Abstract
Allergic asthma is a chronic disease of the airways associated with airway hyperresponsiveness, a variable degree of airflow obstruction, airway remodelling and a characteristic airway inflammation. Factors of the vitamin D axis, which include vitamin D metabolites and vitamin D binding protein (VDBP), have been linked to asthma, but only few data exist about their regulation in the lung during acute allergen-induced airway inflammation. Therefore, we analysed the regulation of factors of the vitamin D axis during the early- and late-phase reaction of allergic asthma. Fifteen patients with mild allergic asthma underwent segmental allergen challenge. VDBP was analysed in bronchoalveolar lavage fluid (BALF) and serum using the enzyme-linked immunosorbent assay (ELISA) technique. 25-hydroxyvitamin D3 [25(OH)D3] and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] were analysed by a commercial laboratory using the liquid chromatography–mass spectrometry (LC/MS) technique. VDBP (median 2·3, range 0·2–7·1 μg/ml), 25(OH)D3 (median 0·060, range < 0·002–3·210 ng/ml) and 1,25(OH)2D3 (median < 0·1, range < 0·1–2·8 pg/ml) were significantly elevated in BALF 24 h but not 10 min after allergen challenge. After correction for plasma leakage using the plasma marker protein albumin, VDBP and 25(OH)D3 were still increased significantly while 1,25(OH)2D3 was not. VDBP and 25(OH)D3 were correlated with each other and with the inflammatory response 24 h after allergen challenge. Serum concentrations of all three factors were not influenced by allergen challenge. In conclusion, we report a significant increase in VDBP and 25(OH)D3 in human BALF 24 h after allergen challenge, suggesting a role for these factors in the asthmatic late-phase reaction.
Keywords: asthma, bronchoalveolar lavage fluid, segmental allergen challenge, vitamin D, vitamin D binding protein (VDBP)
Introduction
The steroid hormone vitamin D plays an important role in the regulation of calcium and phosphate metabolism. The effects on bone health are well established. In addition, during the last decades, there has been a great deal of interest in the additional functions of vitamin D. Research on vitamin D3 has revealed its diverse biological action, including respiratory health and immunity.
Only a few foods contain vitamin D3; the main source of vitamin D is sunlight-induced synthesis. In a photolytic reaction, which takes place in the skin, ultraviolet B (UVB) radiation converts 7-dehydrocholesterol to vitamin D3 (cholecalciferol) 1. Hydroxylation to 25(OH)D3 (calcidiol), the major circulating metabolite of vitamin D, is catalysed mainly in the liver by vitamin-D-25-hydroxylase 2. The second hydroxylation to the most active form 1,25(OH)2D3 is traditionally thought to take place in the kidneys, but increasing numbers of tissues and cells have been described to express 1α-hydroxylase (Cyp27B1), which catalyzes this reaction 3.
In peripheral blood, most vitamin D metabolites are bound to both vitamin D-binding protein (VDBP) and albumin. Because the affinity of VDBP for 25(OH)D3 and 1,25(OH)2D3 is much higher than that of albumin, the majority of these molecules is bound to VDBP 4. Uptake of vitamin D metabolites into cells takes place by endocytosis of bound metabolites by binding of VDBP to megalin and cubulin on target cells or by diffusion of unbound metabolites across cell membranes 5–7.
Allergic asthma is a chronic disease of the airways which is associated with airway hyperresponsiveness, a variable degree of airflow obstruction, airway remodelling and a characteristic airway inflammation 8. Several epidemiological studies have linked vitamin D deficiency in children and adults with asthma. While some studies showed a reduction in asthma excacerbations following vitamin D supplementation, others postulated an increased asthma risk after vitamin D supplementation (reviewed in 1 and 2). As a consequence of these data, there is currently no recommendation for or against vitamin D supplementation in asthma, and further information about the physiological role of the vitamin D axis in the lung during an asthmatic response is needed. Therefore, we analysed the release of VDBP and vitamin D metabolites 25(OH)D3 and 1,25(OH)2D3 into the endobronchial lumen during the early and late asthmatic response using the human asthma model of segmental allergen challenge.
