Abstract
In different bioassays, functional antibodies reacting with the human muscarinic acetylcholine receptor M3(mAchR3) have been detected in sera from patients with Sjögren's syndrome (SS), and there is strong evidence that those antibodies may have pathogenetic relevance. However, depending on the method of detection, their prevalence varied. Furthermore, those bioassays are difficult to standardize. We report on the development and optimization of a novel test system based on a luminometric method to determine downstream signalling of mAchR3 which produces specific and reproducible results. Chinese hamster ovarian (CHO) cells were transfected with plasmids encoding mAchR3 and a green fluorescence protein (GFP)/aequorin fusion protein. Incubation of cells with carbachol resulted in an increase in intracellular [Ca2+], which was detected by measuring light emission with a luminometer, and the effect of incubation with patients' immunoglobulins (Ig) was evaluated. Optimal cell density, Ig preparation and time of incubation with patients' sera were determined. Sera from patients with primary Sjögren's syndrome (pSS; n = 40), systemic sclerosis (SSc; n = 47), myasthenia gravis (MG; n = 133) and 50 blood donors were analysed. Optimal assay conditions were obtained with a cell density of 100 000 cells/ml, isolation of Ig by ammonium sulphate precipitation and short-term incubation. Based on this highly reliable assay, 50% of the pSS patients had antibodies which inhibited carbachol-induced activation of mAchR3; none of the SSc patients, 6% of the patients with MG and 12% of the blood donors had antibodies which reacted with the mAchR3. This method facilitates the determination of functional anti-mAchR3 antibodies in patients' sera, confirmed their high prevalence in pSS patients and may, therefore, help to analyse their pathogenetic and clinical relevance in more detail.
Keywords: aequorin/GFP, anti-mAchR3 antibodies, CHO cells, luminometer, Sjögren's disease
Introduction
The existence of functional autoantibodies reacting with cell surface receptors and thereby inducing clinical symptoms is well known, and has been described especially in organ-specific autoimmune disorders such as Graves' disease, myasthenia gravis or idiopathic cardiomyopathy 1–3. However, in recent years it has emerged that in systemic autoimmune disorders functional antibodies also occur, which may help to explain at least some of their clinical symptoms. For instance, in patients with systemic sclerosis (SSc), antibodies to the platelet-derived growth factor-receptor on human fibroblasts or in patients with primary Sjögren's syndrome (pSS) autoantibodies to the muscarinic acetylcholine receptors, especially of the M3-type (mAchR3), have been described 4–6. Muscarinic receptors are expressed on the surface of salivary acinar glands 6,7, and there is now convincing evidence that perturbation of the muscarinic receptor function by the presence of those antibodies accounts in large part for the glandular hypofunction, but also for some of the reported extraglandular features of pSS 4,8–15.
The mAchR group consists of five subtypes (m1–5), which are encoded by different genes. Subtypes 1, 3 and 5 are coupled to G-proteins of the Gq/G11 family leading after activation to phospholipase Cβ and inositol-1,4,5-trisphosphate-mediated increases in intracellular free Ca2+ 16–20.
Antibodies to mAchR3 have been demonstrated by different methods. The ‘gold standard’ for their detection have been bioassays using the inhibition of smooth muscle from bladder or colon as a detection system 8,15,21–24. Binding studies with radioactively labelled ligands have also been performed to determine the potency and efficacy of autoantibodies with regard to their ability to inhibit binding of ligands to mAchR3 4,25,26. Other authors used pharmacological systems such as microfluorimetric assays measuring agonist-evoked changes in [Ca2+] influx in human salivary gland cells or Chinese hamster ovary (CHO) cells transfected with human mAchR3 cDNA 10,11,27–30. The existence of anti-mAchR3 antibodies in patients with primary or secondary SS was confirmed with all these methods, but the reported prevalences varied from 40 to 100% of patients, and the number of patients analysed was low.
For examining the role of functional autoantibodies towards the mAchR3 with respect to pathogenesis and prognosis of a disease, reproducible ‘high-throughput’ assays would be essential, but several factors limit the application of bioassays to large populations. These include their time-consuming nature, the presence of other inhibitory substances (for instance drugs) in patients' sera, and low sensitivity, reproducibility and validity as well as their susceptibility to interference 31.
Therefore, in further approaches, immunodominant epitopes within the mAchR3 have been identified and applied in enzyme-linked immunosorbent assays (ELISA) 25,26,28,29,32–40. However, it soon became evident that the functional antibodies are directed against conformational epitopes, so that no correlation was observed with assays using linear epitopes or recombinant antigens 21,29,40.
In this study we present a novel test system for the demonstration of functional anti-mAchR3 antibodies resembling a microfluorometric assay while addressing several of the above-mentioned shortcomings. Thus, we used CHO cells transfected with the mAchR3 gene and a calcium-sensitive bioluminescent fusion protein consisting of aequorin and green fluorescence protein (GFP) 41,42. This mimics a phenomenon in nature, where aequorin occurs in the luminiferous organs of the jellyfish Aequora Victoria in association with GFP 43,44. The intermolecular distances of these two proteins allow radiationless energy transfer to GFP in a process known as bioluminescence resonance energy transfer (BRET) 44,45. Ca2+ released by mAchR3 activation in CHO cells forms a complex with aequorin, leading to the emission of blue light; this stimulates GFP to emit green light (509 nm) which can then be measured luminometrically.
With this test system we wanted to confirm the presence of functional anti-mAchR3 antibodies in pSS, and we wanted to determine whether they may occur also in sera from patients with other disorders known to be associated with antibodies affecting membrane receptors.
Material and methods
Patients
Sera from 40 patients with primary Sjögren's syndrome (pSS; 38 females, two males: mean age 56 years, range 31–77 years) were analysed. Diagnosis was based on the typical clinical manifestations of sicca syndrome, a positive Schirmer's test, elevation of erythrocyte sedimentation rates and immunoglobulin G (IgG), the presence of rheumatoid factor and of anti-SSA and/or anti-SSB antibodies, according to the criteria of the American College of Rheumatology 46. Twenty of the patients were still untreated at time of analysis; the remaining 20 were under low-dose steroids. As disease controls, sera from 47 patients with untreated systemic sclerosis (SSc; 42 females, five males; mean age 52 years, range 18–88 years) who all fulfilled the 2013 classification criteria for systemic sclerosis were included 47. All patients with pSS and SSc had been seen by one of the authors (J. H. or R. K.). Sera were obtained for diagnostic purposes. The study was approved by the local ethics committee and was performed in accordance with the Helsinki declaration.
Furthermore, sera from 50 patients with early-onset myasthenia gravis (EOMG), 33 patients with late-onset myasthenia gravis (LOMG) and 50 patients with thymoma were analysed. The characteristics of these patients have been published in previous studies 48,49.
Sera from 50 healthy blood donors (40 females, 10 males; mean age 51 years, range 37–62 years) were kindly provided by Dr D. Wernet (Department of Transfusion Medicine, University of Tuebingen). All sera had been stored at −20°C.
