Abstract
Pregnancy involves remarkable orchestration of physiologic changes. The kidneys are central players in the evolving hormonal milieu of pregnancy, responding and contributing to the changes in the environment for the pregnant woman and fetus. The functional impact of pregnancy on kidney physiology is widespread, involving practically all aspects of kidney function. The glomerular filtration rate increases 50% with subsequent decrease in serum creatinine, urea, and uric acid values. The threshold for thirst and antidiuretic hormone secretion are depressed, resulting in lower osmolality and serum sodium levels. Blood pressure drops approximately 10 mmHg by the second trimester despite a gain in intravascular volume of 30% to 50%. The drop in systemic vascular resistance is multifactorial, attributed in part to insensitivity to vasoactive hormones, and leads to activation of the renin-aldosterone-angiostensin system. A rise in serum aldosterone results in a net gain of approximately 1000 mg of sodium. A parallel rise in progesterone protects the pregnant woman from hypokalemia. The kidneys increase in length and volume, and physiologic hydronephrosis occurs in up to 80% of women. This review will provide an understanding of these important changes in kidney physiology during pregnancy, which is fundamental in caring for the pregnant patient.
Keywords: Pregnancy, Kidneys, Renal physiology, Women
Introduction
Pregnancy affects essentially all aspects of kidney physiology. The orchestration of changes that occur is a physiologic feat. Kidney and systemic hemodynamics are marked by significant volume expansion and vasodilation. Glomerular filtration rate (GFR) increases 50% and renal plasma flow (RPF) increases up to 80% as compared with nonpregnant levels.1 Tubular function and handling of water and electrolytes are altered, leading to mild increases in proteinuria, glucosuria, lower serum osmolality, and reductions in serum sodium levels. The kidneys are larger during pregnancy because of fluid retention, and physiologic hydronephrosis is common. Typical laboratory changes are depicted in Table 1. This article will review important changes in kidney physiology during pregnancy, the understanding of which is fundamental in caring for the pregnant patient.
Table 1.
Typical laboratory values during pregnancy
| Variable | Average Values in Pregnancy |
|---|---|
| Plasma osmolality | 270 mOsm/kg |
| Serum sodium | 135 mEq/L |
| Serum potassium | 3.8 mEq/L |
| Serum bicarbonate | 18-20 mEq/L |
| Serum creatinine | 0.5 mg/dL |
| Blood urea nitrogen | 9.0 mg/dL |
| Uric acid | 2-3 mg/dL |
Anatomic Changes
Anatomic changes during pregnancy have long been appreciated.2,3 Hydronephrosis during pregnancy occurs in 43% to 100% pregnant women, and it is more prevalent with advancing trimester.4 Serial quantitative measurements by ultrasonography demonstrate that the maximal incidence of hydronephrosis is reached at 28 weeks, with a 63% overall incidence of hydronephrosis.5 The dilated collecting system can hold 200 to 300mL of urine, leading to urinary stasis and a 40% increased risk for pyelonephritis in pregnant women with asymptomatic bacteriuria versus nonpregnant women.3
The length of the kidneys also increases by 1 to 1.5 cm during pregnancy and decreases in size over a period of 6 months postpartum.6 Although this growth was initially attributed to hydronephrosis, Christensen and colleagues were able to demonstrate growth in kidney volume in 24 healthy women without evidence of pelviectasis using computerized ultrasonography,7 Although ultrasound remains the imaging modality of choice for evaluation of hydronephrosis in pregnancy, various magnetic resonance techniques can be used when ultrasound is non-diagnostic and can determine the level of obstruction and whether it is intrinsic or extrinsic in nature.7,8 Overall, the volume of kidneys during pregnancy increases up to 30%.9 The growth is attributed to increased kidney vascular and interstitial volume rather than any changes in the number of nephrons.10,11
