Abstract
The reconstruction of a joint's maximum range of mobility (ROM) often is a first step when trying to understand the locomotion of fossil tetrapods. But previous studies suggest that the ROM of a joint is restricted by soft tissues surrounding the joint. To expand the limited informative value of ROM studies for the reconstruction of a fossil species’ locomotor characteristics, it is moreover necessary to better understand the relationship of ex vivo ROM with the actual in vivo joint movement. To gain insight into the relationship between ex vivo mobility and in vivo movement, we systematically tested for the influence of soft tissues on joint ROM in the hip of the modern lizard Iguana iguana. Then, we compared the ex vivo mobility to in vivo kinematics of the hip joint in the same specimens using X-ray sequences of steady-state treadmill locomotion previously recorded. With stepwise removal of soft tissues and a repeated-measurement protocol, we show that soft tissues surrounding the hip joint considerably limit ROM, highlighting the problems when joint ROM is deduced from bare bones only. We found the integument to have the largest effect on the range of long-axis rotation, pro- and retraction. Importantly, during locomotion the iguana used only a fragment of the ROM that was measured in our least restrictive dissection situation (i.e. pelvis and femur only conjoined by ligaments), demonstrating the discrepancy between hip joint ROM and actual in vivo movement. Our study emphasizes the necessity for caution when attempting to reconstruct joint ROM or even locomotor kinematics from fossil bones only, as actual in vivo movement cannot be deduced directly from any condition of cadaver mobility in Iguana and likely in other tetrapods.
Keywords: hip joint, Iguana iguana, limb kinematics, range of mobility, range of motion, soft tissue influence, sprawling gait, XROMM
Introduction
Examining the functional morphology of joints is fundamental for the understanding of the locomotor apparatus of terrestrial vertebrates. The morphology of bone articular surfaces reflects the potential mobility of a joint. Thus, for paleontological inference of locomotor capabilities in extinct species, potential joint mobility (i.e. the maximum range of mobility, ROM) has often been considered a useful starting point for the reconstruction of locomotor capabilities in extinct tetrapods (Morbeck, 1976), drawing on data from articular surfaces, ligaments, and muscles (Hankin & Watson, 1914; Bramwell & Whitfield, 1974; Nicholls & Russell, 1985; Bennett, 1991, 1997a,b; Chapman et al. 1999; Senter, 2005, 2007; Senter & Robins, 2005; Schwarz et al. 2007; Lipkin & Carpenter, 2008; Gatesy et al. 2009; Sellers et al. 2009; Holliday et al. 2010; Mallison, 2010a,b, 2012; Hutson & Hutson, 2012, 2013; Pierce et al. 2012). Analysis of soft tissue influence on ROM in extant species serves as a basis to reconstruct fossil characteristics and to formulate reasonable boundaries for paleontological inference. To date, data about soft tissue influence on ROM are rarely published and are available for only a few joints of mammalian and archosaur taxa (Haines, 1942; Roos et al. 1992; Chan, 2006, 2008; Dzemski & Christian, 2007; Bonnan et al. 2010; Fujiwara et al. 2010; Holliday et al. 2010; Hutson & Hutson, 2012, 2013; Cobley et al. 2013). It has been shown in those studies that joint function is strongly influenced by associated soft tissues: muscles, tendons, ligaments, and cartilage. Unfortunately, these soft tissues are only rarely fossilized and thus, even in the ideal situation of a complete, undistorted body fossil, paleontological inference needs to account for their potentially confounding influence.
To our knowledge, there is a specific lack of studies on soft tissue influence on hip joint mobility for sauropsid species (but see studies on archosaur taxa in Holliday et al. 2010; in the supplementary materials of Pierce et al. 2012; also see Tsai & Holliday, 2013). However, particularly in sprawling gaits, the hip joint plays a fundamental role. The sprawling posture is characterized by an approximately horizontal orientation of the stylopodium (Rewcastle, 1983; Russell & Bels, 2001). The femur operates as a biomechanical lever, and forces against the body and the ground are exerted at angles to its mechanical long axis (Russell & Bels, 2001). During complex three-dimensional (3D) motions of the femur, this lever acts in different planes about the oval form and convex surface of the femoral head: predominantly in the horizontal plane during retraction posteriorly and in the vertical plane during adduction ventrally of the hind limb (Rewcastle, 1981). Additionally, long-axis rotation of the femur occurs during locomotion (e.g. Edwards, 1977; Brinkman, 1981; Ashley-Ross, 1994; Karakasiliotis et al. 2012; Nyakatura et al. 2013). Thus, orientation and motion of the femur and the whole hind limb during the step cycle are determined by the morphology of the hip joint (Snyder, 1954). No previous study has systematically quantified how integument, superficial and deep muscles, and ligaments together restrict, or even extend (as shown for the elbow joint of archosaurs by Hutson & Hutson, 2012, 2013), the hip joint ROM in a lizard, although some previous researchers have assumed that they limit ROM in various tetrapods (see Kemp, 1982).
