Abstract
Effective treatment of bladder cancer with bacillus Calmette–Guérin (BCG) depends on the induction of a T helper type (Th) 1 immune response. Interleukin (IL)-10 down-regulates the Th1 response and is associated with BCG failure. In this study, we investigated whether blocking IL-10 signalling could enhance the BCG-induced Th1 response and anti-tumour immunity in a murine orthotopic tumour model. Treatment with BCG and anti-IL-10 receptor 1 monoclonal antibody (anti-IL-10R1 mAb) increased the interferon (IFN)-γ to IL-10 ratio in both splenocyte cultures and urine. Mice bearing luciferase-expressing MB49 (MB49-Luc) tumours were treated and followed for tumour growth by bioluminescent imaging, bladder weight and histology. Mice treated with phosphate-buffered saline (PBS) (group 1), BCG plus control immunoglobulin (Ig)G1 (group 2) or BCG plus anti-IL-10R1 mAb (group 3) showed 0, 6 and 22% tumour regression, respectively. The mean bladder weight of group 3 mice was substantially lower than those of groups 1 and 2 mice. Remarkably, 36% of group 1 and 53% of group 2 mice but no group 3 mice developed lung metastasis (P = 0·02). To investigate the mechanisms underlying the effect of combination therapy, splenocytes were stimulated with S12 peptide (serine mutation at codon 12 of the K-ras oncogene) known to be expressed in MB49-Luc cells. Induction of ras mutation-specific IFN-γ and cytotoxicity was observed in mice treated with combination therapy. These observations indicate that BCG, in combination with anti-IL-10R1 mAb, induces enhanced anti-tumour immunity that is protective against lung metastasis. Anti-IL-10R1 mAb demonstrates systemic effects and may prove useful in clinical practice for treating bladder cancer in high-risk patients.
Keywords: BCG, bladder cancer, IL-10, immunotherapy
Introduction
Bladder cancer is a common malignant disease dominated by a T helper type (Th) 2 polarized immune response 1. Intravesical instillation of bacillus Calmette–Guérin (BCG) is currently a standard therapy employed for non-muscle invasive bladder cancer (NMIBC) after transurethral resection to prevent recurrence and progression of the disease 2. BCG is also the treatment of choice for carcinoma in situ (CIS). BCG therapy can shift the Th2 environment towards a Th1 milieu, leading to effective anti-bladder cancer immunity in the majority of patients. BCG therapy typically results in 55–65% effectiveness against small residual tumours and a 70–75% complete response rate for CIS 3–5. However, BCG therapy is associated with 40–50% disease recurrence and a lack of therapeutic response in some patients 6. Moreover, BCG therapy is ineffective for invasive and metastatic bladder cancer. Furthermore, up to 90% of patients experience various side effects and occasionally even life-threatening complications, such as sepsis 6,7. Therefore, the effort to improve BCG in both efficacy and safety is greatly needed.
Orthotopic implantation of bladder cancer cells in syngeneic immunocompetent animals has been used widely as a model in BCG therapeutic studies. The MB49 cell line was derived from carcinogen-induced male C57BL/6 bladder epithelial cells 8 and replicates human urothelial carcinoma cell lines in many molecular and phenotypical responses to BCG in vitro 9. The MB49 orthotopic tumour model also resembles the key features of human bladder cancer, including proliferation within luminal epithelium, invasion into muscle and metastasis to draining lymph nodes and remote organs such as lung 10–12. Like human bladder cancer, MB49 tumour is dominated by a Th2 immune response. It has been reported that interleukin (IL)-10, a major Th2 cytokine, inhibits the generation of a tumour-specific Th1 immunity and dendritic cell-induced T cell responses at the tumour site 13,14. To support the Th2 dominance, studies have demonstrated that MB49 tumour, despite its expression of the male minor histocompatibility (HY) antigen 13,14, failed to elicit an HY-specific immune response in normal female mice 13. However, when implanted in female mice genetically deficient in IL-10 (IL-10–/–), MB49 tumour primed for a HY-specific immune response, resulting in prolonged survival and increased tumour rejection 13. These observations suggested that blocking IL-10 could overcome the tumour Th2 dominance and enhance BCG treatment for bladder cancer.
It has been known that IL-10 plays an inhibitory role in bladder cancer immunosurveillance and intravesical BCG efficacy 15,16. The development of a dominant Th1 cytokine profile [e.g. interferon (IFN)-γ] has been associated with the therapeutic effect of BCG, whereas the presence of high levels of Th2 cytokines (e.g. IL-10) have been linked with BCG therapy failure 17. A tendency towards higher ratios of IFN-γ versus IL-10 has been observed for BCG responders 17,18. Consistently, animal model studies have also demonstrated that IFN-γ but not IL-10 was required for local tumour surveillance and that IL-10 affected the therapeutic effect of intravesical BCG 19. We previously showed enhanced BCG-induced anti-bladder cancer immunity in IL-10–/– mice or mice with IL-10 neutralization 17. We recently also demonstrated that blocking IL-10 at the receptor level by anti-IL-10 receptor 1 monoclonal antibody (anti-IL-10R1 mAb) enhanced BCG (a Pasteur strain of live BCG)-induced Th1 immune responses and anti-bladder cancer immunity in MB49-Luc orthotopic tumour model 10. In this study we further tested a reduced dose of BCG, a clinically used lyophilized preparation of Tice BCG strain, in combination with anti-IL-10R1 mAb for treating bladder cancer in the MB49-Luc orthotopic tumour model. We have observed that the combination therapy induced enhanced anti-bladder cancer immunity and effectively prevented bladder cancer metastasis to the lung. An additional mechanistic study revealed that the anti-tumour effect of combination therapy was associated with the induction of ras mutation-specific immune responses such as cytotoxic T lymphocyte (CTL) activity.
