Abstract
The enzyme-linked immunospot (ELISPOT) assay is a widely used tool for enumeration of antigen-specific memory B cells in several disciplines, such as vaccination, cancer immunotherapy and transplantation. For the accurate estimation of antigen-specific memory B cell frequencies, a well-defined B cell activation protocol is pivotal. In this study, we aimed to characterize a polyclonal B cell activation protocol to facilitate optimal monitoring of antigen-specific memory B cell frequencies. Total, naive and memory B cells were activated polyclonally with an α-CD40 monoclonal antibody, cytosine–phosphate–guanine (CPG) oligodeoxynucleotide (ODN) 2006, interleukin (IL)-2, IL-10 and IL-21. Polyclonal activation of B cells resulted in equal cell death ratios in naive and memory B cells. When tested in an antigen-specific system, immunoglobulin (Ig)G spots were detected only in the memory fraction. There was no change in B cell polyclonality due to in-vitro activation. Our data show that the current polyclonal activation protocol may be used reliably to estimate the frequency of memory B cells in ELISPOT assays.
Keywords: antibodies, antibody-secreting cells, B cells, ELISPOT
Introduction
The extent of humoral immune responses can be characterized by quantifying the antigen-specific memory compartment using in-vitro assays. Quantification of the humoral immune response is of particular significance in many disciplines, such as vaccination, cancer immunotherapy and transplantation. In the field of vaccination, it is important to characterize the normal immune response to a pathogen as well as to monitor the protective response elicited by vaccination in terms of immunological memory 1,2. Measuring the memory B cell response is crucial to evaluate the efficacy of the vaccine and to eventually identify the risk groups that will not benefit from the vaccine in infectious diseases 3–5 or cancer immunotherapy 6.
In solid organ transplantation, detecting and quantifying memory B cells capable of producing donor-directed anti-human leucocyte antigen (HLA) antibodies in a patient will potentially aid in defining the post-transplant immunological risk 7. Currently available methods detecting anti-HLA antibodies in the serum do not provide any information on the magnitude of the memory response.
Quantification of the humoral immune response in sensitized individuals by detection of HLA-specific B cells has been performed previously by us and others 8,9. However, only a few studies aimed at the detection and enumeration of the relatively low levels of HLA-specific memory B cells 10,11. Our group has recently developed an HLA-specific B cell enzyme-linked immunospot (ELISPOT) assay which allows for the quantification of memory B cell frequencies directed towards defined HLA molecules 11. This technique was recently adapted by Lynch and colleagues to detect B cell memory towards donor-specific HLA class I on cultured fibroblasts from donor origin 12.
Both naive and memory B cells can differentiate into antibody-secreting cells (ASC) upon antigen-specific stimulation 2. In vitro, polyclonal activation can result in the antigen-independent differentiation of B cells into ASC 13,14. There are several protocols for the polyclonal activation of human B cells, most of which favour isotype switching or plasma cell differentiation of naive B cells, particularly after long-term culture 15–19. However, to estimate the actual frequency of antigen-specific memory B cells in a patient, an activation protocol that does not induce immunoglobulin (Ig)G production in the naive B cell population is preferred. In the present study, we aimed to determine the kinetics of in-vitro human B cell activation upon a previously defined activation protocol 20,21. Here, we report the distinct proliferation kinetics and antibody production patterns of naive and memory B cells and show that the current polyclonal B cell activation protocol can be used for specifically enumerating memory B cell frequencies using techniques such as the ELISPOT assay.
Materials and methods
Peripheral blood B cell isolation
Peripheral blood was obtained with informed consent from blood bank donors under guidelines issued by the Medical Ethics Committee of the Leiden University Medical Center (Leiden, the Netherlands). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Hypaque (Pharmacy LUMC, Leiden, the Netherlands) density gradient centrifugation and frozen in liquid nitrogen until further use. After thawing, B cells were isolated by negative selection using the EasySep Human B cell enrichment kit (Stem Cell Technologies, Grenoble, France), according to the manufacturer's instructions. The purity of B cells was found to be >98%, as assessed by CD19 positivity measured by flow cytometry (FCM).
Naive and memory B cell separation
For some experiments, isolated total B cells were further sorted on fluorescence activated cell sorter (FACS) AriaII (BD Biosciences, Breda, the Netherlands) into CD3–CD19+IgD+CD27– naive and CD3–CD19+IgD–CD27+ memory B cells using the following monoclonal antibodies (clone): CD3-Pacific Blue (UCHT1), CD19-allophycocyanin (APC-cyanin 7 (Cy7) (SJ25C1), IgD-phycoerythrin (PE) (IA6-2; all from BD Biosciences) and CD27-fluorescein isothiocyanate (FITC) (CLB-CD27/1, 9F-4; Sanquin, Amsterdam, the Netherlands). Cell sorting purity for both fractions was more than 95% after sorting.
