Abstract
Chronic Pseudomonas aeruginosa lung infection in cystic fibrosis (CF) patients is characterized by persisting mucoid biofilms in hypoxic endobronchial mucus. These biofilms are surrounded by numerous polymorphonuclear leucocytes (PMNs), which consume a major part of present molecular oxygen (O2) due to production of superoxide (O2−). In this study, we show that the PMNs also consume O2 for production of nitric oxide (NO) by the nitric oxide synthases (NOS) in the infected endobronchial mucus. Fresh expectorated sputum samples (n = 28) from chronically infected CF patients (n = 22) were analysed by quantifying and visualizing the NO production. NO production was detected by optode measurements combined with fluorescence microscopy, flow cytometry and spectrophotometry. Inhibition of nitric oxide synthases (NOS) with NG-monomethyl-L-arginine (L-NMMA) resulted in reduced O2 consumption (P < 0·0008, n = 8) and a lower fraction of cells with fluorescence from the NO-indicator 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) (P < 0·002, n = 8). PMNs stained with DAF-FM and the superoxide indicator hydroethidine (HE) and host cells with inducible NOS (iNOS) were identified in the sputum. In addition, the production of the stable end-products of NO in CF sputum was correlated with the concentration of PMNs; NO3− (P < 0·04, r = 0·66, n = 10) and NO2− (P< 0·006, r = 0·78, n = 11). The present study suggests that besides consumption of O2 for production of reactive oxygen species, the PMNs in CF sputum also consume O2 for production of NO.
Keywords: cystic fibrosis, neutrophil biology, nitric oxide, pneumonia, Pseudomonas aeruginosa
Introduction
Cystic fibrosis (CF) is an autosomal recessive inherited disorder characterized by accumulation of large volumes of thick, endobronchial mucus that increase the risk for severe chronic Pseudomonas aeruginosa infections 1. Chronic P. aeruginosa lung infection is characterized by the formation of biofilm aggregates surrounded by numerous polymorphonuclear leucocytes (PMNs) in the endobronchial mucus 2. The biofilm mode of growth protects the bacteria from antibiotics and shields against host defences such as bactericidal defence mechanisms of the activated PMNs 2. Prolonged activation of PMNs in chronic lung infection can cause progressive lung tissue damage by the release of proteolytic enzymes and reactive oxygen species (ROS) 1,3. However, the activity of PMNs in chronic P. aeruginosa lung infection in CF patients is, by far, not fully understood. We recently observed ongoing respiratory burst in PMNs of expectorated endobronchial secretions and have demonstrated that the O2 consumption by PMNs during the respiratory burst is the major cause of O2 depletion, leading to anoxia in the endobronchial mucus in CF patients with chronic P. aeruginosa lung infection 4,5. In addition, only a minute part of the O2 is consumed by aerobic respiration in endobronchial secretions from CF patients [5], and the production of nitrous oxide (N2O) in CF sputum 6 indicates that P. aeruginosa adapts to the anoxia by employing anaerobic respiration by denitrification to obtain energy for growth. Ongoing denitrification by P. aeruginosa in the infected CF lung is corroborated further by the increased expression of genes involved in denitrification in the virulent mucoid isolates 7. Besides O2 consumption during the respiratory burst, where the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX-2) reduces O2 to superoxide (O2−) 8, activated PMNs may also consume O2 for nitric oxide (NO) production 9 by incorporating O2 into both NO and L-citrulline during the enzymatic oxidation of L-arginine and reduction of O2 by nitric oxide synthases (NOS) 10–13. Human PMNs exhibit a constitutive expression of inducible nitric oxide synthases (iNOS) 14, which may participate in the NO production in PMNs isolated from bacterial infections 10, as well as in the nitration of bacteria ingested by PMNs in vitro 11 and in the formation of stable nitrogen species such as nitrate (NO3−) and nitrite (NO2−) resulting from the rapid reaction of NO with other molecules, such as O2− 15. Both iNOS and the phagocytic NOX-2 can be activated by bacterial and inflammatory stimuli 16, and we therefore hypothesized that PMNs, in addition to utilizing O2 for the formation of O2−, also consume O2 for the production of NO in the lungs of CF patients with chronic P. aeruginosa lung infection. In view of the fact that P. aeruginosa up-regulates genes for denitrification in response to the presence of NO and to O2 depletion 17, the simultaneous reduction of O2 consumption and NO production without decreasing products from the NOX-2 in stimulated human PMNs by inhibition with L-NMMA 9 motivated our use of NG-monomethyl-L-arginine (L-NMMA) to estimate ongoing NO production and the associated O2 consumption by PMNs in CF sputum.
Because NO is an unstable molecule with the ability to produce various end-products, including NO3− and NO2− 15,18, several methods were applied to display the NO production by PMNs in fresh expectorated sputum from CF patients with chronic P. aeruginosa lung infection. These methods include the demonstration of NO in the sputum samples by NOS-dependent staining of sputum cells with fluorescent indicators for NO, estimation of NOS-dependent O2 consumption, specific staining of iNOS expression in sputum cells as well as correlating the concentration of PMNs to NO3− and NO2− concentrations in sputum.
Materials and methods
Sputum samples
As defined by the Danish Act on Research Ethics Review of Health Research Projects, Section 2, the project does not constitute a health research project and was thus initiated without approval from the Committees on Health Research Ethics in the Capital Region of Denmark. Therefore, verbal informed consent was obtained using waiver of documentation of consent. The study was carried out on 28 anonymous samples of surplus expectorated sputum collected for routine bacteriology from 22 CF patients with chronic P. aeruginosa infections (Table 1). Chronic P. aeruginosa infection was defined as the presence of P. aeruginosa in the lower respiratory tract in each monthly cultivation from sputum samples for > 6 months, or for a shorter time in the presence of increased antibody response to P. aeruginosa (more than two precipitating antibodies, normal: 0–1) 19. The experiments were performed before elective intravenous antibiotic treatment of the CF patients for 2 weeks every 3–4 months 20,21.