Materials and methods
Animals
C57BL/6 female mice, 6–8 weeks old (National Cancer Institute, Frederick, MD, USA), were used. All animals were given free access to water and food throughout the duration of the study. All aspects of the study were approved by the University of Iowa Animal Care and Use Committee.
Bladder cancer cell line
The previously described luciferase-expressing bladder cancer cell line MB49-Luc was used 10. Cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin and 800 μg/ml G418 (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified 5% CO2 incubator.
BCG
A lyophilized preparation of Tice BCG was obtained from Organon (West Orange, NJ, USA) and diluted in phosphate-buffered saline (PBS) to designated doses of colony-forming units (CFU) for use in experiments. Each vial contained 50 mg of lyophilized Tice BCG with 1–8 × 108 CFU. The same lot of BCG preparation was used. The full-strength BCG reflected at least 5 × 106 CFU of Tice BCG.
Splenocyte cytokine production and enzyme-linked immunosorbent assay (ELISA) analysis
Splenocytes were prepared as described previously 20. Cells were resuspended in RPMI-1640 medium supplemented with 10% FBS and seeded in 96-well plates at a density of 8 × 105 cells per 200 μl per well. Cells were cultured in the indicated concentrations of phorbol myristate acetate (PMA)/ionomycin (Sigma, St Louis, MO, USA), BCG, rat immunoglobulin (Ig)G1 (Bio X Cell, West Lebanon, NH, USA), BCG with rat IgG1, anti-IL-10R1 mAb (Bio X Cell; rat IgG1 isotype) or various combinations of BCG with anti-IL-10R1 mAb at 37°C in a humidified 5% CO2 incubator. After 24 h of incubation medium was harvested for ELISA analysis of IFN-γ and IL-10 production. Paired capture and detecting antibodies were used for IFN-γ (Endogen, Woburn, MA, USA; clones R4·6.A2 and XMG1·2) and IL-10 (BD PharMingen, San Diego, CA, USA; clones JES5-2A5 and SXC-1). The concentrations of cytokines were calculated utilizing standard mass/volume format with standard curve derived from purified recombinant (r) IFN-γ (BD PharMingen) or rIL-10 (Genzyme, Cambridge, MA, USA).
Bladder immune response and analysis
Groups of five mice were treated intravesically (i.b.) and intraperitoneally (i.p.) with PBS, i.b. BCG (full-strength) plus intraperitoneal (i.p.) rat IgG1 (200 μg) or i.b. BCG (one-ninth, one-third and full-strength) plus i.p. anti-IL-10R1 mAb (200 μg) biweekly for a total of four treatments. The i.b. administration was performed via urethral catheterization into the bladders of anaesthetized mice with a 24-gauge IV catheter for a dwell time of 1 h, as described previously 10. After the last treatment mice were placed in metabolic cages for 20-h urine collection, followed by ELISA analysis of urinary IFN-γ and IL-10, as described above and previously 10.
Tumour implantation and treatment
As described previously 10, mice (n = 60) were anaesthetized and the bladders catheterized. The bladders were then traumatized with 5 μl of 0·2 M silver nitrate, rinsed with PBS and instilled with a 50 μl cell suspension of 1 × 106 MB49-Luc cells mixed in 50% normal mouse serum. Mice were left undisturbed for 1 h, randomized into three groups (n = 20) and injected i.p. with PBS, rat IgG1 (200 μg) or anti-IL-10R1 mAb (200 μg). Beginning on day 1, mice were treated with i.b and i.p. PBS for group 1, i.b. BCG (one-third strength) plus i.p. rat IgG1 (200 μg) for group 2, and i.b. BCG (one-third strength) plus i.p. anti-IL-10R1 mAb (200 μg) for group 3 biweekly for a total of six treatments. Bioluminescence was utilized as a marker for tumour. Mice were imaged weekly using the Xenogen IVIS 200 imaging system after i.p. injection of 1·5 mg luciferin (Xenogen, Alameda, CA, USA). A cut-off of 5 × 104 p/s on imaging was used to establish the presence of bladder tumour. Mice were followed for 76 days. Post-mortem bladders were weighed and processed for histological haematoxylin and eosin (H&E) staining. The lungs were also collected and processed for histological analysis to verify the metastatic tumours detected by bioluminescent imaging.
