Abstract
Background
Quality of life (QOL) is an important issue for cancer survivors; few studies are able to consider elderly populations, address long term survival (≥ 5 years), examine different cancers, or include a valid non-cancer comparison group.
Methods
We assessed QOL in 2004 among women participating in the Iowa Women's Health Study (IWHS), a prospective cohort of older women followed since 1986. Cancer occurrence during follow-up was identified through the State Health Registry of Iowa. We compared unadjusted, and age- and comorbidity-adjusted mean scores for eight SF-36 scales among women with and without cancer by all cancer types, stage, and survival. Analyses were repeated after excluding women who developed a second primary cancer or reported cancer treatment in 2004.
Results
Among 17,385 respondents aged 71–89 years, 2717 (16.6%) had been diagnosed with cancer since 1986. Compared to women without cancer, survivors fared worse on the General Health scale, regardless of cancer type (except colorectal), stage, or survival. Except for lower scores among the longest survivors, Mental Health scores did not differ significantly between women with and without cancer. Women with genitourinary, lung, hematopoietic, lymphoma, or other gastrointestinal cancers, distant stage, or who survived at least ten years consistently experienced significantly lower QOL scores than cancer-free women for most scales.
Conclusions
Differences in QOL depended upon the specific SF-36 scale and which aspect of cancer survivorship we examined. Our findings underscore the complexity of factors contributing to quality of life among cancer survivors.
Keywords: cancer survivors, elderly, quality of life
Introduction
Approximately 60% of the estimated 11,000,000 persons living with cancer in the U.S. are 65 years or older, yet just 9% of survivorship research funded by the National Institutes of Health in 2006 included elderly cancer survivors.1 For older persons with cancer, a concern is that cancer treatment may lead to new chronic conditions or exacerbate pre-existing comorbidity or the aging process to adversely affect their quality of life as a cancer survivor. Studies that assess how a cancer diagnosis and treatment affects physical, mental and social functioning among older persons, beyond other health-limiting conditions and aging, are lacking.2
To help separate the effect of cancer diagnosis and therapy from comorbidity and aging on quality of life, a similar-aged, non-cancer comparison group is needed. However, quality of life and cancer studies have tended to rely for comparison on population norms or a convenience sample of persons without cancer, who may differ in important and unmeasured ways from cancer survivors. In addition, most investigations of quality of life among survivors of adult-onset cancers have been limited by small samples and inclusion of only a few cancers, predominantly, breast, colorectal and prostate cancers.3–5 Athough 68% of older cancer survivors have survived at least 5- , 38% at least 10- , and 17% at least 20 years, few studies have focused on survival beyond five years.2
The Iowa Women's Health Study (IWHS) is a prospective cohort established in 1986 to assess risk factors for cancer development among women ages 55 to 69 at baseline. Prospective cohorts, by allowing for an internal comparison group, strengthen inferences about quality of life among cancer survivors relative to persons without cancer. Although cancer survivors have been compared to persons without cancer in national cross-sectional health surveys,6–11 we identified just three other prospective cohort studies that examined cancer survivorship. One of these was limited to breast cancer, and two relied on self-report of a cancer diagnosis.12–18
Using quality of life data collected from IWHS participants in 2004, when the women were 71 to 89 years old, we sought to answer the following basic questions about quality of life and cancer survivorship. First, does quality of life differ by type of cancer, by stage at diagnosis or by time since diagnosis among older women with cancer relative to similar-aged women without cancer? Second, do these relationships change after accounting for differences in age and cumulative chronic conditions between cancer survivors and women without cancer? And third, what is the effect of cancer treatment or development of a second primary cancer on quality of life of cancer survivors compared to women without cancer? Among cancer survivors, we included women diagnosed with any type of cancer, any stage, and survival from one month to 18 years.
Methods
The Iowa Women's Health Study (IWHS)
In 1986, a self-administered questionnaire was sent to 99,826 women, ages 55 to 69, randomly selected from the Iowa drivers license list. Questions addressed anthropometrics, lifestyle characteristics, prior health conditions, diet, family history of cancer, and demographics. The 41,837 women (42%) who returned the questionnaire formed the IWHS. Annually, the cohort is matched against the State Health Registry of Iowa, a member of the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) program, to identify all incident cancers including diagnosis date, cancer type, stage, and first course of therapy. Additional questionnaires were administered in 1987, 1989, 1992, 1997, and 2004.
Quality of life Measurement
In 2004, 30,501 IWHS women were sent a questionnaire that included the SF-36--a reliable and valid instrument for global assessment of quality of life across various physical and psychosocial domains--that has been used to assess quality of life for more than 200 diseases.19–22 It comprises eight scales (two to ten items each) and measures quality of life in the past four weeks. The General Health scale provides a self-assessment of overall health. The Physical Functioning scale assesses physical limitations (e.g., lifting/carrying groceries, climbing stairs, walking distances), while the Role Physical scale refers to the effect of physical limitations on activities. The Bodily Pain scale measures pain intensity and interference with normal activities. The Vitality scale assesses energy and fatigue. The Mental Health scale assesses anxiety, depression, loss of control and psychological well-being; the Role Emotional scale measures how mental health status affects activities. The Social Functioning scale measures how current health affects the ability to engage in social activities. Although these scales can be reduced to two summary scales for physical and mental health, we analyzed the individual scales to better capture specific aspects of quality of life and cancer survivorship.
Using established algorithms,19,22 we standardized each scale to age-specific 1998 population norms by using z-score standardization and norm-based transformation of each scale’s z-score. Consequently, a mean of 50 represents the population average for a given scale, with each point below or above the mean indicating 1/10th of a standard deviation. Use of norm-based scoring allows direct comparison across scales and eliminates bias due to ceiling or floor effects that may differ between scales when only a 0–100 transformation is used. For all scales, a higher value indicates a higher quality of life.
Comorbidity Measures
IWHS participants reported various health conditions at baseline and each follow-up according to whether a physician had ever indicated that she had diabetes, hypertension, cardiovascular disease (including stroke or myocardial infarction), rheumatoid arthritis or Parkinson's disease. Women were also asked if they had ever had bone fractures that required medical treatment, including arm, wrist, ribs, hip or vertebra fractures.