Table 1.
Demographic data of cystic fibrosis (CF) patients.
| Characteristic | |
|---|---|
| Number (male) | 22 (9) |
| Age (years)† | 38 (23–47) |
| Duration of chronic infection (years)† | 9 (4–19) |
| FEV1 (%)† | 48 (24–82) |
| FVC (%)† | 84 (27–138) |
Values are medians (range). FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity.
Concentration of dissolved O2
Sputum samples were equilibrated to normoxic conditions by diluting 10 times with Krebs–Ringer buffer [130 mM NaCl, 5 mM KCl, 0·9 mM CaCl2, 1·2 mM MgCl2, 15 mM NaH2PO4 (pH = 7·4)] with 10 mM glucose equilibrated in ambient air. Dissolved O2 in the sample was measured in a reaction chamber fixed on top of a luminescent dissolved oxygen (LDO) sensor connected to an HQ40d meter (Hach Company, Loveland, CO, USA), as described previously 5. To separate the O2 consumed by NOS from total consumption, sputum was pretreated with the NOS-inhibitor, 2 mM NG-monomethyl-L-arginine (L-NMMA) (M7033; Sigma, St Louis, MO, USA) for 10 min at 37°C before recording the concentration of O2 in the samples for 30 min.
Visualization of NO production
Sputum was stained with 20 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) (D23842; Life Technologies, Copenhagen, Denmark) and shaken for 30 min at 37°C. To verify that the NO content was related to NOS, sputum samples were pretreated with 2 mM L-NMMA for 10 min before the addition of DAF-FM. Detection of PMNs engaged in NO production in sputum was performed by staining with 5 μM DAF-FM and 5 μM hydroethidine (HE) (D-23107, Life Technologies) to identify PMNs with an ongoing production O2−. After incubation the samples were examined with a fluorescence microscope (BX40; Olympus, Tokyo, Japan) equipped with a fluorescein isothiocyanate-propidium iodide (FITC-PI) filter.
Quantification of intracellular NO
DAF-FM-stained sputum was washed in Krebs–Ringer buffer and fixated in 2% paraformaldehyde (PFA) (P-6148; Sigma-Aldrich, St Louis, MO, USA) in phosphate-buffered saline (PBS), pH 7·4. Cells in the fixed sputum were stained with PI (100 μg ml−1) (P-4170; Sigma-Aldrich) before flow cytometry. To avoid influence of the time-period between the fixation and flow cytometry, fixated samples were analysed 15 min after fixation.
Intracellular staining of iNOS in sputum
Sputum was fixated in 2% PFA in PBS (126101; USB Corporation, Cleveland, OH, USA). Fixed cells were permeabilized with 0·2 % Triton X-100 (45H04811; Sigma) and washed in PBS before incubation with immunoglobulin (Ig)G2a FITC-labelled anti-iNOS (BD 610331; Becton Dickinson, Albertslund, Denmark) and the FITC-labelled mouse isotype control (BD 340459; Becton Dickinson) diluted in mouse sera (X0910; Dako, Glostrup, Denmark) overnight on ice. Cells were stained with PI before flow cytometry.
PMN concentration
The concentration of PMNs was estimated as described previously 5. Briefly, the concentration of leucocytes in the sputum was determined by adding 100 μl diluted sputum to a TrueCount tube (BD 340334; Becton Dickinson) with 400 μl fluorescence activated cell sorter (FACS) lysis solution (BD 349202; Becton Dickinson) with PI (100 μg ml−1) and the samples were incubated for at least 10 min prior to flow cytometry. The frequency of PMNs was estimated by the addition of 5 μl of each of the following mouse anti-human monoclonal antibodies from BD Bioscience: phycoerythrin-labelled CD11b (555388), peridinin chlorophyll A protein-labelled CD14 (345786), FITC-labelled CD15 (555401) and allophycocyanin (APC)-labelled CD45 (555485) to 100 μl diluted sputum. The samples were incubated on ice for 30 min, washed with cold PBS and fixed in PBS with 2% PFA before they were analysed by flow cytometry. The concentration of PMNs was calculated by multiplying the concentration of leucocytes with the fraction of PMNs.
Flow cytometry
The samples were analysed using a FACSort (BD Bioscience, La Jolla, CA, USA) equipped with a 15 mV argon-ion laser tuned at 488 nm, and a red diode laser emitting at 635 nm for excitation. Light-scatter, time and exponentially amplified fluorescence parameters from at least 10 000 events were recorded in list mode. Host cells were discriminated according to their DNA content and their morphology by gating on the PI fluorescence intensity and light-scatter. Leucocytes were discriminated by gating on CD45 and the PMNs were discriminated by gating on CD11b, CD14 and CD15, as described previously 5. The instrument was calibrated using Calibrite beads (BD Bioscience).
NO3− and NO2− quantification
The concentration of NO3− and NO2− in sputum was measured in 11 samples using the Griess colorimetric reaction (no. 780001; Cayman Chemicals, Ann Arbor, MI, USA) according to the manufacturer's recommendations. From each sputum sample, 100 μl were immediately diluted ×10 in PBS and stored at −20°C for later analysis. Frozen sputum samples were transferred to a 96-well microtitre plate. NO2− concentration was estimated by addition of the Griess reagent for 10 min, whereby NO2− was converted into a purple azo-compound, which was quantitated by the optical density at 540–550 nm measured with an enzyme-linked immunosorbent assay (ELISA) plate reader (Thermo Scientific Multiskan EX; Thermo Fisher Scientific Inc., BioImage, Søborg, Denmark). Total NO3− and NO2− levels were estimated by a two-step analysis process: the first step converted NO3− to NO2− utilizing NO3− reductase. After incubation for 2 h, the next step involved the addition of the Griess reagent, whereby NO2− was converted into a purple azo-compound. After incubation with Griess reagent for 10 min the optical density at 540–550 nm was measured with an ELISA plate reader (Thermo Scientific Multiskan EX; Thermo Fisher Scientific Inc., BioImage). A NO3− standard curve was used for determination of total NO3− and NO2− concentration, while a NO2− standard curve was used for determination of NO2− alone. The concentration of NO3− was calculated as the difference between the NO3− concentration and the total NO3− and NO2− concentration.