The kidney pelvis and calyceal systems dilate under mechanical compressive forces on the ureters and possibly because of the effects of progesterone. Progesterone can reduce ureteral tone, peristalsis, and contraction pressure. However, the strongest etiologic evidence for hydronephrosis is for mechanical compression of the ureters themselves.12 There is a right-sided preponderance of hydronephrosis in up to 86% of pregnant women.13 This is attributed to the right ureter crossing the iliac and ovarian vessels at an angle before entering the pelvis, whereas the left ureter travels at less acute an angle and travels in parallel with the ovarian vein. This asymmetry could not be explained by a hormonal effect. Studies have shown administration of progesterone in nonpregnant women fails to cause hydronephrosis, and there is no correlation between progesterone or estrogen levels and severity of calyceal dilatation.12,14 In women with pelvic kidney or ureteral diversion where the ureter enters the conduit above the pelvic brim hydronephrosis is not observed. The greater incidence of hydronephrosis in primigravidas also supports the hypothesis that mechanical compression underlies hydronephrosis in pregnancy. Magnetic resonance imaging allows for characterization of the ureter compressed between the iliopsoas and gravid uterus at the level of the sacral promontory.15,16
Changes in Kidney Hemodynamics
Pregnancy is a state of volume expansion and vasodilation, which occurs in association with careful coordination of several hormones (Fig 1). One of the earliest changes observed in pregnancy is a decrease in blood pressure, approximately 10 mmHg by the second trimester, with mean values of 105/60 mmHg. There are probably various causes, including alterations in the renin-angiotensin-aldosterone system (RAAS) and other hormonal fluctuations. Maternal hormones may influence hemodynamic changes in pregnancy. Mean arterial pressure is decreased in the midluteal phase of menstruation compared with the midfollicular phase in association with a decrease in vascular resistance and rise in cardiac output.17 Progesterone can produce an increase in RPF and GFR, but it cannot account for the magnitude of increase seen in pregnancy. Relaxin is a vasodilating hormone produced by the corpus luteum, decidua, and placenta. It is implicated in the kidney physiology of pregnancy in rodents via upregulation of vascular gelatinase activity, which acts through the endothelial endothelin B receptor-nitric oxide pathway.18 Ogueh and colleagues showed a steady rise in relaxin throughout pregnancy with a decrease postpartum. However, clinical correlations between relaxin levels and hemodynamic parameters, at least in late gestation and postpartum, have not been demonstrated.19,20
Figure 1.
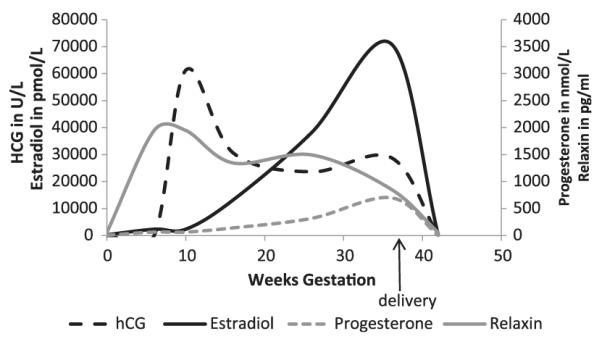
Hormonal variation throughout pregnancy and early postpartum. Mean data are of plasma human chorionic gonadotropin, estradiol, progesterone, and relaxin levels, before, during, and 6 weeks after pregnancy. Adapted from Ogueh and his colleagues.19
Normal pregnancy is marked by an upregulation of RAAS. Renin is released from extrarenal sources, specifically the ovaries and decidua. The placenta produces estrogen, which increases angiotensinogen synthesis by the liver, leading to increased angiotensin II (ANG II). Despite this, systolic blood pressure is known to decrease during pregnancy, likely because of several factors. Refractoriness to ANG II occurs during pregnancy and may explain the vasodilated state.21,22 This insensitivity may be explained by the presence of other substances such as progesterone and vascular endothelial growth factor mediated prostacyclins and/or the monomeric state of angiotensin 1 (AT1) receptors.23 Dysregulation of RAAS occurs in preeclampsia with return of ANG II sensitivity, less aldosterone, heterodimeric AT1 receptors and the presence of autoantibodies to AT1 (AT1-AA). In normal pregnancy, aldosterone levels rise at gestational week 8 and continue to increase 3- to 6-fold the upper limit of normal (80-100 ng/dL) in the third trimester. The result is a net gain of 1.1 to 1.6L and a 30% to 50% increase in blood volume compared with nonpregnant women.