A premise for the reconstruction of locomotor capabilities in fossil sprawling species requires knowledge of how ROM and actual kinematics during locomotion are related to one another. Modern animals do not utilize the full ROM during cyclic locomotion (e.g. Gatesy et al. 2009), thus investigating this discrepancy is critical for inference of locomotor behaviour.
The objectives of this study were two-fold. First, we tested for the influence of soft tissues on ROM in the hip joint of an extant lizard using step-wise removal of soft tissues and repeated measurement of ROM ex vivo. Subsequently, we compared this data with the previously measured in vivo hip joint kinematics from the same individuals to gain insight into the mobility vs. movement relationship. Here, the focus was on the soft tissues that surround the hip joint in contrast to those that are within the joint (e.g. studied in Holliday et al. 2010). Steady-state treadmill locomotion as well as ROM of the femur were recorded using high-speed X-ray videography, and hip kinematics and ROM were quantified using the X-ray reconstruction of moving morphology (XROMM) method (Brainerd et al. 2010; Gatesy et al. 2010). Thus, we used an experimental approach for reconstructing the ROM of disarticulated bones.
Material and methods
Examined animals
Two green iguanas (Iguana iguana Linnaeus 1758) were examined in this study. First, specimen 1 [adult female, overall length of 118 cm and snout–vent length (SVL) of 43 cm] and specimen 2 (adult male, 125 cm overall length and 44 cm SVL) were studied as they walked on a treadmill (see below). The animals were housed in two 1.5 × 2 × 1 m terrariums with several quiescent and climbing possibilities. The animals had access to food and water ad libitum. The required temperature and humidity were automatically regulated. Specimen 1 died of unknown causes. Specimen 2 was euthanized with 100 mg kg−1 pentobarbital intra-peritoneally (i.e. 250 mg). Both cadavers were frozen immediately after death. All experiments were approved by the Thuringian committee for animal research (licence for husbandry: J-SHK-2684-05-04/10-8; licence for experiments: Reg.-Nr. 02-008/11) and adhered strictly to pertinent animal welfare regulations of the State of Thuringia, Germany.
Experimental design
Fully digital, biplane X-ray recordings of steady-state treadmill locomotion of the two subjects were taken prior to the ex vivo ROM experiments from lateral and ventral projection to analyse pelvic and femoral kinematics (condition I). Treadmill speed was manually adjusted to allow steady-state locomotion at a speed favoured by the animals. Specimen 1 walked at a relatively slow speed of 0.25 m s−1 (stance phase: 1.1 s; swing phase: 0.37 s). Specimen 2 walked at a moderate speed of 0.53 m s−1 (stance phase: 0.23 s, swing phase: 0.42 s). For the purpose of this study, hip joint movements of a single steady-state trial of each specimen were analysed using the XROMM approach outlined below (complete Iguana three-dimensional limb kinematics will be presented elsewhere). The X-ray movies were recorded in a 1536 × 1024 pixel image format with 500 fps at 50 kV and 15 mA (for details of X-ray facility cf. Nyakatura et al. 2010).
Postmortem ROM experiments were recorded synchronously from two projections (50° and −15° to vertical axis, respectively) with the same facility. Based on the method of Chan (2006, 2008) and analogous to the approach used by Hutson & Hutson (2012, 2013), the pelvic girdles and right thighs of both animals were dissected by step-wise removal of external tissue around the hip joint (Fig. 1). Following every removal step, ROM was recorded. We measured four subsequent ex vivo conditions: intact cadaver (condition II), integument removed (condition III), extrinsic muscles removed (condition IV) and intrinsic muscles removed, i.e. only ligaments spanning the joint (condition V). Intrinsic muscles were defined following Snyder (1954) as deep one-joint muscles acting upon the hip joint originating from pelvic bone and inserting on the femoral head and proximal third of the femur. Thus, intrinsic muscles are the caudifemoralis brevis and longus, iliofemoralis, ischiotrochantericus, puboischiofemoralis externus and internus. Consequently, all other superficial muscles crossing the hip joint and not inserting on the femoral head and proximal third of femur were defined as extrinsic (the adductor femoris, ambiens, flexor tibialis externus and internus, iliofibularis, iliotibialis, puboischiotibialis, pubotibialis).
