Abstract
A direct water intake study was conducted for one year, involving 423 individuals from arsenic (As) affected villages of West-Bengal, India. Average direct water intake per person was found to be 3.12±1.17 L/day and 78.07±47.08 mL/kg/day (±SD). Average direct water intakes for adult males, adult females and children (age <15 years) were 3.95, 3.03 and 2.14 L/day, respectively. Significant sex differentials were observed between ages 16–55 years. For all participants, a sharp increase in water intake up to age 15 years was observed followed by a plateau at a higher intake level. Significant monthly, seasonal, regional, and occupational variability was also observed. Another study involving 413 subjects determined the amount of indirect water intake. Average indirect water intake per person was 1.80±0.64 L/day; for adult males, females and children, intake was 2.15, 1.81, and 1.10 L/day, respectively. Average total (direct + indirect) water intake was 4.92 L/person/day; for adult males, females and children total intake was 6.10, 4.84, and 3.24 L/person/day, respectively. The overall contribution of indirect water intake to total water consumption is 36.6% for all participants. This study additionally elucidated several factors that contribute to variable water intake, which can lead to better risk characterization of subpopulations and water contaminant ingestion. The study reveals that the water intake rates in the three studied populations in West Bengal are greater than the assumed water intake rates utilized by the World Health Organization (WHO) in the establishment of drinking water quality guidelines; therefore, these assumed intake values may be inappropriate for the study population as well as similar ones.
Keywords: Arsenic-affected population of rural West Bengal, Direct and indirect water consumption, Water consumption patterns, Factors contributing to water consumption, WHO guideline value
1. Introduction
In West Bengal, India and Bangladesh, it is estimated that 100 million people in As-affected areas are potentially at risk from groundwater As contamination above the WHO guideline value of 10 µg/L (Chakraborti et al., 2009; Chakraborti et al., 2010). One of the important parameters needed to determine appropriate drinking water quality guidelines, especially for chemical substances, is the quantity of water intake. In the establishment of guideline values for drinking water contaminants, the WHO and some national agencies use 2 L of water intake per day for 60 kg adults as default assumptions (WHO, 2011; NHRMC, 2004). For many populations in developing countries, particularly those with warmer climates and livelihoods that require significant manual labor, this assumed water intake might underestimate actual individual consumption. In India and Bangladesh for example, people often consume considerably more than 2 L of water per day (Milton, 2006; Watanabe, 2004) while their national standards for As in drinking water remain five times higher than the WHO guideline value (WHO, 2001). Due to differences in water intake levels, the WHO has acknowledged that “local adjustments to the daily water consumption value may be needed in setting local standards” (WHO, 2011).
Many researchers worldwide have reported the adverse health effects associated with the ingestion of elevated levels of As through drinking water (IARC, 2004), emphasizing the importance of setting protective standards. The determination of human water intake and factors contributing to variation is a fundamental component of the risk assessment for As and other contaminant intake. Additionally, there is an increasing literature on the presence of inorganic As in food, particularly rice (http://www.abdn.ac.uk/biologicalsci/staff/details/a.meharg). Although rice often contains As, the concentrations may be particularly elevated in areas where As-rich groundwater is utilized for the irrigation of agricultural fields; furthermore, the water absorbed during cooking may serve as a significant contribution to water intake. This dietary source of As is also a key aspect of risk assessment and has been found to become increasingly important to the ingested dose as drinking water As concentrations decrease (Kile et al. 2007).
A number of previous studies have evaluated the water intake of human populations from USA (Binkowitz and Wartenberg, 2001; Ershow et al., 1991; Michaud et al., 1999; Ryan et al., 2000; USEPA, 2004), Canada (Levallois et al., 1998), Israel (Kristal-Boneh et al., 1995), Sweden (Westrell et al., 2006), Mexico (Del Razo et al., 2002), Taiwan (Abernathy et al., 1989; Chen et al., 1992; USEPA, 1988), Bangladesh (Kile et al., 2007; Milton et al., 2006; Ohno et al., 2007; Watanabe et al., 2004; Ahsan et al., 2006), and West Bengal, India (Chowdhury et al., 2001; Mondal et al. 2010).
Direct intake, the consumption of drinking water alone, is not the only source of water intake, however. Indirect water intake, the consumption of water mixed with food and beverages, can also substantially contribute to total intake. In one study conducted with UK adolescents aged 11–12 years (Zohouri et al., 2004), direct water intake contributed only 65% of the total water consumption (1130 g/day), while another group reported (Sichert-Hellert et al., 2001) an even lower contribution of 49–55%. For US adolescents aged 11–19 years, the direct water intake reported was 715 g/day (Heller et al., 1999), and in a separate study 609 g/day for children aged 1–10 years (Sohn et al., 2001). In the United States, the population was estimated to consume 64% of water as direct water intake (USEPA, 2004).
While most of the available epidemiologic data related to chronic As toxicity are from contaminated areas in developing countries, much of the available water intake data are from developed countries. Daily direct and indirect water intake, water source, lifestyle, nutritional status and climate can significantly differ between these countries. As a result, adopting estimated water intake values from developed countries may not be suitable for the risk characterization of water contaminant intake in human populations from tropical regions of developing countries like rural India and Bangladesh. A detailed longitudinal analysis of the patterns of individual water intake, direct and indirect, has been lacking for the As-affected populations in West Bengal, India. In this study we explored these patterns and the contributing factors over the course of an entire year. Due to the relatively similar geographic location, weather conditions, and socio-economic status of the inhabitants throughout the Bengal Delta, we anticipate that the findings of this study may be applicable to much of the region.
2. Materials and Methods
2.1. Study area
To conduct the direct water intake study, three As-affected villages (Dangapara, Golabari Chandpur and Ambikanagar), each from three blocks of two districts [Tehatta I (Nadia district), Bashirhat II and Deganga (North 24 Parganas district)] of West Bengal, India were selected due to the presence of permanent School of Environmental Studies, Jadavpur University (SOES) field staff in these villages. The map of the study area with the distribution of As contamination is provided in Supplementary Information Fig. 1(a–c). The indirect water intake study was conducted in two villages (Golabari Chandpur and Ambikanagar). As the study areas were selected purposively depending on the availability of permanent staff in the area, the major limitation of the present study is that the sample is not widely geographically representative of the region; for the direct water intake study however, households from the selected study areas were selected randomly.
