Abstract
Prior studies have demonstrated that elevated aldosterone concentrations are an independent risk factor for death in patients with cardiovascular disease. Limited studies, however, have evaluated systematically the association between serum aldosterone and adverse events in the setting of chronic kidney disease (CKD). We investigated the association between serum aldosterone and death and end-stage renal disease (ESRD) in 3,866 participants from the Chronic Renal Insufficiency Cohort. We also evaluated the association between aldosterone and incident congestive heart failure (CHF) and atherosclerotic events in participants without baseline cardiovascular disease. Cox proportional hazards models were used to evaluate independent associations between elevated aldosterone concentrations and each outcome. Interactions were hypothesized and explored between aldosterone and sex, race, and the use of loop diuretics and RAAS inhibitors. Over a median follow-up period of 5.4 years, 587 participants died, 743 developed ESRD, 187 developed CHF, and 177 experienced an atherosclerotic event. Aldosterone concentrations (per standard deviation of the log transformed aldosterone) were not an independent risk factor for death (adjusted HR 1.00, 95% CI [0.93–1.12]), ESRD (adjusted HR 1.07, 95% CI [0.99–1.17]), or atherosclerotic events (adjusted HR 1.04, 95% CI [0.85–1.18]). Aldosterone was associated with CHF (adjusted HR 1.21, 95% CI [1.02–1.35]). Among participants with CKD, higher aldosterone concentrations were independently associated with the development of CHF, but not for death, ESRD, or atherosclerotic events. Further studies should evaluate whether mineralocorticoid receptor antagonists may reduce adverse events in individuals with CKD since elevated cortisol levels may activate the mineralocorticoid receptor.
Keywords: Aldosterone, Chronic kidney disease, Outcomes, Death, Congestive Heart Failure
Chronic kidney disease (CKD) is a major problem that affects 13% of the US population.1 Large-scale epidemiologic studies have demonstrated that CKD patients have an increased risk of cardiovascular events including death, congestive heart failure (CHF), myocardial infarction and stroke.2 Multiple biologic pathways have been implicated in this established association between kidney disease and cardiovascular outcomes including activation of the renin-angiotensin-aldosterone system (RAAS). Impaired kidney function results in RAAS activation, which in turn leads to multiple, deleterious cardiovascular effects including increased volume and salt resorption, vasoconstriction, and fibrosis.3, 4 An understanding of these mechanisms has resulted in the advent of multiple therapies including angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and aldosterone antagonists or mineralocorticoid receptor blockers (MRBs), which reduce cardiorenal complications.5 In particular treatment with ACE inhibitors and ARBs slow the progression of kidney disease;6, 7 however, these drugs have not been proven to reduce cardiovascular morbidity and mortality in CKD patients.8 These medications partially suppress RAAS activation and can result in “aldosterone escape”9 or the ongoing induction of aldosterone production. As a result, experimental studies are seeking to understand whether more complete suppression of the RAAS with aldosterone blockers or MRBs can improve cardiorenal outcomes. This recognition has also motivated evaluation of serum aldosterone as a risk marker and potential therapeutic target especially in patients with CKD.
Activation of the RAAS has been hypothesized to explain some of the racial disparities observed in the incidence of hypertension, left ventricular hypertrophy (LVH), end-stage renal disease (ESRD), and CHF. In particular, the higher rate of hypertension-related complications seen in Blacks including CKD, CHF and death may be attributed to greater activity of downstream mediators of the RAAS including angiotensin II and aldosterone.10 The International Society on Hypertension in Blacks consensus statement suggests that Blacks may be particularly susceptible to the effects of RAAS activity and may have an improved response to RAAS blockade compared to Whites.11 Limited studies, however, have evaluated systematically whether aldosterone is a risk factor for death and cardiorenal complications in a racially diverse cohort of CKD participants. We hypothesized that elevated aldosterone concentrations would be an independent risk factor for death, ESRD, incident heart failure, and atherosclerotic events among CKD participants. In addition, we hypothesized that this association would be stronger in Blacks compared to Whites.
Methods
Study Population
The Chronic Renal Insufficiency Cohort (CRIC) study is a large, multi-center, multiracial, cohort study established to understand the progression of cardiovascular and renal disease among individuals with chronic kidney disease. The study enrolled subjects between June 2003 and September 2008 and was designed to include a racially and ethnically diverse group of adults. Participants who were between 21 to 74 years and had an estimated glomerular filtration rate (eGFR) between 20 and 70 ml/min/1.73m2 were eligible for the study. The age and eGFR criteria were specifically designed to facilitate evaluation of the progression and implications of CKD across a wide spectrum of mild to moderate kidney dysfunction and age. Approximately 20% of participants had an eGFR between 60–70 ml/min/1.73m2, 60% had an eGFR between 60–70ml/min/1.73m2, and 20% had an eGFR <30 ml/min/1.73m2. Specific details on recruitment and design have been published previously.12 The study complies with the Declaration of Helsinki. The institutional review boards at all participating centers approved the study protocol, and study participants provided written informed consent.