Mechanistic study
Groups of five mice were instilled i.b. with 1 × 106 MB49-Luc cells and treated i.p. with PBS, rat IgG1 (200 μg) or anti-IL-10R1 mAb (200 μg), as described above. Beginning on day 1, mice were treated with i.b and i.p. PBS for group 1, i.b. PBS plus i.p. anti-IL-10R1 mAb (200 μg) for group 2, i.b. BCG (one-third strength) plus i.p. rat IgG1 (200 μg) for group 3 and i.b. BCG (one-third strength) plus i.p. anti-IL-10R1 mAb (200 μg) for group 4 biweekly for a total of six treatments. Mice were killed for analysis on day 21. The spleens were collected from mice bearing bladder tumours (n = 3–5) verified by bioluminescent imaging and bladder weight. As described previously 21, splenocytes were prepared, pooled and cultured in the presence of 100 μg/ml S12 peptide (residues 5–17 with mutated serine at codon 12 of the K-ras oncogene) or 100 μg/ml G12 peptide (residues 5–17 with wild-type glycine at codon 12 of the K-ras oncogene) for 3 days, followed by ELISA (R&D Systems, Minneapolis, MN, USA) analysis of IFN-γ production in culture supernatants. In a parallel experiment, splenocytes were stimulated with S12 or G12 peptide (100 μg/ml) for 5 days. T cells were then isolated using a T Cell Enrichment Column (R&D Systems) and used as effector cells in a cytotoxicity assay against MB49-Luc target cells. Cells were incubated at 6·25:1, 12·5:1, 25:1, 50:1 and 100:1 of effector : target (E : T) ratios for 4 h, followed by analysis of cell lysis using a CytoTox 96™ Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA), according to the manufacturer's instructions.
Statistical analysis
Fisher's exact test was used to compare differences among the groups for metastasis. Pairwise group comparisons were performed with P-values adjusted for multiple tests by Bonferroni's method. The Kruskal–Wallis test was used to compare bladder weight among the groups, with P-values again adjusted for multiple tests by Bonferroni's method. Log-rank test and Kaplan–Meier curves were used to compare survival among the groups. Student's t-test was used to compare IFN-γ and IL-10 levels in splenocyte cultures and urine as well as cytotoxicity among the groups. A P-value of < 0·05 was considered statistically significant.
Results
Treatment with anti-IL-10R1 mAb and BCG increases the IFN-γ to IL-10 ratio both in vitro and in vivo
To evaluate the effect of anti-IL-10R1 mAb on BCG induction of Th1 immune responses in vitro, we incubated splenocytes with BCG, control rat IgG1, anti-IL-10R1 mAb or BCG in combination with rat IgG1 or anti-IL-10R1 mAb at the indicated concentrations for 24 h, and then analysed IFN-γ and IL-10 production in culture supernatants by ELISA (Fig. 1). Splenocytes without treatment provided baseline levels of the cytokines. Splenocytes incubated with PMA/ionomycin served as positive controls. BCG alone or with rat IgG1 induced both IFN-γ and IL-10 at similar levels. Splenocytes incubated with rat IgG1 or anti-IL-10R1 mAb alone produced no or a marginal level of the cytokines. Remarkably, when incubated with BCG plus anti-IL-10R1 mAb, splenocytes produced significantly increased IFN-γ by 1·7–5·8-fold in a dose-dependent manner (Fig. 1, top). Interestingly, the same splenocytes also produced significantly increased IL-10 but in a reversed dose-dependent manner (Fig. 1, bottom).
Fig. 1.
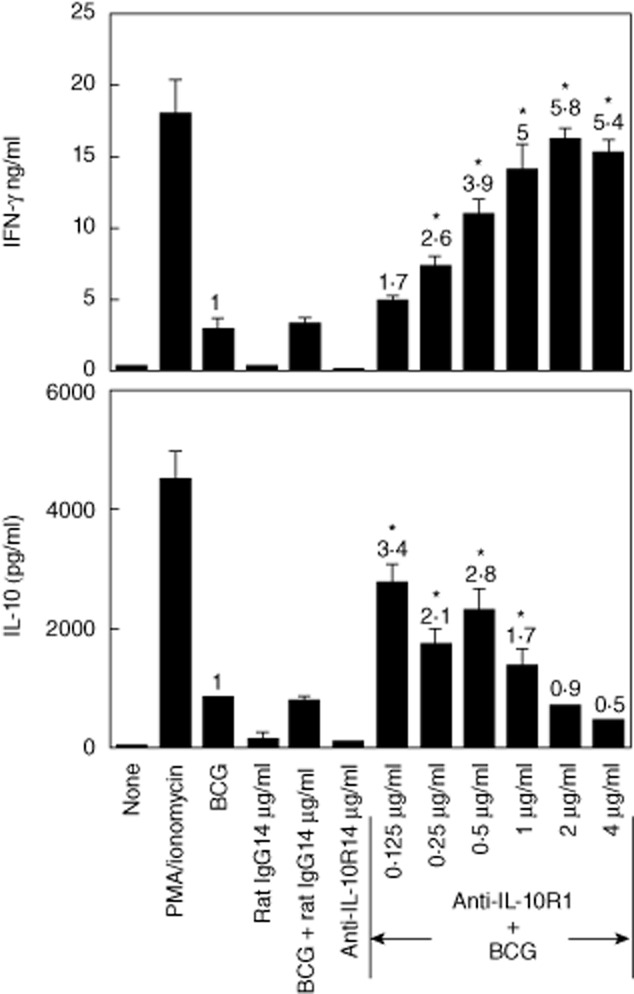
Treatment with increasing doses of anti-interleukin (IL)-10R1 monoclonal antibody (mAb) and bacillus Calmette–Guérin (BCG) increases the interferon (IFN)-γ : IL-10 ratio in cultured splenocytes. Mouse splenocytes were prepared and incubated with BCG [2·5 × 105 colony-forming units (CFU)/ml], control rat immunoglobulin (Ig)G1 (4 μg/ml), BCG (2·5 × 105 CFU/ml) with rat IgG1 (4 μg/ml), anti-IL-10R1 mAb (4 μg/ml) or BCG (2·5 × 105 CFU/ml) with anti-IL-10R1 mAb at twofold escalating dosages ranging from 0·125 to 4 μg/ml for 24 h. Cells incubated with phorbol myristate acetate (PMA) (100 ng/ml)/ionomycin (1500 ng/ml) served as positive controls, whereas cells without treatment served as baseline levels. IFN-γ (top) and IL-10 (bottom) production in the conditioned culture supernatants were evaluated by enzyme-linked immunosorbent assay (ELISA) and shown as mean ± standard deviation (s.d.) of duplicate determinants. Numerical values indicate the fold changes with respect to the levels of IFN-γ and IL-10 produced in response to BCG alone. The results are representative of two separate experiments. *Significantly changed compared to BCG-treated splenocyte cultures.
