Abstract
Background
Poor response to erythropoiesis stimulating agents (ESA) is associated with morbidity and mortality among dialysis patients. It is unclear whether the risk associated with poor ESA response during dialysis extends beyond kidney transplantation. We examined pretransplant ESA response and its effect on allograft failure and mortality.
Methods
The cohort included all adult Medicare recipients from the United States Renal Data System who had received a kidney transplant during years 2000–2007 and had at least 6 months of hemodialysis immediately prior to transplant. ESA hyporesponsiveness was primarily defined as a monthly ESA dose ≥75,000 units and hematocrit ≤33% for at least 3 consecutive months in the pretransplant period. Crude and adjusted Cox-proportional hazards models and Kaplan-Meier methods were used to estimate the effect of ESA hyporesponsiveness on allograft failure and all-cause mortality.
Results
The study group consisted of 36,450 patients; 1,004 exhibited hyporesponsiveness. The adjusted hazard ratios for allograft failure and mortality post-transplant were 1.23 (95%CI 1.10, 1.42) and 1.61 (1.43, 1.81), respectively, supporting that poor ESA response during hemodialysis is associated with adverse post-transplant outcomes.
Conclusions
ESA hyporesponsiveness may be useful in identifying potential allograft recipients who are at high-risk for subsequent morbidity and mortality, and may benefit from more intensive pre- and post-transplant follow-up.
Keywords: Allograft failure, allograft loss, end-stage kidney disease, ESA, mortality
Introduction
Kidney transplantation is the preferred form of renal replacement therapy for individuals with end-stage kidney disease (ESKD). While overall allograft survival has improved over the past two decades (1, 2), early allograft loss remains an important cause of morbidity and mortality (3, 4). Previous studies assessing risk factors for allograft loss have identified donor specific variables (5–8) and recipient-dependent characteristics that are either not modifiable (9–11) or only apparent just prior to transplantation (12, 13). With an average waiting time for deceased donor kidney transplant ranging from 3–5 years (14), the pretransplant dialysis period provides a substantial exposure interval to examine the effect of dialysis specific interventions on post-transplant outcomes. Dialysis associated medications represent an understudied area with regard to post-transplant outcomes.
Erythropoiesis stimulating agents (ESA) are commonly used to treat anemia in patients receiving dialysis (15). In the United States, the average weekly ESA dose ranges between 17,000–18,000 units (1). Their use has likely benefited allograft survival by reducing the need for blood transfusions (1), and consequently reducing pretransplant sensitization (16). Enthusiasm for ESA use has been tempered by studies reporting increased risk associated with high target hemoglobin levels (17–20) and administration of large doses of ESA (21). Subsequent analyses of these studies have shown that diminished responsiveness to ESA most strongly predicts morbidity and mortality in chronic kidney disease and dialysis patients (22–24). While the leading explanation for these observations has been that ESA hyporesponsiveness is an indicator of underlying inflammation in the dialysis population it is not well understood if the effect persists post-transplant.
Chronic inflammation has been known to increase the risk of adverse outcomes in the transplant population (25). Assessment of ESA response may therefore identify high-risk future transplant recipients.
A previous study demonstrated an increased risk of graft failure associated with ESA hyporesponsiveness (26). While the findings were compelling, the study cohort ran from 1995–2002, and may not be reflective of contemporary patients undergoing transplantation; furthermore, the study used a fixed definition of ESA hyporesponsiveness. To take this observation further we examined the effect of diminished responsiveness to ESA in the pretransplant period on post-transplant morbidity and mortality in a contemporary cohort of transplant recipients, using varying definitions of ESA hyporesponsiveness. Specifically, we hypothesized that ESA hyporesponsiveness during the dialysis period may predict allograft failure after transplantation. Furthermore, we postulated that there would be a graded dose-response impact on post-transplant outcomes within the group of individuals hyporesponsive to ESA. We tested these hypotheses in a large retrospective cohort of transplant recipients who were receiving hemodialysis immediately prior to transplant.
Results
Baseline cohort characteristics
There were 36,450 participants that met inclusion criteria, the majority were White, male, and had a deceased donor type. There was a high degree of HLA (human leukocyte antigen) mismatch and a low PRA (panel reactive antibody) in the study population. Of the included individuals there were 31,632 receiving at least 1 unit of ESA and with monthly hematocrit values available for bivariate analysis. Using the ESA dose limit of ≥75,000 units we identified 1,004 hyporesponsive individuals that remained hyporesponsive for 3 months prior to transplant. Hyporesponsive individuals were more often of younger age, African-American, had longer hemodialysis vintage, and were more likely to have a hemodialysis catheter (Table 1). Similar results were seen for individuals that were hyporesponsive for 6 months using the same ESA dose criteria (n=363). The mean ESA monthly dose for individuals with a hematocrit of <=33% group was higher when compared to those with a hematocrit of >33% (see digital content Figure 1).
Table 1.