Cell cultures
Cell cultures were carried out in Iscove's modified Dulbecco's medium (IMDM) (Gibco Invitrogen, Paisley, UK) containing 10% fetal calf serum (FCS) (Gibco Invitrogen), supplemented with 50 μM 2-mercaptoethanol (Sigma-Aldrich, Zwijndrecht, the Netherlands), 2 mM L-glutamine (Gibco Invitrogen), ITS (5 μg/ml insulin, 5 μg/ml transferrin, sodium selenite 5 ng/ml (Sigma-Aldrich) and 100 U/ml penicillin with 100 μg/ml streptomycin (Gibco Invitrogen). Cells were activated with an activation cocktail consisting of 500 ng/ml α-CD40 monoclonal antibody (R&D Systems, Minneapolis, MN, USA), 2·5 μg/ml Toll-like receptor-9 (TLR-9) ligand oligodeoxynucleotides (ODN)-2006 cytosine–phosphate–guanine (CpG) (Hycult Biotechnology, Uden, the Netherlands), 600 IU/ml interleukin (IL)-2 (Proleukin, Amsterdam, the Netherlands), 25 ng/ml IL-10 (R&D Systems) 100 ng/ml IL-21 (Gibco Invitrogen), as described previously 21, and cultured at 37°C in a 5% CO2 humidified incubator. Total, naive and memory B cell fractions were cultured separately for various lengths of time by seeding 1 × 104 cells/well in 96-well U-bottomed plates (BD Falcon, Breda, the Netherlands). In experiments aiming to detect antigen-specific responses, B cell fractions were activated at 2·5 × 105 cells/well in 24-well flat-bottomed plates (Corning Inc., Corning, NY, USA). Cell viability at each harvest time-point was defined by 7-aminoactinomycinD (7-AAD) exclusion (BD Biosciences) by FCM, as well as eosin staining using light microscopy.
Analysis of activation markers
Total, naive and memory B cells were monitored for the expression of activation markers before and after polyclonal activation at various time-points using the following monoclonal antibodies (clone): CD25-PE-Cy7 (M-A251) (BD Biosciences) and CD69-ECD (TP1·55·3; Beckman Coulter). 7-AAD exclusion and side-scatter (SSC) were used to gate on living cells. Cells were acquired on an LSRII flow cytometer (BD Biosciences) and analysed using FlowJo software (version 10, Tree Star Inc., Ashland, OR, USA).
Proliferation assay
B cell proliferation was measured by pulsing with 1 μCi tritiated thymidine ([3H]-TdR; Amersham International, Amersham, UK) per well for the last 18 h of each culture time-point. [3H]-TdR incorporation was measured using a liquid scintillation counter (Perkin-Elmer, Groningen, the Netherlands). Results are expressed as the mean counts per minute (cpm) of triplicate wells.
ELISPOT assays
Total IgM and IgG-producing cell numbers were measured by ELISPOT assays, as described previously 20. In brief, 96-well ELISPOT plates (Millipore, Billerica, MA, USA) were coated with either goat α-IgM or α-IgG antibodies (both from Jackson Immunoresearch Laboratories, Inc., Baltimore, PA, USA) diluted in phosphate-buffered saline (PBS) and incubated overnight. To detect antigen-specific responses, plates were coated with 1 Lf/ml tetanus toxoid (TT) antigen (Dutch Vaccine Institute, Bilthoven, the Netherlands) diluted in PBS. Plates were blocked for at least 1 h with 5% FCS/IMDM, after which activated B cells were added at various cell concentrations for 6 h at 37°C in a 5% CO2 humidified incubator. After washing, biotinylated goat α-IgM and α-IgG antibodies (both from Invitrogen) were added to the appropriate wells and incubated at 4°C overnight. Following washing, plates were incubated with streptavidin-conjugated alkaline phosphatase (Sigma-Aldrich) for 1 h at room temperature. 5-Bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (BCIP/NBT) substrate (Mabtech, Nacka Strand, Sweden) was added to visualize the spots. The reaction was stopped by cold tap-water, and after drying the plates were analysed by an automated ELISPOT reader (AID, Straßberg, Germany).
Complementarity determining region 3 (CDR3) fragment analysis
Non-activated and activated B cells were preserved in RNAlater solution (Qiagen, Venlo, the Netherlands) following isolation and harvesting. Total RNA was extracted using the NucleoSpin miRNA kit (Bioké, Leiden, the Netherlands), according to the manufacturer's instructions. Complementary DNA synthesis was carried out using SuperScript III reverse transcriptase (Life Technologies, Paisley, UK), as described previously 22. Polymerase chain reaction (PCR) assays were performed with each of seven forward primers covering the different VH1–7 chains in combination with a fluorophore-labelled reverse primer specific for the constant region of IgM (μ) or IgG (γ). Primer sequences have been described in a previous paper 23. Amplified fragments were separated and visualized using a 3130xl Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). Genescan 400HD (ROX) size standard (Applied Biosystems) was used for accurate determination of the size of the DNA fragments. Fragment analysis was performed using Peak Scanner software (version 1; Applied Biosystems).