To investigate whether anti-IL-10R1 mAb could also affect BCG induction of Th1 immune responses in the bladder, we treated mice biweekly with PBS, BCG (full-strength) plus control rat IgG1 or BCG (one-ninth, one-third and full-strength) plus anti-IL-10R1 mAb. Treatment with anti-IL-10R1 mAb alone was not included, due to its lack of the induction of urinary cytokines based on a preliminary experiment. After four treatments, urine was collected and analysed for IFN-γ and IL-10 production by ELISA (Fig. 2). Under the experimental setting, BCG plus rat IgG1 induced no urinary IFN-γ. However, BCG plus anti-IL-10R1 mAb induced significantly increased urinary IFN-γ by 1·7–3·3-fold (Fig. 2, left). Interestingly, the one-third strength BCG induced the same level of urinary IFN-γ as the full-strength BCG. In contrast to urinary IFN-γ, mice treated with BCG plus rat IgG1 produced IL-10 in the urine (Fig. 2, right). However, BCG plus anti-IL-10R1 mAb induced only a marginally increased level of urinary IL-10 (1·1–1·6-fold) compared to BCG plus rat IgG1. These observations indicate that BCG in combination with anti-IL-10R1 mAb can increase the IFN-γ : IL-10 ratio, favouring Th1 immune responses.
Fig. 2.
Systemic anti-interleukin (IL)-10R1 monoclonal antibody (mAb) enhances intravesical bacillus Calmette–Guérin (BCG)-induced urinary (IFN)-γ production. Groups of five mice were treated with intravesical (i.b.) phosphate-buffered saline (PBS) plus intraperitoneal (i.p.) PBS for group 1, i.b. BCG (full-strength) plus i.p. control rat immunoglobulin (Ig)G1 (200 μg/dose) for group 2, i.b. BCG (one-ninth strength) plus i.p. anti-IL-10R1 mAb (200 μg/dose) for group 3, i.b. BCG (one-third strength) plus i.p. anti-IL-10R1 mAb (200 μg/dose) for group 4, or i.b. BCG (full-strength) plus i.p. anti-IL-10R1 mAb (200 μg/dose) for group 5 biweekly for a total of four treatments. After the last treatment mice were placed in metabolic cages for 20-h urine collection. Enzyme-linked immunosorbent assay (ELISA) was used to evaluate IFN-γ and IL-10 in the urine. Data are shown as mean ± standard deviation (s.d.) of duplicate determinants. Numerical values indicate the fold changes with respect to the levels of urinary IFN-γ and IL-10 produced in response to BCG plus rat IgG1. *Significantly increased compared to BCG plus rat IgG1-treated group.
Treatment with anti-IL-10R1 mAb and BCG induces enhanced anti-bladder cancer immunity and prevents lung metastases in a MB49-Luc orthotopic tumour model
We used our previously described MB49-Luc orthotopic tumour model to evaluate the effect of anti-IL-10R1 mAb on BCG treatment for bladder cancer 10. To minimize the side effects, we used a reduced BCG dose (one-third strength). Of three randomized groups of 20 mice, 11, 17 and nine mice developed tumours, respectively. Mice were treated biweekly with PBS for group 1, BCG plus control rat IgG1 for group 2 or BCG plus anti-IL-10R1 mAb for group 3 for a total of six treatments. Treatment with anti-IL-10R1 mAb alone was not included due to its lack of inhibition on MB49-Luc tumour growth based on a preliminary experiment. Mice were monitored weekly by bioluminescent imaging and followed for 76 days. Groups 1, 2 and 3 mice showed 0% (none of 11), 6% (one of 17) and 22% (two of nine) tumour regression, respectively (Table 1). The mean bladder weights were 773 ± 484 mg for group 1, 862 ± 563 mg for group 2 and 459 ± 418 mg for group 3 (Fig. 3). However, both tumour-free rates and mean bladder weights were not statistically different between the groups. Survival also showed no statistical difference between the groups (data not shown). Unexpectedly, groups 1 and 2 mice developed lung metastasis at overall rates of 36% (four of 11) and 53% (nine of 17), respectively, while group 3 mice showed no metastasis (none of nine) (P = 0·02) (Table 1). The lung metastasis was identified by both bioluminescent imaging and histological haematoxylin and eosin (H&E) staining (Fig. 4). These observations indicate that anti-IL-10R1 mAb enhances BCG-induced anti-tumour immunity and is protective against bladder cancer metastasis to the lung.
Table 1.
Bladder tumour responses
| Group | No. of mice with bladder tumours† | No. of mice with metastasis† | No of tumour-free mice at day 76 |
|---|---|---|---|
| i.b. PBS/i.p. PBS | 11 | 4/11 (36%)‡ | 0/11 (0%) |
| i.b. BCG/i.p. IgG1 | 17 | 9/17 (53)‡ | 1/17 (6%) |
| i.b. BCG/i.p. anti-IL-10R1 | 9 | 0/9 (0%)‡ | 2/9 (22%) |
Based on bioluminescent imaging or histology.