Baseline cohort characteristics & distribution for ESA hyporesponsiveness among covariates
| N (%), Mean± SD, or Median (IQR) * | p-value | |||
|---|---|---|---|---|
| All N=36450 |
ESA Hyporesponsive † | |||
| Yes (n=1004) | No (n=30628) | |||
| Male gender | 22415 (62) | 603 (60) | 18639 (61) | 0.62 |
| White | 20672 (57) | 500 (50) | 17387 (58) | <0.01 |
| African-American | 13041 (36) | 438 (44) | 10884 (36) | <0.01 |
| Asian | 1821 (5) | 42 (4) | 1620 (5) | 0.12 |
| Other ‡ | 916 (2) | 24 (2) | 737 (2) | 0.19 |
| Hispanic | 5963 (17) | 127 (13) | 5037 (16) | <0.01 |
| Deceased donor type | 29094 (80) | 800 (80) | 24497 (80) | 0.92 |
| Diabetic | 15269 (42) | 364 (36) | 12885 (42) | <0.01 |
| Hemodialysis catheter | 4388 (12) | 197 (20) | 3558 (12) | <0.01 |
| HLA match (0–6) | 2.0 ± 1.6 | 2.0 ± 1.6 | 2.0 ± 1.6 | 0.92 |
| PRA (%) | 6.6 ± 18.8 | 7.6 ± 20.1 | 6.6 ± 18.8 | 0.13 |
| Age (18–87 years) § | 46.8 ± 13.8 | 44.5 ± 13.3 | 47.0 ± 13.8 | <0.01 |
| Iron dose (mg/month) | 166.7 (281.3-33.3) | 166.7 (291.7-10.2) | 166.7 (281.3-33.3) | 0.88 |
| Weight (40–175 kg) § | 76.4 (90.9-65.0) | 79.0 (95.0-67.0) | 76.0 (90.0-65.0) | <0.01 |
| Hemodialysis vintage (0.5–33 years) | 3.6 (5.3-2.2) | 4.0 (5.7-2.6) | 3.6 (5.3-2.2) | <0.01 |
Values reported as %, means ± standard deviations, or median (interquartile range) as appropriate.
ESA hyporesponsiveness was defined as hematocrit ≤33% and erythropoiesis stimulating agent dose ≥75K units/month for 3 consecutive months. 31,632 patients had received at least 1 unit of ESA and had documented monthly hematocrit values.
Includes Native-Americans and unknown race.
HLA: human leukocyte antigen PRA: panel reactive antibody.
Age and weight at the start of hemodialysis.
The mean ESA doses for each month prior to transplant increased consistently from month 6 to month 1 pretransplant in the <33% hematocrit group, whereas opposite results where seen in the >33% hematocrit group (see supplemental digital content Table 1). The most common cause of ESKD was diabetes mellitus (34%), followed by hypertension (24%), and glomerulonephritis (9%).
Allograft failure & loss
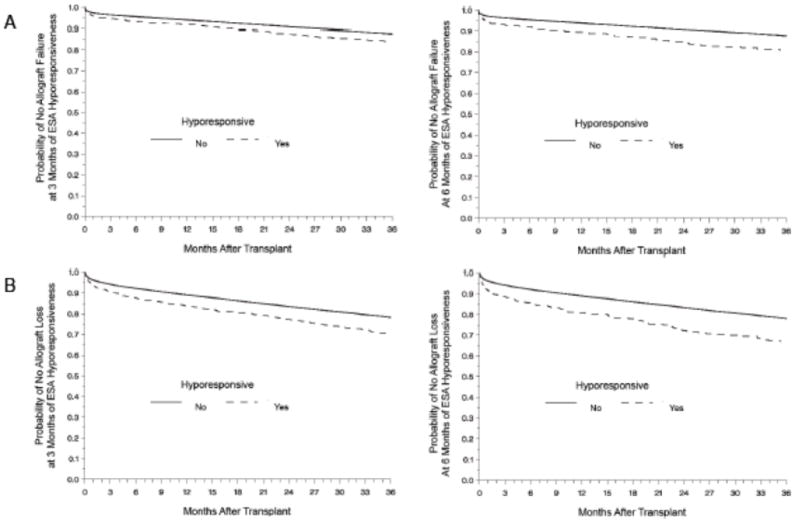
Allograft failure occurred in 17% of the study population (n=6,070). When including participant’s death as a cause of allograft failure (allograft loss) the event occurrence increased to 32% (n=11,666). Crude and adjusted Cox models showed the ESA hyporesponsive group was more likely to experience allograft failure and loss when compared to those who did not meet criteria for hyporesponsiveness regardless of the duration of hyporesponsiveness (Table 2). Kaplan-Meier methods showed similar results (Figure 1). The results were not affected when censoring at 3 years post-transplant or when including all available follow-up time.
Table 2.
Crude and adjusted hazard ratios for post-transplant outcomes according to the duration of ESA hyporesponsiveness
| ESA Hyporesponsiveness * | Allograft Failure | Allograft Loss | All-Cause Mortality | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Crude HR † (95%CI) | Adjusted ‡ HR † (95%CI) | Crude HR † (95%CI) | Adjusted ‡ HR † (95%CI) | Crude HR † (95%CI) | Adjusted ‡ HR † (95% CI) | |
| 3-months | 1.39 (1.21, 1.59) | 1.23 (1.10, 1.42) | 1.44 (1.31, 1.58) | 1.39 (1.25, 1.54) | 1.52 (1.36, 1.69) | 1.61 (1.43, 1.81) |
| 6-months | 1.65 (1.35, 2.03) | 1.43 (1.15, 1.78) | 1.60 (1.38, 1.86) | 1.59 (1.36, 1.87) | 1.61 (1.35, 1.91) | 1.85 (1.55, 2.23) |
Allograft failure, present if: a second transplant, or return to dialysis. Allograft loss, present if: a second transplant, or return to dialysis, or death with a functioning graft.
