Abstract
RATIONALE
Chemical modification of a rare gas cluster ion beam (GCIB) to increase the intensity of desorbed molecular ions in secondary ion mass spectrometry experiments relative to the pure Ar cluster.
METHODS
Doping of the GCIB by mixing small concentration levels (1–3% relative partial pressure) of CH4 into the Ar gas driving the cluster ion source.
RESULTS
Mass spectra were measured on a trehalose film using the doped GCIB exhibit enhanced molecular ion signals. From depth profiling experiments, the results are shown to arise from an increase in the ionization efficiency of the sputtered molecules rather than a change in the sputtering yield of neutral species.
CONCLUSION
Tuning of the chemistry of mixed clusters is suggested as a general approach to enhancing the ionization probability of sputtered molecules.
Introduction
Cluster secondary ion mass spectrometry (SIMS) is now a versatile and widely used tool for the characterization of many types of materials. With primary ion projectiles consisting of Bi3+, C60+ or SF5+, molecular depth profiling and molecule-specific imaging with sub-micron resolution are now routinely possible.[1] The emergence of gas cluster ion beams (GCIBs) has opened even more opportunities since the degree of molecular fragmentation during sputtering and the accumulation of chemical damage in the sample is reduced to almost zero with these projectiles.[2] The most commonly employed class of GCIBs consists of Arx+, with × between 500 and 5000. At the moment, the GCIB is employed largely as an erosion tool rather than a spectral acquisition and imaging tool since it is inconvenient to acquire spectra using conventional time-of-flight SIMS equipment and it has not yet been possible to focus these GCIBs to a sub-micron spot. Moreover, the secondary ion yield of molecules sputtered by GCIBs decreases dramatically as the cluster size increases, presumably since the relatively slow speed does not impart enough energy to adequately shake up the electronic system.[2] Problems associated with beam focus and mass spectral acquisition will undoubtedly be resolved for GCIBs, but the ionization issue remains problematic.
There have been many attempts to increase the secondary ion yield associated with SIMS experiments. Sensitivity is always an issue, especially when attempting to interrogate a sub-micron pixel where the number of available molecules is severely limited. A simple method involves addition of protons to the sample in the form of water-ice, which has been shown to increase the [M+H]+ ion signal by up to an order of magnitude for a limited number of samples.[3] A more sophisticated approach has been to try to increase the probability of [M+H]+ formation by flooding the primary ion impact area with protons. By injection of water vapor ~1 mm above the target surface at a pressure of ~10−3 mbar, an order of magnitude enhancement of the ion signal was observed for various amino acids and peptides.[4] Our laboratory has proposed that dynamically created protons formed during depth profiling experiments can provide a path for ion enhancement via [M+H]+ formation.[5],[6] Finally, it has recently been demonstrated that direct bombardment of a surface with [H20]1000+ can lead to more than an order of magnitude enhancement of a variety of molecules, ranging from lipids to peptides to drugs compared with Ar1000+ bombardment.[7] These experiments are important since they demonstrate that chemical ionization is indeed feasible during SIMS and that strategic thinking along these lines can lead to new pathways for enhanced sensitivity. In this work, we show that it is feasible to tune the chemistry of an Ar-GCIB by mixing a small percentage of other gases – in this case CH4 - into the mix during supersonic expansion. Using trehalose as a model, we show that as little as 3% CH4 incorporated into Ar4000+ enhances the [M+H]+ ion yield by a factor of 4 while leaving the sputtering yield of neutral molecules nearly unchanged. These results are important since they suggest that this mixing strategy can be tested using a variety of other gases to fully optimize the chemical ionization efficiency.