Cancer Survivorship
Among 20,844 respondents to the 2004 questionnaire, 1511 women who reported a cancer diagnosis at baseline in 1986 were not eligible for this study. Otherwise eligible women who did not return the questionnaire (n=8624), who did not complete the quality of life scales (n = 1863), whose questionnaire was completed by proxy (n = 3), or who reported cancer on the 2004 questionnaire that was not recorded in the State Health Registry (n = 82), were classified as non-respondents. Among the remaining 17,385 women, 2717 were identified by the cancer registry as having a new first primary cancer between study enrollment in 1986 and when the 2004 follow-up was conducted.
Cancer survivors were grouped by first primary cancer type, including breast, colorectal, genitourinary tract (bladder, kidney, ureter), gynecologic (endometrium, ovarian, cervix, other genital), lung, hematopoietic (leukemias, myeloma), lymphomas (any site), other gastrointestinal (pancreas, esophagus, stomach, small intestine, liver, anal, gallbladder, biliary tract), skin (primarily melanoma), and other (oropharyngeal, other respiratory, bone, retroperitoneum, soft tissue, eye, endocrine, thyroid, unknown). The occurrence of second primary cancers among survivors was also obtained from the cancer registry. Women with a history of cancer were asked on the 2004 questionnaire if they were currently receiving any cancer-related therapy.
Data Analysis
To examine how non-response, due to death, or inability or unwillingness to return the questionnaire, may have biased our results, we compared all women who completed the 1986 baseline survey, women who died before the 2004 questionnaire, non-respondents, and eligible respondents, for a variety of lifestyle characteristics and chronic conditions collected at baseline, and for cancer occurring during follow-up.
For each SF-36 scale, we compared means among cancer survivors according to cancer type, stage or time since diagnosis relative to women without cancer, using generalized linear regression. We first report unadjusted means for each scale comparing cancer groups to the non-cancer group. We next calculated means for each scale across groups, simultaneously adjusting for age (as a continuous measure) and comorbidity (separate dichotomous measures for each type of condition previously described). We then repeated the analysis in which we accounted for age and comorbidity, but also restricted cancer survivors to those who neither reported cancer treatment in 2004, nor had developed a second primary cancer.
Results
Description of study population
Among 38,006 women who completed a baseline questionnaire and did not report a prior cancer diagnosis in 1986, 10,049 or 26.4%, had died prior to administration of the 2004 questionnaire; 27.8% were excluded from the analysis due to non-response as defined above (Table 1). Women who responded to the 2004 questionnaire and met eligibility criteria (n = 17,385) were younger at baseline, reported higher levels of education, were more likely at baseline to report excellent health, to be less obese, to be never smokers, to be more physically active, and to be less likely to have ever had diabetes, hypertension, cardiovascular disease, or fractures compared to the cohort overall at baseline, or to non-respondents. Approximately 16.6% of women who completed the 2004 questionnaire had been diagnosed with cancer since 1986, slightly higher than non-respondents, but much lower compared to women who had died prior to 2004. In our analytic sample, cancer survivors were older and reported more comorbid conditions than women without cancer (Table 2). For diabetes, rheumatoid arthritis and hypertension, the differences between cancer survivors and women without cancer were statistically significant.
Table 1.
Selected characteristics of cohort members at baseline and by response to the 2004 follow-up questionnaire, Iowa Women’s Health Study
| At 2004 follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|
| All cohort members at baseline 19861 (n=38,006) |
Died before 2004 follow-up (n=10,049) |
Non-respondents/ Ineligible respondents (n=10,572) |
Eligible Respondents (n=17,385) |
|||||
| n | % | n | % | n | % | n | % | |
| Baseline characteristics | ||||||||
| Age > 65 years | 11,094 | 29.2 | 4500 | 44.8 | 3218 | 30.4 | 3376 | 19.4 |
| Post secondary education | 14,720 | 38.7 | 3524 | 35.1 | 3626 | 34.3 | 7570 | 43.5 |
| General health, excellent | 9272 | 24.4 | 1517 | 15.1 | 2435 | 23.0 | 5320 | 30.6 |
| Body mass index >=30 | 8928 | 23.5 | 2763 | 27.5 | 2439 | 23.1 | 3726 | 21.4 |
| Cigarette smoking, never | 24,642 | 64.8 | 5383 | 53.6 | 7181 | 67.9 | 12,078 | 69.5 |
| Physical activity, low | 17,632 | 46.4 | 5188 | 51.6 | 4803 | 45.4 | 7641 | 44.0 |
| Diabetes | 2363 | 6.2 | 1330 | 13.2 | 480 | 4.5 | 553 | 3.2 |
| High blood pressure | 13,982 | 36.8 | 4681 | 46.6 | 3823 | 36.2 | 5478 | 31.5 |
| Heart disease | 3391 | 8.9 | 1519 | 15.1 | 789 | 7.5 | 1083 | 6.2 |
| Heart attack | 1189 | 3.1 | 689 | 6.9 | 207 | 2.0 | 293 | 1.7 |
| Fracture | 5236 | 13.8 | 1680 | 16.7 | 1413 | 13.4 | 2143 | 12.3 |
| Rheumatoid arthritis2 | 3011 | 7.9 | 904 | 9.0 | 784 | 7.4 | 1323 | 7.6 |
| Parkinson’s disease2 | 144 | 0.4 | 87 | 0.9 | 31 | 0.3 | 26 | 0.2 |
| Cancer characteristics 1986–2004 | ||||||||
| Cancer type, | ||||||||
| None | 29,836 | 78.5 | 6104 | 60.7 | 9064 | 85.7 | 14668 | 84.4 |
| Breast | 2563 | 6.7 | 711 | 7.1 | 619 | 5.9 | 1233 | 7.0 |
| Colorectal | 1280 | 3.4 | 562 | 5.6 | 247 | 2.3 | 471 | 2.7 |
| GU | 352 | 0.9 | 165 | 1.6 | 64 | 0.6 | 123 | 0.7 |
| Gynecologic | 964 | 2.5 | 454 | 4.5 | 170 | 1.6 | 340 | 2.0 |
| Lung | 824 | 2.2 | 711 | 7.1 | 47 | 0.4 | 66 | 0.4 |
| Hematopoietic | 374 | 1.0 | 256 | 2.6 | 37 | 0.4 | 74 | 0.4 |
| Lymphoma | 384 | 1.0 | 225 | 2.2 | 52 | 0.5 | 107 | 0.6 |
| Other GI | 455 | 1.2 | 401 | 4.0 | 23 | 0.2 | 31 | 0.2 |
| Skin | 255 | 0.7 | 57 | 0.6 | 59 | 0.6 | 139 | 0.8 |
| Other | 628 | 1.7 | 403 | 4.0 | 92 | 0.9 | 133 | 0.8 |
| Stage at diagnosis3 | ||||||||
| In situ | 607 | 7.4 | 115 | 2.9 | 153 | 10.1 | 339 | 12.5 |
| Local | 3534 | 43.2 | 1037 | 26.3 | 843 | 55.9 | 1654 | 60.9 |
| Regional | 1669 | 20.4 | 890 | 22.6 | 276 | 18.3 | 503 | 18.5 |
| Distant | 1780 | 21.8 | 1515 | 38.4 | 102 | 6.8 | 163 | 6.0 |
| Unknown | 580 | 7.1 | 388 | 9.8 | 134 | 8.9 | 58 | 2.1 |
| Cancer Therapy Received3 | ||||||||
| % surgery | 6001 | 73.5 | 2229 | 56.5 | 1259 | 83.5 | 2513 | 92.5 |
| % chemotherapy | 1837 | 22.5 | 1166 | 29.6 | 206 | 13.7 | 465 | 17.1 |
| % radiation therapy | 1735 | 21.2 | 893 | 22.6 | 297 | 19.7 | 545 | 20.1 |
Excluding participants who reported cancer at baseline
Measured in 1992
Among women diagnosed with cancer
Table 2.