Plasmid DNA purification
The high-copy plasmid pcDNA 3·1(+) (Invitrogen, Carlsbad, CA, USA) harbouring the complete cDNA of the human mAchR3 was obtained from the Guthrie cDNA Resource Center (Rolla, MI, USA). It was propagated in OneShot Top 10 Escherichia coli (Invitrogen) and plasmid DNA purification was performed according to the manufacturer's protocol using a commercially available kit (Qiagen, Hilden, Germany).
Cell culture
CHO-K1 (Chinese hamster ovary) cells were stably transfected with a calcium-sensitive bioluminescent fusion protein consisting of aequorin and green fluorescence protein (GFP) 41. The cells were maintained in Ham's F-12 (Gibco, Gaithersburg, MD, USA), supplemented with 10% (v/v) fetal calf serum (FCS; Gibco), 100 units/ml penicillin, 100 μg/ml streptomycin (Invitrogen) and 300 μg/ml G418 sulphate (Biochrom, Berlin, Germany).
Transfection of CHO-K1 cells
The GFP/aequorin-transfected cells were transfected transiently with 0·5 μg/ml mAchR M3 plasmid DNA (Fig. 1) using FuGENE 6 reagent (Promega, Madison, WI, USA) with a FuGENE 6 : DNA ratio of 3:1. The optimal FuGENE 6 : DNA ratio had been determined in previous experiments (data not shown). To determine the optimal cell density, 3000–400 000 transfected cells/ml were seeded onto 96-well plates (Thermo Fisher Scientific, Rockford, IL, USA) and incubated at 37°C. Non-transfected cells served to assess the efficiency of transfection.
Fig. 1.
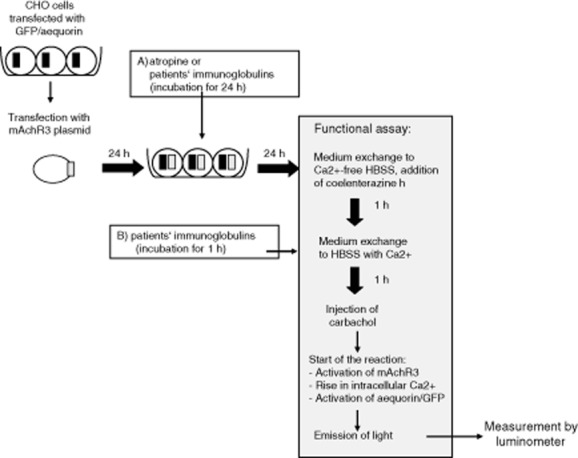
Scheme of the functional assay for the demonstration of functional antibodies to the muscarinic acetycholine receptor type 3 (mAchR3). Chinese hamster ovary cells (CHO) stably transfected with a green fluorescence protein (GFP)/aequorin plasmid were transiently transfected with a mAchR3 plasmid. After 48 h the functional assay was started. (a) Time-point when atropine was added to the inhibition test or when long-term effect of patients' immunoglobulins on the mAchR3 activity was analysed. (b) Time-point when patients' immunoglobulins were added for analysis of their short-term effect on the mAchR3.
Purification of immunoglobulin from patients' serum
Immunoglobulins were isolated from patients' sera by two different methods, namely the Melon Gel IgG purification method and by ammonium sulphate precipitation.
Isolation of immunoglobulins by Melon Gel IgG spin purification
The Melon Gel IgG spin purification kit was obtained from Thermo Fisher Scientific; 50 μl serum were diluted 1:10 in Melon Gel purification buffer and the purification was performed according to the manufacturer's protocol. As indicated by the manufacturer, the gel support of the kit can be regenerated up to three times. To assess whether using regenerated gel supports had an influence on the functional assay, we tested immunoglobulins isolated from fresh gel support or from gel support which had been regenerated three times prior to use. All the immunoglobulin preparations were stored at −20°C.
Ammonium sulphate precipitation of patients' sera
To 300 μl serum an equal amount of a saturated ammonium sulphate solution (76·7 g/100 ml H2O) was added slowly 31,50. After precipitation overnight at 4°C the sample was centrifuged at 5000 g for 30 min. The supernatant was discarded and the precipitate was washed twice with a 60% ammonium sulphate solution and finally dissolved in 300 μl Hanks's balanced salt solution (HBSS). To assess the influence of residual ammonium sulphate in the sample the functional assay was performed with dialysed and non-dialysed samples. Dialysis was performed with HBSS using Amicon Ultra-0·5 centrifugal filter devices (Millipore, Cork, Ireland). All samples were stored at −20°C.
Assay for the determination of mAchR3 functionality
Forty-eight h after transfection, cells were loaded with 5 μM coelenterazine h (Biotium, Hayward, CA, USA) in calcium-free HBSS containing 10 mM HEPES, pH 7·4, for 1 h at 37°C (Fig. 1); 1 h before experiments, the buffer was replaced with HBSS containing 2 mM CaCl2. The mAchR agonist carbachol (10 μM; Sigma-Aldrich, Steinheim, Germany) was added to the cells (final concentration of carbachol: 2 μM). This concentration had been determined by dilution studies prior to the study to give optimal results, as also reported by other research groups using CHO cells 15,19. The change in intracellular [Ca2+] during 20 s was then determined by measuring the emitted light with a 2460 MicroBeta2 LumiJET luminometer (Perkin Elmer, Downers Grove, IL, USA). Measurements were performed in quadruplicate. Results were given as absolute relative light units (RLUs) or – when applying serum immunoglobulins – as a percentage of RLUs without added protein. As a positive control for the validity of the assay, the mAchR antagonist atropine (1% injection solution; Dr F. Köhler Chemie, Bensheim, Germany) was added to the cells 24 h after transfection in final concentrations ranging from 10 ng/ml to 1 mg/ml.
Analysis of the effect of patients' immunoglobulins on the mAchR3 reactivity
Twenty-four h after transfection of the cells or 1 h before injection of carbachol, immunoglobulins isolated from patients' sera either by Melon Gel IgG spin purification or ammonium sulphate precipitation were added to the cells (Fig. 1) to give a final dilution of 1:100 (corresponding to about 0·16 mg/ml). The optimal dilution of the proteins had been determined previously (data not shown).
In order to determine whether the purification buffer itself (according to the manufacturer, 45–65% of the buffer: phosphoric acid, monosodium salt monohydrate, physiological pH) may influence the mAchR3 activity, purification buffer of the Melon Gel IgG spin purification kit was added to the cells to give final dilutions of 1:100, 1:500 or 1:1000.
Western blotting
The purity of the immunoglobulins isolated by the Melon Gel IgG spin purification kit or ammonium sulphate precipitation was analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 4·5% stacking and a 10% running gel 51. Twenty μg of the immunoglobulin preparations were applied to each lane. After electrophoresis, the gels were either stained with Coomassie Blue or the proteins were transferred to nitrocellulose membranes (Amersham Biosciences, Freiburg, Germany) 52. The membranes were incubated with peroxidase-conjugated anti-human IgG, IgA or IgM antibodies (Dakopatts A/S, Copenhagen, Denmark) at a dilution of 1:1000.
Statistics
The results obtained with patients' immunoglobulins were given as the ratio of RLU obtained with carbachol-activated mAchR3-expressing cells incubated with immunoglobulins divided by RLU obtained with these cells without immunoglobulins (multiplied by 100 in order to estimate the percentage).