Experimental
The experiments were performed using a J105 chemical imager system (Ionoptika Ltd, Chandlers Ford, UK) described in detail elsewhere[8]. The system is equipped with a 40-keV C60+ and a 20-keV gas cluster ion source, but only the gas cluster ion source was used here. Under normal conditions, this source is operated with pure Ar and generates an Arn+ cluster ion beam with a size distribution centered around n = 4000 at a gas pressure of 18 bar. To prepare a mixed-composition cluster, the gas inlet of the source was modified by inserting a stainless steel mixing chamber into the primary gas line. In order to generate heterogeneous clusters composed of different particles, multiple pressurized gas tanks were connected via supply lines leading to the bottom of the mixing chamber. The gas line leading to the cluster ion source - along with a pressure gauge and a venting line - was connected to the top of the chamber. This tubing scheme on opposite ends of the chamber was found to be important in order to ensure a proper mixing of the gas before it was introduced into the ion source. The gas mixture was prepared by first introducing the dopant gas into the chamber. At the desired partial pressure, the valve at the pressure regulator was closed and the chamber was backfilled with Ar until the working pressure of the ion source (here 18 bar) was reached. This pressure was then kept constant by leaving the valve between the mixing chamber and the Ar pressure regulator open. With the volume of the mixing chamber being about 2 liter, this procedure allowed a stable operation of the ion source up to several hours.