Fisher's exact test comparing among the groups: P = 0·02. PBS = phosphate-buffered saline; Ig = immunoglobulin; i.b. = intravesical; i.p. = intraperitoneal; BCG = bacillus Calmette–Guérin; IL = interleukin.
Fig. 3.
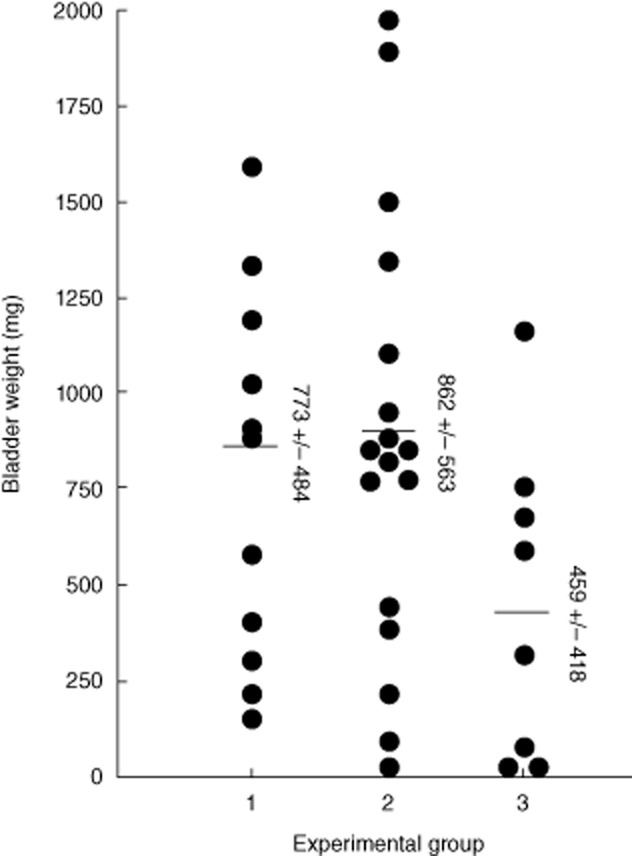
Systemic anti-interleukin (IL)-10R1 monoclonal antibody (mAb) enhances intravesical bacillus Calmette–Guérin (BCG) treatment of bladder tumour. Groups of 20 mice were instilled intravesically (i.b.) with MB49-Luc cells and monitored for tumour growth by bioluminescent imaging. Eleven, 17 and nine mice developed bladder tumours in the three groups, respectively. Mice were treated i.b and intraperitoneal (i.p.) phosphate-buffered saline (PBS) for group 1, i.b. BCG (one-third strength) plus i.p. control rat immunoglobulin (Ig)G1 (200 μg/dose) for group 2 or i.b. BCG (one-third strength) plus i.p. anti-IL-10R1 mAb (200 μg/dose) for group 3 biweekly for a total of six treatments. Mice were followed for 76 days. Post-mortem bladders were weighed. Data are shown as mean ± standard deviation (s.d.) of bladder weights for each group (one bladder in group 3 was not recorded due to severe decay).
Fig. 4.
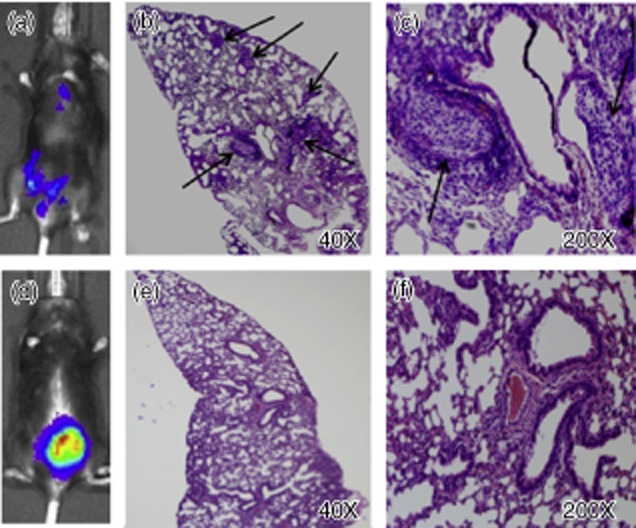
Representative bioluminescent imaging and histological haematoxylin and eosin (H&E) staining of localized MB49-Luc tumour as well as lung metastasis. (a–c) A mouse treated with phosphate-buffered saline (PBS) developed both invasive and lung metastatic tumours; arrows indicate the lung metastatic nodules. (d–f) A mouse treated with bacillus Calmette–Guérin (BCG) plus rat immunoglobulin (Ig)G1 developed a localized bladder tumour and showed normal lung histology.
