheral blood is obviously unique for the LEW.1AR1-iddm strain. Neither its control strain LEW.1AR1 nor the LEW.1WR1 and the LEW strains showed significant variations in T cell frequency. A stable T cell frequency could not only be shown in the congenic strains with the LEW background, but also in the genetically different strains BN and PAR 8. The BBdp rat, another rat model of T1D, was characterized by a severe reduction of peripheral T cells 16. In contrast to the mild reduction of T cells in LEW.1AR1-iddm rats CD8+ T cells were more affected than CD4+ T cells in the BBdp rats 16–18. Reports about the Komeda rat described changes of the regulation in T cell activation, but nothing is known about the T cell frequency in peripheral blood 19.
In humans, several studies provide evidence for changes of the frequency of T cells in peripheral blood at the time of diagnosis of T1D and during ongoing autoimmunity when compared to healthy controls 20–22. However, there is still controversy about the T cell subpopulations that are increased or decreased 23–28. Interestingly, some of these studies from patients with T1D reported a decrease of the CD3+ and CD4+ T cells which was similar to that in the LEW.1AR1-iddm rat 25–28.
As shown previously, changes in the different T cell frequencies in the LEW.1AR1-iddm rat had a direct effect on the CD4+ : CD8+ T cell ratio 8. The strong variabilities led to individual changes of the CD4+ : CD8+ T cell ratio over time in each LEW.1AR1-iddm rat. Changes in the CD4+ : CD8+ T cell ratio were also observed in human studies on patients with T1D during the prediabetic and diabetic period 25,26,28,29.
Regarding the immune cell repertoire, the average frequency of the NK cells was increased significantly in both non-diabetic and diabetic LEW.1AR1-iddm rats compared to LEW.1AR1, LEW.1WR1 and LEW rats. Only diabetic LEW.1AR1-iddm rats exhibited a significantly higher variability of the NK cell frequency. An increased number of NK cells was also observed in acutely diabetic BBdp rats 30. Several studies on NK cells in diabetic patients reported a decreased NK cell frequency at diabetes onset, but also during disease progression 27,31,32. Thus, a dependence on diabetes duration seems not to exist. One study considered that these abnormalities could be persistent or genetically determined 33 but the opposite finding, with an increase of NK cells at diabetes onset, has also been reported 34. During diabetes development in the LEW.1AR1-iddm rat, NK cells were found in the immune cell infiltrate with a frequency of <10% in severely infiltrated islets along with a high number of apoptotic ß cells 2. Thus, NK cells are involved in the process of ß cell destruction in the LEW.1AR1-iddm rat 2.
In contrast to the reduction of the T cells, the average of the B cell frequency over time was increased significantly in peripheral blood of non-diabetic and diabetic LEW.1AR1-iddm rats. The B cells were somewhat more increased and showed a higher variability in the non-diabetic LEW.1AR1-iddm rats than in the diabetic rats. The functional implications of the B cell increase in non-diabetic LEW.1AR1-iddm rats compared to diabetic rats remain unknown. The same pattern of an increased B cell frequency was evident in LEW.1WR1 rats. Interestingly, in the BBdp rat, in spite of T cell lymphopenia, the frequency of B cells in peripheral blood as well as in the control rat strains was in a normal range 16,35. Monocytes were the only immune cell subpopulation without differences between the rat strains studied.
Although all strains in this study are inbred strains maintained under the same environmental conditions as the LEW.1AR1-iddm rats, all analysed immune cell subpopulations, with the exception of the monocytes in peripheral blood, showed great abnormalities and variability.
All immune cell types of the innate and adaptive immune system might be involved in the pathogenesis of autoimmune diabetes 36–38. This has been proposed for monocytes/macrophages, T cells and B cells 39–42. However, the interactions of these immune cell populations during diabetes development remain unknown.
The LEW.1AR1-iddm rat arose through a spontaneous mutation within the background strain LEW.1AR1. Therefore, all diabetic and non-diabetic LEW.1AR1-iddm rats carry the mutation leading to a variable immune cell frequency in peripheral blood (100%), with only 60% of these rats developing diabetes. This variability of the immune cell frequencies might induce imbalances between autoaggressive and regulatory T cell populations leading to diabetes manifestation. The mutation of the LEW.1AR1-iddm rat on RNO1 affected the frequencies of CD3+ T cells, B cells and NK cells in peripheral blood in an opposite fashion. The average number of CD3+ T cells, both CD4+ and CD8+ T cells, was decreased strongly in all LEW.1AR1-iddm rats, both in diabetic and non-diabetic rats, while the B cells and NK cells were slightly increased. Currently, it is still unknown whether the changes in all immune cell subpopulations are a direct result of the mutation or whether some of the changes may also be indirect compensatory changes.