ESA hyporesponsiveness defined as hematocrit ≤33% and erythropoiesis stimulating agent dose ≥75K units/month.
Hazard ratios derived from Cox proportional hazards models comparing ESA hyporesponsiveness to appropriate ESA response. All hazard ratios were statistically significant with p-value <0.01.
Adjusted for age, gender, weight, race/ethnicity, hemodialysis vintage, hemodialysis catheter, diabetes status, and donor type.
Figure 1.
Kaplan-Meier estimates of allograft survival according to ESA response, for allograft failure (present if: a second transplant, or return to dialysis, shown in A) and allograft loss (present if: a second transplant, or return to dialysis, or death with a functioning graft shown in B) at 3 (left) and 6 (right) months of ESA hyporesponsiveness. The probability of allograft loss and failure was higher in the ESA hyporesponsive group (Log-Rank p <0.01).
Mortality
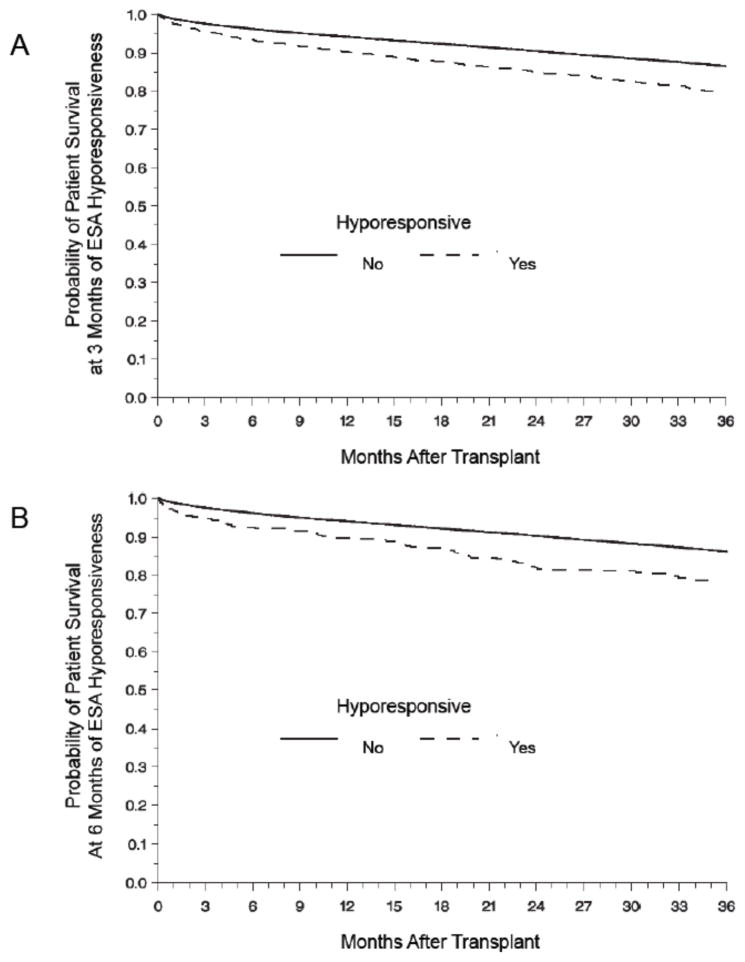
All-cause mortality was 21% (n=7,679) for the entire study population. The most common causes of death were cardiovascular events (39%), infectious complications (21%) and malignancies (8%). ESA hyporesponsiveness was associated with increased all-cause mortality regardless of duration of hyporesponsiveness (Table 2). The probability of survival was lower in the hyporesponsive group (Figure 2).
Figure 2.
Kaplan-Meier estimates of patient survival according to ESA response comparing 3 months (A) to 6 months (B) of persistent ESA hyporesponsiveness. The probability of survival was lower in the ESA hyporesponsive group for both 3 and 6 months periods (Log-Rank p <0.01).
Analyses using alternate ESA hyporesponsiveness definitions
Our results were sensitive to changing ESA minimum monthly dose parameters for the definition of ESA hyporesponsiveness. In both adjusted and unadjusted analyses the hazard ratios for allograft failure, loss and all-cause mortality increased consistently as the minimum monthly ESA dose given increased with one exception. In case of marked hyporesponsiveness for 3 months (ESA ≥200,000) and allograft failure the effect estimate decreased and was not statistically significant (Table 3). A separate analysis using lower dose ESA (<75,000), showed no meaningful increase in risk in those with a hematocrit response of <33%, but increasing estimated risk as the ESA dose increased with the same exception noted above (see supplemental digital content Table 2).
Table 3.