Changes in GFR
One of the earliest kidney changes is the impressive rise in GFR. In an elegant study of 11 healthy women, Davison and Noble documented serial measurements of creatinine clearance using 24-hour urine collections during the menstrual cycle through conception and up to 16 weeks of gestation. They demonstrated that creatinine clearance increases during the luteal phase of the normal menstrual cycle and that increases in GFR during pregnancy occurred as a continuation of these changes. Of the 9 successful pregnancies in this study, creatinine clearance increased 20% at 4 weeks post-menses, 25% as early as gestational week 4, and 45% by gestational week 9. In the 2 women with spontaneous, uncomplicated abortions, GFR did not rise as much and it was not sustained. These changes remarkably occurred 3 weeks before the abortion was clinically evident.24 Chapman and colleagues documented early rises in GFR and kidney blood flow by inulin and paminohippurate clearances in association with systemic and kidney vasodilation in a series of 10 pregnant women.25 A series of studies suggests an overall progressive increase in GFR approximating 40% to 50% with peak increases sustained at term in uncomplicated pregnancies.26,27 Hyperfiltration has been shown to continue at levels 20% above normal at postpartum week 228 and resolve by 1 month postpartum.27
Measurement of GFR
The ability to estimate GFR is critical in the care of the pregnant patient. Accurate estimation of GFR remains an active area of research in pregnancy, as in general nephrology. The Modification or Diet in Renal Disease (MDRD) equation underestimates GFR in pregnant women with and without preeclampsia, consistent with its known tendency to underestimate when GFR is greater than 60 mL/minute.29,30 The CKD Epidemiology Collaboration equation appears to underestimate GFR to a similar degree as the MDRD equation in a study comparing both equations to 24-hour urine collections in preeclamptic patients.31 In a retrospective study, MDRD and cystatin-C-based equations result in mean GFR greater than 120 mL/minute, but cystatin C produced higher first and second trimester GFRs followed by a fall in GFR in the third trimester despite evidence from early studies that GFR increases steadily until term. Although the MDRD equation showed a fall in GFR postpartum, the cystatin C equation showed that GFR rose postpartum.32 Recently, no correlation was found in a prospective study comparing cystatin-C-based GFR calculations to inulin clearances at 3 time points in 12 pregnant patients.33 Thus, 24-hour urine collection for calculation of creatinine clearance remains the preferred standard for estimating GFR in pregnant women.
Mechanisms of Increased GFR
During pregnancy, the GFR increases 50% compared with prepregnancy levels. The precise mechanisms behind this increase are incompletely understood. It is helpful to remember the expression of GFR and appreciate the components that vary during pregnancy.
where the net hydraulic pressure in the glomerulus is (ΔP) and oncotic pressure at the glomerulus is (πGC). Transcapillary hydraulic pressure cannot be directly measured in humans, but it can be measured in animal models using micropuncture techniques. πGC is the mean of the afferent oncotic pressure (πA) and the efferent oncotic pressure (πE). πE is an expression of the oncotic pressure entering the afferent arteriole, divided by 1 minus the filtration fraction (FF), the portion of plasma filtered along the glomerulus:
FF is an expression of the GFR divided by the RPF:
The glomerular ultrafiltration coefficient, Kf, is the product of the surface area available for filtration and the hydraulic permeability (k) such that permeability is the ability to ultrafiltrate across the 3 layers of the glomerulus. Estimates for permeability are derived from autopsy samples and modeling.
In pregnancy, oncotic pressure is substantially decreased because of expansion of the plasma volume, thus contributing to a rise in GFR. There may also be modest changes to Kf due to changes in the surface area for filtration and the hydraulic permeability.34 There is still no consensus as to whether ΔP increases in human pregnancy. In early studies on the 12-day pregnant rat, Baylis found no change in the hydrostatic or oncotic pressure and attributed rise in GFR to increases in RPF.35 A study by Roberts and colleagues showed that glomerular size selectivity appeared to be altered in pregnant women and oncotic pressure was decreased, but they were not able to find evidence of increased ΔP.36 They concluded that much of the increased GFR is due to increases in RPF. This logic would not explain continued elevation GFR later in gestation whereas RPF falls consistently toward term (Fig 2). In a human postpartum study, an analysis of the glomerular filtration dynamics suggested that sustained increase in GFR postpartum is due to either a rise in ΔP up to 16%, an approximate 50% increase in Kf, or a combination of smaller increments of ΔP and Kf.28 Odutayo and Hlaudunewich reason that given that estimated changes in Kf and measured changes in πGC are modest, it is difficult to exclude the possibility that ΔP does change.37
Figure 2.