Age and self-reported cumulative comorbid conditions from 1986 to 2004 among cancersurvivors relative to women without cancer in the Iowa Women’s Health Study
| Cancer Survivor (n=2717) | No Cancer (n=14,668) | ||||
|---|---|---|---|---|---|
| n | % | n | % | p-value | |
| Age in 2004 | |||||
| 71-<77 | 745 | 27.4 | 4665 | 31.8 | |
| 77-<83 | 1269 | 46.7 | 6700 | 45.7 | |
| 83–89 | 703 | 25.9 | 3303 | 22.5 | <0.0001 |
| Total number of comorbid conditions | |||||
| 0 | 388 | 14.3 | 2288 | 15.6 | |
| 1 | 703 | 25.9 | 4235 | 28.9 | |
| 2 | 728 | 26.8 | 3578 | 24.4 | |
| 3+ | 898 | 35.0 | 4567 | 31.1 | <0.0005 |
| Type of comorbid conditions | |||||
| Diabetes | 442 | 16.3 | 1977 | 13.5 | 0.0001 |
| Rheumatoid Arthritis | 529 | 19.5 | 2586 | 17.6 | 0.022 |
| Parkinson’s Disease | 31 | 1.1 | 188 | 1.3 | n.s. |
| Fractures | 1079 | 39.7 | 5643 | 38.5 | n.s. |
| Hypertension | 1760 | 64.8 | 9035 | 61.6 | 0.002 |
| Heart Disease | 748 | 27.5 | 3820 | 26.0 | n.s. |
| Heart Attack | 254 | 9.4 | 1341 | 9.1 | n.s. |
| Stroke | 201 | 7.4 | 1172 | 8.0 | n.s. |
| Total CVD | 909 | 33.5 | 4712 | 32.1 | n.s. |
Approximately 83.7% of cancer survivors were 65 years of age or older when first diagnosed. Breast cancer was the most common cancer, followed by colorectal and gynecologic cancers (Table 3). Every cancer type included women with ten or more years as a cancer survivor. Similarly, each cancer stage was represented across each survivorship group. Overall, 13.8% of cancer survivors had developed at least one additional primary cancer. Reported current cancer treatment on the 2004 questionnaire was particularly frequent among women whose first primary cancer was breast (15.9%), hematopoietic (31.5%), lung (18.2%) or lymphoma (17.5%) (data not shown). Altogether, 23.1% of survivors reported either current cancer treatment in 2004 or had experienced a second primary cancer.
Table 3.
SEER-reported cancer type, cancer stage, first course of therapy, subsequent cancer diagnosis and self-reported cancer treatment among 2717 cancer survivors in the Iowa Women’s Health Study, 2004
| Time since diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| < 2 years | 2–4.99 years | 5–9.99 years | 10+ years | |||||
| n | % | n | % | n | % | n | % | |
| Total N, % of total | 412 | 15.2 | 525 | 19.3 | 779 | 28.7 | 1001 | 36.8 |
| Type of cancer | ||||||||
| Breast | 148 | 12.0 | 237 | 19.2 | 365 | 29.6 | 483 | 39.2 |
| Colorectal | 72 | 15.2 | 87 | 18.5 | 136 | 28.9 | 176 | 37.4 |
| Genitourinary | 14 | 20.0 | 16 | 22.9 | 22 | 31.4 | 18 | 25.7 |
| Gynecologic | 43 | 12.7 | 60 | 17.7 | 93 | 27.3 | 144 | 42.3 |
| Lung | 15 | 22.7 | 9 | 13.6 | 25 | 37.9 | 17 | 27.8 |
| Hematopoietic | 23 | 31.1 | 24 | 32.4 | 15 | 20.3 | 12 | 16.2 |
| Lymphoma | 25 | 23.4 | 17 | 15.9 | 35 | 32.7 | 30 | 28.0 |
| Other GI | 10 | 32.3 | 4 | 12.9 | 8 | 25.8 | 9 | 29.0 |
| Skin | 25 | 18.0 | 30 | 21.6 | 41 | 29.5 | 43 | 30.9 |
| Other | 37 | 19.9 | 41 | 22.0 | 39 | 21.0 | 69 | 37.1 |
| Stage | ||||||||
| In situ | 43 | 12.7 | 76 | 22.4 | 100 | 29.5 | 120 | 35.4 |
| Local | 220 | 13.3 | 312 | 18.9 | 487 | 29.4 | 635 | 38.4 |
| Regional | 88 | 17.5 | 85 | 16.9 | 139 | 27.6 | 191 | 40.0 |
| Distant | 46 | 28.2 | 44 | 27.0 | 39 | 23.9 | 34 | 20.9 |
| Unknown | 15 | 25.9 | 8 | 13.8 | 14 | 24.1 | 21 | 36.2 |
| First course of therapy, % of total | ||||||||
| Surgery | 353 | 85.7 | 475 | 90.5 | 733 | 94.1 | 952 | 95.1 |
| Chemotherapy | 79 | 19.2 | 127 | 24.2 | 137 | 17.6 | 122 | 12.2 |
| Radiation therapy | 96 | 23.3 | 113 | 21.5 | 185 | 23.8 | 151 | 15.1 |
| Subsequent cancer diagnosis, % of total | 16 | 3.9 | 46 | 8.8 | 121 | 15.5 | 192 | 19.2 |
| Reported current cancer treatment, % of total | 108 | 26.2 | 107 | 20.4 | 55 | 7.1 | 52 | 5.2 |
General Health
Compared to women without cancer, cancer survivors fared worse on the General Health scale regardless of cancer type (colorectal cancer, being the one exception), stage at diagnosis, or time since diagnosis (Figures 1a, 2a, 3a). Mean unadjusted scores ranged from 1.5 to 8 points lower relative to the mean for women without cancer and all observed differences were statistically significant. Adjustment for age and other chronic health conditions reduced the difference in mean scores among cancer survivors compared to women without cancer, but differences remained statistically significant. Although differences in General Health mean scores between women with and without cancer were further attenuated after excluding women with a second primary cancer or who reported current treatment, they remained statistically significant except for women with skin or other cancer, in situ or regional stage cancer, or who had survived less than two, or 5 to 10, years. For lung, hematopoietic, lymphoma, and other gastrointestinal cancers, and for distant stage, General Health scores were near or below ½ standard deviation of the mean score for women without cancer regardless of adjustments or exclusions; these differences may be considered clinically significant.23
Figure 1.