Methods
Subjects
Fifteen adult patients with mild to moderate allergic asthma from the region of Rostock (Germany) were included into the study (Table 1) using the following criteria: (1) airway hyperresponsiveness, (2) positive allergen skin prick tests and (3) elevated total or specific immunoglobulin (Ig)E concentrations. Inhaled allergen provocation and calculation of the individual provocation dose were performed as described previously 9. Inhaled and segmental allergen challenge were separated by at least 4 weeks. Cromoglycate and corticosteroids were withdrawn at least 7 days before challenge. All patients gave their written informed consent and the study was approved by the Ethics Committee from the University Medical Clinic Rostock (HV-2011-0012).
Table 1.
Patient characteristics.
| Sex | Age (years) | Duration of asthma (years) | FEV1 (%pred) | Total IgE (kU/l) | Specific IgE (kU/l) | Allergen | Dose (AU) | Medication |
|---|---|---|---|---|---|---|---|---|
| F | 20 | 4 | 92 | 83 | 26·4 | D. pter. | 2·9 | BA |
| F | 23 | 10 | 102 | 141 | 23·2 | D. pter. | 2300 | BA |
| F | 22 | 6 | 101 | 192 | 46·9 | D. pter. | 1920 | BA |
| F | 23 | 11 | 88 | 207 | 1·7 | Rye | 58 | BA, IC |
| M | 23 | 18 | 103 | 262 | 79·8 | Birch | 1070 | BA, IC |
| F | 23 | 3 | 85 | 116 | 14·4 | Birch | 156 | BA |
| F | 27 | 17 | 81 | 88 | 16·8 | D. pter. | 560 | BA, IC |
| M | 26 | 2 | 90 | 130 | 19·1 | Birch | 44 | AH, BA |
| M | 21 | 18 | 93 | 29 | 6·5 | D. pter. | 230 | BA, IC |
| F | 20 | 15 | 95 | 66 | 1·7 | Birch | 450 | AH, BA, IC |
| F | 28 | 21 | 89 | 243 | 20·1 | Rye | 500 | BA |
| M | 21 | n.d. | 94 | 189 | 25·1 | D. pter. | 270 | BA, CR |
| M | 42 | 29 | 91 | 1103 | 35·3 | D. pter. | 260 | BA, CR |
| F | 24 | 1 | 80 | 243 | 89·9 | Birch | 105 | AH |
| M | 32 | n.d. | 87 | 67 | 39·2 | Birch | 325 | BA |
Sex, age, duration of asthma of the patients, prebronchodilator FEV1, medication prior to the study (AH, BA, CR, IC), serum levels of total (normal range < 100 kU/l) and allergen-specific (normal range < 0·7 kU/l) IgE and the allergen and dose used for segmental allergen challenge. AH = oral antihistamine; AU = allergen units; BA = inhaled β2 agonist; CR = cromoglycate; D. pter. = Dermatophagoides pteronyssinus; FEV1 = forced expiratory volume in 1 s; IC = inhaled corticosteroid; IgE = immunoglobulin E; n.d. = not determined; M = male; F = female.
Segmental allergen challenge
Segmental allergen challenge was performed as described 9. Briefly, 2·5 ml of saline was instilled into the left S8 and S4 or S5 segments, and the left S8 was lavaged 10 min later using 100 ml of prewarmed saline. Subsequently, the allergen (diluted in 2·5 ml saline) was instilled into the right S8 and S4 or S5 segments, and the right S8 was lavaged by using 100 ml prewarmed saline after 10 min, or earlier if substantial bronchoconstriction was observed endoscopically. The second bronchoalveolar lavage was performed in the left and right S4 or S5 segments 24 h after challenge. Before each bronchoscopy, venous blood samples were obtained.