Statistics
Correlations between the distributions of VH families before and after activation of B cells for IgM and IgG were analysed by Spearman's rank test using Graphpad Prism software (version 6·02). The statistical level of significance was defined as P < 0·05.
Results
Both naive and memory B cells are activated upon polyclonal activation
In order to assess whether our activation protocol led to activation of both naive and memory B cells, we assessed the expression of the activation markers CD69 and CD25 on both subsets, as well as on total B cells. As shown in Fig. 1, all B cell fractions up-regulated CD69 and CD25 by day 1 of polyclonal activation. The percentage of the early activation marker CD69 decreased to baseline levels by day 2 in the memory fraction, followed by naive B cells at day 4. The percentage of CD25+ cells remained constantly high in both naive and memory B cell fractions.
Fig. 1.
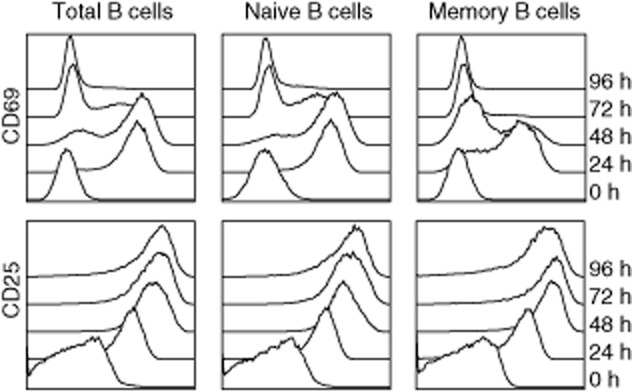
Immunophenotyping for cell surface activation markers. Total, naive and memory B cells were monitored for the expression of CD69 and CD25 before (0 h) and at several time-points (24–96 h) after polyclonal activation. All cells up-regulated both activation markers as early as 24 h following activation. Results are representative of two experiments with different donors.
Naive and memory B cells have similar cell death kinetics upon polyclonal activation
Polyclonal B cell activation is accompanied by a certain degree of cell death 20. We wanted to know whether the induction of cell death by activation is distributed equally among naive and memory B cells. Upon polyclonal activation, there was no difference in the proportion of dead cells among the total, naive and memory B cell populations throughout the culture period. In time, the dead : alive cell ratio increased sharply in all fractions after day 6 (Fig. 2). We obtained similar results when assessing cell death by eosin exclusion using light microscopy (data not shown).
Fig. 2.
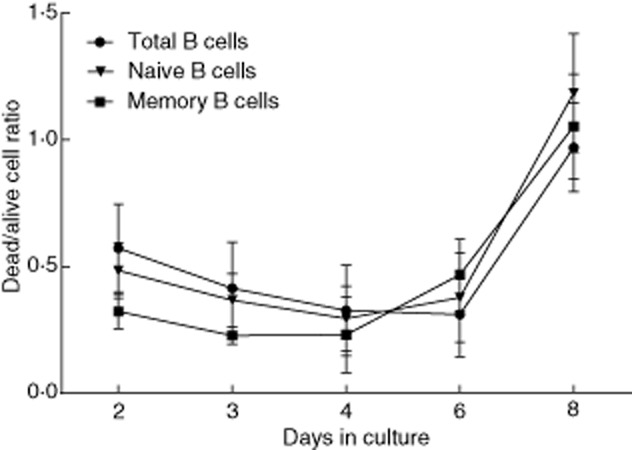
Dead : alive cell ratios among B cell subsets upon polyclonal activation. Total, naive and memory B cells were activated polyclonally for varying lengths of time. At each time-point, cells were harvested and cell viability was determined by 7-aminoactinomycin D (7-AAD) exclusion by flow cytometry. Dead : alive cell ratios for each time-point in all B cell fractions were obtained from percentages of dead (7-AAD-positive) and alive (7-AAD-negative) cells. Dead : alive cell ratios were similar among total, naive and memory B cells. Results are expressed as mean ± standard deviation (s.d.) of seven independent experiments performed with a minimum of three and a maximum of seven donors for each time-point.
Naive B cells proliferate more strongly upon polyclonal activation compared to memory B cells
To address the proliferation kinetics of the naive and memory B cell subsets, an equal number of total, as well as sorted naive and memory B cells, were activated polyclonally and analysed for proliferation by [3H]-TdR incorporation on consecutive days. Upon polyclonal activation, the total B cell fraction proliferated increasingly up to day 6, after which the proliferation gradually declined (Fig. 3, white bars). Similarly, the naive B cell fraction proliferated increasingly up to day 6, with a steep increase from days 3 to 4. After day 6, a steep decline in the proliferation was also observed (Fig. 3, grey bars). Finally, memory B cells started proliferating earlier upon activation compared to naive B cells, with peak proliferation at day 3, after which proliferation gradually declined (Fig. 3, black bars). The maximum proliferation of the two B cell fractions at their respective peak proliferation days was 1·96-fold higher for naive B cells compared to memory B cells.