In brief, CRIC recruited 3939 men and women aged 21 to 74 years. The participants underwent extensive clinical evaluations at study entry, during annual research clinic visits and via telephone interviews midway between clinic visits. Of the 3939 participants enrolled at baseline, we excluded individuals with insufficient blood specimens for aldosterone measurements (n=73) resulting in a final study sample of 3,866 participants. Information on baseline confounders was obtained during the initial visit and included sociodemographics, lifestyle risk factors, medical history and medication use. Blood pressure,13 anthropometrics, ankle-brachial index (ABI),14 and other clinical variables were collected using standard protocols. Blood was obtained from participants in the fasting state. Measurements were made of creatinine, cystatin C, sodium, potassium, total cholesterol, low density lipoprotein, triglycerides, albumin, hemoglobin, serum calcium, phosphate, N-terminal pro-B-type natriuretic peptide (NT-pro BNP) and fibroblast growth factor 23 (FGF-23). 24-hour urine samples were obtained and urine sodium, potassium, albumin, and creatinine were measured.
Measurements
Most measurements were performed at a central laboratory at the University of Pennsylvania. Aldosterone was measured using an ELISA kit (BioVendor, www.biovendor.com) from baseline EDTA specimens, which were collected in the fasting state and stored at −70 degrees Celsius until 2010. All measurements were made after a single thaw. Our estimates of the intra-assay and inter-assay coefficients of variation (CVs) ranged from 6.5 to 8.7%. NT-pro BNP was measured using a chemilluminescent microparticle immunoassay (www.roche-diagnostics.us) on the ElecSys 2010. The values ranged from 5 to 35,000 pg/ml and the CV was 9.3% at a level of 126 pg/ml and 5.5% at 4,319 pg/ml. FGF-23 was measured in duplicate using stored baseline plasma samples and a C-terminal enzyme-linked immunosorbent assay (Immunotopics). The CV was 7.6%.15 Finally, hemoglobin was measured directly at the laboratory of each CRIC Study clinical center. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine and cystatin C using the CRIC-based equation.16
Outcomes
The outcomes included all-cause mortality, end-stage renal disease, incident congestive heart failure, and incident atherosclerotic events. Deaths were ascertained from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the social security death master file. Study participants were queried for the development of ESRD and cardiovascular events at annual follow-up visits and via semi-annual telephone interviews. ESRD was defined as initiation of dialysis or kidney transplantation. The cardiovascular outcomes included first hospitalization for heart failure or first atherosclerotic event including hospitalization for myocardial infarction, cerebrovascular accident or peripheral vascular disease. An adjudication committee reviewed all relevant medical records and interviews to ascertain these outcomes based on well-defined criteria that have been described previously.12 All outcomes occurred between enrollment and March 31, 2011.
Statistical methods
We first described the baseline characteristics of CRIC participants across aldosterone quartiles. Continuous variables were analyzed by ANOVA and nonparametric methods for skewed variables. Categorical variables were analyzed by the chi-squared test.
Separate multivariable adjusted Cox proportional hazards models were used to assess associations between aldosterone and each outcome: death, ESRD, CHF and the atherosclerotic endpoint. For the analysis evaluating the risk of incident CHF and atherosclerotic events, participants with baseline cardiovascular disease and heart failure were excluded. Death was considered as a censoring event when it was not the outcome. Quadratic splines were used to explore potential non-linearity between the log transformed aldosterone and the outcomes of interest.17 Aldosterone was modeled as a continuous variable, and hazard ratios (HRs) were calculated per standard deviation increment of the log-transformed variable. Aldosterone was also categorized into quartiles with the lowest quartile defined as the reference group.
Multivariable analysis was performed for each outcome adjusting for covariates that were potentially confounders or mediators of the association between aldosterone concentrations and cardiorenal outcomes. Adjusted models included the following covariates: demographics (age, sex, and race), clinical center and cardiorenal risk factors including blood pressure, diabetes, eGFR and proteinuria (24 hour urine specimen). We also adjusted for prevalent cardiovascular disease (history of coronary artery disease, heart failure, peripheral vascular disease and stroke) for the death and ESRD outcomes. Additional adjustment for urinary sodium, the urinary sodium to potassium ratio, serum potassium, serum albumin, hemoglobin, and NT-pro BNP was also performed. Medications known to affect RAAS regulation including ACE inhibitors, angiotensin receptor blockers (ARBs), mineralocorticoid receptor blockers (MRBs), loop diuretics, and statins were added to the multivariable model. Fibroblast growth factor 23 (FGF-23) was included in the ESRD analysis. The proportional hazards assumption was met based on cumulative Martingale residuals. We tested for interactions between aldosterone and sex, race, level of eGFR (<45 and ≥45 ml/min/1.73m2), use of medications that affect RAAS regulation (ACE inhibitors, ARBs, and MRBs), and use of loop diuretics for each of the outcomes assessed in this analysis. We also performed a pre-specified, stratified analysis by race for each of the outcomes. Analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, North Carolina). All statistical tests were 2-sided, and P values < 0.05 were considered significant.