NO-microprofile
A sputum sample was added to a well in a microtitre plate (Nunc, Roskilde, Denmark). A NO-microprofile in the sputum sample was then monitored with an amperometric microsensor (Unisense, Århus, Denmark). The microsensor was prepared and calibrated as described previously 22. The microsensor tip with a tip diameter of 80 μm was adjusted manually to the upper surface of the sample and vertical concentration profiles were measured by using a semi-automated set-up. The microsensor was mounted on a micromanipulator (Translation Stage VT-80; Micos, Irvine, CA, USA) and connected to a picoammeter PA2000 (Unisense). Measurements were controlled by Sensortrace Pro version 2·0 (Unisense). Profile measurements were permitted by movement of the sensor in 100-μm steps through the sputum sample.
Statistical methods
Statistical significance was evaluated by Wilcoxon's signed-rank test and Spearman's rank correlation test. A P-value < 0·05 was considered statistically significant. The tests were performed with Prism version 4·0c (GraphPad Software, La Jolla, CA, USA).
Results
NO in host cells infected sputum from CF patients
Staining cells with the NO indicator DAF-FM, a fluorescent dye that expresses fluorescence after the intracellular reaction with an intermediate of NO formed during the spontaneous oxidation of NO to NO2− 23, allowed quantification of sputum host cells with NO generation. Flow cytometry showed that a median of 75% of the cells (range 25–83%) in untreated sputum were positive for NO expression (Fig. 1a).
Fig. 1.
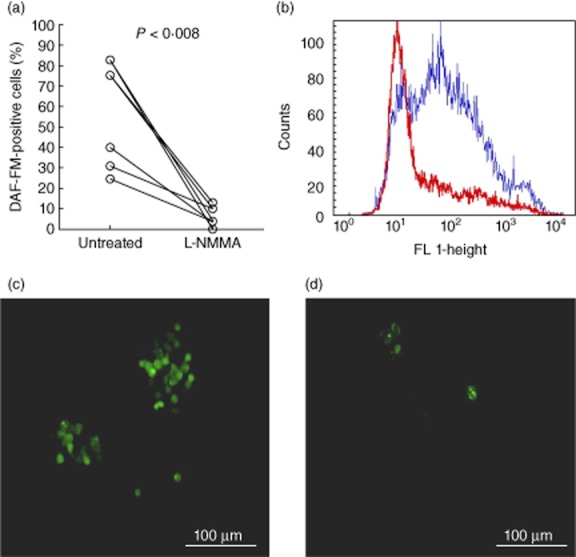
Nitric oxide (NO) expression in sputum samples from cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa lung infection. (a) Mean percentage of cells positive for 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) fluorescence in untreated sputum and in sputum treated with 2 mM NG-monomethyl-L-arginine (L-NMMA) (P < 0·002, n = 8). Statistical analysis was performed by Wilcoxon's signed-rank test. (b) Representative example of intracellular DAF-FM fluorescence (FL h1- height) in untreated sputum cells (blue histogram) and L-NMMA-treated sputum cells (red histogram). (c) Visualization of NO production by fluorescence microscopy (green colour) of cells stained with (DAF-FM) in untreated sputum and (d) sputum treated with 2 mM L-NMMA to inhibit nitric oxide synthase (NOS).
Inhibition of NOS with L-NMMA resulted in an approximately 90% reduction of DAF-FM-positive cells (Fig. 1b), indicating that active NOS in PMNs is the major contributor to the NO formation in sputum. This was confirmed by visual inspection of untreated and L-NMMA-treated sputum samples stained with DAF-FM (Fig. 1c,d).
O2 consumption by NOS- and iNOS-positive cells in infected sputum from CF patients
Initially, the expectorated sputum was diluted 10 times in the Krebs–Ringer buffer equilibrated in ambient air to ensure similar O2 concentrations. Subsequently, O2 consumption was observed in all sputum samples (Fig. 2). When sputum was treated with L-NMMA the rate of O2 consumption decreased, which is in accordance with the O2 consumption by NOS in activated PMNs 9. The decreased O2 consumption indicates that the contribution of NOS to the total O2 consumption approximated 28% (range 9–37%) (Fig. 2).
Fig. 2.
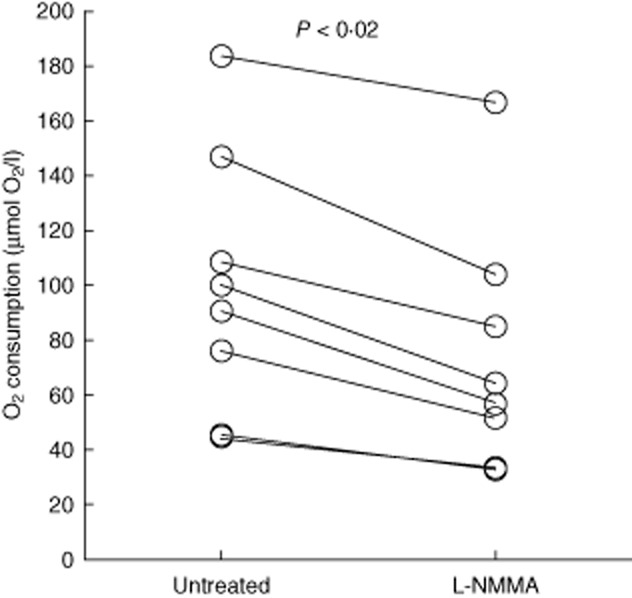
Molecular oxygen (O2) consumption by inducible nitric oxide synthase (iNOS) in sputum from cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa lung infection. Decreased O2 consumption in sputum during treatment with 2 mM NG-monomethyl-L-arginine (L-NMMA) (P < 0·008, n = 8) compared to untreated sputum. Statistical analysis was performed by Wilcoxon's signed-rank test.