2.2. Sampling methodology (direct water intake study)
2.2.1. Household selection
The complete list of households for each of the three selected villages was prepared starting sequentially from one corner of a village and ending at the opposite corner. Forty households were selected from each of the three villages on a systematic random sampling basis for the direct water intake study in order to ensure wide coverage of the village. In the selection process, households having at least three members were included to ensure at least one adult male, one adult female, and a child from each household as study subjects. A total of 120 households were selected for this study. In the selected three villages, there were approximately 820 households (Dangapara 250 households, Ambikanagar 350 households, and Golabari Chandpur 220 households). The selected participants of the selected households were told to drink water from safe tube-wells (As <10µg/L) during the study period.
2.2.2. Study subject selection
At the time of enrollment, each member of the selected 120 households promised their co-operation during the water intake study of one-year duration. Our field staff measured and recorded the water intake, demographic, and anthropometric information, including: age, sex, height, weight, and occupation for each participant.
2.2.3. Determination of sample size
The required sample size was determined following standard statistical methods (Abernathy et al., 1989) and was calculated to be 341 (details of the sample size determination are available in Supplementary Information). To ensure a minimum of 341 individuals for the duration of the water intake study, it was estimated that 90 families (30 from each selected village) would provide the required 341 members, assuming 4 members per family. The study was started with 120 households (40 from each village) having 509 members, anticipating that 10 families from each village may be lost to follow-up for various reasons. At the end of the study period, we had observed 93 households (34 from Dangapara, 30 from Golabari Chandpur and 29 from Ambikanagar village respectively) throughout, having 423 individuals [306 adults, age ≥15 years (154 male, 152 female) and 117 children age <15 years (65 boys, 52 girls)]. This communication presents data related to these households (Dangapara: 153 individuals, 34 households; Golabari Chandpur: 148 individuals, 30 households; Ambikanagar: 122 individuals, 29 households).
2.2.4. Data collection instruments
Two carefully structured questionnaires were used for data collection; one for background information regarding study participants and their households and the other for individual water intake (questionnaires are provided in the Supplementary Information). For the water intake study, food grade polyethylene terephthalate (PET) plastic bottles (1 and 2L size) were provided to each member of the selected households; these bottles were properly labeled to prevent their mix-up among family members. Block replacement of the bottles was completed after six months, and all damaged or missing bottles were replaced in the intervening period, attempting to ensure the possession of an individual bottle by each study participant for the duration of the study.
2.2.5. Study setting
The survey covered one calendar year, starting in March 2006 and ending in February 2007 in order to ascertain monthly and seasonal variations of daily water intake. As far as we know, there is no other longitudinal data on water intake available for the region. Each month, a separate water intake survey covered three consecutive predetermined days (15th–17th day of the month). Consecutive days were considered to minimize variation in the weather conditions between days.
2.2.6. Method of direct water intake data collection
The study participants were advised to refill the bottle with drinking water early in the morning immediately after getting up from bed and asked to drink water exclusively from the bottle provided to him/her for the entire day. They were also advised to refill the bottle completely whenever the bottle became empty and to count the number of refills for a day. On the next day early in the morning, field investigators visited each household and recorded the amount of water drank by each member separately on a printed sheet. Number of refills reported by the participants and the amount of remainder water measured by each investigator was used to calculate the amount of water intake per person per day.
water consumed = (bottle size × no. of refills) − remaining water
The process was continued for three days in a month for 12 months from March 2006 to February 2007. While study participants were asked to drink exclusively from the bottle and other precautions were taken to obtain accurate direct water intake data, carrying a bottle for drinking water is not a common practice in many rural communities; therefore despite best efforts, it is possible that some individuals drank water directly from other sources, attempting, or not attempting, to correct for this later by pouring out water from their bottle and introducing error into the estimates of intake.
2.3. Sampling methodology (indirect water intake study)
2.3.1. Household and study participant selection
For the indirect water intake study, 96 households from two villages were selected conveniently near the residence of SOES field staff to facilitate data collection. The total number of study participants was 413 [327 adults, age ≥15 years (172 male, 155 female) and 86 children age <15 years (45 boys, 41 girls)].
2.3.2. Method of indirect water intake data collection
The amount of indirect water intake by each study participant was calculated as the summation of the water consumption from ‘cooked rice’, ‘prepared food’, ‘beverage’ and ‘dry food’ (details of the different categories of indirect water intake and calculation are available in the Supplementary Information).
2.4. Statistical analysis
Standard statistical techniques were applied to analyze and present the data. The statistical package SPSS 16.0 was used for data entry and analysis. Univariate, bivariate and multivariate analyses were performed according to the merit of the data variables. Simple descriptive measures, test of significance, analysis of variance, and multivariate regression analysis were applied. Bootstrap analysis was also performed to assess the sensitivity of the direct water intake estimates in terms of standard error. Prior to any parametric statistical analysis, data was assessed for normality.
3. Results and Discussion
3.1. Overall direct water intake
The average monthly, seasonal, and yearly water intake per person per day (L/person/day), per unit (kg) body weight (UBW) per day (mL/UBW/day), and associated descriptive statistics are provided in Table 1. The response variable, direct water intake, was found to be normally distributed for the study population (Kolmogorov–Smirnov test, p = 0.532) Average daily water intake for all study participants was 3.12 ± 1.17 L/person/day and average daily intake per unit (kg) body weight was 78.07 ± 47.08 mL/kg/day.
Table 1.