The composition of the gas introduced into the ion source was monitored by means of a residual gas analyzer mounted to the second differentially pumped stage of the ion source, where the generated cluster beam is ionized under high vacuum conditions at a pressure of the order of 10−5 mbar. For every gas mixture, the total primary ion current was measured using a Faraday cup and the beam size was measured by taking a secondary electron image of a 135 mesh Transmission Electron Microscopy (TEM) grid. The cluster size distribution was checked by pulsing the gun and measuring the flight time spectrum of the projectile ions between the pulser and the target surface via the ion induced secondary electron emission signal. The resulting flight time spectrum can be converted into a cluster mass distribution by means of the known flight path (42.9 cm) and kinetic energy (20 keV) of the projectiles. The samples used in this study consisted of a 100 nm trehalose film spin cast onto a silicon substrate. Details of the sample preparation are described elsewhere[9]. The ion beam was pulsed at a 10 kHz repetition rate with a duty cycle of 50% and rastered across an area of 200 × 200 micrometer. The sample stage was kept at ground potential during the analysis, and no sign of surface charging was found for this system. In order to examine for sample homogeneity, the mass spectral data were acquired in the form of images over the quoted raster area consisting of 64 × 64 pixels (thereby matching the pixel size with the beam diameter of about 10 micrometer) with a total fluence of 1.6×1012 ions/cm2 and retrospectively summed over all pixels. Each image was obtained on a fresh surface area that had not been previously subjected to the ion beam.
Results and Discussion
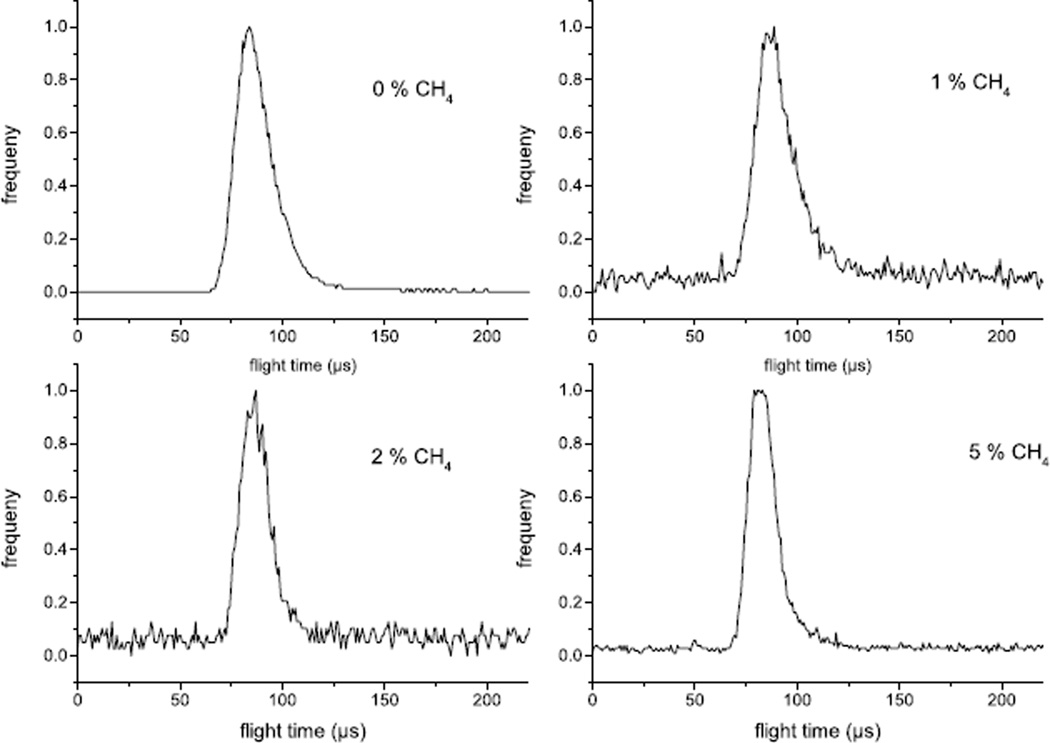