Processing of bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) samples were filtered and centrifuged at 4°C and 400 g for 10 min. Supernatants were removed and stored at −80°C until measured. Cells were resuspended in phosphate-buffered saline. A fraction of the suspension was used for cell counts using a Neubauer chamber and for cytospins. Cytospins were stained with May–Grünwald–Giemsa solution (Merck, Darmstadt, Germany) and differential cell counts determined using standard morphological criteria. Results were expressed as the total number of cells per ml of recovered BALF.
Quantification of 25-hydroxyvitamin D3, 1,25-dihydroxyvitamin D3 and vitamin D-binding protein
Vitamin D metabolites [normal serum range: 25(OH)D3, 20–70 ng/ml; 1,25(OH)2D3, 16–67 pg/ml] were quantified in serum and BALF samples in a commercial laboratory (Labor Limbach, Heidelberg, Germany) by using the liquid chromatography–mass spectrometry (LC/MS) technique [detection limits: 25(OH)D3, 0·002 ng/ml; 1,25(OH)2D3, 0·1 pg/ml]. Vitamin D binding protein was detected in serum and BALF samples using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Wiesbaden, Germany).
Calculation and subtraction of the plasma leakage-induced vitamin D and VDBP concentrations
Because BALF concentrations of vitamin D derivatives and VDBP were much lower compared to serum concentrations, the serum marker protein albumin was measured in BALF and serum samples using a commercially available ELISA kit (AssayPro, St Charles, MO, USA). The hypothetical plasma leakage-induced concentration of vitamin D derivatives and VDBP was then calculated using the following formula:
The concentration of vitamin D metabolites and VDBP in BALF corrected for plasma leakage was calculated using the following formula:
Statistical analysis
Data were analysed using spss version 5·0 software (SPSS, Inc., Chicago, IL, USA). Most parameters were distributed asymmetrically. Comparison of the groups was performed using the Wilcoxon signed-rank test. Correlation analysis was performed using Spearman's correlation coefficient. Probability values of P < 0·05 were regarded as significant. Box-plots show the median (line within the box), interquartile range (edges of the box) and range of values less distant than 1·5 interquartile ranges from the upper or lower quartile (vertical lines).
Results
Vitamin D-binding protein in serum and BALF
Concentrations of VDBP in BALF were increased significantly 24 h after allergen challenge compared with saline-challenged lung segments, while serum concentrations were not affected by allergen challenge (Table 2). Because serum concentrations of VDBP were much higher than BALF concentrations, we measured the plasma marker protein albumin in BALF, which allows us to calculate the potential contamination by plasma leakage (see Methods section for details). After correction of VDBP values for plasma leakage-induced concentrations, there was still a significant increase in VDBP in BALF 24 h after allergen challenge (Fig. 1).
Table 2.
Concentrations of vitamin D3 metabolites, vitamin D binding protein (VDBP) and albumin in bronchoalveolar lavage fluid (BALF) and serum samples.
| Serum | BALF 10 min | BALF 24 h | ||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | Saline | Allergen | Saline | Allergen | |
| VDBP (μg/ml) | 220·3 (75·8–598·6) | 233·7 (74·7–619·6) | 0·2 (0·1–0·7) | 0·1 (0·1–0·3) | 0·2 (0·1–0·6) | 2·3 (0·2–7·1)* |
| 25(OH)D3 (ng/ml) | 22·00 (6·20–39·00) | 22·00 (5·70–41·00) | < 0·002 (<0·002–0·009) | < 0·002 (<0·002–0·005) | < 0·002 (<0·002–0·006) | 0·060 (<0·002–3·210)* |
| 1,25(OH)2 D3 (pg/ml) | 41 (12–62) | 39 (13–63) | < 0·1 | < 0·1 | < 0·1 | < 0·1 (<0·1–2·8)* |
| Albumin (μg/ml) | 65 630 (54 570–70 680) | 65 910 (56 610–72 370) | 39 (11–170) | 26 (13–57) | 55 (16–96) | 211 (39–3420)* |
All parameters are shown as median values (range) (n = 15). Asterisks mark significant differences (P < 0·05) between allergen-challenged and saline-challenged lung segments.