Results
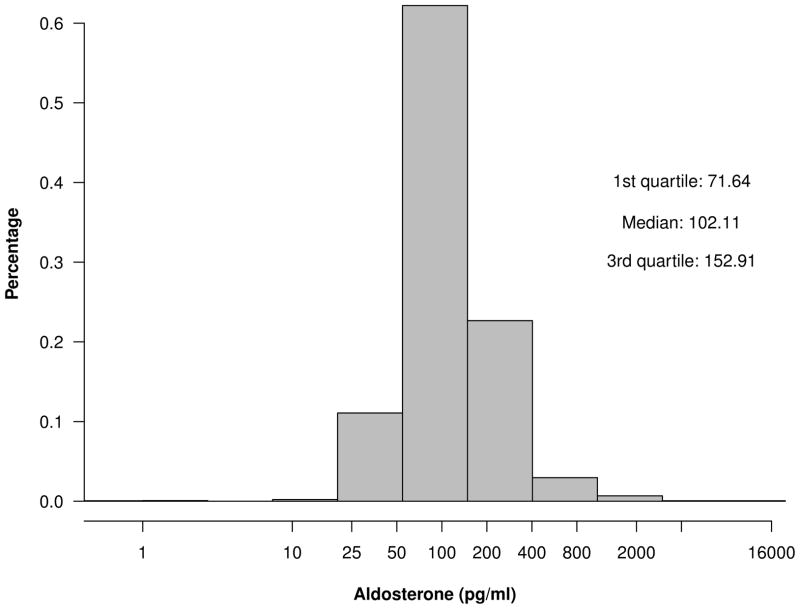
Among the 3866 participants, the mean age was 58±11 years, 45% were female, and 42% were Black. Approximately one-third of CRIC participants had a history of cardiovascular disease at the baseline visit. The median aldosterone concentration was 102.1 [25th – 75th percentile, 71.6–152.9] pg/ml (figure 1). The mean eGFR was 45±17 ml/min/1.73m2 and median level of proteinuria was 0.186 [25th – 75th percentile 0.073–0.92] grams per 24 hours.
Figure 1.
Distribution of aldosterone among CRIC participants
Compared to participants in the lowest aldosterone quartile, those with higher levels were more likely to be male, white, had a lower eGFR, greater proteinuria, a lower urinary sodium, higher urinary potassium, less likely to be taking an ACE inhibitor, ARB or statin, more likely to be taking a MRB or loop diuretic, and had higher levels of NT-pro BNP, FGF-23, and phosphate (Table 1). In the subgroup that was not taking an ACE inhibitor, ARB, or MRB, aldosterone concentrations were associated with most of the same baseline characteristics (Table S1); in addition, they correlated with hypertension, a lower LDL and higher heart rate. There was no association between serum aldosterone level and coronary artery disease, heart failure, or diabetes.
Table 1.
Baseline characteristics among 3,865 participants
| Baseline Variable | Aldosterone Quartiles | p-value | |||
|---|---|---|---|---|---|
| 1 (<72 pg/ml) | 2 (72–102 pg/ml) | 3 (103–153 pg/ml) | 4 (≥153 pg/ml) | ||
| Demographics | |||||
| Age (years) ± SD | 59±11 | 58±11 | 58±11 | 58±12 | 0.072 |
| Female, n (%) | 495 (51%) | 448 (46%) | 373 (39%) | 410 (42%) | <0.001 |
| Race, n (%) | |||||
| Hispanic | 134 (14%) | 136 (14%) | 121 (13%) | 103 (11%) | 0.006 |
| White | 396 (41%) | 369 (38%) | 405 (42%) | 441 (46%) | |
| Black | 403 (42%) | 423 (44%) | 411 (42%) | 370 (38%) | |
| Prevalent Cardiovascular Disease | |||||
| CAD, n (%)a | 231 (24%) | 207 (21%) | 219 (23%) | 195 (20%) | 0.23 |
| Heart failure, n (%) | 78 (8%) | 89 (9%) | 105 (11%) | 105 (11%) | 0.10 |
| PVD, n (%) | 58 (6%) | 75 (8%) | 70 (7%) | 55 (6%) | 0.21 |
| Stroke, n (%) | 87 (9%) | 94 (10%) | 95 (10%) | 107 (11%) | 0.49 |
| CVD Risk Factors | |||||
| Hypertension, n(%) | 822 (85%) | 837 (87%) | 834 (86%) | 839 (87%) | 0.64 |
| Diabetes, n(%) | 479 (50%) | 483 (50%) | 470 (49%) | 442 (46%) | 0.24 |
| BMI (kg/m2) ± SD | 32.1±7.7 | 32.4±7.9 | 32.2±7.7 | 31.8±7.9 | 0.37 |
| Smoking Status, n(%) | |||||
| Current | 128 (13%) | 128 (13%) | 128 (13%) | 120 (12%) | 0.68 |
| Past | 412 (43%) | 411 (43%) | 410 (42%) | 383 (40%) | |
| Never | 427 (44%) | 427 (44%) | 429 (44%) | 463 (48%) | |
| Total chol. (mg/dL) ± SD | 185±46 | 184±46 | 184±48 | 181±42 | 0.15 |
| LDL (mg/dL) ± SD | 104±35 | 102±36 | 102±36 | 101±33 | 0.19 |
| Triglycerides (mg/dL) ± SD | 154±111 | 156±113 | 163±120 | 155±118 | 0.35 |