Fig. 3.
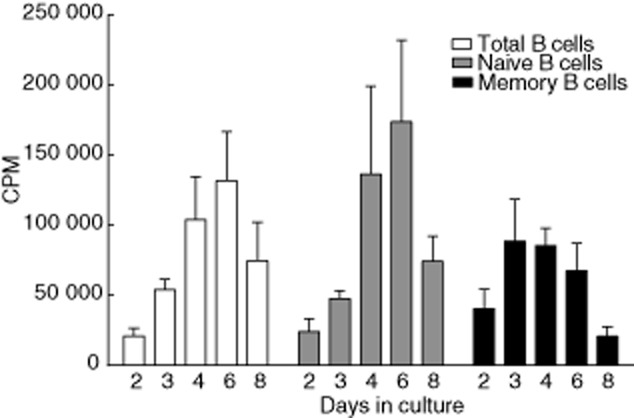
Proliferation kinetics of B cells upon polyclonal activation. Total, naive and memory B cells were activated polyclonally for varying lengths of time. Cell proliferation for each fraction was measured by tritiated thymidine ([3H]-TdR) incorporation. Results are expressed as mean counts per minute (cpm) ± standard deviation (s.d.) of seven independent experiments performed with a minimum of three and a maximum of seven donors for each time-point. Total B cells (white bars); naive B cells (grey bars); memory B cells (black bars).
The frequency estimation of antigen-specific memory B cells in unseparated B cells is not affected by IgG induction in the naive B cell compartment
Next, we wanted to address the kinetics of antibody production, and whether or not polyclonal activation induced any IgG-producing cells from the naive B cell population. The kinetics of antibody production was comparable between naive and memory B cells, having a similar tendency to increase during the course of the activation. Representative for the naive and memory B cell subset distribution in peripheral blood, the number of IgM spots at each harvest day of the culture of the total B cell fraction was higher than the number of IgG spots. Antibody production could be detected as early as day 2 after polyclonal activation (Fig. 4a).
Fig. 4.
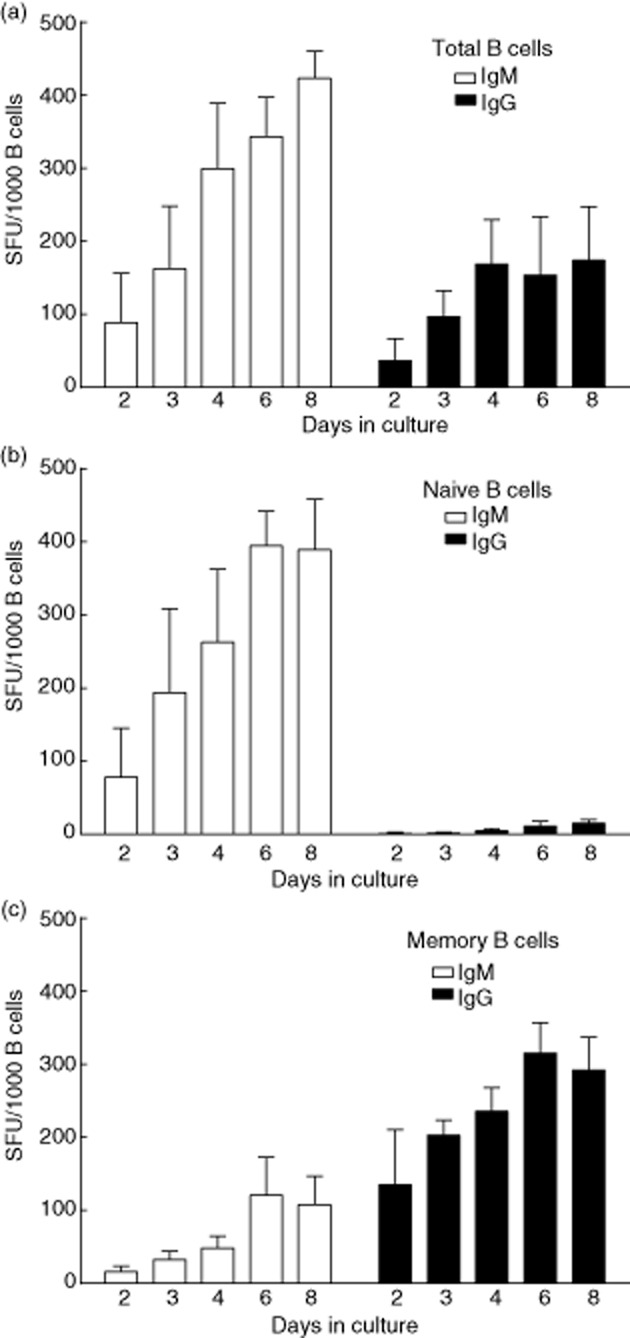
Immunoglobulin (Ig)M and IgG production kinetics of activated B cells. (a) Total, (b) naive and (c) memory B cells were activated polyclonally for varying lengths of time. At each time-point, 1 × 103 activated B cells were transferred to individual wells of enzyme-linked immunospot assay (ELISPOT) plates for IgM (white bars) and IgG (black bars) spot detection. Results are expressed as mean ± standard deviation (s.d.) of seven independent experiments performed with a minimum of three and a maximum of seven donors for each time-point. SFU = spot-forming unit.