In the present study, immune cells from the innate and adaptive immune system were analysed in peripheral blood of LEW.1AR1-iddm rats. Our findings revealed changes of all immune cell types except for monocytes. These alterations of the immune cell frequencies might be a prerequisite for the imbalance, especially between autoreactive and regulatory T cells, resulting in an autoimmune attack upon the pancreatic ß cells.
Acknowledgments
This work was supported by a grant from the European Union (Collaborative Project NAIMIT in the 7th Framework Programme, Contract no. 241447) to S. L. and from the Deutsche Forschungsgemeinschaft (JO 395/2-1) to A. J. The technical assistance of D. Lischke, M. Jentzsch, M. Meyer and S. Eghtessadi is gratefully acknowledged.
Disclosures
None.
Author contributions
T. A. and A. J. performed the research; D. W., S. L. and H.-J. H. designed the research study; T. A. and A. J. analysed the data; all authors wrote the paper.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. The CD4+ : CD8+ T cell ratio was followed from day 30 until day 90 of life for each single animal. In diabetic and non-diabetic LEW.1AR1-iddm rats the ratio was more variable in the LEW.1AR1-iddm strain, independent of diabetes development (a,b). In the LEW.1AR1 strain there was a strain-specific decrease at day 60 of life (c). The LEW.1WR1 and LEW rats showed only small changes in the ratio over the lifetime (c). (d) = diabetic; (nd) = non-diabetic.
References
- 1.Lenzen S, Tiedge M, Elsner M, et al. The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia. 2001;44:1189–1196. doi: 10.1007/s001250100625. [DOI] [PubMed] [Google Scholar]
- 2.Jörns A, Günther A, Hedrich HJ, Wedekind D, Tiedge M, Lenzen S. Immune cell infiltration, cytokine expression, and beta-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes. 2005;54:2041–2052. doi: 10.2337/diabetes.54.7.2041. [DOI] [PubMed] [Google Scholar]
- 3.Jörns A, Arndt T, Meyer zu Vilsendorf A, et al. Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW.1AR1-iddm rat and humans with type 1 diabetes. Diabetologia. 2013;57:512–521. doi: 10.1007/s00125-013-3125-4. [DOI] [PubMed] [Google Scholar]
- 4.Arndt T, Wedekind D, Weiss H, et al. Prevention of spontaneous immune-mediated diabetes development in the LEW.1AR1-iddm rat by selective CD8+ T cell transfer is associated with a cytokine shift in the pancreas-draining lymph nodes. Diabetologia. 2009;52:1381–1390. doi: 10.1007/s00125-009-1348-1. [DOI] [PubMed] [Google Scholar]
- 5.Wedekind D, Weiss H, Jörns A, Lenzen S, Tiedge M, Hedrich HJ. Effects of polyinosinic–polycytidylic acid and adoptive transfer of immune cells in the LEW.1AR1-iddm rat and in its coisogenic LEW.1AR1 background strain. Autoimmunity. 2005;38:265–275. doi: 10.1080/08916930500114321. [DOI] [PubMed] [Google Scholar]
- 6.Weiss H, Bleich A, Hedrich HJ, et al. Genetic analysis of the LEW.1AR1-iddm rat: an animal model for spontaneous diabetes mellitus. Mamm Genome. 2005;16:432–441. doi: 10.1007/s00335-004-3022-8. [DOI] [PubMed] [Google Scholar]
- 7.Weiss H, Arndt T, Jörns A, et al. The mutation of the LEW.1AR1-iddm rat maps to the telomeric end of rat chromosome 1. Mamm Genome. 2008;19:292–297. doi: 10.1007/s00335-008-9102-4. [DOI] [PubMed] [Google Scholar]
- 8.Arndt T, Jörns A, Weiss H, et al. A variable CD3+ T-cell frequency in peripheral blood lymphocytes associated with type 1 diabetes mellitus development in the LEW.1AR1-iddm rat. PloS One. 2013;8:e64305. doi: 10.1371/journal.pone.0064305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 2004;45:278–291. doi: 10.1093/ilar.45.3.278. [DOI] [PubMed] [Google Scholar]