The flight time distribution for pure Ar and three different concentration levels of CH4 mixed into the source gas are shown in Fig. 1. From the known flight distance and cluster kinetic energy, we calculate an average cluster size of 4000 and a half width of about ± 900 constituent units (Ar or CH4, respectively), which corresponds to an impact energy of about 5 eV per constituent. Moreover, it is apparent that the characteristics of the cluster formation process do not seem to change significantly in the dopant concentration range explored here.
Figure 1.
Flight time spectrum of projectile ions as described in the text for three levels of CH4 doped into the gas line driving the cluster ion source.
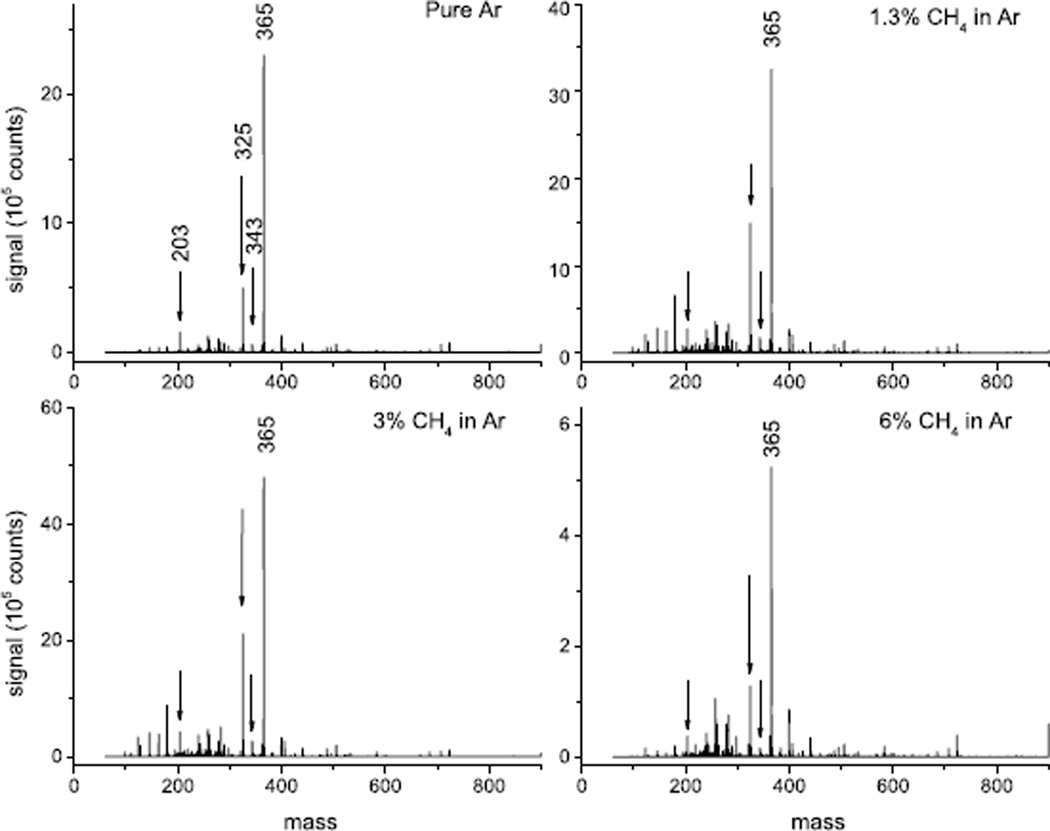
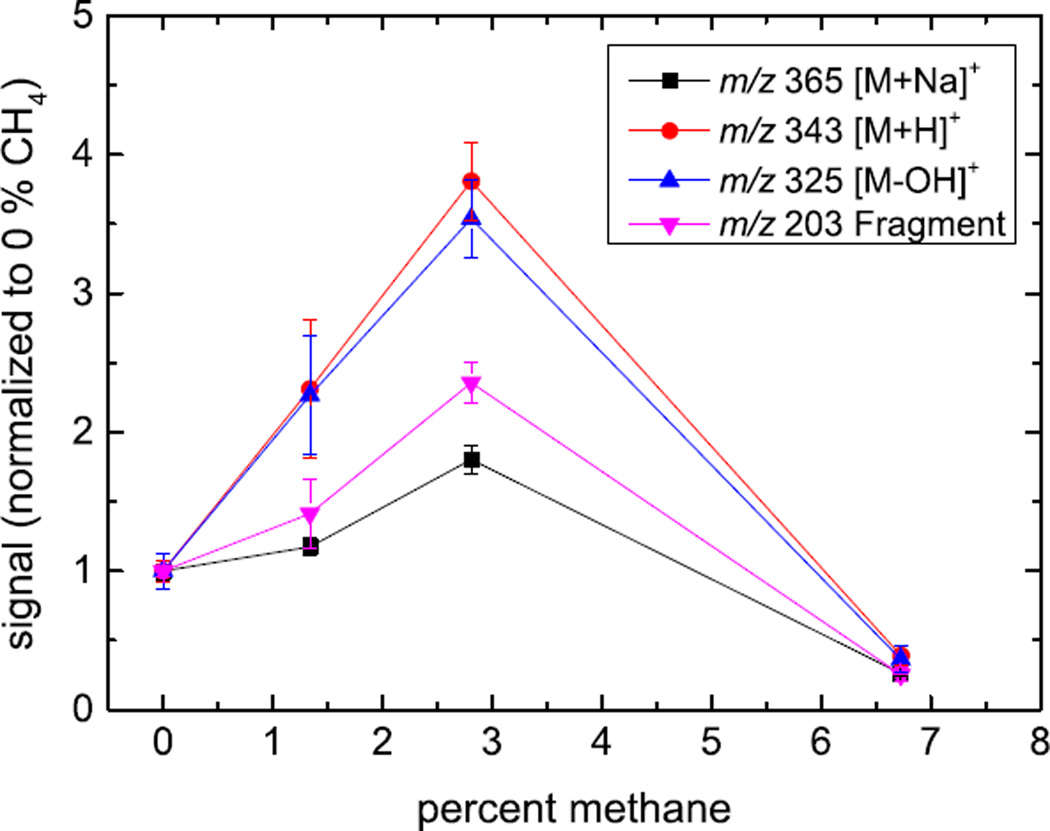
The mass spectra obtained under bombardment with these projectiles are displayed in Fig. 2. The molecular ion signals1 representative of trehalose (M = C12H22O11 with m/z 342.3) are [M+H]+ (m/z 343), [M+Na]+ (m/z 365) and [M-OH]+ or [M+H-H2O]+ (m/z 325); an additional characteristic fragment ion signal is observed at m/z 203 ([C6H12O6Na]+, i.e., a sodiated fragment consisting of one of the two identical rings of the trehalose molecule). The signals of these peaks are plotted vs the CH4 concentration in the gas mixture driving the cluster ion source in Fig. 3. The data have been normalized to the measured projectile ion current and therefore are representative of the respective secondary ion yields. It is obvious that the CH4 admixture leads to an increase of the molecule-specific signals, which appears to be optimized at fairly low concentration levels of the order of only a few percent and drops off at higher concentration.