The high fraction of cells in sputum with specific FITC-labelled anti-iNOS antibody, compared to the low fluorescence intensity of cells stained with FITC-labelled isotype control (Fig. 3), suggests that the majority of sputum cells is equipped with enzymes needed for production of NO. Because PMNs predominate the host cell population in CF sputum and CF epithelial cells have low expression of iNOS 24, this suggests that the PMNs employ iNOS for NO production in CF sputum.
Fig. 3.
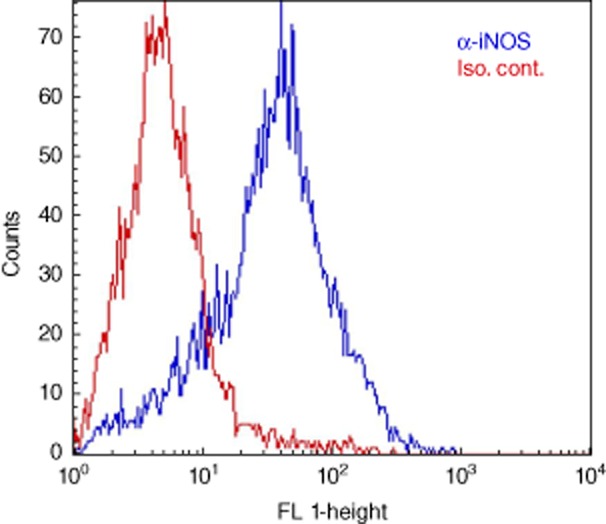
Inducible nitric oxide synthase (iNOS)-positive cells in sputum from cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa lung infection. Representative flow cytrometric histogram of sputum cells demonstrating high fluorescence (FL 1-height) in sputum stained with specific fluorescein isothiocyanate (FITC)-labelled anti-iNOS antibody (blue histogram) and low fluorescence in sputum stained with FITC-labelled isotype control antibody (red histogram).
NO production in PMNs in infected sputum from CF patients
Simultaneous staining with DAF-FM and HE in sputum enabled us to identify sputum PMNs that produce NO simultaneously with O2− due to the green fluorescence from NO reacting with DAF-FM in the cytoplasm and the red fluorescent multi-lobed nuclei resulting from staining of the DNA caused by the product from HE being oxidized by O2− 25 (Fig. 4). To ensure that the chemical reaction between the two probes did not cause an experimental artefact, we used flow cytometry to compare the green and red fluorescence from human PMNs stained with DAF-FM, HE and DAF-FM together with HE. By this means, the cross-interaction was calculated to account for less than 5% of the fluorescence of double-stained PMNs (data not shown). This level of cross-interaction was considered too low to interfere with the interpretation of the results.
Fig. 4.
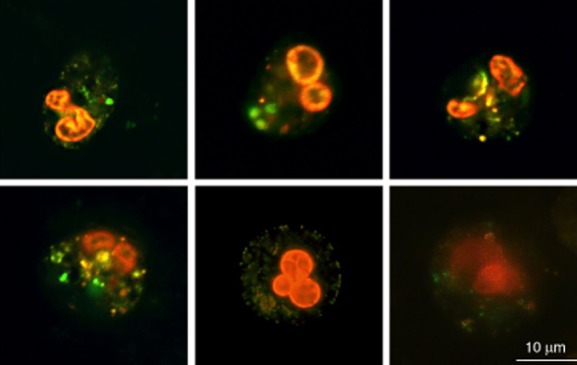
Nitric oxide (NO) and superoxide (O2−) expression in polymorphonuclear leucocytes (PMNs) in sputum from cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa lung infection. Visualization of NO and O2− production in PMNs in sputum by fluorescence microscopy stained with 20 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) (green colour) and 5 μM hydroethidine (red colour).
NO3− and NO2− concentration in infected sputum from CF patients
The simultaneous formation of NO and O2− observed in PMNs in CF sputum (Fig. 4) is likely to cause rapid formation of peroxynitrite (ONOO–) 26. ONOO− may then disintegrate to NO3− and NO2− accumulation in sputum 18. Thus, the concentration of PMNs was compared with the concentration of NO3− and NO2− in infected sputum, resulting in significant correlations (Fig. 5).
Fig. 5.