IgM spot production was mainly by naive B cells (Fig. 4b), but was also detected in the sorted memory population. Virtually all IgG spots were produced by memory B cells (Fig. 4c) with a low, yet detectable number of IgG spots formed in the naive B cell fraction. When determining the frequency of antigen-specific memory B cells, it is pivotal that one does not measure the IgG production induced in the naive B cell population. In order to test whether or not IgG production induced in the naive B cell compartment, if any, would affect the frequency estimation of antigen-specific memory B cells, we set up a TT antigen-specific IgG B cell ELISPOT assay. Total B cells, as well as sorted naive and memory B cell fractions, were activated polyclonally for the detection of TT antigen-specific IgG spots. As shown in Fig. 5, TT-antigen specific spots could be detected in the total B cell population. There was a high interindividual variation in the number of TT-specific B cells within the memory population, as one might expect. Furthermore, we did not observe any spots in the naive B cell fraction, whereas clear spot formation was observed in the memory B cell fraction, indicating that antigen-specific IgG spots were produced only by the pre-existing memory B cells.
Fig. 5.
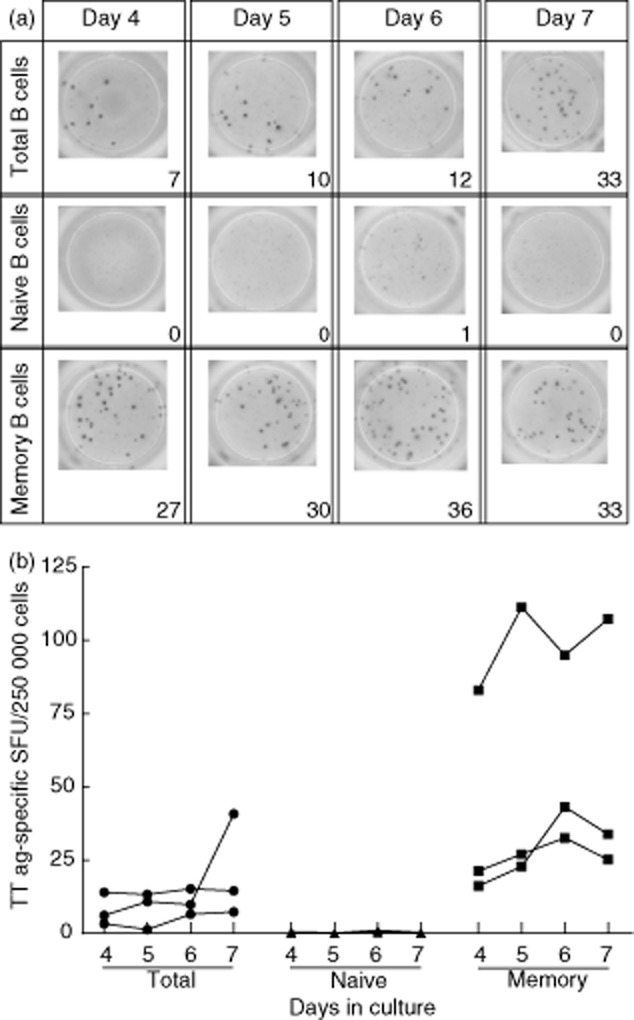
Tetanus toxoid (TT) antigen-specific spots are not detected in naive B cells upon polyclonal activation. (a) Activated naive B cells did not produce TT antigen-specific spots, whereas memory cells produced a robust number of TT specific spots. Total, naive and memory B cells were activated polyclonally for varying lengths of time. At each time-point, 2.5 × 105 activated B cells were transferred to individual wells of TT antigen-coated enzyme-linked immunospot assay (ELISPOT) plates. Representative results of one of three independent experiments are shown. (b) Cumulative data on TT antigen-specific spot-forming units (SFU) per 2·5 × 105 B cells. Total B cells (•), naive B cells (▲) and memory B cells (▪) are shown separately. Data from three independent experiments with different donors are shown.