| SBP (mmHg) ± SD | 129±23 | 129±22 | 129±22 | 127±23 | 0.32 |
| DBP (mmHg) ± SD | 71±12 | 71±12 | 72±14 | 72±13 | 0.10 |
| Pulse (beats/min) ± SD | 68±11 | 68±11 | 68±12 | 68±12 | 0.94 |
| Kidney and Electrolyte Parameters | |||||
| Serum | |||||
| eGFR (mL/min/1.73m2)± SD | 49.6±16.9 | 45.4±16.4 | 42.9±16.5 | 41.6±16.5 | <0.001 |
| eGFR categories (mL/min/1.73m2)± SD | |||||
| >60, n(%) | 232 (24%) | 171 (18%) | 146 (15%) | 136 (14%) | <0.001 |
| 30–60, n(%) | 636 (66%) | 614 (64%) | 580 (60%) | 557 (58%) | |
| <30, n(%) | 99 (10%) | 181 (19%) | 241 (25%) | 273 (28%) | |
| Cystatin C (mg/L) ± SD | 1.4 ± 0.5 | 1.5 ± 0.5 | 1.6 ± 0.6 | 1.6 ± 0.6 | <0.001 |
| Serum Na (mmol/L) ± SD | 139±3 | 139±3 | 139±3 | 139±3 | 0.95 |
| Serum K (mmol/L) ± SD | 4.4±0.5 | 4.4±0.5 | 4.3±0.5 | 4.3±0.6 | <0.001 |
| Serum Ca (mg/dL) ± SD | 9.2±0.5 | 9.2±0.5 | 9.2±0.5 | 9.2±0.6 | 0.30 |
| Serum PO4 (mg/dL) ± SD | 3.7±0.6 | 3.7±0.7 | 3.8±0.7 | 3.8±0.7 | 0.001 |
| Proteinuria (g/24h) | 0.14 [0.07–0.75] | 0.18 [0.07–0.99] | 0.23 [0.08–1.05] | 0.20 [0.07–0.86] | 0.007 |
| Urine | |||||
| Na excretion (mmol/24 h) ± SD | 164 ± 77 | 166 ± 80 | 164 ± 77 | 154 ± 75 | 0.001 |
| K excretion (mmol/24 h) ± SD | 52 ± 24 | 54 ± 24 | 56 ± 26 | 59 ± 31 | <0.001 |
| Na to K ratio | 3.5 ± 1.8 | 3.4 ± 1.6 | 3.2 ± 1.5 | 3.0 ± 1.6 | <0.001 |
| Biological Markers | |||||
| NT-pro BNP (pg/ml) [IQR] | 137 [59–376] | 147 [58–417] | 160 [65–423] | 166 [73–443] | 0.028 |
| FGF-23 (RU/mL) [IQR] | 137 [94–220] | 150 [98–241] | 146 [98–254] | 151 [94–244] | 0.07 |
| Albumin (g/dL) ± SD | 3.9±0.5 | 3.9±0.5 | 4.0±0.5 | 4.0±0.5 | <0.001 |
| Hemoglobin (g/dL) ± SD | 12.5±1.8 | 12.6±1.7 | 12.7±1.8 | 12.7±1.8 | 0.011 |
| Medications | |||||
| ACE Inhibitor/ARB, n(%) | 682 (71%) | 702 (73%) | 662 (69%) | 597 (62%) | <0.001 |
| Aldosterone antagonist n(%) | 9 (1%) | 25 (3%) | 40 (4%) | 84 (9%) | <0.001 |
| Loop diuretics, n(%) | 298 (31%) | 347 (36%) | 408 (43%) | 403 (42%) | <0.001 |
| Beta blockers, n(%) | 450 (47%) | 473 (49%) | 492 (51%) | 483 (50%) | 0.31 |
| Statins, n(%) | 543 (57%) | 563 (58%) | 520 (54%) | 495 (52%) | 0.012 |
CAD, coronary artery disease; PVD, peripheral vascular disease; BMI, body mass index; LDL, low density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; NT-pro BNP, N-terminal pro-B-type natriuretic peptide; FGF, fibroblast growth factor; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blockers.
Over a median follow-up of 5.4±1.6 years, there were 587 deaths, 743 ESRD events, 187 incident CHF cases and 177 incident atherosclerotic events. The annual rates of death and ESRD were 2.8% and 3.9% in the entire study cohort, respectively. We used splines to estimate the risk of each adverse event across aldosterone concentrations (log-transformed). Although the test of linearity for each of the spline functions was significant, we evaluated aldosterone both as a continuous and categorical variable.