Normal values for the functional assay were calculated by receiver operating curves (ROC) comparing the reactivity obtained with immunoglobulins from 40 pSS patients with that of the 280 disease and healthy controls, aiming at a specificity of 95%.
The unpaired Mann–Whitney U-test was used for comparison of antibody reactivities between patient groups, and Wilcoxon's test was used for the paired data. Prevalences were compared using Fisher's exact test. P-values < 0·05 were considered statistically significant.
All statistical analyses were performed with spss version 21.
Results
Optimization of cell density
To optimize the assay conditions, transfected CHO-K1 cells were plated out at densities ranging from 625 to 20 000 cells/well and the activity of the mAchR3 after carbachol stimulation was measured. A good discrimination between non-transfected and transfected cells was obtained using 10 000 cells/well (Fig. 2), and this density was therefore used in subsequent experiments.
Fig. 2.
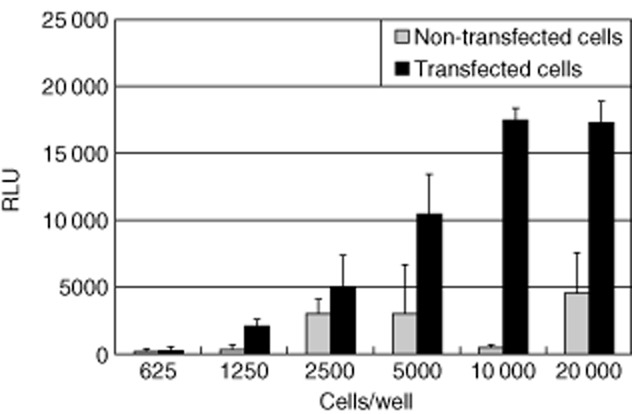
Carbachol-induced changes in [Ca2+]i evoked in Chinese hamster ovary (CHO)-K1 cells expressing human muscarinic acetycholine receptor type 3 (mAchR3). Range of cell density is 625–20 000 cells/well. RLU = relative light units. Shown are mean values (bars) ± standard deviation (s.d.) of four independently performed experiments.
Inhibition of the muscarinic AchR with the antagonist atropine
In a next step we wanted to prove that the reaction observed after incubation of the transfected cells with carbachol was, indeed, related to the enhanced expression of the mAchR3; therefore, the mAchR3 antagonist atropine was added in different concentrations. There was a dose-dependent inhibition of the carbachol-induced [Ca2+] signal with a reduction to 4% of the signal obtained without atropine (Fig. 3).
Fig. 3.
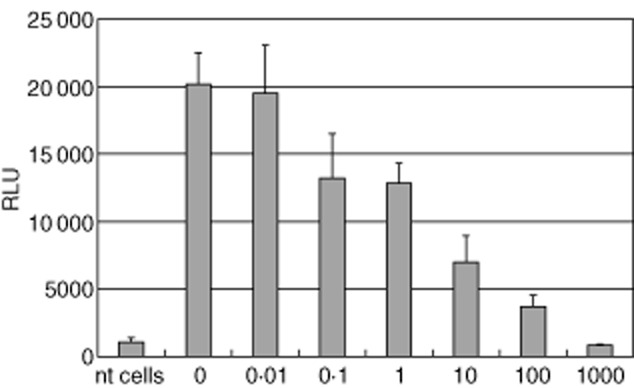
Dose-dependent inhibition of the carbachol-induced [Ca2+]i signal in Chinese hamster ovary (CHO)-K1 cells expressing muscarinic acetycholine receptor type 3 (mAchR3) (10·000 cells/well) by the AchR antagonist atropine (0·01–1000 μg/ml). RLU = relative light units. Shown are mean values (bars) ± standard deviation (s.d.) of four independently performed experiments.
Influence of different kinds of immunoglobulin preparations on mAchR3 reactivity
Immunoglobulins prepared by the Melon Gel IgG spin purification kit
Effect of the immunoglobulin purification buffer
In order to determine whether the purification buffer used for isolation of immunoglobulins from patients' sera by the Melon Gel IgG spin purification kit influences the mAchR3 reactivity, it was added in different concentrations to the transfected CHO cells. There was a significant decrease in the receptor reactivity (Supporting information, Fig. S1) and therefore, for subsequent experiments, buffer exchange towards HBSS was performed prior to use.
Comparison of the effect of immunoglobulins isolated with fresh or regenerated gel support
Immunoglobulins isolated with the Pierce Melon Gel IgG spin purification kit and dialysed against HBSS were then used to analyse whether application of fresh or regenerated gel support from the kit had an impact on the functional assay (immunoglobulins added 24 h after transfection). It became evident that immunoglobulins isolated with regenerated gel support led to generally lower RLUs in the functional assay compared to using fresh gel support (Supporting information, Fig. S2), independent of whether they were derived from patients with Sjögren's syndrome or healthy blood donors. Furthermore, reproducibility of results obtained with regenerated gel support was much worse than with fresh gel support (data not shown).
Immunoglobulins prepared by ammonium sulphate precipitation
Analysis of the purity and immunoglobulin content of fractions obtained by ammonium sulphate precipitation
In order to avoid the high costs and effort associated with the purification of immunoglobulins using the Melon Gel IgG spin purification kit, we investigated whether immunoglobulins prepared by ammonium sulphate (AS) precipitation could be a suitable alternative.
First, the purity of the immunoglobulin fraction obtained by ammonium sulphate precipitation of serum from a healthy donor was analysed by SDS-gel electrophoresis and Western blotting and was compared with immunoglobulins isolated with the Melon Gel IgG spin purification kit (Supporting information Fig. S3). All protein bands in the two fractions visualized by Coomassie staining in the gels (Supporting information Fig. S3a) could be attributed to IgG, IgM or IgA (Supporting information Fig. S3b–d), and no further proteins were detected with this method. Both preparations contained IgG and IgM, while IgA was observed only in the fraction obtained by AS precipitation.
Analysis of the effect of residual ammonium sulphate present in the immunoglobulin fractions on the mAchR3 reactivity
In order to determine whether the residual ammonium sulphate present in the immunoglobulin preparation after precipitation might influence the functional assay, the precipitated proteins from patients' sera were dialysed against HBSS and compared with the results obtained with non-dialysed probes (proteins added 24 h after transfection). There was only a marginal effect, and therefore we used non-dialysed immunoglobulin fractions in further studies (Supporting information Fig. S4).
Comparison of the effect of immunoglobulins isolated by Melon Gel IgG spin purification kit and AS precipitation in the functional mAchR3 assay
Comparing the effect of immunoglobulins prepared by the Melon Gel IgG spin purification kit or AS precipitation from the same probands in the functional mAchR3 assay in five independently performed experiments, it became evident that the median was identical but that the AS precipitation method resulted in much lower variation (Supporting information Fig. S5).
In order to exclude that the inhibitory effect observed with AS-precipitated fractions may be due to other proteins than immunoglobulins which might still be present in the fractions, the same experiments were performed with the supernatants obtained after precipitation of the immunoglobulins. However, these supernatants had no effect on the mAchR3 activity (data not shown).