Monthly, seasonal, and yearly average daily direct water intake irrespective of age, sex, and region of residence
| Monthly water intake information | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | n | Water intake (L/person/day) | Water intake (mL/person/UBW/day) | ||||||
| Min | Max | Mean | SD | Min. | Max. | Mean | SD | ||
| March, ‘06 | 422 | 0.50 | 9.17 | 3.29 | 1.35 | 23.33 | 1100.00 | 83.26 | 58.31 |
| April, ‘06 | 421 | 0.27 | 9.13 | 3.40 | 1.37 | 22.73 | 1111.11 | 84.98 | 58.28 |
| May, ‘06 | 422 | 0.25 | 8.93 | 3.47 | 1.41 | 20.00 | 1027.78 | 85.97 | 53.90 |
| June, ‘06 | 423 | 0.37 | 9.00 | 3.59 | 1.48 | 28.37 | 944.44 | 88.51 | 50.74 |
| July, ‘06 | 423 | 0.30 | 8.50 | 3.40 | 1.29 | 27.27 | 855.56 | 83.71 | 45.50 |
| August, ‘06 | 421 | 0.50 | 7.67 | 3.32 | 1.26 | 25.49 | 833.33 | 82.39 | 45.07 |
| September, ‘06 | 420 | 0.50 | 8.67 | 3.18 | 1.14 | 26.67 | 638.89 | 79.24 | 37.19 |
| October, ‘06 | 418 | 0.40 | 7.83 | 2.92 | 1.19 | 25.64 | 861.11 | 73.11 | 46.05 |
| November, ‘06 | 418 | 0.40 | 6.67 | 2.61 | 1.11 | 19.23 | 777.78 | 65.80 | 42.43 |
| December, ‘06 | 420 | 0.50 | 8.00 | 2.55 | 1.08 | 19.23 | 861.11 | 64.56 | 45.71 |
| January, ’07 | 420 | 0.50 | 8.00 | 2.62 | 1.16 | 19.49 | 933.33 | 66.28 | 48.95 |
| February, 07 | 421 | 0.50 | 9.00 | 3.08 | 1.36 | 18.28 | 1077.78 | 78.93 | 58.37 |
| Seasonal water intake information | |||||||||
| Season | |||||||||
| Summer | 421 | 0.46 | 8.05 | 3.44 | 1.32 | 26.67 | 1045.83 | 85.70 | 53.55 |
| Winter | 418 | 0.50 | 7.92 | 2.72 | 1.14 | 21.51 | 912.50 | 69.04 | 47.98 |
| Monsoon | 418 | 0.48 | 7.82 | 3.21 | 1.17 | 30.15 | 797.22 | 79.69 | 42.15 |
| Yearly water intake information | |||||||||
| All together | 423 | 0.52 | 7.93 | 3.12 | 1.17 | 27.65 | 918.52 | 78.07 | 47.08 |
UBW: Unit Body Weight (kg); SD: Standard Deviation
Average daily direct water intake was recorded as high as 9.17 L by an agricultural labor from Golabari Chandpur; this maximum value is similar to an earlier report from a large population-based cohort study (11,746 population, male = 5042, female = 6704) in Araihazar, Bangladesh (Ahsan et al., 2006) where the maximum daily water consumption was 9.2 L for male and 8.7 L for female. The maximum water intake for an individual from this study is also similar with an earlier report from West Bengal, India (Chowdhury et al., 2001) but much higher than the 6 L/day reported for another Bangladeshi population (Watanabe et al., 2004).
3.1.1. Seasonal, monthly, and daily variability in direct water intake
The direct water intake study was continued for one year to track differences in average water consumption of the study participants among different days of a month, different months, and different seasons of a year. Intake was maximal in the summer months (3.44 L/day), intermediate during the monsoon season (3.21 L/day), and lowest during the winter (2.72 L/day) with a 26.5% difference between the maximum and minimum. Seasonal variations in adult US populations were reported as 6% (Michaud et al., 1999) and 10–30% (Ryan et al., 2000). Monthly water intake data indicate that maximum water was consumed in the month of June (mean 3.59 ± 1.48 L/day) with the lowest consumption in the month of December with mean intake 2.55 ± 1.08 L/day. Average monthly direct water intake for adult males, females and children (age <15 years) show similar trends across months with the total amount of water intake largest for males followed by females and then children (Supplementary Information Fig. 2). One-way ANOVA (Cochran and Cox, 1975) indicated that no significant differences in direct water intake exist between three consecutive days (Table 2). Highly significant differences in average direct water intake were found between different months and seasons. Watanabe et al. (2004) identified a possible positive relationship between activity level and water and subsequently hypothesized that this association could be due to environmental temperature, activity, or other factors. It is plausible that the temporal differences in direct water intake in the present study are also related to these factors.
Table 2.
One way ANOVA Table for testing the homogeneity of average direct water intake in ‘different days’, ‘different months’, and ‘different seasons’ irrespective of age, sex, region of the subjects.
| Testing the homogeneity of average direct water intake in ‘different days’ | |||||
| Sources of Variation |
Sum of Squares |
Degrees of Freedom |
Mean sum of Squares |
F-value | p-value |
| Between days | 7.828 | 2 | 3.914 | 2.155 | 0.135 |
| Within (error) | 27412.953 | 15093 | 1.816 | ||
| Total | 27420.781 | 15095 | 1.817 | ||
| Testing the homogeneity of average direct water intake in ‘different months’ | |||||
| Between | 1840.364 | 11 | 167.306 | 98.644 | <0.0001 |
| Within | 25583.417 | 15084 | 1.696 | ||
| Total | 27423.781 | 15095 | 1.817 | ||
| Testing the homogeneity of average direct water intake in ‘different seasons’ | |||||
| Between | 1385.779 | 2 | 692.889 | 401.635 | <0.0001 |
| Within | 26038.002 | 15093 | 1.725 | ||
| Total | 27423.781 | 15095 | 1.817 | ||
3.1.2. Sex and regional variability of direct water intake
Of the 423 participants in the direct water intake study, 48.2% were female and 51.8% were male. Average water intake by sex is provided in Table 3. Average water intake for all males and females (irrespective of age) was 3.42 L/day and 2.80 L/day, respectively, and was significantly different (p < 0.0001). Similarly, a significant difference for average direct water intake per unit body weight between males and females was also observed. Sex differences in direct water consumption have also been reported among German children (age 9–13 years), 1801 mL/day for boys and 1676 mL/day for girls (Sichert-Hellert et al., 2004), and US adolescents (age 11–19 years), 780 mL/day for boys and 659 mL/day for girls (USEPA, 2004).
Table 3.