Figure 2.
Mass spectra of trehalose obtained under bombardment with 20-keV (Ar+CH4)4000+ cluster ions for different concentration levels of CH4 mixed into the operation gas driving the cluster ion source.
Figure 3.
Signal of molecular ions [M+Na]+ (m/z 365), [M+H]+ (m/z 343) and characteristic fragment ions [M-OH]+ (m/z 325) and [M-X]+ (m/z 203) normalized to the primary ion current vs. CH4 concentration (relative partial pressure) in the gas driving the cluster ion source under otherwise identical experimental conditions. The error bars represent the standard deviation of 3 measurements taken on different areas of the sample surface.
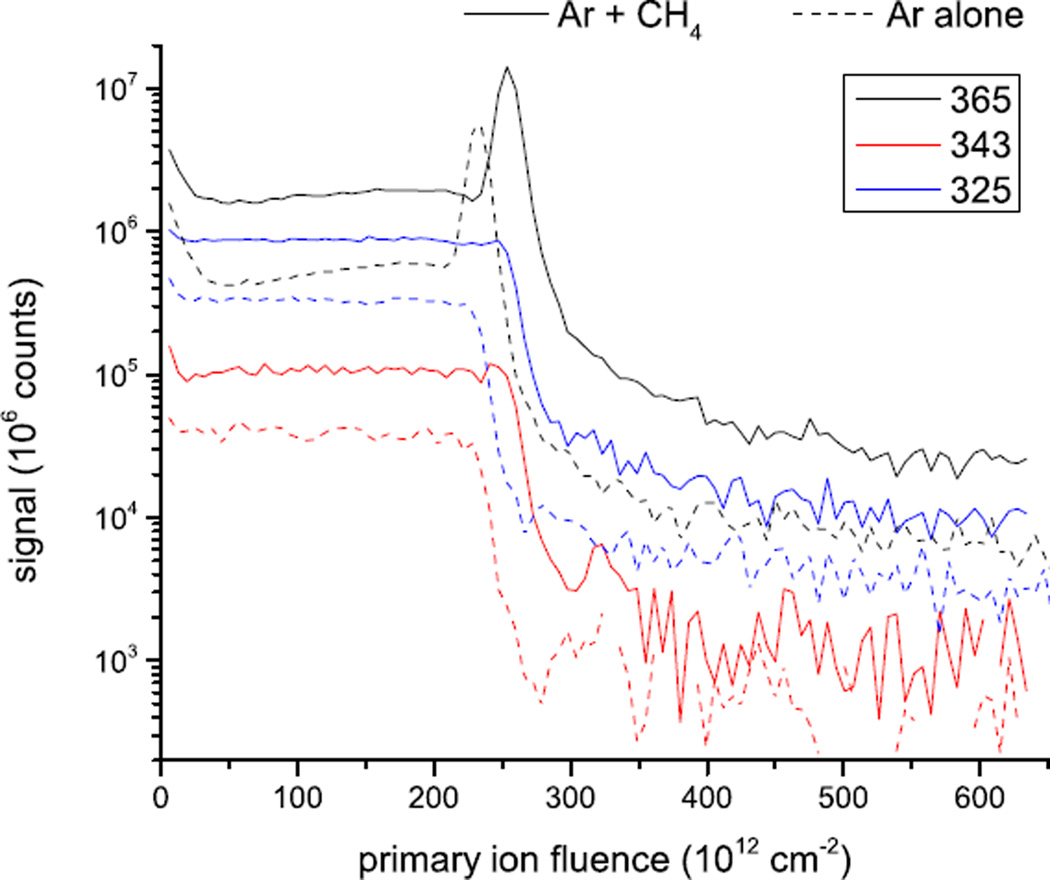
In principle, an increase of the secondary ion yield can be caused either by an enhanced sputtering yield or an enhanced ionization of the sputtered species. In order to distinguish between these two cases, we obtained sputter depth profiles on a thicker (about 200 nm) trehalose film as shown in Fig. 4. If the fluence needed to remove the film is corrected for the film thickness measured via the crater depth obtained by atomic force microscopy (see Supporting Information), it is found that the pure Ar and the mixed cluster beam including 2.2% CH4 remove a volume of 49 nm3 and 43 nm3 per incident ion, respectively, thereby proving that the total sputter yield slightly decreases as a consequence of the CH4 admixture. At the same time, the molecular ion signals are enhanced in the same way as shown in Fig. 3, indicating that the observed signal enhancement must be connected to an enhanced ionization of the sputtered molecules.