Considering that (i) the purity of immunoglobulins isolated by Melon Gel IgG spin purification and AS precipitation was similar, (ii) the ammonium sulphate precipitation is much less expensive and time-consuming and (iii) immunoglobulins isolated by both methods produced nearly identical results, but data obtained with AS precipitation were more reproducible, we decided to use the AS precipitation for isolation of immunoglobulins in our further investigations.
Analysis of the effect of immunoglobulins from patients with pSS on functional activity of the mAchR3
This standardized assay, now showing good reproducibility and validity, was applied to determine whether it was able to confirm the presence of anti-mAchR3 antibodies in sera from patients with pSS. Immunoglobulins isolated by ammonium sulphate precipitation from sera of 40 patients with pSS and 20 healthy donors were added to the cells either 1 or 24 h before starting the functional assay. After incubation for 1 h, immunoglobulins from 20 (50%) of the pSS patients inhibited the mAchR3 function; when they were incubated with the cells for 24 h this effect was observed in only three instances (Fig. 4). Differences were statistically highly significant. Of the healthy donors, only one had inhibitory immunoglobulins, and there was no difference whether the immunoglobulins were incubated for 1 or 24 h.
Fig. 4.
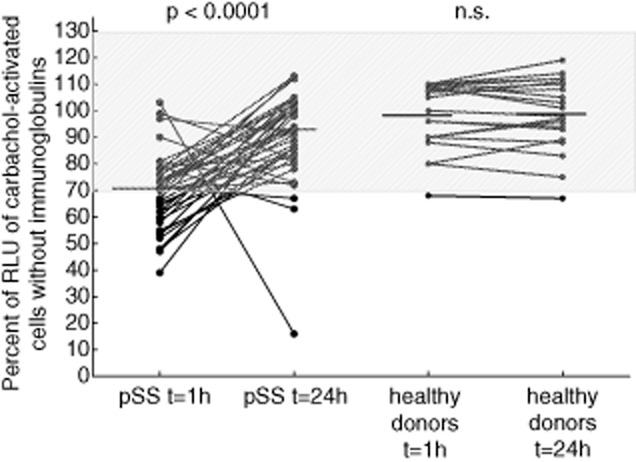
Carbachol-induced [Ca2+]i signal in the functional assay using ammonium sulphate (AS)-precipitated immunoglobulins from sera of 20 healthy donors and 40 patients with Sjögren's syndrome. Proteins were added 1 h or 24 h before measurement to the cells. Data are given as percentage of the results without immunoglobulins. Each data-point represents the mean of four independently performed experiments per patient. Bar indicates median value. Comparison was performed with Wilcoxon's matched-pairs test.
For these experiments, results obtained with probands' immunoglobulins were given as the percentage of RLU obtained with transfected carbachol-stimulated cells without immunoglobulins (see Methods) in order to compensate for the probable differences in transfection efficacy in different experiments. As determined by ROC analysis (Supporting information Fig. S6) comparing the 40 pSS patients and the 230 disease and healthy controls, values ≤ 70% were defined as inhibitory effect on the mAchR3, revealing a test specificity of 95%. Values ≥ 130% were defined as stimulatory.
Comparison of the effect of immunoglobulins from pSS patients and controls on the functional activity of mAchR3
Comparing the inhibitory activity of immunoglobulins from the 40 pSS patients with that from patients with other disorders known to be accompanied by different functional autoantibodies, such as systemic sclerosis or myasthenia gravis, it became evident that it was significantly stronger in the pSS immunoglobulins than in the other disorders or in healthy controls (Fig. 5).
Fig. 5.
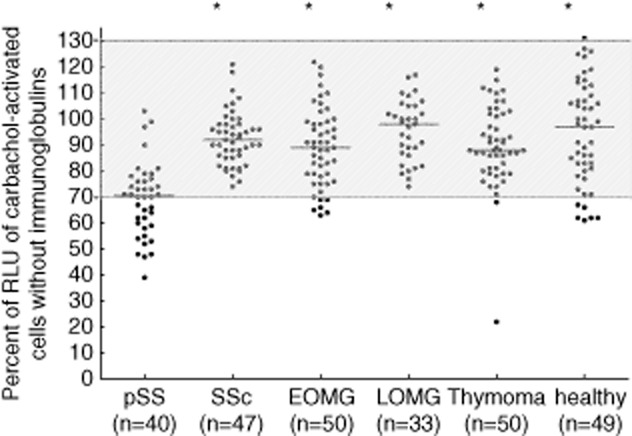
Carbachol-induced [Ca2+]i signal in the functional assay using ammonium sulphate (AS)-precipitated immunoglobulins from sera from patients with primary Sjögren's syndrome (pSS), disease controls and healthy controls. Proteins were added 1 h before measurement to the cells. Data are given as percentage of the results without immunoglobulins. Each data-point represents the mean of four independently performed experiments per patient. Bar indicates median value. Comparison was performed using the Mann–Whitney U-test. *P ≤ 0·001 versus patients with pSS. Stimulatory activity was defined as ≥ 130%, inhibitory activity as ≤ 70%.  = no effect on receptor activity; SSc = systemic sclerosis; EOMG = early-onset myasthenia gravis; LOMG = late-onset myasthenia gravis.
= no effect on receptor activity; SSc = systemic sclerosis; EOMG = early-onset myasthenia gravis; LOMG = late-onset myasthenia gravis.
Based on the normal range of 70–130% (see above), 50% of the pSS patients but only up to 12% of patients with other disorders or healthy controls showed inhibitory antibodies (P < 0·0001, Table 1).
Table 1.
Prevalence of inhibitory and stimulatory anti-human muscarinic acetycholine receptor type 3 (mAchR3) antibodies in patients with different disorders known to be associated with functional autoantibodies
| Stimulatory antibodies (≥ 130%) | Inhibitory antibodies (≤ 70%) | ||
|---|---|---|---|
| Diseases | No. patients tested | Number (%) positive | |
| primary Sjögren's syndrome | 40 | 0 | 20 (50) |
| Systemic sclerosis | 47 | 0 | 0 |
| EOMG | 50 | 0 | 6 (12) |
| LOMG | 33 | 0 | 0 |
| Thymoma | 50 | 0 | 2 (4) |
| Blood donors | 50 | 1 (2) | 6 (12) |
EOMG = early-onset myasthenia gravis; LOMG = late-onset myasthenia gravis.
Analysis of the effect of therapy on anti-mAchR3 activity in sera from pSS patients
Twenty of the pSS patients were under low-dose steroid therapy; 20 patients did not receive any therapy at time of analysis. Comparing the anti-mAchR3 reactivity in these two groups, there was no significant difference (Fig. 6).
Fig. 6.