Sex and regional variability of average direct water intake for both L/person/day and mL/person/UBW/day among the study population
| Sex differentials of water intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | n | Water intake (L/person/day) | Water intake (mL/person/UBW/day) | ||||||
| Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | ||
| Male | 219 | 0.83 | 7.93 | 3.42 | 1.31 | 35.84 | 918.52 | 80.79 | 23.64 |
| Female | 204 | 0.52 | 5.48 | 2.80 | 0.89 | 27.65 | 147.04 | 70.43 | 21.08 |
| Both sex | 423 | 0.52 | 7.93 | 3.12 | 1.17 | 27.65 | 168.89 | 78.07 | 47.08 |
| t-statistic | −5.666 | −4.745 | |||||||
| d.f. | 421 | 421 | |||||||
| p-value | <0.0001 | <0.0001 | |||||||
| Regional variability of water intake | |||||||||
| Village | |||||||||
| Ambikanagar | 122 | 0.83 | 7.01 | 3.37 | 1.15 | 33.59 | 164.05 | 79.94 | 22.64 |
| Golabari Chandpur | 148 | 0.53 | 7.93 | 3.44 | 1.33 | 39.06 | 918.52 | 90.73 | 71.68 |
| Dangapara | 153 | 0.52 | 5.18 | 2.61 | 0.77 | 27.65 | 147.04 | 64.33 | 20.71 |
| All together | 423 | 0.52 | 7.93 | 3.12 | 1.17 | 27.65 | 918.52 | 78.07 | 47.08 |
| ANOVA Results | |||||||||
| F-values | 26.188 | 12.624 | |||||||
| d.f. | 2 and 420 | 2 and 420 | |||||||
| p-values | <0.0001 | <0.0001 | |||||||
UBW: Unit body weight (kg); SD: Standard deviation; d.f.: Degrees of freedom
To grasp the regional variability in direct water intake the study was conducted in three different regions (villages: Ambikanagar, Golabari Chandpur, and Dangapara). Table 3 shows the average direct water intake for different villages. Average water intake among the people of Golabari Chandpur, Ambikanagar and Dangapara village was found to be 3.44, 3.37 and 2.61 L/day, respectively. Irrespective of age and sex the amounts of water intake per person per day per unit body weight were 90.73, 79.94 and 64.33 mL/kg among the subjects from Golabari Chandpur, Ambikanagar and Dangapara villages, respectively. ANOVA indicates that the regional variability of average water intake is significant for both per person per day and per person per unit body weight. Communal and regional variability of water intake was also reported earlier by the researchers (Watanabe et al., 2004). The reason for regional variability in water intake may be due to differences in weather conditions and food intake habits of the population although Mondal et al. (2010) recently reported that there is no significant variation in water intake between As-exposed area (Bhawangola-I block, male - 3.0 ± 0.9, female – 2.7 ± 0.7 L) and unexposed areas (Khejuri-I block, male - 3.3 ± 0.5, female – 2.8 ± 0.8 L) of West Bengal.
3.1.3. Age differentials of direct water intake
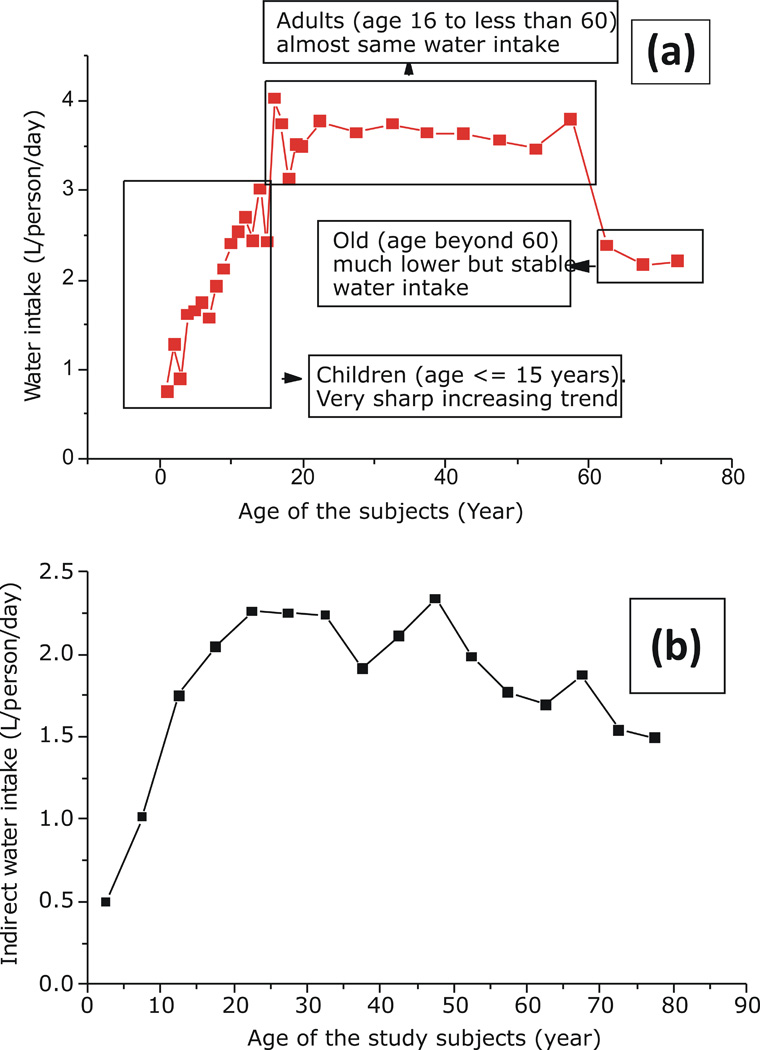
The average age of the study participants was 28.5 ± 18.1 years with an age range from 7 months to 90 years. A sharp linear increase in water consumption (0.154 L/year of age, R2 = 0.92) was observed up to 15 years of age, followed by a stable intake between 16 and 60 years of age (average intake 3.5 L/day), and a lower but stable water intake (average 2.25 L/day) beyond 60 years of age (Fig. 1a). In a previous study with German children and adolescents up to 13 years of age, a similar trend of increasing intake with increasing age was observed (Sichert-Hellert et al., 2001). In the present study, daily average direct water consumption per unit body weight was found to be relatively high up to 11 years of age, decreasing to a steady level up to 60 years of age, and decreasing to a lower but steady intake beyond 60 years of age (Supplementary Information Fig. 3). Variability in average water consumption with age has also been reported earlier (NRC, 2001).
Fig. 1.
(a – b). Average daily (a) direct water intake (L/person/day) of the study population (n= 423) and (b) indirect water intake (L/person/day) of the study population (n = 413) irrespective of their sex and region of residence.
3.1.4. Age-sex differentials of direct water intake
The study findings indicate that during childhood (up to 15 years of age) or beyond 55 years of age there is no sex differential for average direct water intakes; however significant differences were observed between ages 16 and 55 years. Direct water intake per unit body weight for males and females did not differ significantly for all ages with a few exceptions where water intake per unit body weight for males is higher than that of females (Supplementary Information Table 1).