Figure 4.
Sputter depth profile of a ~200-nm Trehalose film obtained with either the Arn (dashed lines) or the mixed (Ar+CH4)n (solid lines) cluster ion beam. Shown are the molecular ion signals of [M+Na]+ (m/z 365), [M+H]+ (m/z 343) and [M-OH]+ (m/z 325).. See the supplemental material for details.
There are several interesting features to note in Fig. 4. While the signals of [M+H]+ and [M-OH]+ clearly reach a steady state, the [M+Na]+ signal exhibits significant variation in this region and also goes through a huge maximum at the interface to the underlying silicon substrate. Since all three signals derive from the same intact sputtered molecules, the difference must be attributed to the ionization mechanism, which is assumed to proceed via the adduction of either H+ or Na+ to a sputtered neutral M molecule. It is well known that the formation probability of adduct ions such as [M+Na]+ can be effectively controlled by the concentration of Na+ in the sample. In this picture, the observed [M+Na]+ yield variation must reflect an inhomogeneous distribution and, in particular, an enrichment of Na+ at the film-substrate interface. The relative signal levels observed at m/z 343 and 365 therefore reveal valuable information about the ionization efficiency of the ejected molecules. The fact that the [M+Na]+ yield is about an order of magnitude larger than that of [M+H]+ and still increases by another order of magnitude at the interface provides an upper estimate of the chemical ionization efficiency of the molecule M via the protonation reaction producing the [M+H]+ ion. In fact, the data show that the probability of [M+H]+ formation must be smaller than 10−2, indicating that there is headroom of more than two orders of magnitude for improving the ionization efficiency via [M+H]+ formation, provided that one finds a more efficient way to promote the protonation reaction.
At present, the detailed mechanism producing the observed yield enhancement is unclear. If the effect is caused by an enhanced chemical ionization of the sputtered molecules, one would in principle expect the yields of different adduct ions to respond in a different way to the CH4 admixture. In fact, the data displayed in Fig. 3 reveal that the signal enhancement is largest for [M+H]+ and smallest for [M+Na]+ ions. In this context, one might note that when the cluster disintegrates upon impact, the hydrogen atoms entrained in the projectile have a very low kinetic energy of only ~0.1 eV per atom. Therefore, it appears feasible that these low energy free hydrogen radicals might stick around in the crater volume for long enough time to facilitate the protonation of a sputtered molecule. While this should act to enhance the [M+H]+ yield, it should evidently be less effective for the formation of [M+Na]+ ions, a trend which is is indeed observed in Fig. 3.
An interesting feature is the relatively strong decay of the signal with increasing methane concentration at dopant levels above a few percent. We think that this finding is caused by massive changes of the gas cluster ion beam at higher dopant levels. In fact, recent data presented by a Japanese group[10] indicate that already at a methane gas concentration of about 10 % the GCIB consists entirely of methane clusters. In the same study, the authors find that the molecular ion signal measured on an insulin film decreases under bombardment with a methane cluster beam compared with the argon cluster beam. These observations are in line with our data and clearly support our suggestion to use small dopant levels in order to preserve the characteristics of the GCIB.