Crude and adjusted analysis for allograft loss & failure and all-cause mortality using alternate ESA dose parameters for the duration of hyporesponsiveness
| ESA Hyporesponsiveness * | Allograft Failure | Allograft Loss | All-Cause Mortality | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Crude HR † (95%CI) | Adjusted ‡ HR † (95%CI) | Crude HR † (95%CI) | Adjusted ‡ HR † (95%CI) | Crude HR † (95%CI) | Adjusted ‡ HR † (95% CI) | ||
| 3-months | ≥75K (n=1004) | 1.39 (1.21, 1.59) | 1.23 (1.10, 1.42) | 1.44 (1.31, 1.58) | 1.39 (1.25, 1.54) | 1.52 (1.36, 1.69) | 1.61 (1.43, 1.81) |
| ≥100K (n=751) | 1.44 (1.24, 1.68) | 1.26 (1.07, 1.48) | 1.46 (1.31, 1.63) | 1.40 (1.24, 1.58) | 1.55 (1.37, 1.76) | 1.64 (1.43, 1.87) | |
| ≥200K (n=233) | 1.49 (1.12, 1.98) | 1.12 (0.81, 1.54)¶1 | 1.64 (1.35, 1.99) | 1.49 (1.20, 1.85) | 1.79 (1.44, 2.23) | 1.92 (1.51, 2.45) | |
| 6-months | ≥75K (n=363) | 1.65 (1.35, 2.03) | 1.43 (1.15, 1.78) | 1.60 (1.38, 1.86) | 1.59 (1.36, 1.87) | 1.61 (1.35, 1.91) | 1.85 (1.55, 2.23) |
| ≥100K (n=293) | 1.57 (1.24, 1.99) | 1.32 (1.03, 1.70) ¶2 | 1.58 (1.33, 1.87) | 1.53 (1.27, 1.84) | 1.64 (1.36, 1.98) | 1.89 (1.53, 2.30) | |
| ≥200K (n=107) | 2.05 (1.43, 2.93) | 1.51 (1.02, 2.24) ¶3 | 1.74 (1.32, 2.31) | 1.67 (1.22, 2.26) | 1.73 (1.25, 2.37) | 2.07 (1.46, 2.95) | |
Allograft failure, present if: a second transplant, or return to dialysis. Allograft loss, present if: a second transplant, or return to dialysis, or death with a functioning graft.
ESA hyporesponsiveness defined as hematocrit ≤33% in combination with various erythropoiesis stimulating agent doses (units/month) as listed.
Hazard ratios derived from Cox proportional hazards models comparing ESA hyporesponsiveness to appropriate ESA response. All hazard ratios were statistically significant with p-value ≤0.01
with exceptions noted p-value 0.5 (1), 0.03 (2,3), respectively.
Adjusted for age, gender, weight, race/ethnicity, hemodialysis vintage, hemodialysis catheter, diabetes status, and donor type.
Discussion
In this cohort of individuals receiving kidney transplantation, we observed that diminished ESA response during the hemodialysis period was associated with allograft failure, loss, and death after a kidney transplant. The presence of low hemoglobin and high ESA dose during the 3 to 6 months immediately prior to transplantation increased the risk of allograft loss and failure by at least 39% in crude analyses; adjustment for demographic and medical factors did not meaningfully attenuate the point estimate. Kaplan-Meier curves demonstrated that the effect appeared relatively soon post-transplant and the trend continued over the entire observation period. Sensitivity analysis using more restrictive definitions of the exposure suggested a dose-response effect. The hazards ratio increased in size as the definition of hyporesponsiveness included a larger dose of ESA.
To our knowledge, this is the first study to demonstrate a dose-related effect of pretransplant ESA hyporesponsiveness on post-transplant survival. Our findings are consistent with the growing body of literature demonstrating that persistent treatment-resistant anemia may be associated with poor outcomes in patients with kidney disease (26). We did not attempt to address the mechanism behind this association, though it is possible that mechanisms postulated for studies in chronic kidney disease might also apply to our results in patients receiving a kidney transplant. Diminished response to ESA is likely to be a marker of underlying inflammation. Inflammation has been observed to predict morbidity and mortality in the general (27–30), chronic kidney disease (31), dialysis (32–35), and transplant populations (25, 36). While inflammation has been routinely assessed by abnormal laboratory parameters such as elevated C-reactive protein & ferritin or low albumin, there is a growing consensus that the clinical scenario of low hemoglobin and high ESA dose also reflects a state of inflammation. Patient comorbidities thought to contribute to inflammation include occult infections (37–41), dialysis catheter use (42), poor dialysate water quality (43–45), uremia (46), intravenous iron use (47–54), and hepicidin-mediated disorders of iron homeostasis and hematopoiesis (55–57). However, many of these sources of inflammation, such as uremia, infections and catheter use, would be absent or less common in the transplant population.