Treatment with anti-IL-10R1 mAb and BCG induces ras mutation-specific immune responses in MB49-Luc orthotopic tumour model
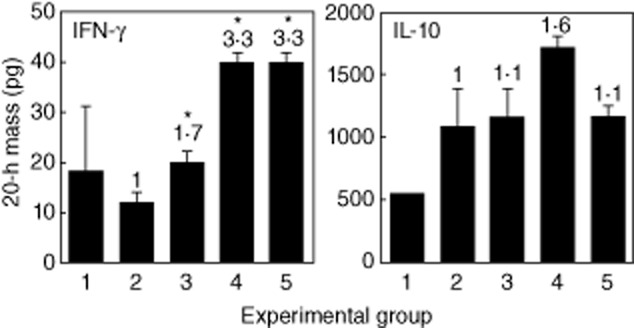
To investigate the mechanisms underlying the effect of BCG in combination with anti-IL-10R1 mAb, we treated mice as described above for the anti-bladder cancer studies. Mice were instilled i.b. with MB49-Luc tumour cells and treated biweekly with PBS for group 1, anti-IL-10R1 mAb for group 2, BCG (one-third strength) plus control rat IgG1 for group 3 or BCG (one-third strength) plus anti-IL-10R1 mAb for group 4 for a total of six treatments. Mice were killed for analysis at day 21. We previously identified a serine mutation at codon 12 of the K-ras oncogene in MB49 cells 21, a parental line of MB49-Luc cells used in this study. This gene mutation resulted in the abundant expression of mutated p21 K-ras protein that was immunogenic and capable of inducing ras mutation-specific immune responses in C57BL/6 mice 21. To investigate whether the ras mutation-specific immune responses could also be induced in mice treated with BCG in combination with anti-IL-10R1 mAb, we stimulated splenocytes prepared from tumour-bearing mice with S12 or control G12 peptide in vitro. Splenocytes from mice treated with combination therapy (group 4) produced significantly increased IFN-γ in response to S12 but not G12 peptide stimulation (Fig. 5a). In contrast, splenocytes from other groups produced no IFN-γ under the same condition.
Fig. 5.
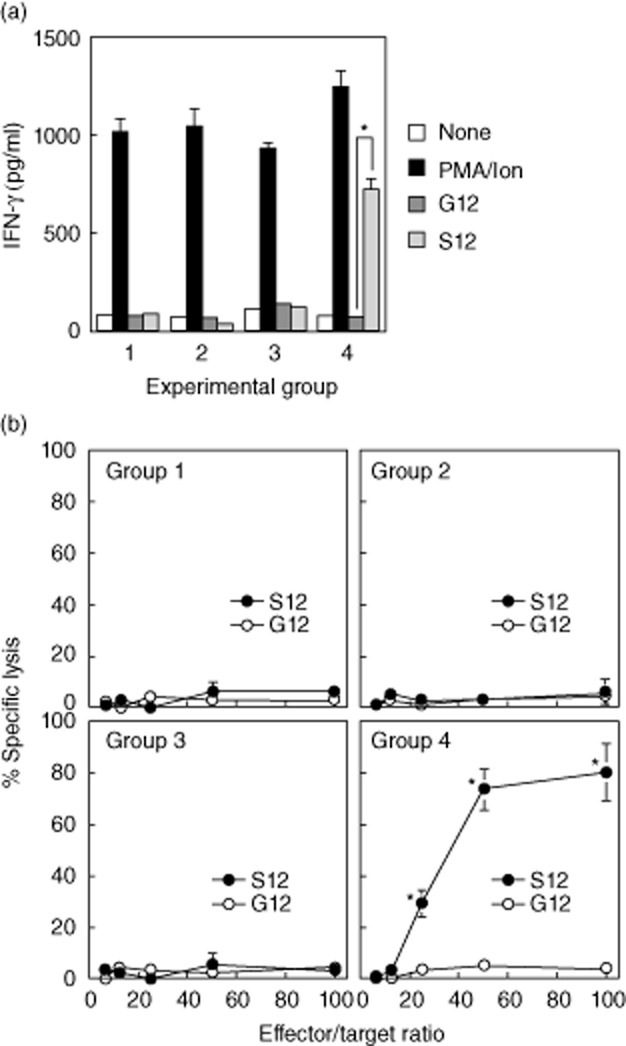
Anti-interleukin (IL)-10R1 monoclonal antibody (mAb) in combination with bacillus Calmette–Guérin (BCG) induces ras mutation-specific immune responses. (a) Groups of five mice were instilled intravesically (i.b.) with MB49-Luc cells and treated i.b and intraperitoneal (i.p.) phosphate-buffered saline (PBS) for group 1, i.b. PBS plus i.p. anti-IL-10R1 mAb (200 μg/dose) for group 2, i.b. BCG (one-third strength) plus i.p. control rat IgG1 (200 μg/dose) for group 3 and i.b. BCG (one-third strength) plus i.p. anti-IL-10R1 mAb (200 μg/dose) for group 4 biweekly for a total of six treatments. Mice were killed on day 21. Splenocytes were prepared and cultured in the presence of G12 or S12 peptide at 100 μg/ml for 3 days, followed by enzyme-linked immunosorbent assay (ELISA) analysis of interferon (IFN)-γ production in culture supernatants. Cells incubated with phorbol myristate acetate (PMA) (100 ng/ml)/ionomycin (1500 ng/ml) served as positive controls, whereas cells without treatment served as baseline levels. Data are shown as mean ± standard deviation (s.d.) of duplicate determinants. *P < 0·05 compared to G12-stimulated splenocytes. (b) In a parallel experiment splenocytes were cultured in the presence of G12 or S12 peptide (100 μg/ml) for 5 days. T cells were then isolated and used as effector cells against MB49-Luc target cells in a cytotoxicity assay. Cells were incubated at 6·2:1, 12·5:1, 25:1, 50:1 and 100:1 of effector : target (E : T) ratios for 4 h and then analysed for lysis. Data are shown as % specific lysis of quadruplicate determinants. *P < 0·05 compared to G12-stimulated splenocytes.