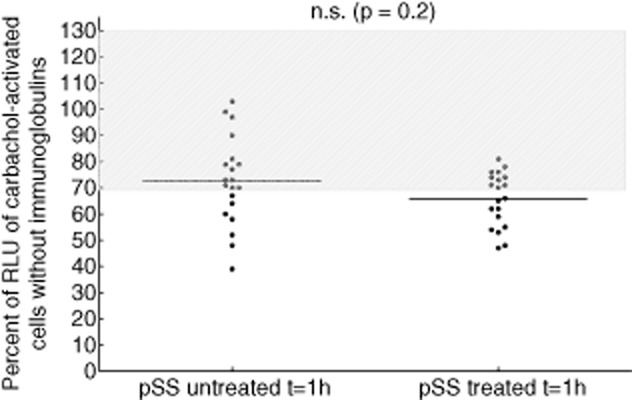
Carbachol-induced [Ca2+]i signal in the functional assay comparing ammonium sulphate (AS)-precipitated immunoglobulins from sera from patients with untreated (n = 20) and treated (n = 20) primary Sjögren's syndrome (pSS).  = no effect on receptor activity. Median values are given. Comparison was performed using the Mann–Whitney U-test.
= no effect on receptor activity. Median values are given. Comparison was performed using the Mann–Whitney U-test.
Discussion
The ability to perform reliable in-vitro inhibition or stimulation assays is a prerequisite for the evaluation of patients with autoimmune disorders for functional antibodies; for instance, those directed against membrane receptors. In this study we have developed and validated a sensitive high-throughput assay for the demonstration of antibodies reacting with the mAchR3. Using this bioassay, inhibitory antibodies were found in 50% of patients with pSS, resembling the prevalence reported by other groups 8,21,23,28,30; there was no difference, whether or not patients were treated. The inhibitory activity and prevalence of these antibodies were significantly higher with the immunoglobulins from pSS patients than with immunoglobulins from patients with other disorders known to be associated with anti-receptor antibodies, such as myasthenia gravis or systemic sclerosis. Inhibitory reactivity values below 70% were determined based on the analysis of all controls by ROC resulting in a specificity of 95%.
The test system presented is based on a pharmacological assay used to evaluate the effect of different substances on mAchR3 19,53,54, and further development of the microfluorometric assays which have been described for the detection of anti-mAchR3 antibodies 10,11,27–30. CHO cells stably transfected with aequorin/GFP were additionally (transiently) transfected with an mAchR3-encoding plasmid which resulted in heterologous expression of the receptor. Although it is not necessarily an advantage to use transiently transfected cells, stable transfection of cells with G-protein-coupled receptors can result in the long-term desensitization of signalling pathways downstream of the receptor or loss of receptor expression. Thus, regular tests for receptor expression and functionality of downstream signalling pathways are required. We therefore decided to perform transient transfections of CHO cells, which are technically easy and result in reliable receptor expression. The effect of mAchR3 agonists or antagonists – and in the present study of patients' immunoglobulins – leading to an alteration of intracellular Ca2+ concentrations with the consequence of a conformational change of aequorin, the emission of blue light and stimulation of GFP to emit green light can be measured luminometrically. The development of bioassays for the demonstration of functional antibodies for routine diagnostic purposes is often hampered by the fact that they are somewhat susceptible to problems and difficult to standardize. Thus, the application of smooth muscles from bladder or colon as a detection system, as described in recent studies, depends upon the quality, amount of excitation and number of muscle cells in those muscle stripes and may vary from one animal to another 8,15,21–24. The application of mAchR3-transfected CHO cells as shown in the present study allows better standardization by using defined and approved transfection protocols, defined cell numbers and uniform environmental conditions. CHO cells were among the first cell lines established for in-vitro cultivation, and their popularity as a host for the production and manufacturing of biological products is still undiminished and undisputed 55. The advantage of aequorin/GFP fusion protein over Fura-1/AM (and other fluorescent calcium indicators) as used in other microfluorometric assays is its huge sensitivity, the absence of background and assay stability 41. Moreover, these fluorescent indicators must be cleaved by the intracellular esterases in order to be active. Some cells have higher levels of esterases than others. Furthermore, the fluorescent dye tends to leak out of cells and thus the assay must be performed within 2–3 h.
The concept that antibodies in sera from pSS patients may have been responsible for the inhibition of receptor activity was proved by using immunoglobulins purified from serum. In a first step the immunoglobins were isolated from the sera using the Melon Gel IgG spin purification kit based on the adsorption of immunoglobulins to coated gel resins. However, in applying these fractions to the functional assay, the results showed great variations, and the buffer necessary for their isolation strongly influenced the CHO cells. These disadvantages could be diminished using only freshly prepared gels for each serum sample and not regenerated gel supports, as suggested by the manufacturer, and dialysing the immunoglobulins prior to further application. However, this rendered this method somewhat costly and time-consuming. We therefore decided to test whether the ‘ancient’ method for separation of serum proteins, namely ammonium sulphate precipitation 56, could be a more suitable and cheaper alternative for the chromatographic method. Indeed, comparing immunoglobulins prepared by both methods and analysing their effects on the mAchR3 reactivity on CHO cells, similar results were obtained with respect to their inhibitory or stimulatory potency, with the advantage of lower variations with the AS-precipitated immunoglobulins. A further advantage over the Melon Gel IgG spin purification kit was that the AS-precipitated immunoglobulin fractions contained all three immunoglobulin classes, while after gel chromatographical purification IgGs were predominantly present and, to a lower extent, IgMs, while IgA was lacking. This may be an important point, as it is well known that autoantibodies are not only of the IgG type but may also belong to the IgA and IgM class 57–62. Of course, it could be argued that the fraction obtained by AS precipitation may contain other proteins besides the immunoglobulins, which could affect receptor function. Although Coomassie blue staining after gel electrophoresis did not reveal further proteins in this fraction, this method may not be sensitive enough to detect small amounts of residual proteins. However, results obtained with the AS-precipitated immunoglobulins were similar to those obtained with the presumably purer fractions obtained by gel chromatography, which is in accordance with observations by other authors analysing patients' sera for functional antibodies 31,50. Furthermore, using the supernatants from these AS-precipitated fractions, which were depleted from immunoglobulins, we did not observe any effect on mAchR3 activity.
Inhibitory or stimulatory effects of immunoglobulins on the mAchR3 may also depend upon the time of their incubation with the M3-expressing cells. Data in this respect are somewhat conflicting in the literature, which may be due to different test systems used in these experiments 23,27. We therefore also performed short-term incubation (1 h) and incubation for 24 h, but found an inhibitory effect of the immunglobulins of pSS patients preferentially when they were added 1 h before starting the functional assay.
Antibodies to the mAchR3 may play an important role in the pathogenesis of different symptoms in pSS, and their determination is therefore of high clinical relevance 4–6,8–15. However, as those functional autoantibodies react with conformational epitopes they cannot be measured simply by ELISA or Western blot using recombinant proteins, or even peptides representing presumably immunodominant epitopes 63–65. Although the bioassay described in this paper may also not be generally available, and restricted to research or specialized laboratories, it represents a reliable and reproducible method which may facilitate the analysis of larger series of patients' sera for inhibitory or stimulatory anti-mAchR3 autoantibodies. Thus, it has been shown that anti-mAchR3 antibodies can also be detected in patients with systemic lupus erythematosus, rheumatoid arthritis and primary biliary cirrhosis 34,36,37,66, all being accompanied by sicca syndromes, the typical clinical manifestation of pSS. However, these analyses had been performed predominantly by ELISA methods using presumably immunodominant epitopes, but not with bioassays, although from preliminary data we have strong evidence that functional – preferentially inhibitory – anti-mAchR3 antibodies can also be observed in patients with autoimmune liver disorders 67.