Fig. 1.
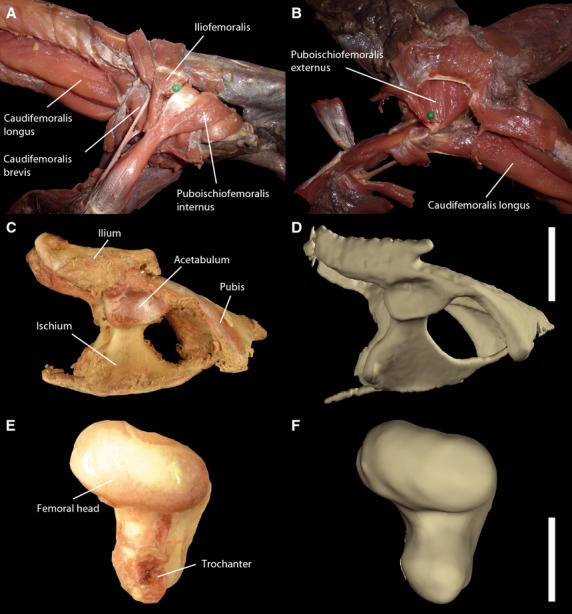
Dissections and similarity between actual bones and CT-based digital bone models. After removal of extrinsic muscles during dissection in lateral (A) and ventral (B) aspect; actual (C) and virtual (D) pelvic bone in lateral aspect (scale bar: 2 cm); actual (E) and virtual (F) femoral head in proximal aspect showing the articular surface (scale bar: 1 cm). Note high degree of similarity of acetabular shape and oval femoral head between actual and virtual bones.
Cadavers were fixed with cable ties allowing free movement of thigh and hip joint during X-ray recordings (Fig. 2A). With the intact cadaver and following every removal step, we performed the same four manipulations: long-axis rotation (LAR) with laterally abducted limb (i.e. an idealized orientation roughly resembling the in vivo orientation at mid-stance), LAR with fully protracted limb, LAR with fully retracted limb and full thigh excursion, including maximum abduction, retraction, adduction and protraction.
Fig. 2.
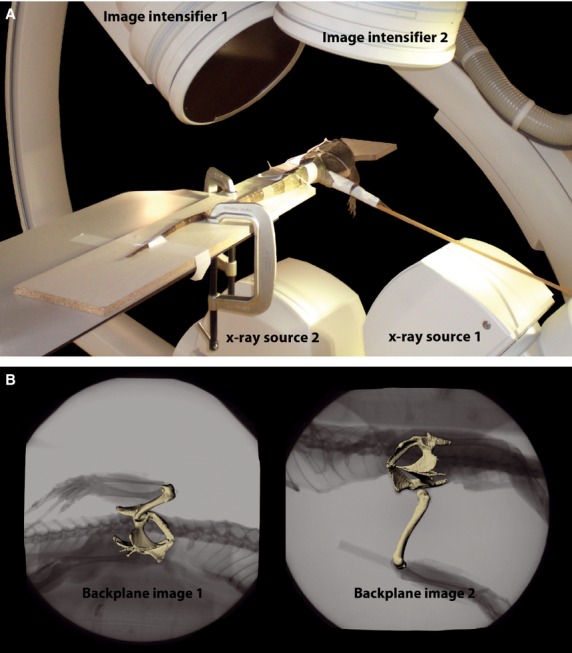
X-ray analysis. (A) Biplane X-ray set up during cadaver manipulations; (B) scientific rotoscoping. The experimental scene is reconstructed (Gatesy et al. 2010) within commercial animation software (maya). Bone models were registered to synchronized X-ray video images (two projections) over a sequence of images (i.e. a trial). With the use of anatomically defined joint coordinate systems, the method is used to obtain high-resolution 3D kinematic data (for further explanation see text and Supporting Information Video S1).