Correlating with the ras mutation-specific IFN-γ production, group 4 splenocytes stimulated with S12 peptide demonstrated profound cytotoxicity against MB49-Luc tumour cells, with 29, 73·7 and 80·9% killing at the E : T ratios of 25:1, 50:1 and 100:1, respectively (Fig. 5b). Splenocytes from other groups stimulated with S12 peptide showed no killing of MB49-Luc tumour cells. There was also no induction of CTL activity in response to G12 peptide stimulation. These observations indicate that BCG in combination with anti-IL-10R1 mAb induces systemic ras mutation-specific immune responses that may contribute to the effect of combination therapy on treating bladder cancer and preventing tumour metastasis in the MB49-Luc orthotopic tumour model.
Discussion
Improving BCG immunotherapy for bladder cancer has been an active area of research, with the ultimate goal of increasing therapeutic efficacy and decreasing side effects. One strategic approach to achieve this goal would be to block the Th2 immune pathway in BCG-induced immune responses. We previously demonstrated that BCG induced enhanced anti-bladder cancer immunity in IL-10–/– mice and mice in which IL-10 had been neutralized 17. We recently also showed that blocking IL-10 at the receptor level by anti-IL-10R1 mAb resulted in reduced tumour burden and improved tumour-free and death rates in BCG (a living Pasteur strain) treatment of bladder cancer in a MB49-Luc orthotopic tumour model 10. In this study (a longitudinal survival study) we further observed that anti-IL-10R1 mAb in combination with Tice BCG, a clinically used lyophilized BCG preparation, induced similar enhanced anti-bladder cancer immunity and, in addition, effectively prevented lung metastasis in the same model. The mechanistic studies revealed that the anti-tumour effect of combination therapy was associated with the induction of systemic ras mutation-specific immune responses including IFN-γ production and CTL activity.
Like the living Pasteur BCG strain, the lyophilized Tice BCG strain is compatible with anti-IL-10R1 mAb for the induction of Th1 immune responses in immunocompetent mice. An increased IFN-γ : IL-10 ratio has been observed in response to BCG plus anti-IL-10R1 mAb both in vitro and in vivo (Figs 1 and 2), which may explain the anti-tumour effect of combination therapy in the MB49-Luc orthotopic tumour model. In addition, we have observed that one-third strength BCG induced the same level of urinary IFN-γ as full-strength BCG (Fig. 2, left), suggesting that a reduced BCG dose could be used for minimized side effects while maintaining therapeutic efficacy. Indeed, we have observed that one-third strength BCG in combination with anti-IL-10R1 mAb was effective on treating local bladder cancer (Fig. 3) and preventing tumour metastasis to the lung (Table 1) in the MB49-Luc orthotopic tumour model. However, unlike our previous study 10, we failed to observe the effect of BCG in combination with control IgG1 on treating MB49-Luc bladder tumour. The lack of the anti-tumour effect was due possibly to the use of a reduced BCG dose and the extended experimental length in this study.
As reported for the variability of orthotopic tumour implantation 12, we have obtained variable tumour take rates in the three treatment groups (55, 85 and 45 for groups 1, 2 and 3, respectively). Also, as in our previous study 10, we have obtained variable bladder weights in tumour-bearing mice (Fig. 3). These variations were probably intrinsic for the orthotopic tumour model, because we performed tumour implantation and housed animals under the same conditions. These variations significantly limited the ability to determine a statistically significant difference in survival and tumour burden between the groups, although the differences were apparent (Fig. 3 and Table 1). However, despite these limitations, we unexpectedly found a statistically significant difference in rates of lung metastasis (Table 1 and Fig. 4). While 36% (four of 11) of group 1 mice (treated with PBS) and 53% (nine of 17) of group 2 mice (treated with BCG plus control IgG1) developed lung metastases, no mice (none of nine) in group 3 (treated with BCG plus anti-IL-10R1 mAb) developed the metastasis (P = 0·02). These data suggest that anti-IL-10R1 mAb is capable of enhancing BCG-induced anti-bladder cancer immunity, leading to effective protection against lung metastasis from orthotopically growing tumours.
The MB49 cell line was derived from carcinogen-induced male C57BL/6 bladder epithelial cells 8 and known to contain a serine mutation at codon 12 of the K-ras oncogene, which leads to the abundant expression of mutated K-ras p21 protein in the cells 21. In addition, the MB49 cell line expresses the HY antigen known to be immunogenic in syngeneic female mice 22–24. However, despite the antigen expressions, we found that mice in control groups developed progressive MB49-Luc tumours. This observation indicated that naturally induced immune responses were not sufficient for tumour rejection even in mice treated with BCG monotherapy. To investigate whether the enhanced anti-tumour effect observed in mice treated with BCG plus anti-IL-10R1 mAb was attributed to the induction of augmented specific anti-tumour immune responses, we analysed splenocytes from treated mice for ras mutation-specific responses in vitro. We have observed that the combination therapy elicited the specific immune responses including IFN-γ production (Fig. 5a) and CTL activity against MB49-Luc cells (Fig. 5b). These observations indicated the systemic effect of anti-IL-10R1 mAb on modulating immune responses to the ras antigen from a localized MB49-Luc tumour. In addition, we have also observed the induction of HY-specific immune responses by the combination therapy (data not shown). As the MB49 cell line is known to express several other tumour-associated antigens (TAA) such as bladder cancer-4 and prostate stem cell antigen 12, it would be interesting to determine whether the combination therapy could also induce these TAA-specific immune responses in the MB49-Luc orthotopic tumour model. Our observations warrant further investigation on this exploration.