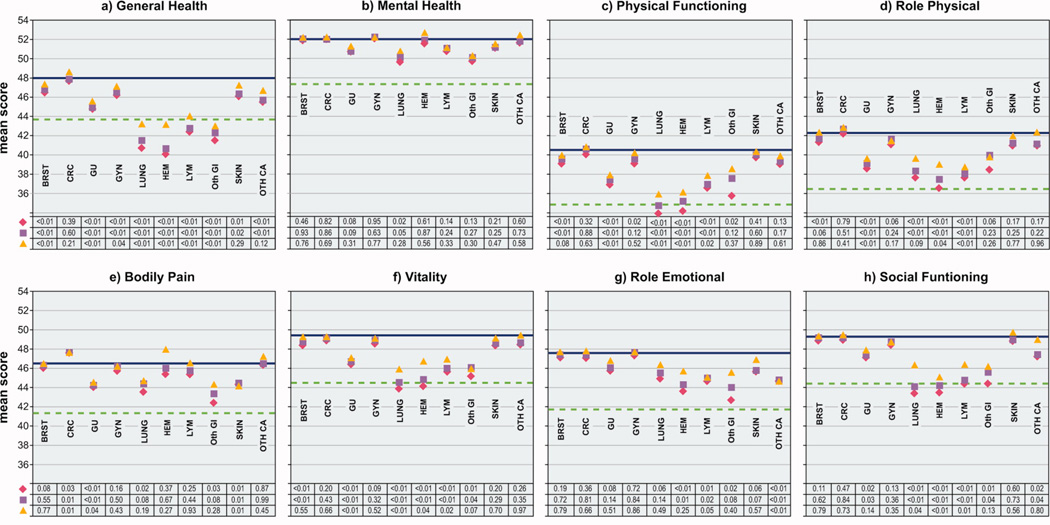
Comparison of mean scores for eight SF-36 scales among women with and without cancer, by cancer type, Iowa Women’s Health Study, 2004
| Values below each graph represent p-values for the difference in mean QOL scores between women with cancer compared to women without cancer |
 Unadjusted, all women Unadjusted, all women |
 Age- and comorbidity-adjusted, all women Age- and comorbidity-adjusted, all women |
 Age- and comorbidity-adjusted, excluding cancer survivors with second primary cancer or reporting cancer treatment in 2004 Age- and comorbidity-adjusted, excluding cancer survivors with second primary cancer or reporting cancer treatment in 2004 |
 mean score for women without cancer mean score for women without cancer |
 1/2 standard deviation below the mean score of women without cancer 1/2 standard deviation below the mean score of women without cancer |
| NO CA | No cancer |
| BRST | Breast |
| CRC | Colorectal |
| GU | Genitourinary |
| GYN | Gynecologic |
| HEM | Hematopoietic |
| LUNG | Lung |
| LYM | Lymphoma |
| OTH GI | Other genitourinary |
| SKIN | Skin |
| OTH CA | Other cancer |
Figure 2.
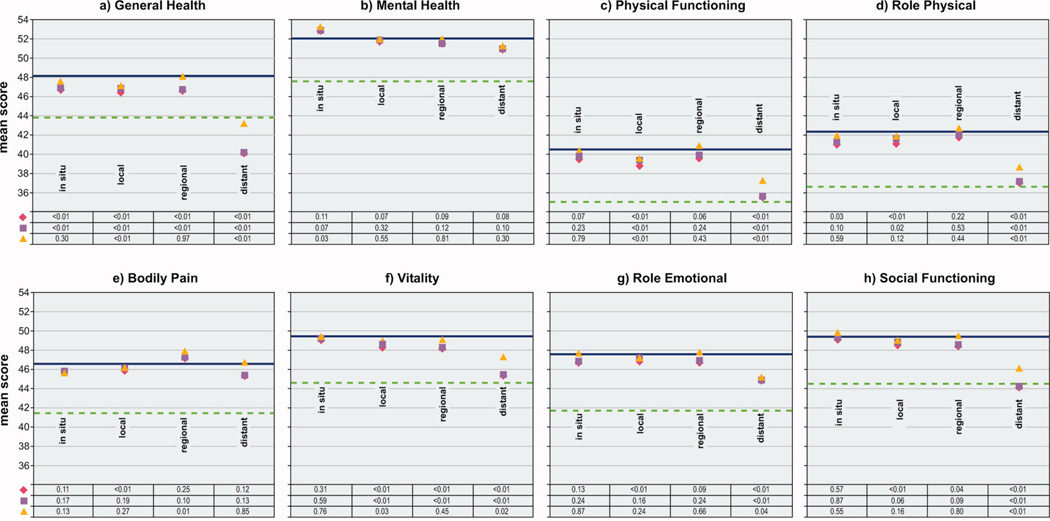
Comparison of mean scores for eight SF-36 scales among women with and without cancer, by cancer stage, Iowa Women’s Health Study, 2004
| Values below each graph represent p-values for the difference in mean QOL scores between women with cancer compared to women without cancer |
 Unadjusted, all women Unadjusted, all women |
 Age- and comorbidity-adjusted, all women Age- and comorbidity-adjusted, all women |
 Age- and comorbidity-adjusted, excluding cancer survivors with second primary cancer or reporting cancer treatment in 2004 Age- and comorbidity-adjusted, excluding cancer survivors with second primary cancer or reporting cancer treatment in 2004 |
 mean score for women without cancer mean score for women without cancer |
 1/2 standard deviation below the mean score of women without cancer 1/2 standard deviation below the mean score of women without cancer |
Figure 3.