Serum aldosterone concentrations were not associated with death or the composite atherosclerotic endpoint in either the unadjusted or adjusted analysis (Table 2). Among the 2,571 CRIC participants without a history of cardiovascular disease at baseline, serum aldosterone concentrations were associated with the development of heart failure in unadjusted analysis. Further adjustment for demographics (age, sex, race), clinical center, cardiorenal risk factors (diabetes, blood pressure, eGFR and proteinuria), urinary and serum factors (urinary sodium, urinary sodium to potassium ratio, hemoglobin, serum albumin, serum potassium, NT-pro BNP), and medications (ACE inhibitors, ARBs, MRBs, loop diuretics, and statins) resulted in a 21% higher risk of heart failure per standard deviation increase in log aldosterone (Table 2). Further, in categorical analysis, the highest aldosterone quartile was associated with a 50% greater risk of heart failure compared with the lowest quartile; however, this association was attenuated to a 36% higher risk in multivariable analysis and non-significant. The risk of ESRD was also higher as aldosterone concentrations increased in unadjusted analysis. We detected a 14% higher risk per standard deviation increase in the log transformed aldosterone levels and a 60% higher risk in the highest aldosterone quartile. These estimates for ESRD risk were attenuated substantially after multivariable adjustment for potential confounders including FGF-23 and did not maintain statistical significance.
Table 2.
Association of Aldosterone with Death, ESRD and Cardiovascular Outcomes in the CRIC Study
| Outcome Variable | Aldosterone Quartiles | Linear model | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Per SD | P-value | |
| Death | ||||||
| Annual event rate (# events/# at risk) | 2.7% (142/967) | 2.6% (133/966) | 3.1% (156/967) | 3.0% (156/966) | ||
| Unadjusted; HR (95% CI) | 1.00 (ref) | 0.95 (0.75, 1.21) | 1.14 (0.91, 1.44) | 1.09 (0.87, 1.37) | 1.03 | 0.48 |
| Adjusted*; HR (95% CI) | 1.00 (ref) | 0.80 (0.62, 1.03) | 0.92 (0.72, 1.18) | 0.89 (0.69, 1.15) | 1.00 | 0.98 |
| ESRD | ||||||
| Annual event rate (# events/# at risk) | 3.0% (145/967) | 3.5% (167/966) | 4.6% (214/967) | 4.6% (217/966) | ||
| Unadjusted; HR (95% CI) | 1.00 (ref) | 1.19 (0.95, 1.48) | 1.58 (1.28, 1.95) | 1.58 (1.28, 1.95) | 1.14 | <0.001 |
| Adjusted*; HR (95% CI) | 1.00 (ref) | 0.96 (0.75, 1.22) | 1.08 (0.85, 1.38) | 1.16 (0.91, 1.48) | 1.06 | 0.15 |
| + FGF-23; HR (95% CI) | 1.00 (ref) | 0.94 (0.74, 1.20) | 1.08 (0.85, 1.37) | 1.17 (0.92, 1.48) | 1.07 | 0.11 |
| CHF | ||||||
| Annual event rate (# events/# at risk) | 1.2% (40/643) | 1.2% (39/643) | 1.5% (50/643) | 1.8% (58/642) | ||
| Unadjusted; HR (95% CI) | 1.00 (ref) | 1.01 (0.65, 1.58) | 1.31 (0.86, 1.98) | 1.49 (1.00, 2.24) | 1.18 | 0.0098 |
| Adjusted†; HR (95% CI) | 1.00 (ref) | 0.82 (0.51, 1.32) | 1.03 (0.64, 1.65) | 1.36 (0.87, 2.13) | 1.21 | 0.012 |
| Combined Atherosclerotic Endpoint | ||||||
| Annual event rate (# events/# at risk) | 1.2% (39/643) | 1.2% (40/643) | 1.5% (47/643) | 1.6% (51/642) | ||
| Unadjusted; HR (95% CI) | 1.00 (ref) | 1.05 (0.67, 1.63) | 1.24 (0.81, 1.90) | 1.34 (0.88, 2.03) | 1.05 | 0.55 |
| Adjusted†; HR (95% CI) | 1.00 (ref) | 0.94 (0.59, 1.50) | 0.95 (0.59, 1.52) | 1.28 (0.81, 2.02) | 1.04 | 0.62 |
SD, standard deviation; HR, hazard ratio; Cl, confidence interval; ESRD, end stage renal disease; FGF, fibroblast growth factor; CHF, congestive heart failure.
adjusted for age, sex, race, clinical center, diabetes, systolic blood pressure, history of cardiovascular disease, eGFR, urinary protein (24 hour sample), urinary sodium excretion (24 hour sample), urinary sodium to potassium ratio (24 hour sample), hemoglobin, serum albumin, serum potassium, ACE inhibitor/ARB use, mineralocorticoid receptor blocker use, loop diuretic use, statin, and log NT-pro BNP.
adjusted for age, sex, race, clinical center, diabetes, systolic blood pressure, eGFR, urinary protein (24 hour sample), urinary sodium excretion (24 hour sample), urinary sodium to potassium ratio (24 hour sample), hemoglobin, serum albumin, serum potassium, ACE inhibitor/ARB use, mineralocorticoid receptor blocker use, loop diuretic use, statin, and log NT-pro BNP.