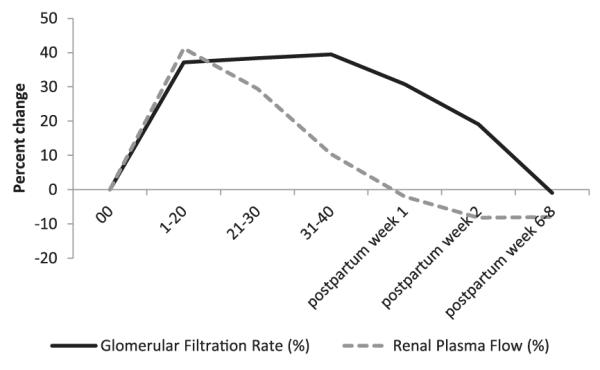
Percentage change from baseline of GFR and RPF throughout pregnancy and postpartum. Mean values are measured by inulin or isothalamate and p-aminohippurate clearance for GFR and RPF, respectively, as synthesized by Odutayo and Hladunewich.37
Changes in Tubular Function
During pregnancy there are alterations in tubular handling of wastes and nutrients. Uric acid excretion increases due to the increases in GFR, decreases in proximal tubular reabsorption, or a combination of both. Serum uric acid levels fall in early pregnancy, reaching a nadir of 2 to 3.0 mg/dL by 22 to 24 weeks, followed by gradual rise to normal by term. The increased clearance is thought to be necessary to handle the increased production from placenta and fetus.
Glucose is freely filtered at the glomerulus and almost completely reabsorbed in the proximal tubule, with a small amount reabsorbed in the collecting tubule. Glucosuria typically indicates that the filtered load of glucose has exceeded the maximal tubular reabsorptive capacity. In pregnancy, there is less effective reabsorption of glucose and variability in glucose excretion. Older studies proposed that increased GFR and thus increased filtered load of glucose overwhelmed the capacity of the proximal tubule to reabsorb glucose, thus leading to glucosuria with normoglycemia or a physiologic glucosuria. In a study of 29 pregnant women, glucose infusion with concomitant measurements of glucose excretion and inulin clearance demonstrated that regardless of presence of glucosuria, pregnant women had reduced glucose reabsorption. Women who had no glucosuria returned to an efficient state of reabsorption 8 to 12 weeks postpartum; however, women with varying degrees of glucosuria during pregnancy continued to have some degree of impaired capacity to reabsorb although they were no longer glucosuric.38 Distal nephron reabsorption may also be less effective in pregnancy.39 Similar to uric acid and glucose handling, there is a decreased fractional reabsorption of amino acids and β-microglobulin, resulting in increased excretion of these substances.