The average direct water intake for adult (age ≥15 years) males, females and children (age <15 years) was 3.95, 3.03 and 2.14 L/day, respectively. Per kg body weight, the direct intake for adult males, females and children direct water intake was 76.09, 66.69 and 87.47 mL/kg/day, respectively (Table 4).
Table 4.
Average direct water intake and amount of indirect water intake by adult (age ≥15 years) male, female, and children (age <15 years)
| Age categories | Sex | Direct water intake | Indirect water intake (L/person/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of subjects (n) |
Water intake (L/person/day) (Mean ± SD) |
Water intake (mL/person/UBW/ day) (Mean ± SD) |
No. of subjects (n) |
Sources | Total indirect water intake |
|||||
| Cooked rice (%) |
Prepared food (%) |
Dry food (%) |
Beverage (%) |
|||||||
| Adult (≥15 years) | Male | 154 | 3.95 ± 0.79 | 76.09 ± 21.47 | 172 | 1.21 ±0.48 (56%) | 0.57 ±0.16 (27%) | 0.08 ±0.06 (4%) | 0.29 ±0.17 (13%) | 2.15 ±0.55 |
| Female | 152 | 3.03 ± 1.11 | 66.69 ± 18.94 | 155 | 1.10 ±0.40 (61%) | 0.46 ±0.12 (25%) | 0.06 ±0.06 (3%) | 0.19 ±0.12 (10%) | 1.81 ±0.42 | |
| Both | 306 | 3.49 ± 1.07 | 71.42 ± 20.76 | 327 | 1.15 ±0.44 | 0.52 ±0.15 | 0.07 ±0.06 | 0.25 ±0.16 | 1.99 ±0.52 | |
| Children (<15 years) | Boys | 65 | 2.15 ± 0.75 | 92.36 ± 24.93 | 45 | 0.62 ±0.37 (56%) | 0.34 ±0.18 (31%) | 0.05 ±0.04 (5%) | 0.10 ±0.11 (9%) | 1.11 ±0.55 |
| Girls | 52 | 2.13 ± 0.81 | 81.37 ± 23.30 | 41 | 0.64 ±0.43 (59%) | 0.31 ±0.12 (29%) | 0.04 ±0.03 (4%) | 0.09 ±0.10 (8%) | 1.08 ±0.55 | |
| Children | 117 | 2.14 ± 0.78 | 87.47 ± 24.69 | 86 | 0.64 ±0.40 | 0.32 ±0.15 | 0.05 ±0.04 | 0.09 ±0.11 | 1.10 ±0.55 | |
| Total | 423 | 3.12 ± 1.17 | 78.07 ± 23.01 | 413 | 1.05 ±0.48 (58%) | 0.48 ±0.17 (27%) | 0.06 ±0.05 (3%) | 0.21 ±0.16 (12%) | 1.80 ±.64 (100%) | |
UBW: Unit body weight (kg); SD: Standard deviation
3.1.5. Occupational variability of direct water intake
Due to a wide variety of occupations identified, reported occupations by the study participants were classified into seven broad categories (service, business, agriculture, day labor, student, house work, and others) (details about the occupational categories are available in Supplementary Information). Among the occupational categories ‘agriculture,’ ‘day labor,’ and some individuals participating in ‘business’ were considered labor intensive; the remaining were considered non-labor intensive. Daily average water intake among agricultural laborers was found to be highest (4.64 L/day), followed by day laborers (4.13 L/day). The lowest direct water intake (2.54 L/day) was reported by the occupational category ‘student,’ which may be due to the fact that most students were children below 15 years of age. Significant differences in mean daily direct water intake were identified between different occupational categories (Supplementary Information Table 2) with participants involved in more labor intensive occupations consuming more water compared to those engaged in relatively less labor intensive occupations.
3.1.6. Association between direct water intake and area of residence, occupation, and body weight of the study population
The association between direct water intake of the study participants and region of residence, occupation, and body weight was examined using contingency table analysis (Supplementary Information Table 3). Chi-squared tests of independence revealed that water intake is strongly dependent on the region of residence (χ2 = 59.74, df = 6), occupational category (χ2 = 163.27, df = 18), and body weight of the study participants (χ2 = 211.91, df = 12).
3.1.7. Determination of factors influencing average direct water intake (L/person/day) of the study population: a multivariate regression approach
For each study participant, daily water intake was measured at 36 time points (3 days per month × 12 months). In addition to water intake measurements, information on place of residence, age, sex, height, weight, and occupation was also collected. Details of the model are provided in the Supplementary Information.
Two models were considered: one for the individuals below 15 years of age and the other for individuals 15 years and above due to the finding that the water intake patterns for these two age groups were different. The estimates of the regression parameters and standard errors for both models are presented in Supplementary Information Table 4. The covariates: region of residence, occupation, sex, age, and body mass index (BMI) were considered in the models. Among the explanatory variables only age is a continuous variable for which both linear and quadratic terms were considered in the models. The variable occupation categories was: labor intensive and non-labor intensive. The variable body mass index (BMI) categories was: underweight (BMI below 18.5), normal (BMI between 18.5 and 25), and overweight (BMI above 25).
For model I (age <15 years), the variable region was found to have a significant effect on water intake. Individuals from Ambikanagar and Golabari Chandpur villages consume significantly more water than individuals from Dangapara village. Individuals engaged in labor intensive jobs take significantly more water than individuals with non-labor intensive jobs. Similarly, for Model II (age ≥15 years) people of Dangapara village consume significantly less water than the individuals of Ambikanagar and Golabari Chandpur villages.
Age had a significant effect on water intake for individuals below 15 years age, but no such effect was observed for individuals with an age ≥15 years even after considering a quadratic function. Water intake by males was significantly higher than females among the individuals ≥15 years of age; however, no sex differential was observed for individuals below 15 years of age. Underweight individuals consumed significantly less water than overweight individuals (Model II), but BMI categories were not found to affect direct water intake for individuals below 15 years (Model I).