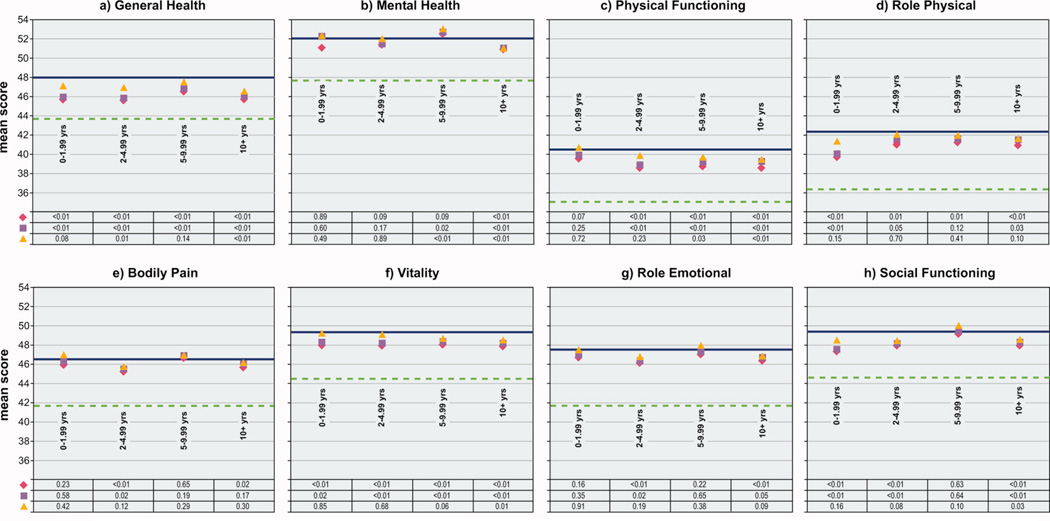
Comparison of mean scores for eight SF-36 scales among women with and without cancer, by length of survival, Iowa Women’s Health Study, 2004
| Values below each graph represent p-values for the difference in mean QOL scores between women with cancer compared to women without cancer |
 Unadjusted, all women Unadjusted, all women |
 Age- and comorbidity-adjusted, all women Age- and comorbidity-adjusted, all women |
 Age- and comorbidity-adjusted, excluding cancer survivors with second primary cancer or reporting cancer treatment in 2004 Age- and comorbidity-adjusted, excluding cancer survivors with second primary cancer or reporting cancer treatment in 2004 |
 mean score for women without cancer mean score for women without cancer |
 1/2 standard deviation below the mean score of women without cancer 1/2 standard deviation below the mean score of women without cancer |
Mental Health
In contrast to General Health scores, cancer survivors reported Mental Health mean unadjusted scores that were similar to or higher than the mean score for women without cancer for almost all cancer types (except lung, and the age- and comorbidity-adjusted difference for this cancer was no longer statistically significant after exclusions), all stages of cancer, and regardless of length of survival, except for women surviving the longest (Figures 1b – 3b). The statistical significance of the lower Mental Health score for women surviving ten or more years, compared to women without cancer, persisted regardless of adjustments or exclusions.
Findings for the other six SF-36 scales are described below, separately by cancer type (Figure 1), stage (Figure 2) and survival (Figure 3).
Breast cancer
Mean unadjusted scores for Physical Functioning, Role Physical, and Vitality scales were each statistically significantly lower among breast cancer survivors than women without cancer. After adjusting the mean scores for age and comorbidity, small differences for these scales between women with and without breast cancer remained, but the difference for the Role Physical scale was no longer statistically significant. The Vitality mean adjusted score among breast cancer survivors became nearly identical to the score for women without cancer after excluding those survivors who had experienced a second primary cancer or who reported current treatment. After these exclusions, a small but non-significant difference remained for the Physical Functioning scale.
Colorectal cancer
Colorectal cancer survivors reported equal to, or in the case of the Bodily Pain scale, higher mean unadjusted and adjusted scores, than women without cancer for all six remaining scales. For the Bodily Pain scale, a higher age- and comorbidity-adjusted score for colorectal cancer survivors compared to women without cancer was statistically significant.
Genitourinary cancer
Women with genitourinary cancers fared worse than women without cancer for Physical Functioning, Role Physical, Bodily Pain, Vitality and Social Functioning scales, even after adjusting for age and comorbidity. Only slight improvement in scores occurred after exclusions, and all differences, except for the Social Functioning scale, remained statistically significant.
Gynecologic cancers
Survivors of gynecologic cancers reported lower unadjusted mean scores for the Physical Functioning scale than women without cancer, however, the statistical significance of this difference did not hold after accounting for age and comorbidity. For the other five quality of life scales, small to no differences were observed between women with these cancers compared to women without.
Lung cancer
Women with lung cancer reported statistically significantly lower mean unadjusted scores than women without cancer for Physical Functioning, Role Physical, Bodily Pain, Vitality, and Social Functioning scales. Adjustment for age and other chronic health conditions led to improvements in these scores relative to women without cancer, but differences remained statistically significant, except for the Bodily Pain scale. Scores further increased among lung cancer survivors relative to women without cancer for Physical Functioning, Role Physical, Vitality and Social Functioning scales after exclusions were made. However, scores for Physical Functioning and Vitality scales still remained near the ½ standard deviation demarcation and the differences for these scales between women with and without lung cancer were statistically significant.