In normal pregnancy there is an increase in total urinary protein and albumin excretion, especially notable after 20 weeks. The protein content in urine is mostly Tamm-Horsfall, with a small amount of albumin and other circulating proteins. The rise in proteinuria during pregnancy is often attributed to the rise in GFR, although the timing does not fall within the peak increase in GFR. There is evidence that the amount of albuminuria increases in late pregnancy, albeit with levels that do not exceed the upper limit of normal.40 Circulating soluble antiangiogenic factors, which are found at unusually high levels in preeclampsia and disrupt the slit diaphragm, are also increased late in normal pregnancy and may explain late-term elevations in proteinuria.41 Another potential factor includes selective alterations in glomerular charge or the presence of other protein material, which is seen in the third trimester.42-44
Abnormal proteinuria in pregnant women is defined as protein levels of 300 mg/24 hours or more, which is twice the normal limit in nonpregnant women.45,46 This value was derived from a relatively small sample, and further studies have shown that mean levels of protein excretion generally do not exceed 200 mg/24 hours.47-49 Although the use of urine protein/creatinine for quantification of proteinuria in nonpregnant patients has become more routine and can be used to screen for presence or absence of proteinuria, the 24-hour urine collection is still the gold standard for quantification of pregnant proteinuria patients. Unfortunately, a substantial percentage of timed urine collections in pregnant women are incomplete because of errors in timing and retention because the dilated systems can contain large volumes.50
Kidney Handling of Water and Electrolytes
The threshold for stimulating the osmoreceptors for antidiuretic hormone (ADH) and thirst are lowered during pregnancy. Plasma osmolality approximates 270 mOsm/kg, and serum sodium levels decrease an average of 4 to 5 mEq/L. This change may be mediated by increased β-human chorionic gonadotropin, a pattern also seen to a lesser degree in menstruating women during the luteal cycle.51 It is hypothesized that the drop in serum sodium is related to the occurrence of vasodilation, arterial underfilling, and subsequent ADH release.52 Relaxin may play a role here because it has been shown to cause ADH secretion and water drinking in animal studies, and levels are increased during human pregnancy.53,54 Mild hyponatremia occurs at the same time there is a rise in aldosterone and its antinatriuretic forces. Deoxycorticosterone also promotes sodium retention and upregulation of sodium pumps across various membranes. These forces are balanced by the natriuretic forces of an increased GFR and increases in atrial natriuretic peptide and progesterone levels. Although the total sodium gain during pregnancy is estimated to be 900 to 1000 mEq, as mentioned in the Clinical Summary, the net balance between these factors is a preferential retention of water over salt and lower osmolality. Total body potassium stores increase by approximately 320 mEq by the end of gestation. This occurs despite the sodium retention from aldosterone because of the antikaliuretic effects of progesterone. Potassium excretion is held constant throughout pregnancy, with changes in tubular reabsorption adapting to alterations in filtered load. In an early study by Brown and colleagues, progesterone was not found to be involved in the acute regulation of potassium or sodium excretion.55
It is interesting to note that the metabolic clearance of ADH increases at week 10 and through midpregnancy because of an enzyme produced by the placenta, vasopressinase. Peak enzyme activity is during the third trimester, remaining high during labor and delivery and then falling to undetectable levels at 2 to 4 weeks postpartum. However, plasma ADH concentrations are usually kept normal in pregnancy because of increased secretion. A few women develop polyuria due to transient diabetes insipidus (DI), manifested by polydipsia, polyuria, high-normal serum sodium, and inappropriately low urine osmolality. These women may have more vasopressinase activity than women without DI. It can be managed with desmopressin (DDAVP), which is resistant to degradation by vasopressinase.52 Although frequent urination is often reported in pregnant women, true polyuria (>3 L/day) is rare.
Summary
The kidneys face remarkable demands during pregnancy, and it is critical that the practicing nephrologist understands the normal kidney adaptations to pregnancy. GFR rises early to a peak of 40% to 50% that of prepregnancy levels, resulting in lower levels of serum creatinine, urea, and uric acid. There is a net gain of sodium and potassium, but a greater retention of water, with gains of up to 1.6 L. Through effects of progesterone and alterations in RAAS, the systemic vascular resistance falls, leading to lower blood pressure and an increased RPF. The precise orchestration of hemodynamic changes and balance of fluids and electrolytes are essential to the development and maintenance of a successful pregnancy for mother and child.
CLINICAL SUMMARY.
Glomerular filtration rate increases 40% to 50% in normal pregnancy.
Creatinine clearance by 24-hour urine collection is the current standard for measurement of glomerular filtration rate in pregnant women.
Pregnancy is a vasodilated state mediated by alterations in sensitivity to angiotensin II and elevated levels of progesterone.
Pregnancy is a volume-expanded state characterized by net sodium retention of 900 to 1000 mEq and 1.1 to 1.6 L water mediated by a balance between natriuretic and antinatriuretic forces.
Footnotes
The authors have no financial disclosures to report.