- 10.Yokoi N, Hidaka S, Tanabe S, et al. Role of major histocompatibility complex class II in the development of autoimmune type 1 diabetes and thyroiditis in rats. Genes Immun. 2012;13:139–145. doi: 10.1038/gene.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mordes JP, Guberski DL, Leif JH, et al. LEW.1WR1 rats develop autoimmune diabetes spontaneously and in response to environmental perturbation. Diabetes. 2005;54:2727–2733. doi: 10.2337/diabetes.54.9.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunstýr I. Diagnostic microbiology for laboratory animals. Stuttgart: Gustav Fischer Verlag; 1992. [Google Scholar]
- 13.Hedrich HJ. Genetic monitoring of inbred strains of rat. Stuttgart: Gustav Fischer Verlag; 1990. [Google Scholar]
- 14.Wedekind D, Reifenberg K, Hedrich HJ. Genetic monitoring of inbred strains of mice. In: Hedrich H, editor. The laboratory mouse. New York: Academic Press Elsevier; 2012. pp. 621–637. [Google Scholar]
- 15.Jörns A, Rath KJ, Terbish T, et al. Diabetes prevention by immunomodulatory FTY720 treatment in the LEW.1AR1-iddm rat despite immune cell activation. Endocrinology. 2010;151:3555–3565. doi: 10.1210/en.2010-0202. [DOI] [PubMed] [Google Scholar]
- 16.Elder ME, Maclaren NK. Identification of profound peripheral T lymphocyte immunodeficiencies in the spontaneously diabetic BB rat. J Immunol. 1983;130:1723–1731. [PubMed] [Google Scholar]
- 17.Woda BA, Like AA, Padden C, McFadden ML. Deficiency of phenotypic cytotoxic-suppressor T lymphocytes in the BB/W rat. J Immunol. 1986;136:856–859. [PubMed] [Google Scholar]
- 18.Hernandez-Hoyos G, Joseph S, Miller NG, Butcher GW. The lymphopenia mutation of the BB rat causes inappropriate apoptosis of mature thymocytes. Eur J Immunol. 1999;29:1832–1841. doi: 10.1002/(SICI)1521-4141(199906)29:06<1832::AID-IMMU1832>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Yokoi N. Identification of a major gene responsible for type 1 diabetes in the Komeda diabetes-prone rat. Exp Anim. 2005;54:111–115. doi: 10.1538/expanim.54.111. [DOI] [PubMed] [Google Scholar]
- 20.Richens ER, Jones WG. T-lymphocyte subpopulations in type I diabetes mellitus. A longitudinal study. Acta Diabetol Lat. 1985;22:229–238. doi: 10.1007/BF02590774. [DOI] [PubMed] [Google Scholar]
- 21.Pontesilli O, Chase HP, Carotenuto P, Herberger MJ, Hayward AR. T lymphocyte subpopulations in insulin-dependent (type I) diabetes mellitus. Clin Exp Immunol. 1986;63:68–72. [PMC free article] [PubMed] [Google Scholar]
- 22.Legendre CM, Schiffrin A, Weitzner G, Colle E, Guttmann RD. Two-color flow cytometry analysis of activated T-lymphocyte subsets in type I diabetes mellitus. Diabetes. 1988;37:792–795. doi: 10.2337/diab.37.6.792. [DOI] [PubMed] [Google Scholar]
- 23.Petersen LD, Duinkerken G, Bruining GJ, van Lier RA, de Vries RR, Roep BO. Increased numbers of in vivo activated T cells in patients with recent onset insulin-dependent diabetes mellitus. J Autoimmun. 1996;9:731–737. doi: 10.1006/jaut.1996.0095. [DOI] [PubMed] [Google Scholar]
- 24.Peakman M, Warnock T, Vats A, et al. Lymphocyte subset abnormalities, autoantibodies and their relationship with HLA DR types in children with type 1 (insulin-dependent) diabetes and their first degree relatives. Diabetologia. 1994;37:155–165. doi: 10.1007/s001250050087. [DOI] [PubMed] [Google Scholar]
- 25.Al-Sakkaf L, Pozzilli P, Bingley PJ, et al. Early T-cell defects in pre-type 1 diabetes. Acta Diabetol. 1992;28:189–192. doi: 10.1007/BF00778996. [DOI] [PubMed] [Google Scholar]
- 26.Faustman D, Eisenbarth G, Daley J, Breitmeyer J. Abnormal T-lymphocyte subsets in type I diabetes. Diabetes. 1989;38:1462–1468. doi: 10.2337/diab.38.11.1462. [DOI] [PubMed] [Google Scholar]