ESA hyporesponsive patients in this study and other studies have considerably higher cumulative exposure to ESA. Direct adverse effects of high doses of ESA cannot be excluded as a potential factor for the resulting adverse outcomes. Experimental models and clinical studies have shown that ESA induce physiological changes in two key areas; the coagulation cascade and the blood pressure. Among hemodialysis patients, ESA promotes activation of the coagulation cascade at a dose as small as 2000 units three times weekly (58). Additionally, cultured human endothelial cells exposed to recombinant erythropoietin showed a dose dependent increase in expression of tissue factor, an important mediator of the coagulation cascade (59). Dialysis patients with hemoglobin > 13 and exposed to ESA demonstrate thrombocytosis (60). ESA administration increases platelet reactivity, thrombopoiesis, and endothelial activation (61). ESA also increase systemic arterial blood pressure, most likely from increased blood volume and impaired blood nitric oxide production (62), though only one of the randomized studies of ESA administration showed a significant increased risk of hypertension (63). Taken in sum, ESA use may lead to a physiologic environment that would promote adverse cardiovascular events. However, it is unclear whether these effects would linger into the post-transplant period. A 1-year follow-up study of the Normal Hematocrit Study found that risks between the two treatment groups were identical after the discontinuation of the study intervention (19).
The findings of this study should be interpreted in the context of the following limitations. While basic demographic and medical variables at baseline suggested that the two groups were roughly equivalent, information was not available on many potentially important time-dependent variables such as serum albumin and serum C-reactive protein. The lack of measures on such variables clouds our ability to determine what the underlying cause of the poor treatment response may be. Additionally our study was not designed to examine a direct effect from ESA, thus we cannot determine whether the association that we have observed might be partially or completely attributable to ESA exposure. We also did not have information on ESA use after the transplant occurred and cannot exclude the possibility that ESA use during this period could have impacted the observed effects. Recent studies, documenting ESA use in the post transplantation period have demonstrated varying effects (64–66). One study in the immediate post-transplant period did not demonstrate an association of ESA use with transplant (kidney) function (64). A second study showed that treatment with ESA was associated with mortality throughout the post-transplant period (65). Another potential confounding factor is the unknown effect of blood transfusions; data on transfusion rates among those who were ESA resistant was not reliably available and was not included in any analysis.
The findings of the study should not be interpreted as a reason to avoid or delay kidney transplantation among patients found to be hyporesponsive to ESA. It is well established that among patients with end-stage renal disease, transplantation remains the best option for long-term survival; even among individuals with diminished responsiveness to ESA, the benefits of transplantation likely outweigh the risks of continued hemodialysis. Rather, such patients would benefit from close surveillance during the post-transplant period with the goal of reducing their risk of adverse events.
Our study had several important strengths that should be noted. The assembled cohort, representative of the transplant population in the United States (US), allows for high generalizability of the results among US transplant and dialysis centers. Additionally, the sample size afforded the opportunity to vary the definition of the exposure for multiple analyses; the findings of which were highly consistent across the board. Lastly the study potentially opens up novel areas for investigation—pretransplant medications as potentially modifiable predictors of transplant outcomes. Strategies to identify individuals in the pretransplant period who are at high risk for allograft loss and death could prevent significant morbidity after kidney transplantation. Indirectly such strategies could help to reduce the problematic shortage of organs.
In summary we conclude that ESA response appears to be a strong predictor of poor post-transplant outcomes. Additional confirmatory studies are necessary. In the meantime the finding of low ESA response may help identify patients for greater surveillance and to optimize therapy during the pre and post-transplant period.
Methods
Study population
Participants were ≥18 years of age recipients of a first kidney transplant between January 1st 2000 and December 31st 2007 included in the United States Renal Data System (USRDS). This database provides baseline demographic information and follow-up data for all Medicare patients with ESKD receiving dialysis or a kidney transplant. The included participants were required to have at least 6 months of in-center hemodialysis immediately prior to the transplant date and to have a minimum of 12 months follow-up after transplant. Participants had Medicare as a primary payer source in order to obtain the information on ESA dosing from monthly billing claims. All participants had a monthly billing claim for ESA administration for 6 months prior to transplant. Recipients of combined organs were excluded. Follow-up data was available through the end of 2008. The study was reviewed and approved by the University of North Carolina Institutional Review Board.
Exposure definition
ESA hyporesponsiveness was defined as a total ESA dose ranging from ≥75,000 to ≥200,000 units per month and a preceding corresponding hematocrit value of ≤33% (approximately equivalent to a hemoglobin of 11 g/dl) for 3 or 6 consecutive months immediately prior to the transplant date. The ESA total dose criteria was varied to examine the potential for a dose-related response and to ensure the results were consistent. Additionally, since ESA dosing is based on weight, and we were expecting a wide range of weight measurements in this population, this precaution decreased the potential of misclassifying insufficient ESA doses, particularly given that participant’s weight was only available at the time of dialysis start as recorded in the End Stage Renal Disease Medical Evidence Report (also known as CMS-2728 form). To simplify reporting, results are presented using the ESA hyporesponsiveness definition dose of ≥75,000 units and a hematocrit of ≤33% (for either 3 or 6 months) as the main exposure and result differences for analyses using variable ESA dosing criteria will be mentioned when appropriate.
Outcome ascertainment
The primary outcome was allograft failure, defined as one of the following: 1) evidence of a second transplant, 2) return to dialysis or 3) transplant nephrectomy at any time after transplant. Secondary outcomes were 1) allograft loss, defined as death with a functioning graft in addition to all causes of allograft failure, and 2) mortality defined as patient death from any cause after transplant.