References
- 1.Dunlop W. Serial changes in renal hemodynamics during normal human pregnancy. Br J Obstet Gynaecol. 1981;88(1):1–9. doi: 10.1111/j.1471-0528.1981.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 2.Harrow BR, Sioan JA, Salhanick L. Etiology of the hydronephrosis of pregnancy. Surg Gynecol Obstet. 1964;119:1042–1048. [PubMed] [Google Scholar]
- 3.Rasmussen PE, Nielson FR. Hydronephrosis in pregnancy: a literature survey. Eur J Obstet Gynecol Reprod Biol. 1988;27(3):249–259. doi: 10.1016/0028-2243(88)90130-x. [DOI] [PubMed] [Google Scholar]
- 4.Faundes A, Bricola-Filho M, Pinto e Silva JL. Dilatation of the urinary tract during pregnancy: proposal of a curve of maximal caliceal diameter by gestational age. Am J Obstet Gynecol. 1998;178(5):1082–1086. doi: 10.1016/s0002-9378(98)70552-6. [DOI] [PubMed] [Google Scholar]
- 5.Cietak KA, Newton JR. Serial quantitative maternal nephrosonography in pregnancy. Br J Radiol. 1985;58(689):405–413. doi: 10.1259/0007-1285-58-689-405. [DOI] [PubMed] [Google Scholar]
- 6.Bailey RR, Rolleston GL. Kidney length and ureteric dilatation in the puerperium. J Obstet Gynaecol Br Commonw. 1971;78(1):55–61. doi: 10.1111/j.1471-0528.1971.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 7.Christensen T, Klebe JG, Bertelsen V, Hansen HE. Changes in renal volume during normal pregnancy. Acta Obstet Gynecol Scand. 1989;68(6):541–543. [PubMed] [Google Scholar]
- 8.Leyendecker JR, Gorengaut V, Brown JJ. MR imaging of maternal diseases of the abdomen and pelvis during pregnancy and the immediate postpartum. Radiographics. 2004;24(5):1301–1316. doi: 10.1148/rg.245045036. [DOI] [PubMed] [Google Scholar]
- 9.Roy C, Saussine C, Jahn C, et al. Fast imaging MR assessment of ureterohydronephrosis during pregnancy. Magn Reson Imaging. 1995;13(6):767–772. doi: 10.1016/0730-725x(95)00036-g. [DOI] [PubMed] [Google Scholar]
- 10.Beydoun SN. Morphologic changes in the renal tract in pregnancy. Clin Obstet Gynecol. 1985;28(2):249. doi: 10.1097/00003081-198528020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Strevens H, Wide-Swensson D, Hansen A, et al. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG. 2003;110(9):831–836. [PubMed] [Google Scholar]
- 12.Au KKL, Woo JSK, Tang LCH, Liang ST. Aetiological factors in the genesis of pregnancy hydronephrosis. Aust N Z J Obstet Gynaecol. 1985;25(4):248–251. doi: 10.1111/j.1479-828x.1985.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 13.Schulman A, Herlinger H. Urinary tract dilatation in pregnancy. Br J Radiol. 1975;48(572):638–645. doi: 10.1259/0007-1285-48-572-638. [DOI] [PubMed] [Google Scholar]
- 14.Schneider DH, Eichner E, Gordon MB. An attempt at production of hydronephrosis of pregnancy, artificially induced. Am J Obstet Gynecol. 1953;65:660–665. doi: 10.1016/0002-9378(83)90626-9. [DOI] [PubMed] [Google Scholar]
- 15.Lin YJ, Ou YC, Tsang LC, Lin H. Diagnostic value of magnetic resonance imaging for successful management of a giant hydronephrosis. J Obstet Gynaecol. 2012;33(1):89–91. doi: 10.3109/01443615.2012.721820. [DOI] [PubMed] [Google Scholar]
- 16.Spencer JA, Chahal R, Kelly A, Taylor K, Eardley I, Lloyd SN. Evaluation of painful hydronephrosis in pregnancy: magnetic resonance urographic patterns in physiological dilatation versus calculous obstruction. J Urol. 2004;171(1):256–260. doi: 10.1097/01.ju.0000102477.19999.b2. [DOI] [PubMed] [Google Scholar]
- 17.Chapman AB, Zamudio S, Woodmansee W, et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273(5 Pt 2):F777–F782. doi: 10.1152/ajprenal.1997.273.5.F777. [DOI] [PubMed] [Google Scholar]
- 18.Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R267–R275. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogueh O, Clough A, Hancock M, Johnson MR. A longitudinal study of the control of renal and uterine hemodynamic changes of pregnancy. Hypertens Pregnancy. 2011;30(3):243–259. doi: 10.3109/10641955.2010.484079. [DOI] [PubMed] [Google Scholar]