3.1.8. Sensitivity analysis of sampling
In order to assess the sensitivity of the estimates obtained from the direct water intake study, non-parametric bootstrap analysis was performed. One thousand bootstrap iterations produced an estimated average daily water intake of 3.1232 ± 0.0567 L/day (± SD); from the sample, direct intake was determined to be as 3.1186 ± 0.0568 L/day (± SE). Standard errors for the estimated regression coefficients from the sample data and from 1000 bootstrap samples were also calculated (Supplementary Information Table 5). The narrow differences between estimated and sampled direct intake values indicate the robustness of these estimates and appropriateness of the model as well as the sample.
3.2. Overall indirect water intake
Table 4 shows the average daily indirect water intake by adults and children (age <15 years) from different categories of indirect water consumption (different categories of indirect water intake is available in Supplementary Information). The relative percentages of indirect water intake sources was found to be similarly independent of age and sex with on average 58% of indirect water intake contributed by ‘cooked rice’, 27% by ‘prepared food’, 12% by ‘beverage’ and 3% from ‘dry food’ (Table 4). Average indirect water intake by adult males, females and children (age <15 years) from these four sources was 2.15, 1.81 and 1.10 L/day, respectively.
3.2.1. Sex and age-sex differentials of indirect water intake
Out of the 413 study participants included in the indirect water intake study, 217 (52.5%) were male and the remaining 47.5% were female. Irrespective of age, average daily indirect water intake by males and females was found to be 1.93 L and 1.66 L, respectively (Supplementary Information Table 6). This difference was statistically significant (p <0.001). No sex differential of daily indirect water intake was found for 5-year age groups except for individuals 35–40 years of age (Supplementary Information Table 7).
3.2.2. Age differentials of indirect water intake
The average age of the participants included in the indirect water intake study was 31.2 ± 17.6 years, ranging from 1.2 years to 90 years. Irrespective of sex and region of residence, indirect water intake was observed to increase sharply through 20 years of age and gradually decrease with increasing age (Fig. 1b). A similar trend during childhood was also observed for direct water intake in this study.
3.3. Total water intake (direct and indirect)
Average daily water intake data by adults (male and female: age ≥15 years) and children (age <15 years) from both the direct and indirect water studies are presented in Supplementary Information Table 8. The relative contributions of direct and indirect intake to average total water intake (4.92 L/person/day for all participants) appears to roughly characterize water intake of each of the subgroups: 63% direct and 37% from indirect water. For adult males, the total average daily water consumption is 6.1 L, 64.8% (3.95 L) from direct intake and 35.2% (2.15 L) from indirect intake. An adult female consumes 4.84 L of water per day with 3.03 L (62.6%) by direct intake, and 1.81 L (37.4%) by indirect intake. For children (age <15 years), the total average water intake was found to be 3.24 L with 2.14 L (66.0%) by direct water intake and 1.10 L (34.0%) by indirect water intake. The contribution of indirect water intake on total water consumption is highest among adult females (37.4%), followed by adult males (35.2%), and then children (34.0%). The reason for this slightly greater indirect water intake contribution for females may be a result of female members consuming more ‘pantavat,’ which is when water is added to cooked rice at night and eaten the following morning.
4. Concluding remarks
To our knowledge this is the first study that quantifies both direct and indirect daily drinking water intake over the course of a year in rural Bengal. The daily intake rates computed for this study are likely generalizable to many different rural populations in South East Asia.
Accurate exposure data is essential for the establishment of protective guideline values. Exposure factors are also important for accurately characterizing risk as they are variables used in the calculation, and the factors measured in this study could be used for assessing human exposure to drinking water contaminants, including As. The WHO guideline values for chemical contaminants in drinking water are based on the default assumption that adults weighing 60 kg consume on average 2 L of water per day (WHO, 2011). While this assumption may be valid in some settings, the present study shows that adults in the most severely As-affected region in the world (the Bengal Delta) consume on average 3.49 ± 1.07 L/day through drinking water alone and 5.48 ± 1.19 L/day if indirect water intake is considered. Direct water intake estimates for the present study are largely consistent with previous studies (Table 5) from South and Southeast Asia. Considering the indirect water contribution, the findings (total water intake) are the highest among the published reports listed (Table 5) and provide additional evidence that the 2 L/day for a 60 kg adult assumption for drinking water intake may be a substantial underestimation. While the WHO default assumptions for water intake may be used to estimate risk in some populations, they are not appropriate for West Bengal or other similar, rural populations that rely on local water supplies and consume rice as a staple food.
Table 5.
Reported water intake values in several populations
| Study population | Water intake (L/day)a | Reference |
|---|---|---|
| Canada, n = 125 | 1.6 ± 0.1 | Levallois et al., 1998 |
| USA, n = 73 | 0.9 ± 0.6; max = 4.1 | Ryan et al., 2000 |
| USA, n = 20,261 | Female:1.18 ± 0.3; Male:1.30 ± 0.4 | USEPA, 2004 |
| USA, n = 47909 | 1.9 | Michaud et al., 1999 |
| USA, n = 6201 | 1.9 ± 0.7b | Ershow et al., 1991 |
| USA (Review) | 1.3–1.5 | Binkowitz et al., 2001 |
| Israel, n = 5 | 1.1 ± 0.1 | Kristal-Boneh et al., 1995 |
| Sweden, n=10957 | 1.86 | Westrel et al., 2006 |
| Mexico, n=50 | 1.75 (summer); 0.67 (winter) | Del Razo et al., 2002 |
| Taiwan | Male: 3.5; Female: 2 | Abernathy et al., 1989; USEPA, 1988 |
| Bangladesh, n=38 (Male = 19, female = 19) | Male: 3 ± 1.2; Female: 3 ± 0.8 | Watanabe et al., 2004 |
| Bangladesh, (male = 5042, female = 6704) | Male: 2.9 ± 1.2, Female: 3.1 ± 1.1 | Ahsan et al., 2006 |
| Bangladesh, n = 640 | 3.53 ± 0.98 | Milton et al., 2006 |
| Bangladesh (male = 28, female =23, boys = 4, girls = 10) | Male: 2.7 ± 0.7, Female: 2.0 ± 0.5 | Ohno et al., 2007 |
| Bangladesh, n = 47 | 2.55 ± 0.99 | Kile et al., 2007 |
| Laksham, Bangladesh (male = 134, female = 135, children = 104) | Male: 2.62, Female: 2.37, Child: 1.23 | Khan et al., 2009 |
| Sirajdikhan, Bangladesh (male = 125, female = 139, children = 81) | Male: 3.34, Female: 2.67, Child: 1.42 | Khan et al., 2009 |
| Manikganj, Bangladesh (male = 127, female = 123, children = 55) | Male: 3.89, Female: 3.02, Child: 1.58 | Khan et al., 2009 |
| West Bengal, India | Adult male: 4, Adult female: 3 and children (age ≤11 years): 2 | Chowdhury et al., 2001 |
| Bhawangola-I, West Bengal (Male = 28, female = 23) | Male: 3.0 ± 0.9, Female: 2.7 ± 0.7 | Mondal et al., 2010 |
| Chakdah, West Bengal (Male = 52, female = 52) | Male: 3.0 ± 1.2, Female: 2.4 ± 1.0 | Mondal et al., 2010 |
| Khejuri-I, West Bengal (Male = 35, female = 33) | Male: 3.3 ± 0.5, Female: 2.8 ± 0.8 | Mondal et al., 2010 |
| West Bengal, India Total water intake (direct+indirect) | Adult male: 6.1; Adult female: 4.84; Child (age <15 years): 3.24 | Present study |
Direct water intake (mean ± SD).