Fig. 1.
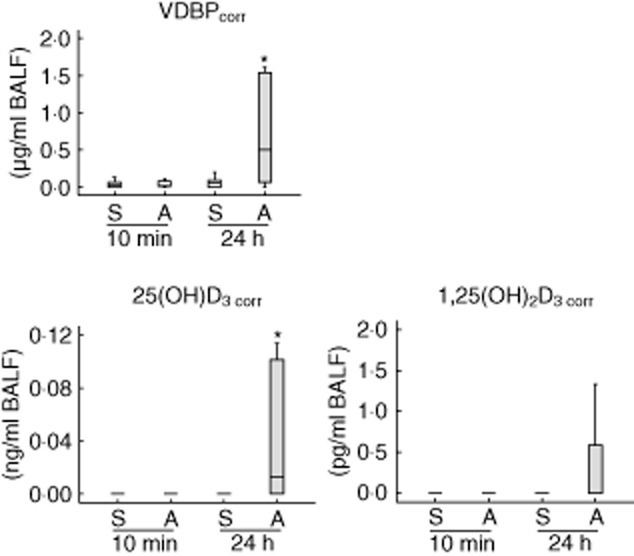
Vitamin D binding protein (VDBP) and vitamin D metabolites corrected for contamination by plasma leakage in human bronchoalveolar lavage fluid (BALF) after allergen challenge (n = 15). A, allergen-challenged lung segment; S, saline-challenged control segment. *P-values < 0·05 were regarded as significant.
25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in serum and BALF
Seven patients analysed had serum levels of 25(OH)D3 lower than 20 ng/ml and were therefore classified as vitamin D-deficient. One patient was analysed in October, one in December, one in February, three in March and one in May. Two patients analysed in October and November, respectively, showed 25(OH)D3 concentrations > 20 ng/ml. After 10 min in saline-challenged lung segments and the allergen-challenged segment, 25(OH)D3 and 1,25(OH)2D3 could hardly be detected, while a significant increase in concentrations of vitamin D derivatives was observed 24 h after allergen challenge (Table 2). Serum concentrations of vitamin D metabolites were not influenced by allergen challenge (Table 2) and no significant association between serum and BALF levels of vitamin D metabolites was detected. After correction of the measured values for potential contamination by plasma leakage, a significant increase in 25(OH)D3 in BALF 24 h after allergen challenge was still observed (Fig. 1). In contrast, 1,25(OH)2 D3 was no longer elevated 24 h after allergen challenge (Fig. 1).
Correlation with the inflammatory response
Numbers of lymphocytes, neutrophils and eosinophils were elevated significantly in BALF 24 h after allergen challenge compared with saline-challenged control segments (Table 3). In BALF 24 h after allergen challenge, a significant correlation between concentrations of 25(OH)D3 and numbers of macrophages (rS = 0·543; P = 0·037), numbers of lymphocytes (rS = 0·885; P = 0·001), numbers of neutrophils (rS = 0·775; P = 0·001), numbers of eosinophils (rS = 0·796; P = 0·001) as well as the allergen dose used for challenge (rS = 0·657; P = 0·008) were found. No significant correlations were found between 1,25(OH)2D3 and inflammatory or clinical parameters. In BALF 24 h after allergen challenge, VDBP was correlated significantly with the number of macrophages (rS = 0·604; P = 0·017), lymphocytes (rS = 0·554; P = 0·032), neutrophils (rS = 0·732; P = 0·002), eosinophils (rS = 0·518; P = 0·048) (Fig. 2) and the allergen dose used for challenge (rS = 0·518; P = 0·048). Furthermore, VDBP was correlated significantly with 25(OH)D3 in BALF 24 h after allergen challenge (rS = 0·643; P = 0·010).
Table 3.