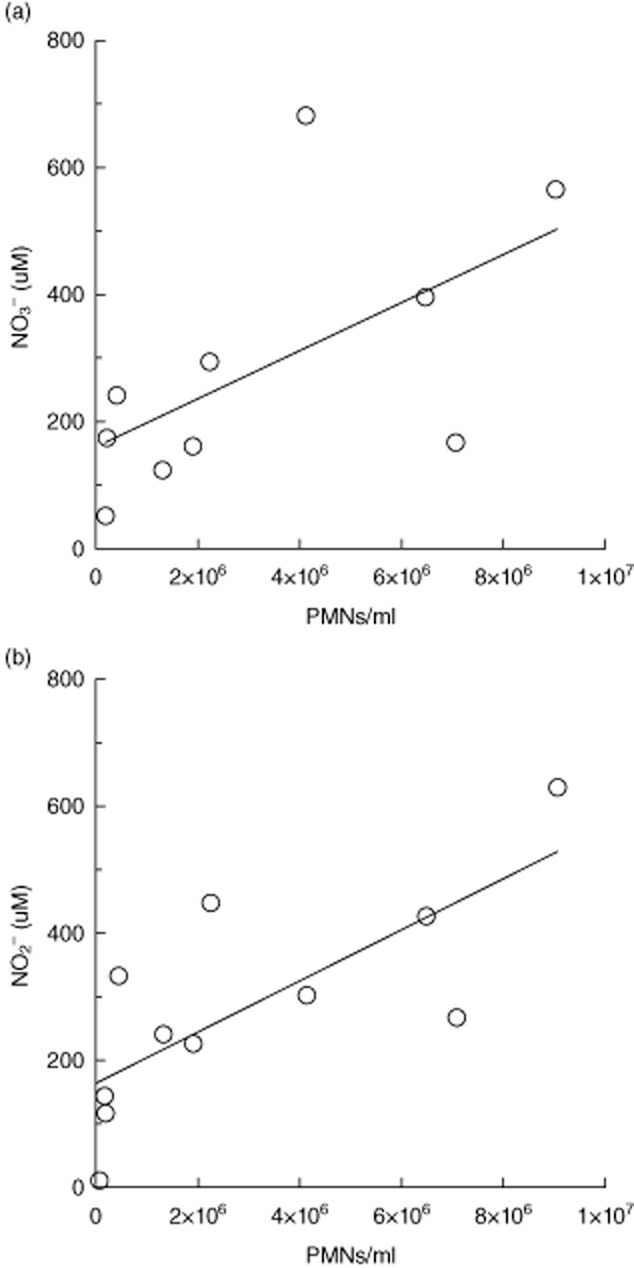
Correlations between the concentration of nitrate (NO3−)and nitrite (NO2−) to the concentration of polymorphonuclear leucocytes (PMNs) in sputum from cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa lung infection. The concentrations of NO3− and NO2− were determined by the Griess reagent system and the concentration of PMNs was estimated by flow cytometry. (a) NO3− concentration versus the concentration of PMNs (P < 0·04, n = 10). (b) NO2− concentration versus the concentration of PMNs (P < 0·006, n = 11). Statistical analysis was performed by Spearman's rank correlation test.
NO in sputum from CF patients with chronic P. aeruginosa lung infection
The sputum occasionally revealed discrete occurrences of NO when we injected a NO microsensor in to the sputum sample (Fig. 6). We measured a concentration of up to 4 μM of NO in the sputum. We believe that the profile demonstrates localized secretion of NO, which is rapidly lost due to the instability of NO 13,15,16.
Fig. 6.
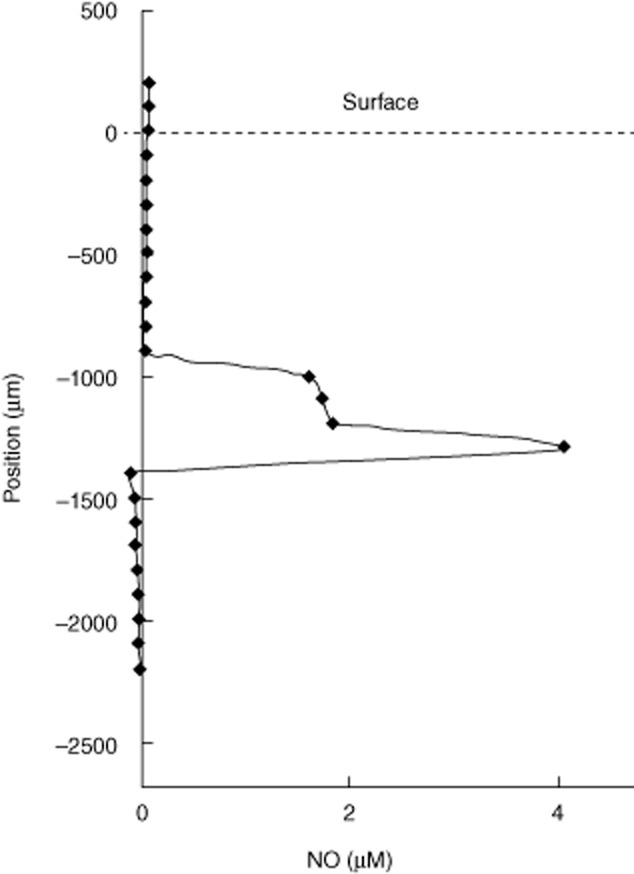
Nitric oxide (NO) profile in sputum from a cystic fibrosis (CF) patient with chronic Pseudomonas aeruginosa lung infection. Representative microprofile of NO detected with a microsensor in CF sputum showing the local distribution of the concentration of NO.
Discussion
NO production by NOS in CF airways infected with P. aeruginosa has been demonstrated previously by increased levels of exhaled NO following L-arginine inhalation 27. However, the cellular sources of NOS activity have so far not been demonstrated firmly, as iNOS expression and NO production is reduced in CF lung epithelial cells during proinflammatory stimulation 28. In the present study we now provide substantial evidence for the presence of ongoing NO production in PMNs in freshly expectorated sputum from CF patients with chronic P. aeruginosa lung infection. iNOS was detected in the majority of sputum cells and iNOS may be activated in human alveolar macrophages 29, neutrophils 14,30,31 and inflammatory cells from CF airways 32. Therefore, other cell types than PMNs may contribute to the observed NO production in sputum. The PMNs, however, constitute the major host cell population in sputum as the host cell population in CF sputum consists of leucocytes and epithelial cells, which rarely exceed 20% of the total host cell population 33, and the PMNs constitute from 96 to 99% of the leucocytes 34,35. In addition, CF epithelial cells produce low amounts of NO 28. Accordingly, we propose that PMNs predominate the population of NO-positive cells found in our study. Other leucocytes or other cells may have substantial effects on the NOS activity, but only PMNs are known to have the ability to employ anaerobic glycolysis to sustain production of energy 36,37 in order to provide NADPH 38, which is needed to fuel NOS activity 12. Thus, the minor fraction of O2 consumed in CF sputum by aerobic respiration and the resistance to treatment with KCN 5 of the luminol-enhanced chemiluminescence, which is dependent upon NOS activity 39, suggests that the influence on the NOS activity in CF sputum by cells dependent upon aerobic respiration is limited.