- 20.Lafayette RA, Hladunewich MA, Derby G, Blouch K, Druzin ML, Myers BD. Serum relaxin levels and kidney function in late pregnancy with or without preeclampsia. Clin Nephrol. 2011;75(3):226–232. doi: 10.5414/cnp75226. [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Karim R. Pressor response to angiotonin in pregnant and non-pregnant women. Am J Obstet Gynecol. 1961;82:246–251. doi: 10.1016/0002-9378(61)90053-9. [DOI] [PubMed] [Google Scholar]
- 22.Schrier RW, Briner VA. Peripheral arterial vasodilation hypothesis of sodium and water retention in pregnancy: implication for pathogenesis of preeclampsia-eclampsia. Obstet Gynecol. 1991;77(4):632–639. [PubMed] [Google Scholar]
- 23.Irani RA, Xia Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin Nephrol. 2011;31(1):47–58. doi: 10.1016/j.semnephrol.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88(1):10–17. doi: 10.1111/j.1471-0528.1981.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 25.Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54(6):2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 26.Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18(2):152–161. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- 27.Krutzén E, Olofsson P, Back S-E, Nilsson-Ehle P. Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand J Clin Lab Invest. 1992;52(5):387–392. doi: 10.3109/00365519209088374. [DOI] [PubMed] [Google Scholar]
- 28.Hladunewich MA, Lafayette RA, Gerby GC, et al. The dynamics of glomerular filtration in the puerperium. Am J Physiol Ren Physiol. 2004;286(3):F496–F503. doi: 10.1152/ajprenal.00194.2003. [DOI] [PubMed] [Google Scholar]
- 29.Smith MC, Moran P, Ward MK, Davison JM. Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG. 2008;115(1):109–112. doi: 10.1111/j.1471-0528.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 30.Alper AB, Yi Y, Webber LS, et al. Estimation of glomerular filtration rate in preeclamptic patients. Am J Perinatol. 2007;24(12):569–574. doi: 10.1055/s-2007-986697. [DOI] [PubMed] [Google Scholar]
- 31.Alper AB, Yi Y, Rahman M, et al. Performance of estimated glomerular filtration rate prediction equations in preeclamptic patients. Am J Perinatol. 2011;28(6):425–430. doi: 10.1055/s-0030-1268712. [DOI] [PubMed] [Google Scholar]
- 32.Larsson A, Palm M, Hannson L, Axelsson O. Cystatin C and modification of diet in renal disease (MDRD) estimated glomerular filtration rate differ during normal pregnancy. Acta Obstet Gynecol Scand. 2010;89(7):939–944. doi: 10.3109/00016341003739559. [DOI] [PubMed] [Google Scholar]
- 33.Saxena AR, Karumanchi SA, Fan SL, et al. Correlation of cystatin-C with glomerular filtration rate by inulin clearance in pregnancy. Hypertens Pregnancy. 2012;31(1):22–30. doi: 10.3109/10641955.2010.507845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng A, Baylis C. Glomerular hemodynamic responses to pregnancy in rats with severe reduction of renal mass. Kidney Int. 1995;48(1):39–44. doi: 10.1038/ki.1995.264. [DOI] [PubMed] [Google Scholar]
- 35.Baylis C. The mechanism of the increase in glomerular filtration rate in the twelve-day pregnant rat. J Physiol. 1980;305(1):405–414. doi: 10.1113/jphysiol.1980.sp013372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol. 1996;270(2):F338–F343. doi: 10.1152/ajprenal.1996.270.2.F338. [DOI] [PubMed] [Google Scholar]
- 37.Odutayo A, Hladunewich M. Obstetric nephrology: renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol. 2012;7(12):2073–2080. doi: 10.2215/CJN.00470112. [DOI] [PubMed] [Google Scholar]