In conclusion, we have developed a reliable high-throughput assay with good sensitivity for measuring functional autoantibodies to mAchR3 in patients' sera. Application of this assay in a larger series of patients and perhaps clinical trials should facilitate studies with respect to their pathogenetic and probably prognostic relevance, and can also be applied in patients with other disorders/symptoms related to disturbances of the parasympathic nerve system. This may allow the definition of subgroups of patients in whom autoimmune mechanisms may play a role and inaugurate the introduction of new therapeutic regimens.
Disclosure
The authors declare no conflicts of interest.
Author contributions
B. P. performed the experiments, S. T and S. O. provided the plasmids and designed the principle test system, J. H. provided sera and clinical data from patients with pSS and SSc, R. K. designed the study and B. P and R. K. wrote the paper.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Inhibitory effect of the purification buffer (pb) from the Melon Gel immunoglobulin (Ig)G purification kit on the carbachol induced [Ca2+]i signal evoked in Chinese hamster ovary (CHO)-K1 cells expressing human muscarinic acetycholine receptor type 3 (mAchR3) (transfected cells – tc). Final dilution of the pb 1:100, 1:500, 1:1000, respectively. RLU = relative light units. Shown are mean values (bars) ± standard error of the mean (s.e.m.) of four independently performed experiments and comparisons using the Mann–Whitney U-test. *P ≤ 0·05 versus transfected cells without purification buffer.
Fig. S2. Effect of immunoglobulins from blood donors (bd; n = 3) and patients with Sjögren's syndrome (SS; n = 3) isolated with fresh or regenerated gel support from the Melon Gel immunoglobulin (Ig)G spin purification kit on carbachol-induced [Ca2+]i signal evoked in Chinese hamster ovary (CHO)-K1 cells expressing human muscarinic acetycholine receptor type 3 (mAchR3). Shown are relative light units (RLU) [mean values (bars) + standard deviation (s.d.) of four independently performed experiments per patient].
Fig. S3. Sodium dodecyl sulphate (SDS)-gel electrophoresis and Western blotting for the demonstration of immunoglobulin (Ig)G, IgM and IgA immunoglobulins in the immunoglobulin fractions isolated by Melon Gel IgG spin purification kit and ammonium sulphate (AS) precipitation from a serum of a healthy donor. (a) Coomassie staining, (b–d) Western blotting with anti-human horseradish peroxidase (HRP)-conjugated antibodies: (b), anti-human IgG, (c) anti-human IgM and (d) anti-human IgA antibodies; M: molecular weight marker, lane 1: Ig purified from serum using Melon Gel IgG purification kit, lane 2: AS precipitated proteins.
Fig. S4. Effect of dialysed and non-dialysed ammonium sulphate-precipitated immunoglobulins from blood donors (bd; n = 3) and patients with Sjögren's syndrome (SS; n = 3) on carbachol-induced [Ca2+]i signal evoked in Chinese hamster ovary (CHO)-K1 cells expressing human muscarinic acetycholine receptor type 3 (mAchR3). Shown are mean values (bars) ± standard deviation (s.d.) of four independently performed experiments per patient.
Fig. S5. Comparison of the effect of immunoglobulins from a healthy donor isolated by Melon Gel IgG spin purification kit (a; fresh gel support) and AS precipitation (b; not dialyzed) on carbachol-induced [Ca2+]i signal evoked in muscarinic acetycholine receptor type 3 (mAchR3) expressing Chinese hamster ovary (CHO)-K1. RLU = relative light units. Each data point represents the mean of four independently performed experiments. Bar indicates median value.
Fig. S6. Receiver operating curve (ROC) comparing the reactivity of immunoglobulins from 40 pSS patients with that from 230 disease and healthy controls. Area under the curve: 0·083; P < 0·001.
References
- 1.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez AM, Van Den Broeck J, Vrolix K, et al. Antibody effector mechanisms in myasthenia gravis-pathogenesis at the neuromuscular junction. Autoimmunity. 2010;43:353–370. doi: 10.3109/08916930903555943. [DOI] [PubMed] [Google Scholar]
- 3.Jahns R, Schlipp A, Boivin V, Lohse MJ. Targeting receptor antibodies in immune cardiomyopathy. Semin Thromb Hemost. 2010;36:212–218. doi: 10.1055/s-0030-1251506. [DOI] [PubMed] [Google Scholar]
- 4.Bacman S, Sterin-Borda L, Camusso JJ, Arana R, Hubscher O, Borda E. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjogren's syndrome. Clin Exp Immunol. 1996;104:454–459. doi: 10.1046/j.1365-2249.1996.42748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 6.Perez Leiros C, Sterin-Borda L, Hubscher O, Arana R, Borda ES. Activation of nitric oxide signaling through muscarinic receptors in submandibular glands by primary Sjogren syndrome antibodies. Clin Immunol. 1999;90:190–195. doi: 10.1006/clim.1998.4640. [DOI] [PubMed] [Google Scholar]
- 7.Watson EL, Abel PW, DiJulio D, et al. Identification of muscarinic receptor subtypes in mouse parotid gland. Am J Physiol. 1996;271:C905–913. doi: 10.1152/ajpcell.1996.271.3.C905. [DOI] [PubMed] [Google Scholar]
- 8.Cha S, Singson E, Cornelius J, Yagna JP, Knot HJ, Peck AB. Muscarinic acetylcholine type-3 receptor desensitization due to chronic exposure to Sjogren's syndrome-associated autoantibodies. J Rheumatol. 2006;33:296–306. [PubMed] [Google Scholar]
- 9.Dawson L, Tobin A, Smith P, Gordon T. Antimuscarinic antibodies in Sjogren's syndrome: where are we, and where are we going? Arthritis Rheum. 2005;52:2984–2995. doi: 10.1002/art.21347. [DOI] [PubMed] [Google Scholar]
- 10.Dawson LJ, Stanbury J, Venn N, Hasdimir B, Rogers SN, Smith PM. Antimuscarinic antibodies in primary Sjogren's syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum. 2006;54:1165–1173. doi: 10.1002/art.21764. [DOI] [PubMed] [Google Scholar]
- 11.Koo NY, Li J, Hwang SM, et al. Functional epitope of muscarinic type 3 receptor which interacts with autoantibodies from Sjogren's syndrome patients. Rheumatology. 2008;47:828–833. doi: 10.1093/rheumatology/ken064. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs L, Feher E, Bodnar I, et al. Demonstration of autoantibody binding to muscarinic acetylcholine receptors in the salivary gland in primary Sjogren's syndrome. Clin Immunol. 2008;128:269–276. doi: 10.1016/j.clim.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Nikolov NP, Illei GG. Pathogenesis of Sjogren's syndrome. Curr Opin Rheumatol. 2009;21:465–470. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orman B, Sterin-Borda L, De Couto Pita A, Reina S, Borda E. Anti-brain cholinergic auto antibodies from primary Sjogren syndrome sera modify simultaneously cerebral nitric oxide and prostaglandin biosynthesis. Int Immunopharmacol. 2007;7:1535–1543. doi: 10.1016/j.intimp.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Park K, Park S, Jackson MW. The inhibitory effects of antimuscarinic autoantibodies in the sera of primary Sjogren syndrome patients on the gastrointestinal motility. Mol Immunol. 2013;56:583–587. doi: 10.1016/j.molimm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 17.Bonner TI, Young AC, Brann MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988;1:403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- 18.Liao CF, Themmen AP, Joho R, Barberis C, Birnbaumer M, Birnbaumer L. Molecular cloning and expression of a fifth muscarinic acetylcholine receptor. J Biol Chem. 1989;264:7328–7337. [PubMed] [Google Scholar]