To test for the sensitivity of our data to the observer's variability during manipulations, one of the authors (P.A.) additionally conducted repeated measurements of the excursion manipulation. To this end, P.A. collected five trials of excursion with the intact cadaver of specimen 2. Measurements within the observer's trials are very similar and show only little mean deviation (Fig. 3). All data analysed in the study were collected by P.A. to avoid potentially confounding inter-observer variability (see Hutson & Hutson, 2012).
Fig. 3.
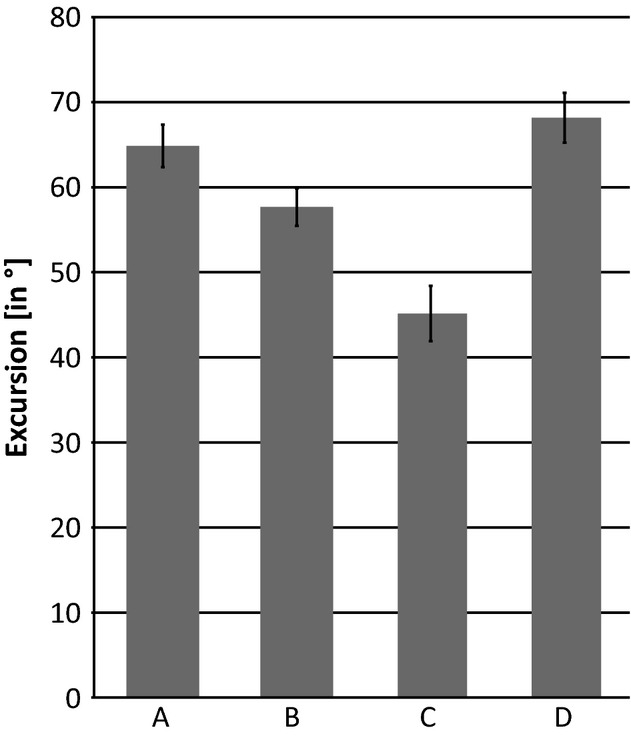
Intra-observer variability in repeated measurements. (A) Maximum protraction; (B) maximum retraction; (C) maximum abduction; (D) maximum adduction. Maximum excursion is influenced by the force applied by observers. Note small standard deviation (black error bars) within the repeated measurements performed by P.A. All subsequent manipulations were performed exclusively by P.A. to avoid potential confounding inter-observer variability.
X-ray reconstruction of moving morphology (XROMM)
Scans of both specimens were taken using medical computer tomography (General Electric CT Lightspeed VCT 64 Pro) at the Universitätsklinikum Jena, Germany (slice thickness: 0.625 mm). To reconstruct virtual bone models of the femur and pelvis, raw data were aligned and bone contours were manually segmented using segmentation editor of amira 5.4.2 (Visage Imaging, Richmond, Australia). Subsequently, the bone models were surface-rendered and saved as .obj files. These CT-derived bone models allowed detailed representation of actual bony morphology (Fig. 1C-F). Moreover, lizard hip joints possess only a minor acetabular lip and lack several soft tissues (menisci, fibroadipose tissue, real transverse ligament) regularly found in mammals and birds (Canillas et al. 2011). Unlike in crocodilians and dinosaurs, the squamate femur also has only a relatively thin layer of hyaline articular cartilage on its proximal condyle (Haines, 1969; Holliday et al. 2010). Overall, there is only little soft tissue internal to the lizard hip joint and thus bone models are suitable for estimating ROM in the hip of iguana. However, the spacing between the articulating surfaces of the femoral head and the acetabulum is still crucial (see below).
The bone models were imported into maya 2012 (Autodesk, San Rafael, CA, USA) and hierarchically connected via virtual maya-joints (Gatesy et al. 2010). The reference pose of the model setup was orientated following Sullivan (2007), with the long axis of the femur parallel to the pelvic longitudinal axis and distal condyles directed ventrally (see Supporting Information Video S1). With a sensitivity analysis of the joint space assumption (between femoral head and acetabulum), we evaluated its influence on the mobility of the reference pose. The virtual femur was angled (adducted) maximally until it collided with the pelvic bone (similar to the approach used in Pierce et al. 2012). Sensitivity to joint space was tested by measuring mobility of the femur in two extreme possible cases of distances between femoral head and acetabulum; we first set up the model without any joint space (steady contact of femoral head and acetabulum) and then used the maximum distance apparent during XROMM analysis of in vivo locomotion (Fig. 4). We then decided to use an intermediate distance in the reference pose of the model setup. This position resulted in no contact between the femoral head and the acetabulum during femoral motion, as evident in the X-ray recordings (see Results).