Including indirect intake
Furthermore, this provisional guideline value and some national standards do not take into account the higher susceptibility of individuals to As poisoning due to poor nutrition (Mitra et al., 2004), genetic factors (Engström et al., 2007), smoking habits (Ferreccio et al., 2000), or lower body weight (average weight of adult male participants in this study = 51.9 kg and females = 45.4 kg). Considering only the negative health effects of chronic As exposure, the WHO has stated that their guideline should be significantly lower than the current value (WHO, 2001), and in fact based on health evidence Australia has lowered their national standard to 7 µg/L. The national standards of India and Bangladesh, however, remain at 50 µg/L. In an attempted compromise, Bureau of Indian Statistics (BIS) has recently published (BIS, 2009) revised drinking water standards, listing 10 µg/L for As, as the desirable level but 50 µg/L as the legally enforceable standard if alternative sources are unavailable.
Through the present yearlong study of water intake patterns, additional factors contributing to variations in individual water intake and consequently possible As exposure were elucidated. Significant differences in study participant direct water intake were found between males and females, children and adults, areas of residence, months, seasons, and occupational categories. Significantly different indirect water intake between study participants was also observed. An understanding of all of these factors can lead to the identification of subpopulations at greater risk of contaminant exposure and potential related health effects.
With the emerging evidence of the negative health effects due to chronic low-doses of As (Chen et al., 2009; McDonald et al., 2007) as well as the presence of additional routes of exposure (Pal et al., 2007), primarily food (Kile et al., 2007; Roychowdhury et al., 2002), we must be proactive in setting protective guideline values and standards to safeguard not only currently at-risk populations but future generations (Raqib et al., 2009; Smith et al., 2006) and those currently suffering. Once the signs and symptoms of arsenicosis emerge, there is little treatment that can be provided other than symptomatic care. As a result, safe drinking water is by far the most effective protective measure, and carefully implemented and enforced standards are vital if we are to slow the effects of this environmental disaster and prevent it and others from occurring in the future.
Supplementary Material
Acknowledgments
The authors are thankful to volunteers of this study and field workers of School of Environmental Studies (SOES). One of the authors (D. Chakraborti) is very grateful to Irene Suzukida Dooley, Environmental Scientist, Office of Groundwater and Drinking Water, USEPA for inspiring us to undertake this study and for reviewing the draft version of the manuscript.
Footnotes
Appendix A. Supplementary data
The supplementary data associated with this article is available.
References
- Abernathy C, Marcus W, Chen C, Gibb H, White P Report on Arsenic (As) Work Group Meetings. Office of Drinking Water, Office of Research and Development, U.S. Environmental Protection Agency. 1989. Memorandum to Cork, P., Preuss, P., Office of Regulatory Support and Scientific Management, U.S. Environmental Protection Agency. [Google Scholar]
- Ahsan H, Chen Y, Parvz F, Argos M, Hussain AZMI, Momotaj H, et al. Health effects of arsenic longitudinal study (HEALS): Description of a multidisciplinary epidemiologic investigation. J Exp Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- Binkowitz BS, Wartenberg D. Disparity in quantitative risk assessment: a review of input distributions. Risk Anal. 2001;21:75–90. doi: 10.1111/0272-4332.211091. [DOI] [PubMed] [Google Scholar]
- BIS. Draft Indian Standard. Drinking Water Specification (Second Revision of IS 10500) 2009 Doc: FAD 25(2047) C. Last Date for Comments: 24/12/2009. [Google Scholar]
- Chakraborti D, Das B, Rahman MM, Chowdhury UK, Biswas B, Goswami AB, et al. Status of groundwater arsenic contamination in the state of West Bengal, India: A 20-year study report. Mol Nutr Food Res. 2009;53:542–551. doi: 10.1002/mnfr.200700517. [DOI] [PubMed] [Google Scholar]
- Chakraborti D, Rahman MM, Das B, Murrill M, Dey S, Mukherjee SC, et al. Status of groundwater arsenic contamination in Bangladesh: A 14-year study report. Water Res. 2010;44:5789–5802. doi: 10.1016/j.watres.2010.06.051. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder, and Kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;66:888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, et al. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: Review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 2009;239:184–192. doi: 10.1016/j.taap.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury UK, Rahman MM, Mandal BK, Paul K, Lodh D, Biswas BK, et al. Groundwater arsenic contamination and human sufferings in West Bengal, India and Bangladesh. Environ Sci. 2001;83:393–415. [Google Scholar]
- Cochran WG, Cox GM. Experimental Designs. New York: John Wiley and Sons; 1975. [Google Scholar]
- Del Razo LM, Garcia-Vergas GG, Garcia-Salcedo J, Sanmiguel MF, Rivera M, Hernandez MC, et al. Arsenic level in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico. Food Chem Toxcol. 2002;40:1423–1431. doi: 10.1016/s0278-6915(02)00074-1. [DOI] [PubMed] [Google Scholar]
- Engström KS, Broberg K, Concha G, Nermell B, Warholm M, Vahter M. Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Perspect. 2007;115:599–605. doi: 10.1289/ehp.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershow AG, Brown LM, Cantor KP. Intake of tap water and total water by pregnant and lactating women. Am J Public Health. 1991;81:328–334. doi: 10.2105/ajph.81.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha A, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiol. 2000;11:673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Heller KE, Sohn W, Burt BA, Eklund SA. Water consumption in the United States in 1994–96 and implications for water fluoridation policy. J Publ Health Dent. 1999;59:3–11. doi: 10.1111/j.1752-7325.1999.tb03228.x. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Some drinking water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risk Hum. 2004;84:67–267. [PMC free article] [PubMed] [Google Scholar]