Hematopoietic cancers, lymphomas
Survivors of these cancers reported statistically significantly lower mean unadjusted scores than women without cancer for Physical Functioning, Role Physical, Vitality, Role Emotional, and Social Functioning scales. Neither age, nor comorbidity adjustments eliminated the statistically significant differences observed. Although scores for these scales tended to improve after exclusions for treatment or second primary cancers, the differences remained statistically significant for all scales, except for the Role Emotional scale among survivors of hematopoietic cancers.
Other gastrointestinal, skin, other cancers
Women with other gastrointestinal cancers experienced lower mean unadjusted scores for Physical Functioning, Bodily Pain, Vitality, Role Emotional and Social Functioning scales, compared to women without cancer. Scores for each of these scales improved after adjustments for age and comorbidity, but statistically significant differences remained for the Vitality and Social Functioning scales. With exclusions, scores for these two scales remained lower than for women without cancer, but the differences were no longer statistically significant. Survivors of skin cancer reported lower mean unadjusted scores for Bodily Pain, and women with other cancer types experienced lower mean unadjusted scores for the Role Emotional and Social Functioning scales; scores for these scales changed only slightly, and except for the Social Functioning scale, the statistical significance of the differences persisted regardless of adjustments and exclusions.
Cancer stage
Survivors of in situ stage cancer generally reported scores that were slightly lower or similar to scores of women without cancer for all six remaining quality of life scales; scores changed very little after adjustments or exclusions (Figure 2). In contrast, women with local stage disease reported statistically significantly lower mean unadjusted scores than women without cancer for all six scales. Adjustment for age and comorbidity or exclusions only marginally attenuated the differences in scores between these survivors and women without cancer. For two scales, Physical Functioning and Vitality, the differences remained statistically significant even after excluding women reporting current treatment or with a second primary cancer.
Survivors of regional stage cancers reported higher mean unadjusted scores for the Bodily Pain and lower mean unadjusted scores for the Vitality and Social Functioning scales than women without cancer. After adjustments and exclusions, Vitality and Social Functioning scores between women with and without cancer were nearly identical. Except for the Bodily Pain scale, women surviving distant stage cancer had statistically significantly lower quality of life scores than women without cancer for the other five scales. None of these statistically significant differences were eliminated by adjustments or exclusions.
Length of survival
Compared to women without cancer, small, statistically significant age- and comorbidity-adjusted differences were observed for Role Physical, Vitality, and Social Functioning scales among women diagnosed within two years of the 2004 questionnaire, and for all six remaining scales among women surviving two to five years (Figure 3). After survivors with current treatment or a second primary were excluded, none of the remaining small differences for women surviving less than five years were statistically significant.
Women who had survived five to ten years reported a statistically significantly lower Physical Functioning score relative to women without cancer, despite adjustments and exclusions. These women also reported lower mean unadjusted scores on the Role Physical and Vitality scales. For the Role Physical scale, the difference was reduced and no longer statistically significant after adjustment for age and comorbidity; this was also observed for Vitality once exclusions were made. Women surviving the longest, ten or more years, reported lower mean unadjusted scores than women without cancer on all six remaining scales; for Physical Functioning, Vitality and Social Functioning scales, the small differences observed between these longest surviving women and women without cancer remained statistically significant whether or not adjustments or exclusions were made.
Discussion
Our analysis of quality of life and cancer survivorship is among the first to describe the physical, mental and social functioning of older female cancer survivors--an understudied but substantial proportion of all cancer survivors--across all cancer types, all cancer stages and from very recent to very long-term survival. By comparing older cancer survivors to similar-aged women without cancer within a prospective cohort, we were able to isolate the effect of a cancer diagnosis on quality of life from the effects of comorbidity and aging. And by removing cancer survivors who reported current therapy or who had developed a second primary cancer, we were able to present, within the limits of our data, the best case scenario for quality of life among cancer survivors in the IWHS population.
Despite these strengths, we measured quality of life at only one point, and therefore, could not describe changes in quality of life before and after a cancer diagnosis or throughout the survivorship experience. We were unable to examine the effect of specific cancer treatments or recurrence on quality of life because the cancer registry does not record this information. By the nature of the study design, this analysis was based on a generally healthier group of women and relied on self-reported comorbidity, but these limitations applied to both the cancer survivors and women without cancer. We examined eight scales across ten cancer types, four stages, and four survivorship groups, but did not adjust for multiple comparisons, so some statistically significant differences between cancer and non-cancer groups may be due to chance. And while we tried to identify quality of life concerns specific to cancer type, stage or length of survival by stratification on these aspects of cancer survivorship, heterogeneity undoubtedly exists within each group. Nevertheless, our findings, point to several notable conclusions regarding quality of life and cancer survivorship—lower General Health and comparable Mental Health scores among survivors relative to cancer-free women, and quality of life decrements across most scales for survivors of specific cancers, with advanced stage, or who had survived the longest.
Other than colorectal cancer, we found that lower scores for perceived General Health among cancer survivors persisted across cancer types, stage and length of survival, and were generally not explained by age, comorbidity, current treatment or second primary cancers. Cancer survivors commonly report worse general health than persons without cancer, a finding which has been associated with older age, presence of other chronic conditions, type of cancer and treatment status.6,8–12 However, few previous studies have been able to account for age or comorbidity, as we were able to do. The perception of poorer health among IWHS cancer survivors could be due, in part, to observed decrements in Physical Functioning, which also has been widely reported among cancer survivors.8,9,11,12,16,24–27 In our study, women diagnosed with colorectal cancer consistently reported higher quality of life across most scales relative to women without cancer. Although we have no explanation for this finding, others have also shown that colorectal cancer survivors fare better than population norms for the majority of SF-36 scales.28,29
Prevalence of depression among cancer patients has been reported to range from 0 to 58%, compared to 4.5–9.3% among healthy persons, varying by cancer site.30 In our study, cancer survivor scores for Mental Health were remarkably similar to scores among women without cancer, regardless of which aspect of cancer survivorship we examined. In contrast, a study of 22,000 cancer survivors receiving Medicare found that survivors reported small but statistically significant decrements in mental health function relative to persons without cancer.6 Although cancer survivors reported more psychological problems and greater use of mental health services than persons without cancer in one large national sample,7,8 these concerns were much more common among younger than older survivors. Others have found that older women with breast cancer tend to fare better on psychosocial measures than their younger counterparts,31–33 perhaps due to perspective gained with age, more experience with chronic illness, or fewer demands such as family and employment, on older compared to younger women. Two reports from the Health and Retirement Study found depression among cancer survivors to be similar to persons without cancer, but only if they had survived four or more years.12,17
That women with metastatic cancer fared worse on six of eight scales than women with less advanced cancer, and relative to women without cancer, is not surprising, but that these women reported Bodily Pain and Mental Health scores similar to women without cancer was somewhat unexpected. Cancer stage as a marker for disease severity has seldom been examined in relation to quality of life. In a study of 395 patients with incurable cancer, differences in reported pain between those with and without lung, liver or bone metastases, depended on where the cancer had metastasized.34 Since we were unable to identify where cancer had metastasized, our largely negative findings for the Bodily Pain scale among survivors with distant disease may reflect metastases that are not associated with pain. Alternative explanations include the possibility that pain had diminished or was well-controlled, that the SF-36 Bodily Pain scale is not a sensitive measure of cancer-related pain, or that survivors with significant pain did not respond to the questionnaire. Depression and pain are often viewed as co-occurring manifestations.30 IWHS cancer survivors were similar to women without cancer for both Bodily Pain and Mental Health scales, suggesting adaptation or adjustment to cope with the disease.