Interaction Testing and Subgroup Analysis
In the evaluation of incident heart failure, we did not detect an interaction between aldosterone and race (p interaction > 0.10). Subgroup analysis, however, demonstrated that the association between aldosterone and heart failure was modestly stronger in Blacks compared with Whites. For every standard deviation increase in log transformed aldosterone levels, the adjusted hazard ratio for CHF was 1.35, 95% CI [1.08 – 1.69] in Blacks and 1.19, 95% CI [0.93 – 1.53] in Whites. In addition, aldosterone was a stronger risk factor for incident heart failure in men compared with women (HR in men 1.43, 95% CI [1.16 – 1.76], HR in women 0.98, 95% CI (0.78 – 1.22); p interaction 0.015).
For the evaluation of ESRD, we did not detect an interaction between aldosterone and race (p interaction > 0.10). Subgroup analyses, however, also demonstrated that an association between aldosterone and ESRD was present in Blacks and not Whites. The hazard ratio for ESRD was 1.18, 95% CI [1.04 – 1.35] per SD increase in aldosterone in Blacks and 1.03, 95% CI [0.88 – 1.21] in Whites. All other interaction tests, which have not already been described above, between aldosterone and race, sex, eGFR, or use of RAAS medications or loop diuretics for the prediction of each of the four outcomes (death, ESRD, incident CHF, and incident atherosclerotic events) were not significant.
Discussion
Among participants enrolled in the CRIC Study, serum aldosterone concentrations were marginally associated with incident heart failure; however, they were not an independent risk factor for all-cause mortality, ESRD, or atherosclerotic events. In exploratory analysis, aldosterone was a stronger risk factor for CHF in men compared with women. Although the race interactions were not significant, aldosterone concentrations appeared to be a stronger risk factor for heart failure and ESRD in Blacks compared with Whites. To our knowledge this study is one of the first to evaluate systematically aldosterone’s associations with cardiorenal events in a well-characterized population of CKD participants.
Our data are in contrast to prior studies that have reported an association between aldosterone and mortality and cardiovascular events in patients with advanced heart failure,18, 19 acute myocardial infarction20 and coronary artery disease.21–23 The lack of a strong association between aldosterone and death and cardiorenal events in our study may stem from underlying RAAS dysregulation that is already present in the setting of CKD. Animal models of kidney disease have demonstrated upregulation of adrenal CYP11β2 (aldosterone synthase) messenger RNA expression.24 These models have elevated aldosterone concentrations and adrenal hypertrophy that precede the development of hypertension, proteinuria, and glomerulosclerosis.25 Although cross-sectional, we detected a reduction in eGFR and increase in proteinuria across aldosterone quartiles. Since all participants in CRIC have CKD, it is possible that merely universal dysregulation in RAAS limits the use of serum aldosterone to prognosticate mortality risk in individuals with CKD.
Aldosterone levels are an independent risk factor for incident heart failure. RAAS activation and elevations in aldosterone concentrations induce inflammatory and oxidative stresses that result in cardiac fibrosis.26, 27 These effects are known to occur despite high or low renin levels.28 In addition, although the RAAS is regulated by cardiac function and intravascular volume, our findings suggest that the aldosterone-incident heart failure link is independent of cardiac strain, kidney function, and total body water. We adjusted for NT-pro BNP, which represents neurohormonal activation and is secreted from cardiac myocytes in response to increased myocardial pressures and volume.29 In addition, we assessed volume status by adjusting for loop diuretic use, twenty-four hour sodium excretion and the twenty-four hour sodium to potassium ratio. Sodium excretion partially reflects total body sodium and water content although recent work suggests that rhythmic changes in the neuro-endocrine axis regulate aldosterone concentrations and subsequently sodium content.30 Further work should assess whether aldosterone profiling can identify CKD patients that are at the highest risk of developing CHF.
Most of the previous large-scale studies that have evaluated the association between aldosterone and mortality and cardiovascular risk have enrolled predominantly White participants with relatively preserved kidney function.18–22 Our analysis included 1,606 Black participants in whom stratified analysis demonstrated a slightly higher risk of incident heart failure and ESRD. Although the interaction testing was not significant, previous small-scale studies have demonstrated the significance of aldosterone in individuals of African ancestry with hypertension.31 Specifically, plasma aldosterone levels have correlated with blood pressure and left ventricular mass in Blacks but not Whites.32 More extensive work is necessary to determine whether RAAS activation comprises one of the primary pathways implicated in the disproportionately higher rates of ESRD, CHF, hypertension, and LVH observed in Blacks.