Covariates
Age, gender, race and ethnicity were obtained from the CMS-2728 form. Hemodialysis vintage was derived using the date of start of dialysis and the date of the first transplant. Hemodialysis catheter information was examined during the 6 months immediately prior to transplant. Medicare billing claims for hemodialysis catheter placement (Current Procedural Terminology codes 36558, 36565, 36575, 36581, 36489, & 36491) in combination with access type listed at the time of initiation of hemodialysis were used to ascertain hemodialysis catheter status. Diabetes status was obtained from co-morbidity information listed both at the initiation of hemodialysis and at the time of the first transplant. Donor type was defined as either living or deceased, as listed at the time of transplant and reported to USRDS by the United Network for Organ Sharing.
Statistical analysis
Participant characteristics are presented for the full cohort and according to ESA response status using proportions and frequencies for categorical variables, and means with standard deviations for continuous variables. Medians with interquartile range are reported for skewed distributions. Independent group t-tests, Wilcoxon-Mann-Whitney, and chi-square tests were used for bivariate comparisons as appropriate. Kaplan-Meier methods were used to present the probability of allograft failure, loss and all-cause mortality in a time to event analysis. Log-rank tests were used to compare survivor functions. Cox proportional hazards models were used in crude and adjusted analysis to test for covariate effects. Final models for the analyses planned were determined using a backwards-stepwise modeling strategy. The models were adjusted for recipient age, gender, weight, race/ethnicity, hemodialysis duration, hemodialysis catheter, diabetic status, and donor type. Proportionality was tested with Wald and partial Likelihood Ratio tests in a series of models using time-varying variables. The ESA minimum total monthly dose parameter for the hyporesponsiveness definition was adjusted from values ranging between 75,000 – 200,000 units for multiple analyses. For each analysis participants were censored at the time the outcome was present or at 3 years after transplant whichever occurred earlier. Additionally a separate analysis was conducted including all available follow-up time. All analyses were performed using SAS statistical software (SAS Institute Inc., SAS® 9.2, Cary, NC, USA).
Supplementary Material
Acknowledgments
Dr. Costa was supported by a training grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases while conducting this study. Dr. Brookhart was supported by a career development award from the National Institute on Aging. Drs. Costa and Brookhart have full access to all the data used for this study and take responsibility for the integrity and accuracy of the data analysis. The funding source had no active role in the study design, conduct, and reporting.
Dr. Brookhart has received research support from Amgen and has sat on advisory boards for Amgen and Pfizer (honoraria declined or paid directly to institution). Part of the data from this study have been presented at the American Society of Nephrology Kidney Week 2011 in Philadelphia, Pennsylvania. Results from this study were presented at the American Transplant Congress 2012 in Boston, Massachusetts. No part of the work described in this manuscript has been previously published. The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Abbreviations
- ESA
erythropoiesis stimulating agents
- ESKD
end stage kidney disease
- HLA
human leukocyte antigen
- PRA
panel reactive antibody
- USRDS
United States Renal Data System
Footnotes
Nadiesda A. Costa: participated in research design, performance of the research, data analysis, and writing of the paper.
Abhijit V. Kshirsagar: participated in research design, performance of the research, and writing of the paper.
Lily Wang: participated in research design, performance of the research, and data analysis
Randal K. Detwiler: participated in research design, and writing of the paper.
M. Alan Brookhart: participated in research design, performance of the research, data analysis, and writing of the paper
There are no conflicts of interest to report for Drs. Kshirsagar, Wang and Detwiler.
References
- 1.USRDS. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System, National Institutes of Health, National Institute of Diabetes and Digestive Diseases; Bethesda, MD: 2010. [Google Scholar]
- 2.United Network for Organ Sharing. United States Department of Health and Human Services, Organ Procurement and Transplantation Network data as of march 16. 2011 available at http://optn.transplant.hrsa.gov.
- 3.Woo YM, Jardine AG, Clark AF, et al. Early graft function and patient survival following cadaveric renal transplantation. Kidney Int. 1999;55 (2):692. doi: 10.1046/j.1523-1755.1999.00294.x. [DOI] [PubMed] [Google Scholar]
- 4.Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2008. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD.: 2009. [Google Scholar]
- 5.Port FK, Dykstra DM, Merion RM, Wolfe RA. Trends and results for organ donation and transplantation in the United States, 2004. Am J Transplant. 2005;5 (4 Pt 2):843. doi: 10.1111/j.1600-6135.2005.00831.x. [DOI] [PubMed] [Google Scholar]
- 6.Sellers MT, Velidedeoglu E, Bloom RD, et al. Expanded-criteria donor kidneys: a single-center clinical and short-term financial analysis--cause for concern in retransplantation. Transplantation. 2004;78 (11):1670. doi: 10.1097/01.tp.0000144330.84573.66. [DOI] [PubMed] [Google Scholar]