The experience of persons surviving cancer five years and beyond is an area of intense research interest.4–5 Studies that have repeated quality of life measures before or during cancer treatment, and up to 8–9 years afterwards, show that quality of life improves or declines, depending on the specific scale, and whether comparisons are made between persons with and without cancer, or repeated over time among cancer survivors.16,24,26 Some studies have found that longer-term survivors experience greater problems with physical function but regain normal mental health function, while others have found either no relationship between length of survival and quality of life, or even that survivors fare better than normal comparison groups.13,16,24–26,35–37 Our finding for the General Health scale among women surviving cancer ten or more years is consistent with two other studies that had internal non-cancer comparison groups.8,11 These longest-surviving IWHS women also reported lower Physical Functioning, Vitality, and Social Functioning scores relative to women without cancer that were not explained by adjustments or exclusions, and they were the only cancer survivors to report statistically significantly lower scores for the Mental Health scale. Although chemotherapy and radiation therapy have been shown to adversely affect some aspects of quality of life,24 ten or more-year survivors in the IWHS were the least likely to have received these treatments. Thus, our findings represent preliminary evidence that a cancer diagnosis may have long term consequences for quality of life.
After excluding women with a second primary or who reported current cancer treatment in 2004, we still observed lower Vitality scores among cancer survivors for several types of cancer, with local or distant stage cancers, and for the longest surviving women, compared to women without cancer. Cancer patients are known to experience higher levels of fatigue than the general population, and cancer-related fatigue is apparent even among cancer patients who do not experience treatment-related anemia, possibly due to treatment effects on other body systems, sleep disturbances, stress or depression.38 Some reports also suggest that fatigue, depression and pain tend to cluster among cancer patients.17 For example, a study of breast cancer survivors, two to five years after diagnosis, found that depression and pain predicted fatigue, leading to a lower quality of life for survivors relative to population norms.39 We did not examine clustering among our measures of pain, fatigue and mental health, but of these three areas, only fatigue seemed to be an issue for IWHS cancer survivors.
About 85% of IWHS women reported at least one chronic disease, with cancer survivors reporting a higher frequency of comorbid conditions than women without cancer, similar to observations from other nationally representative samples or cohorts.8,10–12,18,40,41 Unfortunately, due to irregular timing of the follow-up questionnaires, we were unable to link the presence of comorbidity before or after a cancer diagnosis to quality of life, and thus, could not evaluate whether a cancer diagnosis affects risk of other chronic conditions. While presence and number of other chronic conditions and their severity have been shown to affect functioning or increase mortality among cancer survivors,13,42,43 our approach was to examine quality of life by removing the influence of comorbidity (and age) through statistical adjustment. Quality of life scores among survivors tended to improve after these adjustments, although the fact that most (82%) of the differences remained statistically significant suggests that cancer exerts an effect, albeit often small, on quality of life beyond these factors. However, our results could be compromised by the limited number of chronic health conditions assessed, the possibility of inaccurate reporting of comorbidity, and lack of information regarding severity or treatment of the chronic conditions reported.
Our report underscores the complexity of understanding quality of life among cancer survivors. Conclusions about quality of life and cancer survivorship depend upon which aspect of quality of life—type of cancer, stage, or time since diagnosis—we examine. Despite concerns that development or exacerbation of chronic conditions or aging reduces cancer survivors' quality of life, we found these factors to be of only marginal consequence. For women diagnosed with certain cancers, like genitourinary, lung, hematopoietic, lymphoma, or other gastrointestinal cancers, or metastatic disease, the reduction in quality of life across multiple scales is likely to have clinical relevance. But for most of the IWHS cancer survivors, the statistically significant differences we observed between survivors and women without cancer were fairly small. Thus, while a cancer diagnosis unquestionably affects some dimensions of quality of life, and the effect of the cancer diagnosis is present even among those surviving the longest, many aspects of elderly women's lives appear to be only mildly compromised by a cancer diagnosis.
Acknowledgements
The authors would like to thank Dr. Aaron Folsom, Principal Investigator of the Iowa Women’s Health Study, and Dr. Mary Jo Nissen, a cancer outcomes researcher, for their helpful comments on earlier versions of this manuscript.
This research was supported by a National Cancer Institute award, R01 CA39742.
Footnotes
Financial disclosures: none.