The activation protocol does not affect the polyclonality of activated B cells
Another important aspect of an activation protocol to determine the frequency of antigen-specific memory B cells is that there is no preferential expansion of certain clones. We addressed this issue by determining the B cell receptor (BCR) repertoire of B cells by immunoscope methodology 23,24. In order to define if the repertoire diversity in IgM and IgG remained similar before and after polyclonal activation, we performed CDR3 fragment analysis on non-activated and 6-day-activated B cells. As depicted in Fig. 6, there was a strong correlation between the distribution of the peaks in all VH gene families before and after activation for IgM, indicating the persistence of the repertoire diversity. Concerning IgG, VH3 and VH4 represent the majority of all rearrangements, whereas the smallest gene families such as VH6 and VH7 are rarely used 24. In the present study, the distribution of the peaks for the commonly used VH3 and VH4 were correlated significantly before and after activation. The correlation analysis could not be performed for IgG VH6 and VH7 due to their rarity in usage.
Fig. 6.
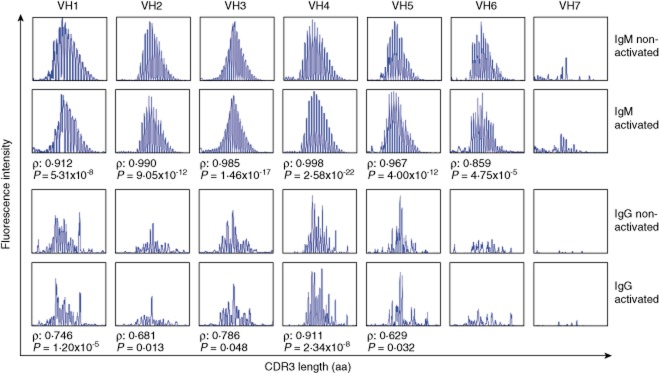
B cell receptor CDR3 length distribution is not altered significantly upon polyclonal B cell activation. Each panel shows the CDR3 length distribution profile for one of seven VH families, as determined for non-activated B cells as well as 6-day activated B cells. The x-axis indicates CDR3 length (amino acids), and the y-axis displays fluorescence intensity of polymerase chain reaction (PCR) products. Representative profiles of three independent experiments with different donors are shown. ρ = Spearman's rank correlation coefficient; CDR3 = complementarity determining region 3; aa = amino acids.
Discussion
In vivo, memory B cells provide a more rapid and higher-affinity antibody response compared to primary responses initiated by naive B cells 25. While serum antibodies are produced mainly by bone marrow-residing plasma cells 26,27, their serum levels may not be representative for the peripheral memory B cell pool. Indeed, Lynch et al. recently showed the presence of donor-specific memory B cells in the absence of donor-specific serum IgG antibodies in kidney transplant recipients 12. These, and other data 11, made clear that in-vitro polyclonal B cell activation may be used to provide a snapshot of the B cell memory pool at a given time-point. By focusing upon defined antigens, such as viral antigens or HLA molecules, the actual frequency of antigen-specific memory B cells can be determined.
The accuracy of such assays is dependent in part upon the activation protocol used. Several protocols exist to activate B cells polyclonally. Variations in cytokines, the source of CD40 ligation, if any, and the inclusion of a BCR trigger have been described 13,15,28–32. These variations, as well as differences in cell source and B cell purity, may cause different kinetics of naive versus memory B cells, both in terms of proliferation and antibody production. In this study, we were able to demonstrate the distinct proliferation kinetics and antibody production patterns of naive and memory B cells in response to a previously defined in-vitro polyclonal activation protocol based on CD40 ligation using an α-CD40 monoclonal antibody in combination with CpG DNA and B cell-activating cytokines 20,21. Our main interest is on HLA-specific memory B cells in the context of (solid organ) transplantation. As HLA-specific memory B cells are present at extremely low frequencies 11, our protocol includes a B cell purification step prior to activation to increase assay sensitivity. For B cell activation, we have selected the CD40–CD40L pathway as surrogate for T cell help in combination with ODN2006 CpG and IL-21, which are potent drivers of B cells into ASC plasma cells 18,33. In our hands, this activation protocol was superior to several other protocols, including B cell receptor ligation, Staphylococcus aureus and pokeweed mitogen (data not shown). Additionally, the high variability of the commercially available form of pokeweed mitogen, as described by Crotty et al., may render standardization difficult 34.
Many in-vitro B cell assays to assess immunoglobulin production require prolonged stimulation of B cells of up to 14 days 8,35. Such long B cell activation periods invariably lead to a certain degree of cell death, and may therefore influence clonal distribution. In the present study, dead : alive cell ratios between naive and memory B cells, as well as in total B cells, were indistinguishable at all times throughout the culture process. When we analysed the CDR3 length distribution, we found no statistically significant differences before and after activation, indicating that the clonality of activated B cells at 6 days of culture is not significantly different from the starting population.