Fig. 4.
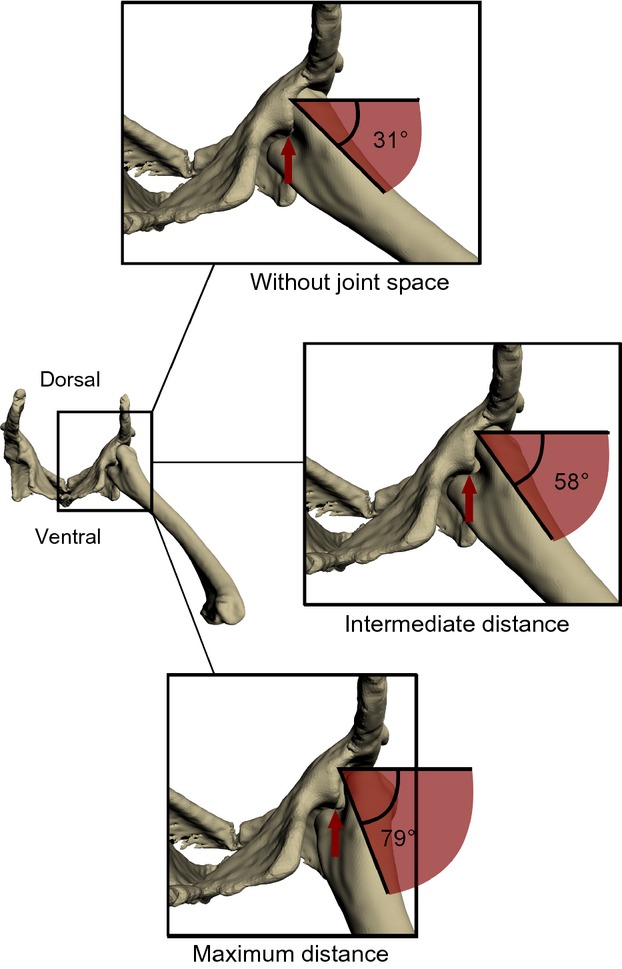
Sensitivity to the joint space assumption in the reference pose between femoral head and acetabulum demonstrated by the example of femoral adduction in caudal aspect. At a given joint space, the femur was adducted until collision of the two bones. Red arrows point to the point of collision between femur and pelvis bone models.
The hierarchical chain of maya-joints comprised just two virtual joints in this study. The higher hierarchy virtual joint determined pelvic movement and the lower hierarchy joint movement of the femur. Thus, when the pelvis is moved, the femur is moved along with it, but femoral movement did not influence pelvis position or orientation. Movement of the femur relative to the pelvis was measured using an anatomically defined joint coordinate system placed into the centre of rotation. To approximate the centre of rotation in a reproducible fashion, the joint coordinate system was placed into the centre of a sphere which was positioned in the centre of the femoral head. However, this does not imply that the joint itself is represented by a sphere, rather that all measurements are taking the actual anatomy of the joint into account and rotations as well as translations can be captured relative to the initial positioning of the joint coordinate system during scientific rotoscoping (SR, see below and Gatesy et al. 2010).
The x-axis was aligned along the long axis of the femur, thus rotation about this axis was defined as femoral LAR. Rotation about the y-axis reflects abduction/adduction and z-axis rotation reflects retraction (see Video S1 for an animation that shows the definitions of these axis rotations and Fig. 5 for the measured movements). Translatory movements, if apparent on the X-ray recordings, also were animated, but not quantified (see Results). Additionally, a maya locator was fixed to the distal femur (intercondylar fossa), enabling documentation of femoral excursion relative to the hip via a coxofemoral excursion field (similar to the glenohumeral excursion field used by Chan, 2006). All X-ray recordings were undistorted and corrected with matlab 7.11.0 (Mathworks, Natick, MA, USA) using a reference grid (see Brainerd et al. 2010). Recordings were transferred to greyscale and squared via batch processing in matlab. Afterwards, recordings were saved as .jpg image sequences in AfterEffects 6.5 (Adobe, San Jose, CA, USA). A calibration object (20 × 12 × 12 cm) with metal beads inserted at 1-cm distances was used for calibration of 3D space covered by both X-ray devices. In maya, two virtual cameras were created and their relative position in virtual 3D space calibrated via recordings of a calibration object in matlab imitating the actual X-ray sources (necessary matlab scripts and maya embedded language files available at http://www.xromm.org). Undistorted X-ray image sequences from both projections were put in the backplane of the recreated X-ray cameras. The model setup was imported into the video setup enabling SR (Gatesy et al. 2010). To measure and animate movement using SR, the orientation of bone models was approximated to match both synchronized X-ray projections (Fig. 2B) every 10 frames, i.e. every 0.08 s. Subsequently, the whole motion was spine-interpolated. After finishing a trial, six degree of freedom data were exported into Microsoft excel. The translation of the maya locator fixed to the distal femur relative to the acetabulum (representing the coxofemoral excursion field) was exported, too.