It is unknown how iNOS is activated in PMNs in CF sputum, although the exposure of PMNs to bacterial lipopolysaccharide (LPS), interferon (IFN)-γ and tumour necrosis factor (TNF)-α has been suggested as triggers for iNOS induction 40. In fact, LPS, IFN-γ and TNF-α are present in CF sputum 41–43, where they have the potential to activate PMNs for both the respiratory burst and NO production.
We have previously demonstrated ongoing rapid O2 uptake during the respiratory burst of PMNs in sputum from CF patients chronically infected with P. aeruginosa 5. Both the inhibition of O2 consumption and NO generation with L-NMMA and DAF-FM demonstrated in the present study are indications of an ongoing NO generation by the PMNs in sputum from chronically infected CF patients. The process of equilibrating the sputum samples to ambient air by dilution in Krebs–Ringer buffer is unlikely to have activated iNOS in the sputum cells, as it has been shown previously that human PMNs are not activated by resuspension in Krebs–Ringer buffer 5. We thus argue that iNOS was already active in the endobronchial mucus prior to equilibration of the sputum samples. Our demonstration of ongoing iNOS activity in PMNs from CF sputum thus suggests strongly that iNOS in PMNs in the endobronchial mucus of CF patients converts O2 and L-arginine to NO and L-citrulline, which contributes to the O2 depletion in sputum. It may be argued that our results are influenced by endothelial and neuronal NOS activity, as L-NMMA inhibits the NOS subtypes with low selectivity 10, but NOS expression in neutrophils is predominated by iNOS 14,30–32. Because NO is highly reactive and diffusible, it is recommended to apply more than one method for measuring and visualizing the production of NO 44. In the present study, we selected methods allowing us to demonstrate that inhibition of NOS results in both reduced consumption of the substrate O2 and reduced fluorescence of the NO indicator, DAF-FM. We found that ∼28% of the O2 consumption in infected sputum samples were used by NOS activity, which is in agreement with the O2 consumption by NOS activity in stimulated human peripheral PMNs 9. However, in addition to production of NO, active iNOS may also mediate the formation of minor amounts of O2− 12. We have shown previously that the majority of the O2 consumption in infected sputum is caused by the respiratory burst in PMNs, accounting for approximately 60% of the O2 consumption in sputum 5. The results from the present study thus corroborate that activated PMNs are the major consumers of O2 in infected endobronchial secretions in CF.
We still need to demonstrate in-vivo NO production by PMNs directly in the lungs of the CF patients, and our findings do not necessarily reflect the situation of all PMNs in the endobronchial mucus of chronically infected CF patients, as the O2 supply can vary 4. Nevertheless, NO can persist at micromolar concentrations in solutions for several minutes before it reacts with other molecules 26, and a few preliminary measurements of NO microprofiles with NO microelectrodes in fresh sputum indicated localized concentrations of up to 4 μM NO, which was rapidly degraded. Furthermore, the large percentage of cells positive for NO generation in sputum samples strongly suggests that iNOS in PMNs was active in the lungs even before expectoration. This implies that the iNOS in PMNs enabled the production of NO from O2, as demonstrated experimentally 9.
To determine if sputum PMNs consume O2 for simultaneous NO and O2− production, the co-expression of these reactive species in the PMNs from the sputum samples further indicates ongoing activity of both iNOS and NOX-2. The respiratory burst produces O2− that combines rapidly with NO to generate ONOO− 18,45. In fact, O2− released by activated PMNs can thus decrease NO 46, and we suggest that NO consumption by the reaction with O2− contributes to the decreased levels of exhaled NO seen in CF patients 47. In addition, nitrosative lesions in the lung tissue of CF patients can occur as a consequence of ONOO− reacting with tyrosine residues, leading to the increased content of 3-nitrotyrosine in CF lungs 48. The combination of NO with O2− to generate ONOO− dramatically enhances the bactericidal effect of NO 49, and P. aeruginosa relies on the nitric oxide reductase (NOR) to obtain tolerance against phagocytic NO 50. Interestingly, NOR is up-regulated in mucoid clinical isolates, which is the most virulent phenotype 7, and during hypoxic growth utilizing physiological levels of NO3− P. aeruginosa may transiently release NO in the micromolar range 51, which may contribute to the DAF-FM fluorescence that remained during inhibition with L-NMMA.
PMNs can reduce ONOO–, resulting from the reaction of NO with O2−, to the stable end-products NO3− and NO2− as the protonated form of ONOOH is decomposed 52. Accordingly, our demonstration of a correlation between the density of PMNs with the concentration of NO3− and NO2− in the sputum is in agreement with the spontaneous release of NO3− and NO2− by PMNs isolated from CF sputum 53. We therefore suggest that activated PMNs are involved in the accumulation of the NO3− and NO2− in CF sputum 47,54,55 due to the combined action of iNOS and NOX-2. Furthermore, the finding of elevated NO3− and NO2− in CF sputum samples caused by inhalation of NO 56 suggests that O2− from activated PMNs 5 is able to react with the inhaled NO.