Conclusions
We have demonstrated that the admixture of small amounts of a chemically reactive species to the Ar gas driving the GCIB leads to an improvement of the molecular ion signals measured on the trehalose model sample. Similar results are found using other classes of molecules including lipids, peptides and various drug molecules with details to be published later. We have also shown that this signal enhancement is not caused by a simple increase of the total sputtering yield and must therefore be attributed to an enhanced ionization of the ejected molecules. Although the effect observed here is limited to a 4-fold increase, the data shown in Fig. 4 indicate that there is headroom of at least two orders of magnitude for improving the ionization efficiency of a sputtered molecule compared with [M+H]+-formation under bombardment of a pure Ar cluster ion beam. The admixture technique described here in principle allows introduction of a virtually unlimited number of chemically reactive species into the gas cluster projectiles and may therefore provide a versatile tool to tailor the surface chemistry exactly in the impact zone, where it is needed in order to facilitate the chemical ionization process. Going to the extreme, one could, for instance, envision the addition of salts or even acids (like, for instance, HCl), thereby directly delivering protons and adduct ions (like Na+ or Cl−) as a means to enhance the ionization efficiency via the formation of adduct ions like [M+X]+, [M+Y]− in a similar way as the [M+Na]+ shown here.
Supplementary Material
Acknowledgement
Financial support from the National Institutes of Health under Grant No. 2R01 EB002016-19, the U.S. Department of Energy, Division of Chemical Sciences, Basic Energy Sciences, under grant number DE-FG02-06ER15803 and Novartis is gratefully acknowledged.
Footnotes
we use the term "molecular ion" here for any secondary ion that derives from the intact sputtered parent molecule M
References
- 1.Mahoney CM. Cluster Secondary Ion Mass Spectrometry: Principles and Applications. Hoboken, NJ, USA: John Wiley & Sons; 2013. [Google Scholar]
- 2.Gnaser H, Ichiki K, Matsuo J. Strongly reduced fragmentation and soft emission processes in sputtered ion formation from amino acid films under 8 large Arn+ (n<= 2200) cluster ion bombardment. Rapid Commun. Mass Spectrom. 2012;26:1. doi: 10.1002/rcm.5286. [DOI] [PubMed] [Google Scholar]
- 3.Piwowar AM, Fletcher JS, Kordys J, Lockyer NP, Winograd N, Vickerman JC. Effects of Cryogenic Sample Analysis on Molecular Depth Profiles with TOF-Secondary Ion Mass Spectrometry. Anal. Chem. 2010;82:8291. doi: 10.1021/ac101746h. [DOI] [PubMed] [Google Scholar]
- 4.Mouhib T, Delcorte A, Poleunis C, Bertrand P. Organic Secondary Ion Mass Spectrometry: Signal Enhancement by Water Vapor Injection. J. Am. Soc. Mass Spectrom. 2010;21:2005. doi: 10.1016/j.jasms.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Willingham D, Brenes DA, Wucher A, Winograd N. Strong-Field Photoionization of Sputtered Neutral Molecules for Molecular Depth Profiling. J. Phys. Chem. C. 2010;114:5391. doi: 10.1021/jp9054632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrison BJ. Molecular ions in cluster bombardment: What clues do the molecular dynamics simulations provide? Surf. Interf. Anal. 2011;43:134. [Google Scholar]
- 7.Sheraz S, Barber A, Fletcher JS, Lockyer NP, Vickerman JC. Enhancing Secondary Ion Yields in Time of Flight-Secondary Ion Mass Spectrometry Using Water Cluster Primary Beams. Anal. Chem. 2013;85:5654. doi: 10.1021/ac4013732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher JS, Rabbani S, Henderson A, Blenkinsopp P, Thompson SP, Lockyer NP, Vickerman JC. A New Dynamic in Mass Spectral Imaging of Single Biological Cells. Anal. Chem. 2008;80:9058. doi: 10.1021/ac8015278. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J, Wucher A, Winograd N. Molecular Depth Profiling with Cluster Ion Beams. J. Phys. Chem. B. 2006;110:8329. doi: 10.1021/jp0573341. [DOI] [PubMed] [Google Scholar]
- 10.Moritani K, Kanai M, Ihara I, Mochiji K. Data presented at the 19th International Conference on Secondary Ion Mass Spectrometry SIMS-19. Jeju, Korea: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.