Fig. 5.
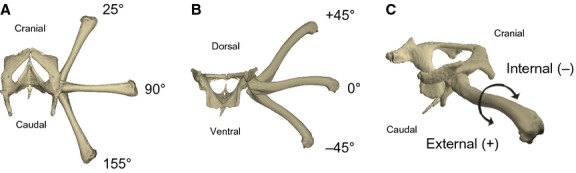
Rotational degrees of freedom of the bone model. (A) Retraction in dorsal aspect (given in absolute values), (B) abduction/adduction in caudal aspect, and (C) long-axis rotation (LAR) with internal and external rotation in lateral aspect.
Results
Range of LAR
Overall, in both specimens the range of LAR increased with step-wise removal of soft tissues in all analysed thigh orientations (i.e. in lateral abducted, maximum retracted and maximum protracted orientation). With condition II, LAR varied consistently between the different thigh positions in both specimens (Fig. 6). The range was largest with the lateral abducted limb, whereas a maximum protracted limb allowed very little rotation. Rotation at the maximal retracted orientation was about half that of the abducted limb orientation. Condition III resulted in a distinct increase in femoral LAR in all limb positions of both animals. The rotation at the protracted thigh position especially was increased. Rotation at the retracted thigh position also more than doubled after the removal of the integument. Removing extrinsic muscle (condition IV) further increased the range of LAR. Condition V leads to the largest range of LAR in all three analysed orientations. When only hip ligaments remained, similar rotational ranges regardless of femoral orientation were allowed.
Fig. 6.
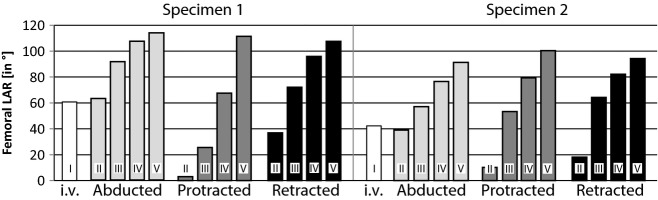
Femoral long-axis rotation during in vivo locomotion and cadaver manipulations after successive tissue removal steps. White: in vivo (i.v., condition I) LAR. Light grey: possible LAR with lateral abducted thigh orientation. Dark grey: possible LAR with protracted thigh orientation. Black: possible LAR with retracted thigh orientation. Conditions II–V, stepwise removal of soft tissues: II, intact cadaver; III, integument removed; IV, extrinsic muscles removed; V, intrinsic muscles removed (only ligaments conjoin the femur and pelvis). Note that rotation with laterally abducted thigh of the intact cadaver is nearly the same as in vivo. Possible rotation increased with step-wise tissue removal and led to similar mobility in all positions with condition V.
When the proportions of internal (i.e. clockwise rotation when viewed from lateral and head pointing to the right) and external (i.e. anti-clockwise rotation when viewed from lateral) rotation of range of LAR (cf. Fig. 5) are compared, the internal rotation predominated with all conditions and femoral orientations in both specimens (Fig. 7). Although external rotation increased slightly with tissue removal, it never represented more than 35% of the total range, even if only hip ligaments remained to limit rotation.
Fig. 7.