Implications for future clinical trials
Our study was an observational cohort designed to provide novel insights into the relationship of the RAAS and clinical outcomes in individuals with CKD. Although aldosterone has limited ability in prognosticating risk among CKD individuals, clinical trials to evaluate the use of MRBs in the CKD population are strongly needed. Activation of the mineralocorticoid receptor may still occur through circulating cortisol and subsequently contribute to cardiovascular injury in individuals with CKD.33, 34 In CKD, the enzyme 11 beta hydroxysteroid dehydrogenase type 2 (11βHSD2), which usually inactivates cortisol to its metabolite, has reduced activity and results in cortisol concentrations that are 100 to 1000 fold higher than aldosterone concentrations.35 As a result, the mineralocorticoid receptor is activated and can result in cardiovascular injury through multiple mechanisms described previously. MRBs block the actions of both cortisol and aldosterone and might explain why they remain effective in heart failure populations with normal aldosterone levels.36, 37 Further, a randomized controlled trial conducted in the early stages of CKD demonstrated that adding spironolactone to an ACE inhibitor or ARB reduced left ventricular mass and arterial stiffness.38 More recently, the efficacy and safety of spironolactone was evaluated in an open label randomized trial among ESRD patients on hemodialysis in the Dialysis Outcomes Heart Failure Aldactone Study. Although this trial enrolled a limited number of participants and was not blinded, it demonstrated a reduction in death or hospitalization from cardiovascular events.39 Future, prospective studies should evaluate the use of MRBs in patients with a greater range of CKD and not just those individuals on dialysis.
Several limitations of this study deserve mention. We did not measure serum renin and, therefore, could not use the aldosterone-to-renin ratio to differentiate cases of primary versus secondary aldosteronism. However, we have highlighted prior work that suggests a direct link between kidney disease and increases in aldosterone production. In addition, we did not measure cortisol in the CRIC study and have specified how mineralocorticoid receptor activation and a subsequent rise cardiovascular risk may still occur despite the current findings. Furthermore, aldosterone was only measured at one visit and a single time point in this study, making it impossible to assess immediate and long-term changes. Variations in aldosterone concentrations occur throughout the day based on salt intake, body positioning and other physiologic parameters. The use of a 24 hour urine collection for aldosterone determination at serial intervals throughout the study follow-up period would be the most reliable means of assessing aldosterone levels in CRIC. Finally, we did not collect the duration of ACE inhibitor/ARB therapy among the majority of participants that were on such therapies at the baseline visit. As a result, we could not compare the significance of elevated aldosterone concentrations between participants that had been on RAAS inhibitors and subsequently developed the aldosterone escape phenomena to those that had never been on an ACE inhibitor/ARB.
The strengths of our study include the use of a large, well-characterized, multiracial cohort with rigorous adjudication of cardiorenal endpoints and detailed characterization of markers of renal physiology and function. Aldosterone was measured at a single laboratory using a well-validated assay.
Perspectives
The current analyses suggest that higher aldosterone concentrations had a modest, independent association with incident heart failure in Black and White participants with CKD. Aldosterone, however, was not an independent risk factor for death, ESRD, or atherosclerotic events. This assessment is one of the first systematic evaluations of aldosterone as a potential risk factor for cardiorenal events in the CKD population. Further research is necessary to understand whether inhibition of RAAS activity can prevent heart failure and other cardiorenal events in the CKD population and whether RAAS activity can be implicated in explaining the disproportionately higher risk of cardiorenal events in Blacks compared with Whites.
Supplementary Material
Novelty and Significance.
What is New?
This project from the Chronic Renal Insufficiency Cohort study is one of the first to systematically evaluate the association between serum aldosterone levels and adverse events in Blacks and Whites with chronic kidney disease (CKD).
What is Relevant?
Activation of the renin-angiotensin-aldosterone system and subsequent increases in aldosterone levels are implicated in hypertension and CKD.
Elevations in aldosterone may explain the higher risk of cardiorenal events observed in individuals with CKD.
Aldosterone concentrations may also explain the disproportionately higher risk of cardiorenal events in Blacks compared with Whites.
Summary
Among individuals with CKD, higher aldosterone concentrations were independently associated with the development of CHF, but not for death, ESRD, or atherosclerotic events.
Acknowledgments
The CRIC Study investigators include The CRIC Study Investigators include Lawrence J. Appel, MD, MPH, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, and Mahboob Rahman, MD.