Cell counts in bronchoalveolar lavage fluid (BALF) after allergen challenge.
| 10 min | 24 h | |||
|---|---|---|---|---|
| Saline | Allergen | Saline | Allergen | |
| Recovery (ml) | 52 (17–74) | 35 (8−69) | 57 (43−76) | 50 (20–75) |
| Macrophages (103/ml) | 47 (1–85) | 18 (2–36) | 68 (13–122) | 78 (2–223) |
| Lymphocytes (103/ml) | 3 (0–13) | 1 (0–4) | 4 (1–12) | 11 (1–111)* |
| Neutrophils (103/ml) | 0 (0–2) | 0 (0–1) | 3 (0–111) | 8 (1–154)* |
| Eosinophils (103/ml) | 0 (0–4) | 0 (0–3) | 1 (0–4) | 60 (0–654)* |
The table displays the recovery and number of cells in BALF after saline and allergen challenge, respectively. All parameters are shown as median values (range) (n = 15). Asterisks mark significant differences (P < 0·05) in cell counts between allergen-challenged and saline-challenged lung segments.
Fig. 2.
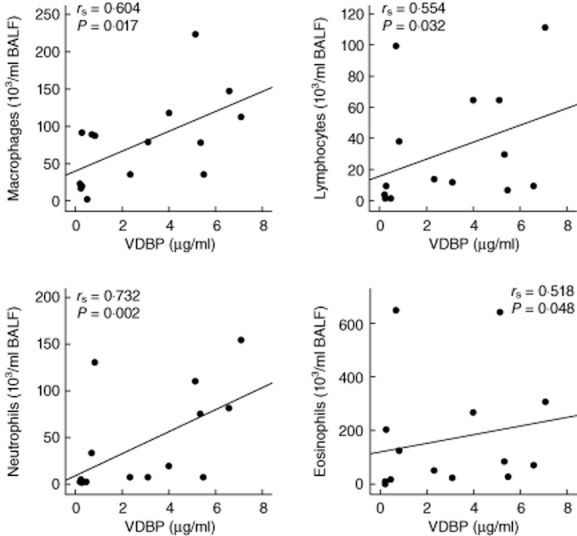
The scatterplots display the association of soluble vitamin D binding protein (VDBP) with leucocyte subpopulations in human bronchoalveolar lavage fluid (BALF) 24 h after allergen challenge (n = 15). Correlation analysis was performed using Spearman's correlation coefficient (rS). P-values < 0·05 were regarded as significant.
Discussion
This is the first study to analyse VDBP in human BALF during acute allergen-induced inflammation. VDBP is of interest because this protein is not only a carrier protein for vitamin D metabolites, but has additional functions in inflammation: it has a high affinity for actin monomers, and one of its major functions is actin clearance after cellular damage to avoid the formation of F-actin networks which could occlude the vasculature 7,10. In addition, VDBP can contribute to macrophage activation after conversion to macrophage-activating factor by enzyme activity present on B and T lymphocytes 11. Finally, VDBP enhances the chemotactic effects of complement factor C5a to neutrophils 12–14.
We found increased levels of VDBP in BALF 24 h after allergen challenge, but not 10 min after allergen challenge. Due to the immediate bronchoconstriction 10 min following allergen challenge, the recovery at this time-point was reduced and it cannot be fully excluded that this reduction in recovery might have influenced the obtained results. While some of the VDBP in BALF seems to be derived from plasma leakage, our data provide evidence that other active mechanisms might contribute to this observation. So it has been shown that VDBP bound to neutrophils, which are elevated in our model of asthma, can be released unaltered into the extracellular space by serine protease activity 15. Our findings are in line with a previous study which described elevated VDBP concentrations in BALF of children with severe therapy-resistant asthma 16. In the latter investigation, there was also no impact of the asthmatic status on serum VDBP levels 16, suggesting that VDBP is up-regulated locally in the asthmatic lung. In our study, the best correlation was detected between VDBP and neutrophils in BALF. Thus, it could be speculated that VDBP contributes to neutrophil recruitment in this human model of allergic asthma. It has already been shown in a mouse model of lung inflammation that VDBP is involved in neutrophil recruitment into lung 17.