- 7.Woo YM, Gill JS, Johnson N, Pereira BJ, Hariharan S. The advanced age deceased kidney donor: current outcomes and future opportunities. Kidney Int. 2005;67 (6):2407. doi: 10.1111/j.1523-1755.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 8.Schold JD, Kaplan B, Baliga RS, Meier-Kriesche HU. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5 (4 Pt 1):757. doi: 10.1111/j.1600-6143.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Evans I, Joseph R, et al. Comorbid conditions in kidney transplantation: association with graft and patient survival. J Am Soc Nephrol. 2005;16 (11):3437. doi: 10.1681/ASN.2005040439. [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Pozzoni P, Del Vecchio L. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol. 2004;15(Suppl 1):S25. doi: 10.1097/01.asn.0000093239.32602.04. (Journal Article) [DOI] [PubMed] [Google Scholar]
- 11.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65 (2):597. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 12.Hill GS, Nochy D, Bruneval P, et al. Donor-Specific Antibodies Accelerate Arteriosclerosis after Kidney Transplantation. J Am Soc Nephrol. 2011 doi: 10.1681/ASN.2010070777. (Journal Article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4 (3):438. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 14.Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008;68 (Suppl 1):3. doi: 10.2165/00003495-200868001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kinney R. Centers for Medicare and Medicaid Services;2005 Annual Report: ESRD Clinical Performance Measures Project. Am J Kidney Dis. 2006;48 (4 Suppl 2):S1. doi: 10.1053/j.ajkd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Sautner T, Gnant M, Banhegyi C, et al. Risk factors for development of panel reactive antibodies and their impact on kidney transplantation outcome. Transpl Int. 1992;5 (Suppl 1):S116. doi: 10.1007/978-3-642-77423-2_38. [DOI] [PubMed] [Google Scholar]
- 17.Singh AK, Szczech L, Tang KL, et al. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N Engl J Med. 2006;355 (20):2085. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Burdmann EA, Chen CY, et al. Baseline characteristics in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT) Am J Kidney Dis. 2009;54 (1):59. doi: 10.1053/j.ajkd.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Besarab A, Bolton WK, Browne JK, et al. The Effects of Normal as Compared with Low Hematocrit Values in Patients with Cardiac Disease Who Are Receiving Hemodialysis and Epoetin. N Engl J Med. 1998;339 (9):584. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 20.Brookhart MA, Schneeweiss S, Avorn J, Bradbury BD, Liu J, Winkelmayer WC. Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA. 2010;303 (9):857. doi: 10.1001/jama.2010.206. [DOI] [PubMed] [Google Scholar]
- 21.Cotter DJ, Thamer M, Zhang Y. Relative mortality and epoetin alpha dose in hemodialysis patients. Am J Kidney Dis. 2008;51 (5):865. doi: 10.1053/j.ajkd.2007.12.045. author reply 865. [DOI] [PubMed] [Google Scholar]
- 22.Szczech LA. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney international. 2008;74 (6):791. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363 (12):1146. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 24.Kilpatrick RD, Critchlow CW, Fishbane S, et al. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3 (4):1077. doi: 10.2215/CJN.04601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abedini S, Holme I, Marz W, et al. Inflammation in renal transplantation. Clin J Am Soc Nephrol. 2009;4 (7):1246. doi: 10.2215/CJN.00930209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campise M, Mikhail A, Quaschning T, Snyder J, Collins A. Impact of pre-transplant anaemia correction and erythropoietin resistance on long-term graft survival. Nephrol Dial Transplant. 2005;20(Suppl 8):viii8. doi: 10.1093/ndt/gfh1110. [DOI] [PubMed] [Google Scholar]
- 27.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350 (14):1387. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R, Sr, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1 (2):92. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375 (9709):132. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151 (7):483. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K. Inflammatory marker mania in chronic kidney disease: pentraxins at the crossroad of universal soldiers of inflammation. Clin J Am Soc Nephrol. 2007;2 (5):872. doi: 10.2215/CJN.02750707. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291 (4):451. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 33.Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12 (7):1549. doi: 10.1681/ASN.V1271549. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann J. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55 (2):648. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 35.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35 (3):469. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 36.Van Ree RM, Gross S, Zelle DM, et al. Influence of C-reactive protein and urinary protein excretion on prediction of graft failure and mortality by serum albumin in renal transplant recipients. Transplantation. 2010;89 (10):1247. doi: 10.1097/TP.0b013e3181d720e3. [DOI] [PubMed] [Google Scholar]
- 37.Rahmati MA, Craig RG, Homel P, Kaysen GA, Levin NW. Serum markers of periodontal disease status and inflammation in hemodialysis patients. Am J Kidney Dis. 2002;40 (5):983. doi: 10.1053/ajkd.2002.36330. [DOI] [PubMed] [Google Scholar]
- 38.Chen LP, Chiang CK, Chan CP, Hung KY, Huang CS. Does periodontitis reflect inflammation and malnutrition status in hemodialysis patients? Am J Kidney Dis. 2006;47 (5):815. doi: 10.1053/j.ajkd.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Kshirsagar AV, Craig RG, Beck JD, et al. Severe periodontitis is associated with low serum albumin among patients on maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2007;2 (2):239. doi: 10.2215/CJN.02420706. [DOI] [PubMed] [Google Scholar]