Sources of Funding
This work was supported by National Institutes of Health (NIH) grant K23DK089118 to RD. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
Disclosures: None
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Ku E, Campese VM. Aldosterone in the pathogenesis of chronic kidney disease and proteinuria. Curr Hypertens Rep. 2010;12:303–306. doi: 10.1007/s11906-010-0116-4. [DOI] [PubMed] [Google Scholar]
- 4.Weber KT, Anversa P, Armstrong PW, Brilla CG, Burnett JC, Jr, Cruickshank JM, Devereux RB, Giles TD, Korsgaard N, Leier CV. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol. 1992;20:3–16. doi: 10.1016/0735-1097(92)90130-f. [DOI] [PubMed] [Google Scholar]
- 5.Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005;18:44–49. doi: 10.1016/j.amjhyper.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Kent DM, Jafar TH, Hayward RA, Tighiouart H, Landa M, de Jong P, de Zeeuw D, Remuzzi G, Kamper AL, Levey AS. Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol. 2007;18:1959–1965. doi: 10.1681/ASN.2006101081. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Kawachi K, Saito Y, Saito T, Morishita K, Hoshino J, Hosoi T, Iwasaki T, Ohyama Y, Kurabayashi M. Effects of ARB or ACE-inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J. 2009;50:501–512. doi: 10.1536/ihj.50.501. [DOI] [PubMed] [Google Scholar]
- 10.Michel FS, Norton GR, Majane OH, Badenhorst M, Vengethasamy L, Paiker J, Maseko MJ, Sareli P, Woodiwiss AJ. Contribution of circulating angiotensinogen concentrations to variations in aldosterone and blood pressure in a group of African ancestry depends on salt intake. Hypertension. 2012;59:62–69. doi: 10.1161/HYPERTENSIONAHA.111.181230. [DOI] [PubMed] [Google Scholar]
- 11.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Jr, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 13.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 15.Ky B, Shults J, Keane MG, Sutton MS, Wolf M, Feldman HI, Reese PP, Anderson CA, Townsend RR, Deo R, Lo J, Gadegbeku C, Carlow D, Sulik MJ, Leonard MB. FGF23 modifies the relationship between vitamin D and cardiac remodeling. Circ Heart Fail. 2013;6:817–824. doi: 10.1161/CIRCHEARTFAILURE.112.000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J. 2004;25:292–299. doi: 10.1016/j.ehj.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Stork S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 20.Beygui F, Collet JP, Benoliel JJ, Vignolles N, Dumaine R, Barthelemy O, Montalescot G. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114:2604–2610. doi: 10.1161/CIRCULATIONAHA.106.634626. [DOI] [PubMed] [Google Scholar]
- 21.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31:1237–1247. doi: 10.1093/eurheartj/ehq019. [DOI] [PubMed] [Google Scholar]
- 22.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, Marz W. Association of plasma aldosterone with cardiovascular mortality in patients with low estimated GFR: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am J Kidney Dis. 2011;57:403–414. doi: 10.1053/j.ajkd.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, Collet JP, Beygui F, Hennache B, Ennezat PV, Juthier F, Richard F, Dallongeville J, Hillaert MA, Doevendans PA, Jude B, Bertrand M, Montalescot G, Van Belle E. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 24.Endemann DH, Wolf K, Boeger CA, Riegger GA, Kramer BK. Adrenal aldosterone biosynthesis is elevated in a model of chronic renal failure--role of local adrenal renin-angiotensin system. Nephron Physiol. 2004;97:37–44. doi: 10.1159/000078409. [DOI] [PubMed] [Google Scholar]
- 25.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young MJ. Mechanisms of mineralocorticoid receptor-mediated cardiac fibrosis and vascular inflammation. Curr Opin Nephrol Hypertens. 2008;17:174–180. doi: 10.1097/MNH.0b013e3282f56854. [DOI] [PubMed] [Google Scholar]
- 27.Michea L, Villagran A, Urzua A, Kuntsmann S, Venegas P, Carrasco L, Gonzalez M, Marusic ET. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and prevents oxidative stress in uremic rats. Hypertension. 2008;52:295–300. doi: 10.1161/HYPERTENSIONAHA.107.109645. [DOI] [PubMed] [Google Scholar]
- 28.Ritz E, Tomaschitz A. Aldosterone and kidney: a rapidly moving frontier (an update) [Accessed January 1, 2014];Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft035. http://ndt.oxfordjournals.org/content/early/2013/11/04/ndt.gft035.full. [DOI] [PubMed]
- 29.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 30.Titze J, Dahlmann A, Lerchl K, Kopp C, Rakova N, Schroder A, Luft FC. Spooky sodium balance. [Accessed November 1, 2013];Kidney International. 2013 doi: 10.1038/ki.2013.367. http://www.nature.com/ki/journal/vaop/ncurrent/full/ki2013367a.html. [DOI] [PubMed]
- 31.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Aldosterone contributes to blood pressure variance and to likelihood of hypertension in normal-weight and overweight African Americans. Am J Hypertens. 2009;22:1303–1308. doi: 10.1038/ajh.2009.167. [DOI] [PubMed] [Google Scholar]
- 32.Grim CE, Cowley AW, Jr, Hamet P, Gaudet D, Kaldunski ML, Kotchen JM, Krishnaswami S, Pausova Z, Roman R, Tremblay J, Kotchen TA. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005;45:766–772. doi: 10.1161/01.HYP.0000154364.00763.d5. [DOI] [PubMed] [Google Scholar]
- 33.Drechsler C, Ritz E, Tomaschitz A, Pilz S, Schonfeld S, Blouin K, Bidlingmaier M, Hammer F, Krane V, Marz W, Allolio B, Fassnacht M, Wanner C. Aldosterone and cortisol affect the risk of sudden cardiac death in haemodialysis patients. Eur Heart J. 2013;34:578–587. doi: 10.1093/eurheartj/ehs361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 35.N’Gankam V, Uehlinger D, Dick B, Frey BM, Frey FJ. Increased cortisol metabolites and reduced activity of 11beta-hydroxysteroid dehydrogenase in patients on hemodialysis. Kidney International. 2002;61:1859–1866. doi: 10.1046/j.1523-1755.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 36.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 37.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 38.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, Yakushigawa T, Sugiyama H, Shimada Y, Nojima Y, Shio N. Spironolactone reduces cardio- and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63:528–536. doi: 10.1016/j.jacc.2013.09.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.