References
- 1.Rowland JH, Bellizzi KM. Cancer survivors and survivorship research: a reflection on today's successes and tomorrow's challenges. Hematol Oncol Clin North Am. 2008;22(2):181–200. doi: 10.1016/j.hoc.2008.01.008. v. [DOI] [PubMed] [Google Scholar]
- 2.Bellizzi KM, Rowland JH. Role of comorbidity, symptoms and age in the health of older survivors following treatment for cancer. Aging Health. 2007;3(5):625–635. [Google Scholar]
- 3.Hewitt M, Greenfield S, Stovall EE. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 4.Gotay C, Muraoka M. Quality of life in long-term survivors of adult-onset cancers. J Natl Cancer Inst. 1998;90(9):656–667. doi: 10.1093/jnci/90.9.656. [DOI] [PubMed] [Google Scholar]
- 5.Bloom J, Petersen D, Kang S. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology. 2007;16(8):691–706. doi: 10.1002/pon.1208. [DOI] [PubMed] [Google Scholar]
- 6.Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97(3):674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt M, Rowland J. Mental health service use among adult cancer survivors: analyses of the National Health Interview Survey. J Clin Oncol. 2002;20(23):4581–4590. doi: 10.1200/JCO.2002.03.077. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 9.Stafford RS, Cyr PL. The impact of cancer on the physical function of the elderly and their utilization of health care. Cancer. 1997;80(10):1973–1980. doi: 10.1002/(sici)1097-0142(19971115)80:10<1973::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Yabroff KR, McNeel TS, Waldron WR, Davis WW, Brown ML, Clauser S, et al. Health limitations and quality of life associated with cancer and other chronic diseases by phase of care. Med Care. 2007;45(7):629–637. doi: 10.1097/MLR.0b013e318045576a. [DOI] [PubMed] [Google Scholar]
- 11.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96(17):1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 12.Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53(12):2145–2152. doi: 10.1111/j.1532-5415.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 13.Garman K, Pieper C, Seo P, Cohen H. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci. 2003;58(12):M1119–M1124. doi: 10.1093/gerona/58.12.m1119. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22(10):1849–1856. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 15.Michael YL, Berkman LF, Colditz GA, Holmes MD, Kawachi I. Social networks and health-related quality of life in breast cancer survivors: a prospective study. J Psychosom Res. 2002;52(5):285–293. doi: 10.1016/s0022-3999(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 16.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses’ Health Study. Cancer. 2000;89(11):2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::aid-cncr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Reyes-Gibby CC, Aday LA, Anderson KO, Mendoza TR, Cleeland CS. Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. J Pain Symptom Manage. 2006;32(2):118–128. doi: 10.1016/j.jpainsymman.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo PH, Pieper CF, Cohen HJ. Effects of cancer history and comorbid conditions on mortality and healthcare use among older cancer survivors. Cancer. 2004;101(10):2276–2284. doi: 10.1002/cncr.20606. [DOI] [PubMed] [Google Scholar]
- 19.Ware JJ, Koskinski M, Gandek B. Lincoln, RI: QualityMetric Incorporated; 1993. SF-36® Health Survey: Manual & Interpretation Guide; p. 2000. [Google Scholar]
- 20.SF-36.org. [[Accessed 22 October 2008]];A community for measuring health outcomes using SF tools. SF-36.org Web Site. Available at: http://www.sf-36.org/
- 21.Garratt A, Ruta D, Abdalla M, Buckingham J, Russell I. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ. 1993;306(6890):1440–1444. doi: 10.1136/bmj.306.6890.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JJ, Koskinski M. SF-36® Physical & Mental Health Summary Scales: A Manual for Users of Version 1. Second edition ed. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 23.Norman G, Sloan J, Wyrwich K. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 24.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Deimling GT, Sterns S, Bowman KF, Kahana B. Functioning and activity participation restrictions among older adult, long-term cancer survivors. Cancer Invest. 2007;25(2):106–116. doi: 10.1080/07357900701224813. [DOI] [PubMed] [Google Scholar]
- 26.Schroevers M, Ranchor AV, Sanderman R. Adjustment to cancer in the 8 years following diagnosis: a longitudinal study comparing cancer survivors with healthy individuals. Soc Sci Med. 2006;63(3):598–610. doi: 10.1016/j.socscimed.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98(8):521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 28.Trentham-Dietz A, Remington PL, Moinpour CM, Hampton JM, Sapp AL, Newcomb PA. Health-related quality of life in female long-term colorectal cancer survivors. Oncologist. 2003;8(4):342–349. doi: 10.1634/theoncologist.8-4-342. [DOI] [PubMed] [Google Scholar]
- 29.Mosconi P, Apolone G, Barni S, Secondino S, Sbanotto A, Filiberti A. Quality of life in breast and colon cancer long-term survivors: an assessment with the EORTC QLQ-C30 and SF-36 questionnaires. Tumori. 2002;88(2):110–116. doi: 10.1177/030089160208800206. [DOI] [PubMed] [Google Scholar]
- 30.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 31.Mor V, Malin M, Allen S. Age differences in the psychosocial problems encountered by breast cancer patients. J Natl Cancer Inst Monogr. 1994;(16):191–197. [PubMed] [Google Scholar]
- 32.Cimprich B, Ronis DL, Martinez-Ramos G. Age at diagnosis and quality of life in breast cancer survivors. Cancer Pract. 2002;10(2):85–93. doi: 10.1046/j.1523-5394.2002.102006.x. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22(10):1849–1856. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 34.Jordhoy MS, Fayers P, Loge JH, Saltnes T, Ahlner-Elmqvist M, Kaasa S. Quality of life in advanced cancer patients: the impact of sociodemographic and medical characteristics. Br J Cancer. 2001;85(10):1478–1485. doi: 10.1054/bjoc.2001.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peuckmann V, Ekholm O, Rasmussen N, Møller S, Groenvold M, Christiansen P, et al. Health-related quality of life in long-term breast cancer survivors: nationwide survey in Denmark. Breast Cancer Res Treat. 2007;104(1):39–46. doi: 10.1007/s10549-006-9386-6. [DOI] [PubMed] [Google Scholar]
- 36.Ferrell B, Dow K, Leigh S, Ly J, Gulasekaram P. Quality of life in long-term cancer survivors. Oncol Nurs Forum. 1995;22(6):915–922. [PubMed] [Google Scholar]
- 37.Ramsey S, Berry K, Moinpour C, Giedzinska A, Andersen M. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol. 2002;97(5):1228–1234. doi: 10.1111/j.1572-0241.2002.05694.x. [DOI] [PubMed] [Google Scholar]
- 38.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 39.Meeske K, Smith A, Alfano C, McGregor B, McTiernan A, Baumgartner K, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16(6):947–960. doi: 10.1007/s11136-007-9215-3. [DOI] [PubMed] [Google Scholar]
- 40.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 41.Yancik R, Havlik RJ, Wesley MN, Ries L, Long S, Rossi WK, et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol. 1996;6(5):399–412. doi: 10.1016/s1047-2797(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 42.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 43.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]