Of note, it has to be assumed that the allergen-induced influx of macrophages and lymphocytes can degrade VDBP to macrophage-activating factor (MAF), which is generated by cleavage of carbohydrate residues from VDBP 11. The ELISA used in our experiments has not been tested for cross-reactivity with MAF. Therefore, we cannot rule out completely that some of the measured VDBP might in fact be due to VDBP-derived MAF.
Most of the patients who were classified as vitamin D-deficient were analysed from October to March, suggesting that vitamin D deficiency might be due to low sunlight exposure during these months, as the main source of vitamin D in humans is sunlight-induced synthesis 2. Nevertheless, vitamin D deficiency has been linked to allergic asthma by several studies (reviewed in 1 and 2), and therefore other physiological mechanisms might additionally account for the observed phenomenon in our study. Our results on vitamin D metabolites in human BALF corroborate previous findings which reported a significant increase in 25(OH)D3, but not 1,25(OH)2D3 in human BALF 20–24 h after allergen challenge, after correction for plasma exudation 18. In keeping with this study, we found a significant correlation between 25(OH)D3 and the inflammatory response in BALF 24 h after allergen challenge. However, compared to the investigation by Liu et al., we were able to show that 25(OH)D3 concentrations increase 24 h after allergen challenge, but not 10 min after allergen challenge, suggesting an allergen-dependent up-regulation of vitamin D concentrations in the lungs during the late-phase asthmatic response. While elevated levels of 1,25(OH)2D3 in BALF after allergen challenge can be explained by plasma leakage, our data provide evidence that other mechanisms such as metabolic and/or transport mechanisms may additionally account for the increase in 25(OH)D3. It may be speculated that transport via VDBP, which is also elevated after allergen challenge and correlated with 25(OH)D3, might be one of the mechanisms contributing to this observation.
Concentrations of 1,25(OH)2D3, the most active form of vitamin D, were very low in BALF (< 3·0 pg/ml) even after allergen challenge, and therefore a physiological role of free 1,25(OH)2D3 in allergen-induced endobronchial inflammation seems questionable. Nevertheless, vitamin D3 hydroxylating enzymes have been detected in a variety of other tissues such as the skin, the intestine and the lungs as well as in cells such as lung macrophages, monocytes, dendritic cells and T lymphocytes 3,19,20. This suggests that our finding of allergen-induced elevated levels of 25(OH)D3 in the lung may have a pathophysiological relevance. In bronchial smooth muscle cells vitamin D3 induces genes involved in morphogenesis, cell growth and survival, as well as genes encoding for structural proteins which may be involved in airway remodelling 21. In bronchial and respiratory epithelial cells, vitamin D induces thymic stromal lymphopoietin (TSLP) and expression of the anti-microbial peptide cathelicidin, respectively 3,22. DCs were recognized as a central target for vitamin D: 1,25(OH)2D3 inhibits DC differentiation, maturation and function by decreasing production of IL-12 and increasing secretion of IL-10 23–26. By modulating DC function in that manner, 1,25(OH)2D3 alters T cell activation favouring the induction of regulatory T cells and leading to T cell hyporesponsiveness 24,27. Several studies have shown that VDBP is restricting the bioavailability of 25(OH)D3 to monocytes and DC and therefore suppresses vitamin D receptor signalling 28,29, suggesting that elevated levels of VDBP in our model of allergen-induced lung inflammation might favour a proinflammatory DC phenotype. To date, it is unclear if anti-inflammatory effects through elevated vitamin D metabolites or proinflammatory effects through elevated VDBP predominate our human model of allergen-induced inflammation.
In conclusion, we report significantly elevated levels of VDBP and 25(OH)D3 in BALF 24 h after allergen challenge in a human model of allergic asthma suggesting an as-yet unclear role for both factors in late events of allergen-induced endobronchial inflammation.