- 19.Offermanns S, Wieland T, Homann D, et al. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Mol Pharmacol. 1994;45:890–898. [PubMed] [Google Scholar]
- 20.Abrams P, Andersson KE, Buccafusco JJ, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavill D, Waterman SA, Gordon TP. Antibodies raised against the second extracellular loop of the human muscarinic M3 receptor mimic functional autoantibodies in Sjogren's syndrome. Scand J Immunol. 2004;59:261–266. doi: 10.1111/j.0300-9475.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 22.Goldblatt F, Gordon TP, Waterman SA. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology. 2002;123:1144–1150. doi: 10.1053/gast.2002.36057. [DOI] [PubMed] [Google Scholar]
- 23.Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjogren's syndrome. Arthritis Rheum. 2000;43:1647–1654. doi: 10.1002/1529-0131(200007)43:7<1647::AID-ANR31>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Park K, Haberberger RV, Gordon TP, Jackson MW. Antibodies interfering with the type 3 muscarinic receptor pathway inhibit gastrointestinal motility and cholinergic neurotransmission in Sjogren's syndrome. Arthritis Rheum. 2011;63:1426–1434. doi: 10.1002/art.30282. [DOI] [PubMed] [Google Scholar]
- 25.Bacman S, Perez Leiros C, Sterin-Borda L, Hubscher O, Arana R, Borda E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjogren's syndrome. Invest Ophthalmol Vis Sci. 1998;39:151–156. [PubMed] [Google Scholar]
- 26.Berra A, Sterin-Borda L, Bacman S, Borda E. Role of salivary IgA in the pathogenesis of Sjogren syndrome. Clin Immunol. 2002;104:49–57. doi: 10.1006/clim.2002.5228. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Ha YM, Ku NY, et al. Inhibitory effects of autoantibodies on the muscarinic receptors in Sjogren's syndrome. Lab Invest. 2004;84:1430–1438. doi: 10.1038/labinvest.3700173. [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi H, Matsumoto I, Wakamatsu E, et al. New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren's syndrome. Clin Exp Immunol. 2010;162:53–61. doi: 10.1111/j.1365-2249.2010.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson LJ, Allison HE, Stanbury J, Fitzgerald D, Smith PM. Putative anti-muscarinic antibodies cannot be detected in patients with primary Sjogren's syndrome using conventional immunological approaches. Rheumatology. 2004;43:1488–1495. doi: 10.1093/rheumatology/keh389. [DOI] [PubMed] [Google Scholar]
- 30.Jin M, Hwang SM, Davies AJ, et al. Autoantibodies in primary Sjogren's syndrome patients induce internalization of muscarinic type 3 receptors. Biochim Biophys Acta. 2012;1822:161–167. doi: 10.1016/j.bbadis.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Persson KE, Lee CT, Marsh K, Beeson JG. Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J Clin Microbiol. 2006;44:1665–1673. doi: 10.1128/JCM.44.5.1665-1673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacman S, Berra A, Sterin-Borda L, Borda E. Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjogren syndrome. Invest Ophthalmol Vis Sci. 2001;42:321–327. [PubMed] [Google Scholar]
- 33.Cavill D, Waterman SA, Gordon TP. Failure to detect antibodies to extracellular loop peptides of the muscarinic M3 receptor in primary Sjogren's syndrome. J Rheumatol. 2002;29:1342–1344. [PubMed] [Google Scholar]
- 34.He J, Guo JP, Ding Y, et al. Diagnostic significance of measuring antibodies to cyclic type 3 muscarinic acetylcholine receptor peptides in primary Sjogren's syndrome. Rheumatology. 2011;50:879–884. doi: 10.1093/rheumatology/keq420. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi Y, Nakamura Y, Matsumoto I, et al. Muscarinic-3 acetylcholine receptor autoantibody in patients with systemic sclerosis: contribution to severe gastrointestinal tract dysmotility. Ann Rheum Dis. 2009;68:710–714. doi: 10.1136/ard.2008.096545. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs L, Marczinovits I, Gyorgy A, et al. Clinical associations of autoantibodies to human muscarinic acetylcholine receptor 3(213–228) in primary Sjogren's syndrome. Rheumatology. 2005;44:1021–1025. doi: 10.1093/rheumatology/keh672. [DOI] [PubMed] [Google Scholar]
- 37.Li YN, Guo JP, He J, et al. Serum IgA against type 3 muscarinic acetylcholine receptor is a novel marker in diagnosis of Sjogren's syndrome. Chin Med J. 2011;124:2490–2495. [PubMed] [Google Scholar]
- 38.Marczinovits I, Kovacs L, Gyorgy A, et al. A peptide of human muscarinic acetylcholine receptor 3 is antigenic in primary Sjogren's syndrome. J Autoimmun. 2005;24:47–54. doi: 10.1016/j.jaut.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Naito Y, Matsumoto I, Wakamatsu E, et al. Muscarinic acetylcholine receptor autoantibodies in patients with Sjogren's syndrome. Ann Rheum Dis. 2005;64:510–511. doi: 10.1136/ard.2004.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roescher N, Kingman A, Shirota Y, Chiorini JA, Illei GG. Peptide-based ELISAs are not sensitive and specific enough to detect muscarinic receptor type 3 autoantibodies in serum from patients with Sjogren's syndrome. Ann Rheum Dis. 2011;70:235–236. doi: 10.1136/ard.2010.129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baubet V, Le Mouellic H, Campbell AK, Lucas-Meunier E, Fossier P, Brulet P. Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc Natl Acad Sci USA. 2000;97:7260–7265. doi: 10.1073/pnas.97.13.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waud JP, Bermudez Fajardo A, Sudhaharan T, et al. Measurement of proteases using chemiluminescence-resonance-energy-transfer chimaeras between green fluorescent protein and aequorin. Biochem J. 2001;357:687–697. doi: 10.1042/0264-6021:3570687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakayan A, Vaquero CF, Picazo F, Llopis J. Red fluorescent protein-aequorin fusions as improved bioluminescent Ca2+ reporters in single cells and mice. PLOS ONE. 2011;6:e19520. doi: 10.1371/journal.pone.0019520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- 45.Morise H, Shimomura O, Johnson FH, Winant J. Intermolecular energy transfer in the bioluminescent system of Aequorea. Biochemistry. 1974;13:2656–2662. doi: 10.1021/bi00709a028. [DOI] [PubMed] [Google Scholar]