Flow cytometric analyses showed that both naive and memory B cells were activated by our activation protocol. Expression of CD69 and CD25, generally considered as markers for B cell activation 36,37, was up-regulated in all fractions 1 day after polyclonal activation. These data suggest that the current activation protocol is potent enough to activate both the naive and memory B cells.
A reliable estimate of the size of an antigen-specific B cell pool requires that the culture protocol does not induce significant levels of isotype switching in vitro. As many polyclonal activation strategies induce a certain degree of isotype-switching, particularly after long-term cultures varying between 7 and 12 days 15–19, we aimed to determine whether the selected activation protocol in combination with an incubation time of maximally 8 days induced any IgG production in the naive B cell population. Using total IgM and IgG ELISPOT analysis, very low, yet detectable numbers of IgG spots were found in the naive B cell cultures. The detection of a small proportion of cells in the naive B cell fraction that were capable of producing IgG could be due either to the inability to yield sufficient high purity cells in FACS (average sorting purity: 98% for naive B cells) or due to activation-induced isotype switching of a small number of naive B cells. Whether or not the presence of a small number of IgG-producing cells in sorted naive B cell fractions would influence the frequency estimation of antigen-specific memory B cells, we used TT as a model antigen for ELISPOT. Using antigen-specific B cell ELISPOT assays, we did not observe any spots in polyclonally activated sorted naive B cells, suggesting that the induction of IgG in naive B cells, if any, is not interfering significantly with the estimation of antigen-specific memory B cells. Furthermore, our activation protocol may also be used to study naive B cell responses, as it also results in a potent activation of naive B cells into IgM-producing cells.
In conclusion, we were able to characterize peripheral B cells upon polyclonal activation for the purpose of determining the frequency of antigen-specific memory B cells. Polyclonal activation using α-CD40, TLR-9-triggering, IL-2, IL-10 and IL-21 can be used reliably to estimate the frequency of antigen-specific memory B cells in ELISPOT assays without the interference of IgG-producing cells in the naive B cell population and with retained polyclonality of the total B cell population.
These findings are particularly important in assays aiming at estimating the frequency of antigen-specific memory B cells, such as the HLA-specific memory B cell ELISPOT assay. Whereas in this study we used TT as a model antigen, previously we have used a similar activation protocol for the quantification of HLA-specific memory B cells 11. We are currently performing a clinical study in order to validate the clinical usefulness of the HLA-specific B cell ELISPOT assay. In this Dutch multi-centre study, purified B cells of kidney transplant recipients are activated polyclonally with the present culture system and tested in HLA-specific B cell ELISPOT assays at several time-points before and after transplantation. These assays will be complemented by detailed analysis of the HLA-specific antibody repertoire.
Acknowledgments
The authors thank Edwin de Haas for the technical assistance in cell sorting and Geert W. Haasnoot in statistical analysis. This work was supported by the National Reference Center for Histocompatibility Testing. S. H. is supported by a grant from the Dutch Kidney Foundation (VR.12·01)
Disclosures
Authors have no financial conflicts of interest.
Author contributions
G. E. K designed and performed the experiments, collected and analysed the data and wrote the paper; M. E. designed the experiments; J. D. H. A. performed the experiments and analysed the data; F. H. J. C. analysed the data and wrote the paper; S. H. designed the study, analysed the data and wrote the paper.
References
- 1.Kyu SY, Kobie J, Yang H, et al. Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells. J Immunol Methods. 2009;340:42–47. doi: 10.1016/j.jim.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Halliley JL, Kyu S, Kobie JJ, et al. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–3587. doi: 10.1016/j.vaccine.2010.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss GE, Ndungu FM, McKittrick N, et al. High efficiency human memory B cell assay and its application to studying Plasmodium falciparum-specific memory B cells in natural infections. J Immunol Methods. 2012;375:68–74. doi: 10.1016/j.jim.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titanji K, De Milito A, Cagigi A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 6.Ren S, Yu F, Zuo S, et al. Inhibition of tumor angiogenesis in lung cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine. 2011;29:5802–5811. doi: 10.1016/j.vaccine.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Tait BD, Susal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 8.Mulder A, Kardol MJ, Kamp J, et al. Determination of the frequency of HLA antibody secreting B lymphocytes in alloantigen sensitized individuals. Clin Exp Immunol. 2001;124:9–15. doi: 10.1046/j.1365-2249.2001.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zachary AA, Kopchaliiska D, Montgomery RA, Leffell MS. HLA-specific B cells: I. A method for their detection, quantification, and isolation using HLA tetramers. Transplantation. 2007;83:982–988. doi: 10.1097/01.tp.0000259017.32857.99. [DOI] [PubMed] [Google Scholar]