Acknowledgments
This study was supported by the University of Rostock (FORUN Project no. 889015/2012).
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Paul G, Brehm JM, Alcorn JF, Holguín F, Aujla SJ, Celedón JC. Vitamin D and asthma. Am J Respir Crit Care Med. 2012;185:124–132. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown SD, Calvert HH, Fitzpatrick AM. Vitamin D and asthma. Dermato-Endocrinology. 2012;4:137–145. doi: 10.4161/derm.20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012;523:95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikle DD, Siiteri PJ, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61:969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 5.Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake of and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 6.Nykjaer A, Fyfe JC, Kozyraki R, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proc Natl Acad Sci USA. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chishimba L, Thickett DR, Stockley RA, Wood AM. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–462. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–1372. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 9.Nassenstein C, Braun A, Erpenbeck VJ, et al. The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J Exp Med. 2003;198:455–467. doi: 10.1084/jem.20010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldschmidt-Clermont PJ, Van Alstyne EL, Day JR, et al. Group-specific component (vitamin D binding protein) prevents the interaction between G-actin and profilin. Biochemistry. 1986;25:6467–6472. doi: 10.1021/bi00369a019. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci USA. 1991;88:8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Kew RR. Identification of a region in the vitamin-binding protein that mediates its C5a chemotactic cofactor function. J Biol Chem. 2004;279:53282–53287. doi: 10.1074/jbc.M411462200. [DOI] [PubMed] [Google Scholar]
- 13.McVoy LA, Kew RR. CD44 and annexin A2 mediate the C5a chemotactic cofactor function of the vitamin D binding protein. J Immunol. 2005;175:4754–4760. doi: 10.4049/jimmunol.175.7.4754. [DOI] [PubMed] [Google Scholar]
- 14.DiMartino SJ, Trujillo G, MCVoy LA, Zhang J, Kew RR. Upregulation of vitamin D binding protein (Gc-globulin) binding sites during neutrophil activation from a latent reservoir in azurophil granules. Mol Immunol. 2007;44:2370–2377. doi: 10.1016/j.molimm.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMartino SJ, Shah AB, Trujillo G, Kew RR. Elastase controls the binding of the vitamin D-binding protein (Gc-globulin) to neutrophils: a potential role in the regulation of C5a co-chemotactic activity. J Immunol. 2001;166:2688–2694. doi: 10.4049/jimmunol.166.4.2688. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Dimeloe S, Richards DF, Bush A, Saglani S, Hawrylowicz CM. Vitamin D binding protein and asthma severity in children. J Allergy Clin Immunol. 2012;129:1669–1671. doi: 10.1016/j.jaci.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Ge L, Trujillo G, Miller EJ, Kew RR. Circulating complexes of the vitamin D binding protein with G-actin induce lung inflammation by targeting endothelial cells. Immunobiology. 2014;219:198–207. doi: 10.1016/j.imbio.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu MC, Xiao HQ, Brown AJ, Ritter CS, Schroeder J. Association of vitamin D and antimicrobial peptide production during late-phase allergic responses in the lung. Clin Exp Allergy. 2012;42:383–391. doi: 10.1111/j.1365-2222.2011.03879.x. [DOI] [PubMed] [Google Scholar]
- 19.Baeke F, Korf H, Overbergh L, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–227. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 21.Bosse Y, Maghni K, Hudson TJ. 1α,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics. 2007;29:161–168. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Peng C, Zhao H, et al. Induction of thymic stromal lymphopoietin expression in 16-HBE human bronchial epithelial cells by 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3. Int J Mol Med. 2013;32:203–210. doi: 10.3892/ijmm.2013.1353. [DOI] [PubMed] [Google Scholar]
- 23.D'Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 25.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 26.Mora JR, Iwata M, von Adrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penna G, Roncari A, Amuchastegui S, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+FoxP3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 28.Chun RF, Lauridsen AL, Suon L, et al. Vitamin d-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffery LE, Wood AM, Qureshi OS, et al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155–5164. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]