- 46.Shiboski SC, Shiboski CH, Criswell L, et al. Sjogren's International Collaborative Clinical Alliance Research Group. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein R, Marx A, Strobel P, Schalke B, Nix W, Willcox N. Autoimmune associations and autoantibody screening show focused recognition in patient subgroups with generalized myasthenia gravis. Hum Immunol. 2013;74:1184–1193. doi: 10.1016/j.humimm.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 49.Mussig K, Leyhe T, Holzmuller S, et al. Increased prevalence of antibodies to central nervous system tissue and gangliosides in Hashimoto's thyroiditis compared to other thyroid illnesses. Psychoneuroendocrinology. 2009;34:1252–1256. doi: 10.1016/j.psyneuen.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 52.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cembala TM, Sherwin JD, Tidmarsh MD, Appadu BL, Lambert DG. Interaction of neuromuscular blocking drugs with recombinant human m1-m5 muscarinic receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1998;125:1088–1094. doi: 10.1038/sj.bjp.0702166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang ZL, Puhl HL, May LG, Williams CL, Aronstam RS. Influence of acute and chronic ethanol treatment on muscarinic responses and receptor expression in Chinese hamster ovary cells. Biochem Pharmacol. 1997;54:833–839. doi: 10.1016/s0006-2952(97)00250-5. [DOI] [PubMed] [Google Scholar]
- 55.Geisse S, Voedisch B. Transient expression technologies: past, present, and future. Methods Mol Biol. 2012;899:203–219. doi: 10.1007/978-1-61779-921-1_13. [DOI] [PubMed] [Google Scholar]
- 56.Kekwick RA. The serum proteins in multiple myelomatosis. Biochem J. 1940;34:1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daha NA, Banda NK, Roos A, et al. Complement activation by (auto-) antibodies. Mol Immunol. 2011;48:1656–1665. doi: 10.1016/j.molimm.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 58.Klein R, Lindenborn-Fotinos J, Berg PA. Use of ATPase-associated antigen (M2) for detection of antimitochondrial antibodies in primary biliary cirrhosis by fluorometric immunoassay. J Immunol Methods. 1983;64:227–238. doi: 10.1016/0022-1759(83)90401-5. [DOI] [PubMed] [Google Scholar]
- 59.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520–2524. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 60.Meijide H, Sciascia S, Sanna G, Khamashta MA, Bertolaccini ML. The clinical relevance of IgA anticardiolipin and IgA anti-beta2 glycoprotein I antiphospholipid antibodies: a systematic review. Autoimmun Rev. 2013;12:421–425. doi: 10.1016/j.autrev.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Nann D, Berg CP, Preuss BE, Klein R. Analysis of the clinical relevance of antimitochondrial antibodies to the beta- and gamma-subunits of the F1F0-ATPase in patients with primary biliary cirrhosis. BMC Gastroenterol. 2012;12:152–162. doi: 10.1186/1471-230X-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roggenbuck D, Reinhold D, Werner L, Schierack P, Bogdanos DP, Conrad K. Glycoprotein 2 antibodies in Crohn's disease. Adv Clin Chem. 2013;60:187–208. doi: 10.1016/b978-0-12-407681-5.00006-4. [DOI] [PubMed] [Google Scholar]
- 63.Hausdorf G, Roggenbuck D, Feist E, et al. Autoantibodies to asialoglycoprotein receptor (ASGPR) measured by a novel ELISA – revival of a disease-activity marker in autoimmune hepatitis. Clin Chim Acta. 2009;408:19–24. doi: 10.1016/j.cca.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 64.Herda LR, Felix SB, Boege F. Drug-like actions of autoantibodies against receptors of the autonomous nervous system and their impact on human heart function. Br J Pharmacol. 2012;166:847–857. doi: 10.1111/j.1476-5381.2012.01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michalek K, Morshed SA, Latif R, Davies TF. TSH receptor autoantibodies. Autoimmun Rev. 2009;9:113–116. doi: 10.1016/j.autrev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berg CP, Blume K, Lauber K, et al. Autoantibodies to muscarinic acetylcholine receptors found in patients with primary biliary cirrhosis. BMC Gastroenterol. 2010;10:120–127. doi: 10.1186/1471-230X-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Preuss B, Berg CP, Tunaru S, Offermanns S, Klein R. Demonstration of functional antibodies inhibiting the human muscarinic acetylcholine receptor of the M3 type (hm3AchR) in sera from patients with autoimmune liver disorders. Hepatology. 2013;58:567A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Inhibitory effect of the purification buffer (pb) from the Melon Gel immunoglobulin (Ig)G purification kit on the carbachol induced [Ca2+]i signal evoked in Chinese hamster ovary (CHO)-K1 cells expressing human muscarinic acetycholine receptor type 3 (mAchR3) (transfected cells – tc). Final dilution of the pb 1:100, 1:500, 1:1000, respectively. RLU = relative light units. Shown are mean values (bars) ± standard error of the mean (s.e.m.) of four independently performed experiments and comparisons using the Mann–Whitney U-test. *P ≤ 0·05 versus transfected cells without purification buffer.
Fig. S2. Effect of immunoglobulins from blood donors (bd; n = 3) and patients with Sjögren's syndrome (SS; n = 3) isolated with fresh or regenerated gel support from the Melon Gel immunoglobulin (Ig)G spin purification kit on carbachol-induced [Ca2+]i signal evoked in Chinese hamster ovary (CHO)-K1 cells expressing human muscarinic acetycholine receptor type 3 (mAchR3). Shown are relative light units (RLU) [mean values (bars) + standard deviation (s.d.) of four independently performed experiments per patient].
Fig. S3. Sodium dodecyl sulphate (SDS)-gel electrophoresis and Western blotting for the demonstration of immunoglobulin (Ig)G, IgM and IgA immunoglobulins in the immunoglobulin fractions isolated by Melon Gel IgG spin purification kit and ammonium sulphate (AS) precipitation from a serum of a healthy donor. (a) Coomassie staining, (b–d) Western blotting with anti-human horseradish peroxidase (HRP)-conjugated antibodies: (b), anti-human IgG, (c) anti-human IgM and (d) anti-human IgA antibodies; M: molecular weight marker, lane 1: Ig purified from serum using Melon Gel IgG purification kit, lane 2: AS precipitated proteins.
Fig. S4. Effect of dialysed and non-dialysed ammonium sulphate-precipitated immunoglobulins from blood donors (bd; n = 3) and patients with Sjögren's syndrome (SS; n = 3) on carbachol-induced [Ca2+]i signal evoked in Chinese hamster ovary (CHO)-K1 cells expressing human muscarinic acetycholine receptor type 3 (mAchR3). Shown are mean values (bars) ± standard deviation (s.d.) of four independently performed experiments per patient.
Fig. S5. Comparison of the effect of immunoglobulins from a healthy donor isolated by Melon Gel IgG spin purification kit (a; fresh gel support) and AS precipitation (b; not dialyzed) on carbachol-induced [Ca2+]i signal evoked in muscarinic acetycholine receptor type 3 (mAchR3) expressing Chinese hamster ovary (CHO)-K1. RLU = relative light units. Each data point represents the mean of four independently performed experiments. Bar indicates median value.
Fig. S6. Receiver operating curve (ROC) comparing the reactivity of immunoglobulins from 40 pSS patients with that from 230 disease and healthy controls. Area under the curve: 0·083; P < 0·001.