Both NO3− and NO2− can function as terminal electron acceptors in bacteria either through the assimilatory pathway, where NO3− is reduced to ammonia, or through the dissimilatory pathway, where NO3− is reduced to nitrogen gas (N2) during the denitrification process 57. Interestingly, denitrification in the anoxic endobronchial secretions of CF patients with chronic P. aeruginosa lung infection is indicated by the production of nitrous oxide 6, the presence of OprF porin 58 and isolates with up-regulated genes involved in denitrification 7,59,60. Therefore, we speculate that the activated iNOS and NOX-2 of the PMNs shapes the microenvironment in the infected CF mucus by accelerating O2 depletion and secretion of NO3− and NO2− to a level which favours pathogens with anaerobic survival strategies such as denitrification. P. aeruginosa, which tolerates the assaults from activated PMNs by forming biofilm aggregates 2, is a facultative anaerobe capable of denitrification 57. Therefore, P. aeruginosa is well adaptable to these changes in the lung microenvironment enforced by active PMNs. Furthermore, the microenvironment may have a profound effect on the action of antibiotics, and improvement of treatment is likely to rely on knowledge of the infectious microenvironment 61.
In conclusion, we have demonstrated significant NO production in PMNs in sputum from CF patients with chronic P. aeruginosa lung infection that can further deplete O2 and facilitate growth and persistence driven by denitrification in biofilms aggregates of pathogenic bacteria, and can cause nitrosative lesions in the nearby lung tissue of the patients. Further research on nitrogen metabolism and its regulation, both in PMNs and P. aeruginosa, may thus gain important new insight and yield new targets for anti-microbial treatment of chronic lung infections.
Acknowledgments
We thank Marjan Yousefi for skillful and essential technical assistance. T. B. was funded by the Lundbeck Foundation. M. Kühl was funded by the Danish Council for Independent Research | Natural Sciences.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Koch C, Høiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 2.Jensen PØ, Givskov M, Bjarnsholt T, Moser C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol. 2010;59:292–305. doi: 10.1111/j.1574-695X.2010.00706.x. [DOI] [PubMed] [Google Scholar]
- 3.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 4.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolpen M, Hansen CR, Bjarnsholt T, et al. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax. 2010;65:57–62. doi: 10.1136/thx.2009.114512. [DOI] [PubMed] [Google Scholar]
- 6.Kolpen M, Kühl M, Bjarnsholt T, et al. Nitrous oxide production in sputum from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. PLOS ONE. 2014;9:e84353. doi: 10.1371/journal.pone.0084353. doi: 10.1371/journal.pone.0084353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee B, Schjerling CK, Kirkby N, et al. Mucoid Pseudomonas aeruginosa isolates maintain the biofilm formation capacity and the gene expression profiles during the chronic lung infection of CF patients. APMIS. 2011;119:263–274. doi: 10.1111/j.1600-0463.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- 8.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 1994;341:65–68. doi: 10.1016/0014-5793(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler MA, Smith SD, Garcia-Cardena G, Nathan CF, Weiss RM, Sessa WC. Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Invest. 1997;99:110–116. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans TJ, Buttery LD, Carpenter A, Springall DR, Polak JM, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci USA. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuehr DJ. Enzymes of the L-arginine to nitric oxide pathway. J Nutr. 2004;134:2748S–2751. doi: 10.1093/jn/134.10.2748S. [DOI] [PubMed] [Google Scholar]
- 14.Cedergren J, Follin P, Forslund T, Lindmark M, Sundqvist T, Skogh T. Inducible nitric oxide synthase (NOS II) is constitutive in human neutrophils. APMIS. 2003;111:963–968. doi: 10.1034/j.1600-0463.2003.1111008.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobzik L. Translating NO biology into clinical advances: still searching for the right dictionary? Am J Respir Cell Mol Biol. 2009;41:9–13. doi: 10.1165/rcmb.2009-0156TR. [DOI] [PubMed] [Google Scholar]
- 16.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 17.Arai H. Regulation and function of versatile aerobic and anaerobic respiratory metabolism in Pseudomonas aeruginosa. Front Microbiol. 2011;2:103. doi: 10.3389/fmicb.2011.00103. doi: 10.3389/fmicb.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassett DJ, Cuppoletti J, Trapnell B, et al. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv Drug Deliv Rev. 2002;54:1425–1443. doi: 10.1016/s0169-409x(02)00152-7. [DOI] [PubMed] [Google Scholar]
- 19.Høiby N. Microbiology of cystic fibrosis. In: Hodson ME, Geddes DM, editors. Cystic fibrosis. London, UK: Arnold; 2000. pp. 83–107. [Google Scholar]
- 20.Koch C, Høiby N. Diagnosis and treatment of cystic fibrosis. Respiration. 2000;67:239–247. doi: 10.1159/000029503. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen SS, Jensen T, Hoiby N, Koch C, Flensborg EW. Management of Pseudomonas aeruginosa lung infection in Danish cystic fibrosis patients. Acta Paediatr Scand. 1987;76:955–961. doi: 10.1111/j.1651-2227.1987.tb17271.x. [DOI] [PubMed] [Google Scholar]
- 22.Revsbech NP. An oxygen microsensor with a guard cathode. Limnol Oceanogr. 1989;34:474–478. [Google Scholar]