- Khan NI, Bruce D, Naidu R, Owens G. Implementation of food frequency questionnaire for the assessment of total dietary arsenic intake in Bangladesh: Part B: preliminary findings. Enviro. Geochem. Health. 2009;31:221–238. doi: 10.1007/s10653-008-9232-3. [DOI] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Breton CV, Quamruzzaman Q, Rahman M, Mahiuddin G, et al. Association between total ingested arsenic and biomarker response. J Environ Sci Health Part A. 2007;42:1827–1834. doi: 10.1080/10934520701566819. [DOI] [PubMed] [Google Scholar]
- Kristal-Boneh E, Glusman J, Shitrit R, Chaemovitz C, Cassuto Y. Physical performance and heat tolerance after chronic water loading and heat acclimation. Aviat Space Environ Med. 1995;66:733–738. [PubMed] [Google Scholar]
- Levallois P, Guevin N, Gingras S, Levesque B, Weber JP, Letarte R. New patterns of drinking-water consumption: results of a pilot study. Sci Total Environ. 1998;209:233–241. [PubMed] [Google Scholar]
- McDonald C, Hoque R, Huda N, Cherry N. Risk of arsenic-related skin lesions in Bangladhesi villages at relatively low exposure: a report from Gonoshasthaya Kendra. Bull World Health Organ. 2007;85:668–673. doi: 10.2471/BLT.06.036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Curhan GC, Willett WC, et al. Fluid intake and the risk of bladder cancer in men. N Engl J Med. 1999;340:1390–1397. doi: 10.1056/NEJM199905063401803. [DOI] [PubMed] [Google Scholar]
- Milton AH, Rahman H, Smith W, Shrestha R, Dear K. Water consumption patterns in rural Bangladesh: are we underestimating total arsenic load? J Water Health. 2006;4:431–436. [PubMed] [Google Scholar]
- Mitra SR, Mazumder DN, Basu A, Block G, Haque R, Samanta S, et al. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ Health Perspect. 2004;112:1104–1109. doi: 10.1289/ehp.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Banerjee M, Kundu M, Banerjee N, Bhattacharya U, Giri AK, et al. Comparison of drinking water, raw rice and cooking of rice as arsenic exposure routes in three contrasting areas of West Bengal, India. Environ. Geochem. Health. 2010;32:463–477. doi: 10.1007/s10653-010-9319-5. [DOI] [PubMed] [Google Scholar]
- NHRMC (National Health and Medical Research Council) Australian drinking water guidelines. Chapter 6. Australian Government; 2004. [Google Scholar]
- NRC (National Research Council) Arsenic in drinking water: 2001 update. Washington. DC: National Research Council; 2001. [PubMed] [Google Scholar]
- Ohno K, Yanase T, Matsuo Y, Kimura T, Rahman MH, Magura Y, et al. Arsenic intake via water and food by a population living in an arsenic-affected area of Bangladesh. Sci Total Environ. 2007;381:68–76. doi: 10.1016/j.scitotenv.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Pal A, Nayak B, Das B, Hossain A, Ahamed S, Chakraborti D. Additional danger of arsenic exposure through inhalation of cow dung cakes laced with arsenic as a fuel in arsenic affected villages in Ganga-Meghna-Brahmaputra Plain. J Environ Monit. 2007;9:1067–1070. doi: 10.1039/b709339j. [DOI] [PubMed] [Google Scholar]
- Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AMW, et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol Lett. 2009;185:197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Roychowdhury T, Uchino T, Tokunaga H, Ando M. Survey of arsenic in food composites from an arsenic-affected area of West Bengal, India. Food Chem Toxicol. 2002;40:1611–1621. doi: 10.1016/s0278-6915(02)00104-7. [DOI] [PubMed] [Google Scholar]
- Ryan PB, Huet N, MacIntosh DL. Longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environ Health Perspect. 2000;108:731–735. doi: 10.1289/ehp.00108731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichert-Hellert W, Kersting M, Manz F. Fifteen year trends in water intake in German children and adolescents: results of the DONALD Study. Acta Pædiatr. 2001;90:732–737. [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W, Heller KE, Burt BA. Fluid consumption related to climate among children in the United States. J Publ Health Dent. 2001;61:99–106. doi: 10.1111/j.1752-7325.2001.tb03373.x. [DOI] [PubMed] [Google Scholar]
- USEPA (U.S. Environmental Protection Agency) Estimated per capita water ingestion in the United States - an update based on data collected by the U.S. Department of Agriculture’s 1994–1997 and 1998 Continuing Survey of Food Intakes by Individuals. Washington, DC: U.S. Environmental Protection Agency; 2004. EPA/822/R-00/001. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency) Special report on ingested inorganic arsenic: skin cancer; nutritional essentiality. Washington, DC: U.S. Environmental Protection Agency; 1988. EPA/625/3-87/013. [Google Scholar]
- Watanabe C, Kawata A, Sudo N, Sekiyama M, Inaoka T, Bae M, et al. Water intake in an Asian population living in arsenic-contaminated area. Toxicol Appl Pharmacol. 2004;198:272–282. doi: 10.1016/j.taap.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Westrell T, Andersson Y, Stenstrom TA. Drinking water consumption patterns in Sweden. J Water Health. 2006;4:511–522. [PubMed] [Google Scholar]
- WHO (World Health Organization) World Health Organization Fact Sheet No. 210. Geneva: World Health Organization; 2001. [Revised May 2001]. Arsenic in Drinking Water; pp. 1–6. [Google Scholar]
- WHO (World Health Organization) Guidelines for drinking-water quality. 4th ed. Geneva: World Health Organization; 2011. pp. 164–168. [Google Scholar]
- Zohouri FV, Rugg-Gunn AJ, Fletcher ES, Hackett AF, Moynihan PJ, Mathers JC, et al. Changes in water intake of Northumbrian adolescents 1980 to 2000. Br Dent J. 2004;196:547–552. doi: 10.1038/sj.bdj.4811226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.