- 38.Davison JM, Hytten FE. The effect of pregnancy on the renal handling of glucose. Br J Obstet Gynaecol. 1975;82(5):374–381. doi: 10.1111/j.1471-0528.1975.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 39.Bishop JH, Green R. Glucose handling by distal portions of the nephron during pregnancy in the rat. J Physiol. 1983;336(1):131–142. doi: 10.1113/jphysiol.1983.sp014572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erman A, Neri A, Sharoni R, et al. Enhanced urinary albumin excretion after 35 weeks of gestation and during labour in normal pregnancy. Scand J Clin Lab Invest. 1992;52(2):409–413. doi: 10.3109/00365519209088376. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimatsu J, Matsumoto H, Goto K, Shimano M, Narahara H, Miyakawa I. Relationship between urinary albumin and serum soluble fms-like tyrosine kinase (sFlt-1) in normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):204–208. doi: 10.1016/j.ejogrb.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Cornelius T, Odutayo A, Keunen J, Hladunewich M. The kidney in normal pregnancy and preeclampsia. Semin Nephrol. 2011;31(1):4–14. doi: 10.1016/j.semnephrol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Moran P, Baylis PH, Lindheimer MD, Davison JM. Glomerular ultrafiltration in normal and preeclamptic pregnancy. J Am Soc Nephrol. 2003;14(3):648–652. doi: 10.1097/01.asn.0000051724.66235.e0. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi M, Ueda Y, Hoshimoto K, et al. Changes in urinary excretion of six biochemical parameters in normotensive pregnancy and preeclampsia. Am J Kidney Dis. 2002;39(2):392–400. doi: 10.1053/ajkd.2002.30561. [DOI] [PubMed] [Google Scholar]
- 45.ACOG Committee on Obstetrics Practice ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2002;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 46.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20(1):ix–xiv. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 47.Higby K, Suiter CR, Phelps JY, Siler-Khodr T, Langer O. Normal values of urinary albumin and total protein excretion during pregnancy. Am J Obstet Gynecol. 1994;171(4):984–989. doi: 10.1016/s0002-9378(13)90019-3. [DOI] [PubMed] [Google Scholar]
- 48.Jaschevatzky OE, Rosenberg RP, Shalit A, Zonder HB, Grunstein S. Protein/creatinine ratio in random urine specimens for quantitation of proteinuria in preeclampsia. Obstet Gynecol. 1990;75(4):604–606. [PubMed] [Google Scholar]
- 49.Kuo VS, Koumantakis G, Gallery ED. Proteinuria and its assessment in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1992;167(3):723–728. doi: 10.1016/s0002-9378(11)91578-6. [DOI] [PubMed] [Google Scholar]
- 50.Lindheimer MD, Kanter D. Interpreting abnormal proteinuria in pregnancy: the need for a more pathophysiological approach. Obstet Gynecol. 2010;115(2 pt 1):365–375. doi: 10.1097/AOG.0b013e3181cb9644. [DOI] [PubMed] [Google Scholar]
- 51.Davison JM, Shiells EA, Philips PR, Lindheimer MD. Serial evaluation of vasopressin release and thirst in human pregnancy. Role of human chorionic gonadotropin in the osmoregulatory changes of gestation. J Clin Invest. 1988;81(3):798–806. doi: 10.1172/JCI113386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrier RW. Systemic arterial vasodilation, vasopressin, and vasopressinase in pregnancy. J Am Soc Nephrol. 2010;21(4):570–572. doi: 10.1681/ASN.2009060653. [DOI] [PubMed] [Google Scholar]
- 53.Thornton SN, Fitzsimons JT. The effects of centrally administered porcine relaxin on drinking behaviour in male and female rats. J Neuroendocrinol. 1995;7(3):165–169. doi: 10.1111/j.1365-2826.1995.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhao S, Malmgren CH, Shanks RD, Sherwood OD. Monoclonal antibodies specific for rat relaxin. VIII. Passive immunization with monoclonal antibodies throughout the second half of pregnancy reduces water consumption in rats. Endocrinology. 1995;136(5):1892–1897. doi: 10.1210/endo.136.5.7720635. [DOI] [PubMed] [Google Scholar]
- 55.Brown MA, Sinosich MJ, Saunders DM, Gallery ED. Potassium regulation and progesterone-aldosterone interrelationships in human pregnancy: a prospective study. Am J Obstet Gynecol. 1986;155(2):349–353. doi: 10.1016/0002-9378(86)90824-0. [DOI] [PubMed] [Google Scholar]