- 23.Kojima H, Nakatsubo N, Kikuchi K, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 24.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest. 1998;102:1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Kalivendi S, Zhang H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 26.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 27.Grasemann H, Tullis E, Ratjen F. A randomized controlled trial of inhaled l-arginine in patients with cystic fibrosis. J Cyst Fibros. 2013;12:468–474. doi: 10.1016/j.jcf.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Meng QH, Polak JM, Edgar AJ, et al. Neutrophils enhance expression of inducible nitric oxide synthase in human normal but not cystic fibrosis bronchial epithelial cells. J Pathol. 2000;190:126–132. doi: 10.1002/(SICI)1096-9896(200002)190:2<126::AID-PATH500>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988;27:8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- 30.McCall TB, Feelisch M, Palmer RM, Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991;102:234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yui Y, Hattori R, Kosuga K, et al. Calmodulin-independent nitric oxide synthase from rat polymorphonuclear neutrophils. J Biol Chem. 1991;266:3369–3371. [PubMed] [Google Scholar]
- 32.Warner RL, Paine R, III, Christensen PJ, et al. Lung sources and cytokine requirements for in vivo expression of inducible nitric oxide synthase. Am J Respir Cell Mol Biol. 1995;12:649–661. doi: 10.1165/ajrcmb.12.6.7539274. [DOI] [PubMed] [Google Scholar]
- 33.Sadeghi E, Matlow A, MacLusky I, Karmali MA. Utility of Gram stain in evaluation of sputa from patients with cystic fibrosis. J Clin Microbiol. 1994;32:54–58. doi: 10.1128/jcm.32.1.54-58.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 35.Hector A, Jonas F, Kappler M, Feilcke M, Hartl D, Griese M. Novel method to process cystic fibrosis sputum for determination of oxidative state. Respiration. 2010;80:393–400. doi: 10.1159/000271607. [DOI] [PubMed] [Google Scholar]
- 36.Sbarra AJ, Karnovsky ML. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959;234:1355–1362. [PubMed] [Google Scholar]
- 37.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borregaard N, Schwartz JH, Tauber AI. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984;74:455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang PH, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart – evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 40.Geller DA, Billiar TR. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998;17:7–23. doi: 10.1023/a:1005940202801. [DOI] [PubMed] [Google Scholar]
- 41.Jensen PØ, Moser C, Kharazmi A, Pressler T, Koch C, Høiby N. Increased serum concentration of G-CSF in cystic fibrosis patients with chronic Pseudomonas aeruginosa pneumonia. J Cyst Fibros. 2006;5:145–151. doi: 10.1016/j.jcf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Kronborg G, Shand GH, Fomsgaard A, Høiby N. Lipopolysaccharide is present in immune complexes isolated from sputum in patients with cystic fibrosis and chronic Pseudomonas aeruginosa lung infection. APMIS. 1992;100:175–180. [PubMed] [Google Scholar]
- 43.Paats MS, Bergen IM, Bakker M, et al. Cytokines in nasal lavages and plasma and their correlation with clinical parameters in cystic fibrosis. J Cyst Fibros. 2013;12:623–629. doi: 10.1016/j.jcf.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 46.Jones KL, Bryan TW, Jinkins PA, et al. Superoxide released from neutrophils causes a reduction in nitric oxide gas. Am J Physiol. 1998;275:L1120–1126. doi: 10.1152/ajplung.1998.275.6.L1120. [DOI] [PubMed] [Google Scholar]
- 47.Grasemann H, Michler E, Wallot M, Ratjen F. Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatr Pulmonol. 1997;24:173–177. doi: 10.1002/(sici)1099-0496(199709)24:3<173::aid-ppul2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 48.Jones KL, Hegab AH, Hillman BC, et al. Elevation of nitrotyrosine and nitrate concentrations in cystic fibrosis sputum. Pediatr Pulmonol. 2000;30:79–85. doi: 10.1002/1099-0496(200008)30:2<79::aid-ppul1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Brunelli L, Crow JP, Beckman JS. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch Biochem Biophys. 1995;316:327–334. doi: 10.1006/abbi.1995.1044. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman LR, Richardson AR, Houston LS, et al. Nutrient availability as a mechanism for selection of antibiotic tolerant Pseudomonas aeruginosa within the CF airway. PLOS Pathog. 2010;6:e1000712. doi: 10.1371/journal.ppat.1000712. doi: 10.1371/journal.ppat.1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kakishima K, Shiratsuchi A, Taoka A, Nakanishi Y, Fukumori Y. Participation of nitric oxide reductase in survival of Pseudomonas aeruginosa in LPS-activated macrophages. Biochem Biophys Res Commun. 2007;355:587–591. doi: 10.1016/j.bbrc.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Kissner R, Koppenol WH. Product distribution of peroxynitrite decay as a function of pH, temperature, and concentration. J Am Chem Soc. 2002;124:234–239. doi: 10.1021/ja010497s. [DOI] [PubMed] [Google Scholar]
- 53.Francoeur C, Denis M. Nitric oxide and interleukin-8 as inflammatory components of cystic fibrosis. Inflammation. 1995;19:587–598. doi: 10.1007/BF01539138. [DOI] [PubMed] [Google Scholar]
- 54.Hassett DJ. Anaerobic production of alginate by Pseudomonas aeruginosa: alginate restricts diffusion of oxygen. J Bacteriol. 1996;178:7322–7325. doi: 10.1128/jb.178.24.7322-7325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmer KL, Brown SA, Whiteley M. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J Bacteriol. 2007;189:4449–4455. doi: 10.1128/JB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ratjen F, Gartig S, Wiesemann HG, Grasemann H. Effect of inhaled nitric oxide on pulmonary function in cystic fibrosis. Respir Med. 1999;93:579–583. doi: 10.1016/s0954-6111(99)90158-0. [DOI] [PubMed] [Google Scholar]
- 57.Zumft WG. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon SS, Hennigan RF, Hilliard GM, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell. 2002;3:593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 59.Son MS, Matthews WJ, Jr, Kang Y, Nguyen DT, Hoang TT. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun. 2007;75:5313–5324. doi: 10.1128/IAI.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciofu O, Johansen HK, Aanaes K, et al. P. aeruginosa in the paranasal sinuses and transplanted lungs have similar adaptive mutations as isolates from chronically infected CF lungs. J Cyst Fibros. 2013;12:729–736. doi: 10.1016/j.jcf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Bjarnsholt T, Alhede M, Alhede M, et al. The in vivo biofilm. Trends Microbiol. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]