- 10.Wadia PP, Herrera ND, Abecassis MM, Tambur AR. Mycophenolic acid inhibits maturation and function of human dendritic cells and B cells. Hum Immunol. 2009;70:692–700. doi: 10.1016/j.humimm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Heidt S, Roelen DL, de Vaal YJ, et al. A novel ELISPOT assay to quantify HLA-specific B cells in HLA-immunized individuals. Am J Transplant. 2012;12:1469–1478. doi: 10.1111/j.1600-6143.2011.03982.x. [DOI] [PubMed] [Google Scholar]
- 12.Lynch RJ, Silva IA, Chen BJ, Punch JD, Cascalho M, Platt JL. Cryptic B cell response to renal transplantation. Am J Transplant. 2013;13:1713–1723. doi: 10.1111/ajt.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Rousset F. Growing human B lymphocytes in the CD40 system. Nature. 1991;353:678–679. doi: 10.1038/353678a0. [DOI] [PubMed] [Google Scholar]
- 14.Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huggins J, Pellegrin T, Felgar RE, et al. CpG DNA activation and plasma-cell differentiation of CD27– naive human B cells. Blood. 2007;109:1611–1619. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordani L, Sanchez M, Libri I, Quaranta MG, Mattioli B, Viora M. IFN-alpha amplifies human naive B cell TLR-9-mediated activation and Ig production. J Leukoc Biol. 2009;86:261–271. doi: 10.1189/jlb.0908560. [DOI] [PubMed] [Google Scholar]
- 17.Fecteau JF, Neron S. CD40 stimulation of human peripheral B lymphocytes: distinct response from naive and memory cells. J Immunol. 2003;171:4621–4629. doi: 10.4049/jimmunol.171.9.4621. [DOI] [PubMed] [Google Scholar]
- 18.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 19.Nagumo H, Agematsu K, Kobayashi N, et al. The different process of class switching and somatic hypermutation; a novel analysis by CD27(–) naive B cells. Blood. 2002;99:567–575. doi: 10.1182/blood.v99.2.567. [DOI] [PubMed] [Google Scholar]
- 20.Heidt S, Roelen DL, Eijsink C, van Kooten C, Claas FH, Mulder A. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation. 2008;86:1292–1300. doi: 10.1097/TP.0b013e3181874a36. [DOI] [PubMed] [Google Scholar]
- 21.Heidt S, Hester J, Shankar S, Friend PJ, Wood KJ. B-cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naive B cells. Am J Transplant. 2012;12:1784–1792. doi: 10.1111/j.1600-6143.2012.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eikmans M, Rekers NV, Anholts JD, Heidt S, Claas FH. Blood cell mRNAs and microRNAs: optimized protocols for extraction and preservation. Blood. 2013;121:e81–89. doi: 10.1182/blood-2012-06-438887. [DOI] [PubMed] [Google Scholar]
- 23.Silva HM, Takenaka MC, Moraes-Vieira PM, et al. Preserving the B-cell compartment favors operational tolerance in human renal transplantation. Mol Med. 2012;18:733–743. doi: 10.2119/molmed.2011.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim A, Lemercier B, Wertz X, Pottier SL, Huetz F, Kourilsky P. Many human peripheral VH5-expressing IgM+ B cells display a unique heavy-chain rearrangement. Int Immunol. 2008;20:105–116. doi: 10.1093/intimm/dxm125. [DOI] [PubMed] [Google Scholar]
- 25.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 26.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 27.Manz RA, Arce S, Cassese G, Hauser AE, Hiepe F, Radbruch A. Humoral immunity and long-lived plasma cells. Curr Opin Immunol. 2002;14:517–521. doi: 10.1016/s0952-7915(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 28.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 29.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 30.Eriksen AB, Indrevaer RL, Holm KL, Landskron J, Blomhoff HK. TLR9-signaling is required for turning retinoic acid into a potent stimulator of RP105 (CD180)-mediated proliferation and IgG synthesis in human memory B cells. Cell Immunol. 2012;279:87–95. doi: 10.1016/j.cellimm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol. 2003;170:686–694. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 32.Ireland SJ, Blazek M, Harp CT, et al. Antibody-independent B cell effector functions in relapsing remitting multiple sclerosis: clues to increased inflammatory and reduced regulatory B cell capacity. Autoimmunity. 2012;45:400–414. doi: 10.3109/08916934.2012.665529. [DOI] [PubMed] [Google Scholar]
- 33.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 34.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Han M, Rogers J, Lavingia B, Stastny P. Peripheral blood B cells producing donor-specific HLA antibodies in vitro. Hum Immunol. 2009;70:29–34. doi: 10.1016/j.humimm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Agrawal S, Gollapudi S. Increased activation and cytokine secretion in B cells stimulated with leptin in aged humans. Immun Ageing. 2013;10:3. doi: 10.1186/1742-4933-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamburova EG, Koenen HJ, Boon L, Hilbrands LB, Joosten I. In vitro effects of rituximab on the proliferation, activation and differentiation of human B cells. Am J Transplant. 2011;12:341–350. doi: 10.1111/j.1600-6143.2011.03833.x. [DOI] [PubMed] [Google Scholar]