- 27.Herold KC, Huen A, Gould L, Traisman H, Rubenstein AH. Alterations in lymphocyte subpopulations in type 1 (insulin-dependent) diabetes mellitus: exploration of possible mechanisms and relationships to autoimmune phenomena. Diabetologia. 1984;27(Suppl.):102–105. doi: 10.1007/BF00275660. [DOI] [PubMed] [Google Scholar]
- 28.Galluzzo A, Giordano C, Rubino G, Bompiani GD. Immunoregulatory T-lymphocyte subset deficiency in newly diagnosed type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1984;26:426–430. doi: 10.1007/BF00262214. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Fikrig SM, Khanna S, Orti E. Deficiency of suppressor T-cells in insulin-dependent diabetes mellitus: an analysis with monoclonal antibodies. Immunol Lett. 1982;4:289–294. doi: 10.1016/0165-2478(82)90054-2. [DOI] [PubMed] [Google Scholar]
- 30.Woda BA, Biron CA. Natural killer cell number and function in the spontaneously diabetic BB/W rat. J Immunol. 1986;137:1860–1866. [PubMed] [Google Scholar]
- 31.Rodacki M, Svoren B, Butty V, et al. Altered natural killer cells in type 1 diabetic patients. Diabetes. 2007;56:177–185. doi: 10.2337/db06-0493. [DOI] [PubMed] [Google Scholar]
- 32.Negishi K, Waldeck N, Chandy G, et al. Natural killer cell and islet killer cell activities in type 1 (insulin-dependent) diabetes. Diabetologia. 1986;29:352–357. doi: 10.1007/BF00903343. [DOI] [PubMed] [Google Scholar]
- 33.Hussain MJ, Alviggi L, Millward BA, Leslie RD, Pyke DA, Vergani D. Evidence that the reduced number of natural killer cells in type 1 (insulin-dependent) diabetes may be genetically determined. Diabetologia. 1987;30:907–911. doi: 10.1007/BF00295872. [DOI] [PubMed] [Google Scholar]
- 34.Scheinin T, Maenpaa J, Kontiainen S. Immune responses to insulin and lymphocyte subclasses at diagnosis of insulin-dependent diabetes and one year later. Immunobiology. 1990;180:431–440. doi: 10.1016/s0171-2985(11)80304-9. [DOI] [PubMed] [Google Scholar]
- 35.Ellerman KE, Like AA. Islet cell membrane antigens activate diabetogenic CD4+ T-cells in the BB/Wor rat. Diabetes. 1999;48:975–982. doi: 10.2337/diabetes.48.5.975. [DOI] [PubMed] [Google Scholar]
- 36.Peakman M. Immunological pathways to beta-cell damage in Type 1 diabetes. Diabet Med. 2013;30:147–154. doi: 10.1111/dme.12085. [DOI] [PubMed] [Google Scholar]
- 37.Eisenbarth GS. 2010. Online Edition version 3.0. Available at: http://www.ucdenver.edu/academics/colleges/medicalschool/centers/BarbaraDavis/OnlineBooks/Pages/Type1Diabetes.aspx (accessed 23 April 2014)
- 38.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Chatenoud L. Immune therapy for type 1 diabetes mellitus – what is unique about anti-CD3 antibodies? Nat Rev Endocrinol. 2010;6:149–157. doi: 10.1038/nrendo.2009.275. [DOI] [PubMed] [Google Scholar]
- 40.Chatenoud L. Therapeutic targeting of B cells and T cells in autoimmune diabetes: is it a solution? Diabetes. 2013;62:2659–2661. doi: 10.2337/db13-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietropaolo M, Towns R, Eisenbarth GS. Humoral autoimmunity in type 1 diabetes: prediction, significance, and detection of distinct disease subtypes. Cold Spring Harbor Perspect Med. 2012;2:a012831. doi: 10.1101/cshperspect.a012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zipris D. Innate immunity and its role in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:326–331. doi: 10.1097/MED.0b013e3283073a46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The CD4+ : CD8+ T cell ratio was followed from day 30 until day 90 of life for each single animal. In diabetic and non-diabetic LEW.1AR1-iddm rats the ratio was more variable in the LEW.1AR1-iddm strain, independent of diabetes development (a,b). In the LEW.1AR1 strain there was a strain-specific decrease at day 60 of life (c). The LEW.1WR1 and LEW rats showed only small changes in the ratio over the lifetime (c). (d) = diabetic; (nd) = non-diabetic.