- 40.Ayus JC, Sheikh-Hamad D. Silent infection in clotted hemodialysis access grafts. J Am Soc Nephrol. 1998;9 (7):1314. doi: 10.1681/ASN.V971314. [DOI] [PubMed] [Google Scholar]
- 41.Roberts TL, Obrador GT, St Peter WL, Pereira BJ, Collins AJ. Relationship among catheter insertions, vascular access infections, and anemia management in hemodialysis patients. Kidney Int. 2004;66 (6):2429. doi: 10.1111/j.1523-1755.2004.66020.x. [DOI] [PubMed] [Google Scholar]
- 42.Cappelli G, Tetta C, Canaud B. Is biofilm a cause of silent chronic inflammation in haemodialysis patients? A fascinating working hypothesis. Nephrol Dial Transplant. 2005;20 (2):266. doi: 10.1093/ndt/gfh571. [DOI] [PubMed] [Google Scholar]
- 43.Schiffl H, Lang SM, Stratakis D, Fischer R. Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrol Dial Transplant. 2001;16 (9):1863. doi: 10.1093/ndt/16.9.1863. [DOI] [PubMed] [Google Scholar]
- 44.Arizono K, Nomura K, Motoyama T, et al. Use of ultrapure dialysate in reduction of chronic inflammation during hemodialysis. Blood Purif. 2004;22 (Suppl 2):26. doi: 10.1159/000081870. [DOI] [PubMed] [Google Scholar]
- 45.Rahmati MA, Homel P, Hoenich NA, Levin R, Kaysen GA, Levin NW. The role of improved water quality on inflammatory markers in patients undergoing regular dialysis. Int J Artif Organs. 2004;27 (8):723. doi: 10.1177/039139880402700811. [DOI] [PubMed] [Google Scholar]
- 46.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62 (5):1524. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 47.Parkkinen J, von Bonsdorff L, Peltonen S, Gronhagen-Riska C, Rosenlof K. Catalytically active iron and bacterial growth in serum of haemodialysis patients after i.v. iron-saccharate administration. Nephrol Dial Transplant. 2000;15 (11):1827. doi: 10.1093/ndt/15.11.1827. [DOI] [PubMed] [Google Scholar]
- 48.Brewster UC, Perazella MA. Intravenous iron and the risk of infection in end-stage renal disease patients. Semin Dial. 2004;17 (1):57. doi: 10.1111/j.1525-139x.2004.17115.x. [DOI] [PubMed] [Google Scholar]
- 49.Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol. 2005;43 (3):325. doi: 10.1016/j.femsim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Collins AJ, EJ, Ma JZ, Xia H. Iron dosing patterns and mortality. J Am Soc Nephrol. 1998;9(250A) [Google Scholar]
- 51.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219 (1):1. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr. 1985;5:365. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- 53.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20 (5):707. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 54.Brewster UC. Intravenous iron therapy in end-stage renal disease. Semin Dial. 2006;19 (4):285. doi: 10.1111/j.1525-139X.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 55.Barany P, Divino Filho J, Bergstrom J. High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis. 1997;29 (4):565. doi: 10.1016/s0272-6386(97)90339-5. [DOI] [PubMed] [Google Scholar]
- 56.Gunnell J. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 1999;33 (1):63. doi: 10.1016/s0272-6386(99)70259-3. [DOI] [PubMed] [Google Scholar]
- 57.de Francisco AL, Stenvinkel P, Vaulont S. Inflammation and its impact on anaemia in chronic kidney disease: from haemoglobin variability to hyporesponsiveness. NDT Plus. 2009;2 (Suppl_1):i18. doi: 10.1093/ndtplus/sfn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malyszko J, Malyszko JS, Borawski J, et al. A study of platelet functions, some hemostatic and fibrinolytic parameters in relation to serotonin in hemodialyzed patients under erythropoietin therapy. Thromb Res. 1995;77 (2):133. doi: 10.1016/0049-3848(95)91619-v. [DOI] [PubMed] [Google Scholar]
- 59.Fuste B, Serradell M, Escolar G, et al. Erythropoietin triggers a signaling pathway in endothelial cells and increases the thrombogenicity of their extracellular matrices in vitro. Thromb Haemost. 2002;88 (4):678. [PubMed] [Google Scholar]
- 60.Streja E, Kovesdy CP, Greenland S, et al. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52 (4):727. doi: 10.1053/j.ajkd.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stohlawetz PJ, Dzirlo L, Hergovich N, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000;95 (9):2983. [PubMed] [Google Scholar]
- 62.Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA) Clin J Am Soc Nephrol. 2009;4 (2):470. doi: 10.2215/CJN.05040908. [DOI] [PubMed] [Google Scholar]
- 63.Drueke TB, Locatelli F, Clyne N, et al. Normalization of Hemoglobin Level in Patients with Chronic Kidney Disease and Anemia. N Engl J Med. 2006;355 (20):2071. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 64.Kamar N, Reboux AH, Cointault O, et al. Impact of very early high doses of recombinant erythropoietin on anemia and allograft function in de novo kidney-transplant patients. Transpl Int. 2010;23 (3):277. doi: 10.1111/j.1432-2277.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- 65.Heinze G, Kainz A, Horl WH, Oberbauer R. Mortality in renal transplant recipients given erythropoietins to increase haemoglobin concentration: cohort study. BMJ. 2009;339:b4018. doi: 10.1136/bmj.b4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez Fresnedo G, de Francisco AL, Ruiz JC, Gomez Alamillo C, Arias M. Hemoglobin level variability in renal transplant patients treated with erythropoiesis stimulating agents. Transplant Proc. 2008;40 (9):2919. doi: 10.1016/j.transproceed.2008.08.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.