In summary, intravesical BCG plus systemic anti-IL-10R1 mAb induces enhanced anti-bladder cancer immunity that is protective against lung metastasis in a murine orthotopic tumour model. The anti-tumour effect of combination therapy is associated with the induction of ras mutation-specific immune responses. Anti-IL-10R1 mAb demonstrates systemic effects and may prove useful in clinical practice for the treatment of bladder cancer in high-risk patients.
Acknowledgments
The authors thank Pfizer Inc. for the support of this study and Dr Miriam Zimmerman for statistical analysis.
Disclosure
The authors have no competing interests.
References
- 1.Loskog A, Ninalga C, Paul-Wetterberg G, de la Torre M, Malmström PU, Tötterman TH. Human bladder carcinoma is dominated by T-regulatory cells and Th1 inhibitory cytokines. J Urol. 2007;177:353–358. doi: 10.1016/j.juro.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell MA. Optimizing BCG therapy. Urol Oncol. 2009;27:325–328. doi: 10.1016/j.urolonc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette–Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205–1209. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 5.Morales A, Ottenhof P, Emerson L. Treatment of residual, non-infiltrating bladder cancer with bacillus Calmette–Guerin. J Urol. 1981;125:649–651. doi: 10.1016/s0022-5347(17)55150-2. [DOI] [PubMed] [Google Scholar]
- 6.Williams SK, Hoenig DM, Ghavamian R, Soloway M. Intravesical therapy for bladder cancer. Exp Opin Pharmacother. 2010;11:947–958. doi: 10.1517/14656561003657145. [DOI] [PubMed] [Google Scholar]
- 7.Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol. 2006;175:2004–2010. doi: 10.1016/S0022-5347(06)00264-3. [DOI] [PubMed] [Google Scholar]
- 8.Summerhayes IC, Franks LM. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst. 1979;62:1017–1023. [PubMed] [Google Scholar]
- 9.Chen F, Zhang G, Cao Y, Hessner MJ, See WA. MB49 murine urothelial carcinoma: molecular and phenotypic comparison to human cell lines as a model of the direct tumor response to bacillus Calmette–Guerin. J Urol. 2009;182:2932–2937. doi: 10.1016/j.juro.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Bockholt NA, Knudson MJ, Henning JR, et al. Anti-interleukin-10R1 monoclonal antibody enhances bacillus Calmette–Guérin induced T-helper type 1 immune responses and antitumor immunity in a mouse orthotopic model of bladder cancer. J Urol. 2012;187:2228–2235. doi: 10.1016/j.juro.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y, Chen X, O'donnell MA. Use of prostate specific antigen to measure bladder tumor growth in a mouse orthotopic model. J Urol. 2004;172:2414–2420. doi: 10.1097/01.ju.0000143860.50878.b1. [DOI] [PubMed] [Google Scholar]
- 12.Loskog A, Ninalga C, Hedlund T, Alimohammadi M, Malmström PU, Tötterman TH. Optimization of the MB49 mouse bladder cancer model for adenoviral gene therapy. Lab Anim. 2005;39:384–393. doi: 10.1258/002367705774286475. [DOI] [PubMed] [Google Scholar]
- 13.Halak BK, Maguire HC, Jr, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–917. [PubMed] [Google Scholar]
- 14.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–2157. [PubMed] [Google Scholar]
- 15.Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother. 2007;61:299–305. doi: 10.1016/j.biopha.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Tham SM, Ng KH, Pook SH, Esuvaranathan K, Mahendran R. Tumor and microenvironment modification during progression of murine orthotopic bladder cancer. Clin Dev Immunol. 2011;2011:865684. doi: 10.1155/2011/865684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadler R, Luo Y, Zhao W, et al. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette–Guérin (BCG) mediated antitumour activity. Clin Exp Immunol. 2003;131:206–216. doi: 10.1046/j.1365-2249.2003.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saint F, Patard JJ, Maille P, et al. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guerin treatment for superficial bladder cancer. J Urol. 2002;167:364–367. [PubMed] [Google Scholar]
- 19.Riemensberger J, Böhle A, Brandau S. IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol. 2002;127:20–26. doi: 10.1046/j.1365-2249.2002.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Chen X, O'Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine. 2003;21:17–26. doi: 10.1016/s1043-4666(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, Chen X, Han R, Chorev M, DeWolf WC, O'Donnell MA. Mutated ras p21 as a target for cancer therapy in mouse transitional cell carcinoma. J Urol. 1999;162:1519–1526. [PubMed] [Google Scholar]
- 22.Yang AS, Monken CE, Lattime EC. Intratumoral vaccination with vaccinia-expressed tumor antigen and granulocyte macrophage colony-stimulating factor overcomes immunological ignorance to tumor antigen. Cancer Res. 2003;63:6956–6961. [PubMed] [Google Scholar]
- 23.Melchionda F, McKirdy MK, Medeiros F, Fry TJ, Mackall CL. Escape from immune surveillance does not result in tolerance to tumor-associated antigens. J Immunother. 2004;27:329–338. doi: 10.1097/00002371-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Fry TJ, Shand JL, Milliron M, Tasian SK, Mackall CL. Antigen loading of DCs with irradiated apoptotic tumor cells induces improved anti-tumor immunity compared to other approaches. Cancer Immunol Immunother. 2009;58:1257–1264. doi: 10.1007/s00262-008-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