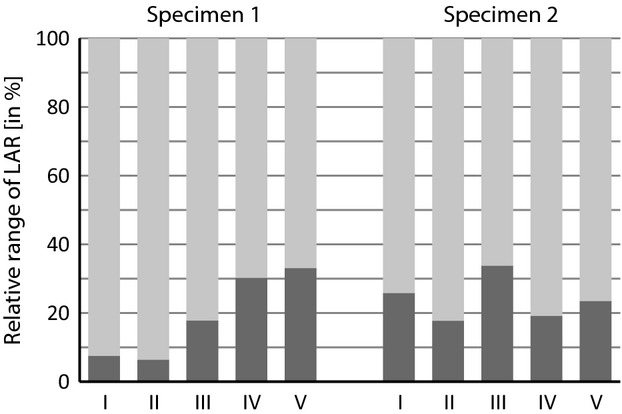
Relative proportion of internal (light grey) and external (dark grey) rotation during in vivo (I) locomotion and stepwise soft tissue removal. II, Intact cadaver; III, integument removed; IV, extrinsic muscles removed; V, intrinsic muscles removed (only ligaments conjoin the femur and pelvis). Note emphasis on internal rotation in in vivo locomotion as well as on all tissue removal steps in both specimens.
Femoral excursion
Overall, femoral movement during a step cycle is similar to published data of other lizards (Snyder, 1952; Sukhanov, 1974; Russell, 1975; Reilly & Delancey, 1997; Higham & Jayne, 2004). In Iguana, the femoral retraction following hindlimb touch down is coupled to adduction until the middle of the stance phase. Afterwards, the femur is abducted for the rest of the stance phase and the whole of swing phase. Femoral protraction starts briefly after lift off of the hindlimb.
Minor translatory movements occurred in the hip during treadmill locomotion. Whereas joint space is small and invariable during the step cycle, the femoral head translates along the long-axis of its oval shape (Fig. 8A,C), especially when the limb is protracted at touch down. When apparent in the X-ray recordings and to consider their influence on the position on rotational axes, these translations were included in the rotoscoping process (Fig. 8 B,D). However, given the resolution of the X-ray recordings those movements were too small to be reliably quantified over the entire stride.
Fig. 8.
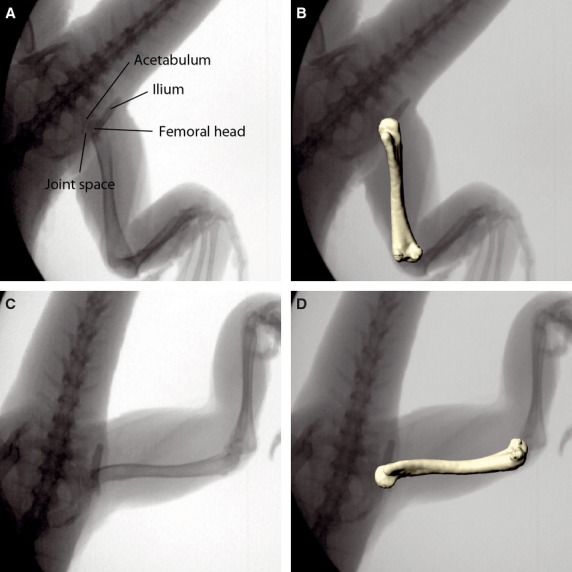
Femoral translation during in vivo locomotion. X-ray images of the hip joint configuration during the step cycle in touch down (A) and lift off (C) position; the same X-ray images with matched virtual femur model (B,D). Note that joint space is small and invariable. Minor femoral translation is apparent along the long-axis of the oval femoral head. This translation is considered in the scientific rotoscoping approach.
Maximum range of retraction and abduction with all conditions for both specimens are illustrated in Table 1. The range of retraction as well as the range of abduction in vivo were about 79° and 71°, respectively, but were both 110° with condition II (Fig. 9). Range of retraction increased to about 130° with condition III. Condition IV resulted in possible retraction of nearly 160° and did not increase any more with condition V. The range of abduction on average was 128°, 132°, and 147° with condition III, IV, and V, respectively. Overall, the range of retraction as well as the range of abduction/adduction generally increased with step-wise removal of soft tissue.
Table 1.
Range of retraction and abduction of all conditions of step-wise soft tissue removal for both specimens
| Condition | Specimen 1 (°) | Specimen 2 (°) | Mean (°) | |
|---|---|---|---|---|
| Retraction | in-vivo/I | 83.2 | 75.4 | 79.3 |
| II | 105.4 | 116.6 | 111.0 | |
| III | 121.2 | 142.8 | 132.0 | |
| IV | 157.8 | 157.9 | 157.8 | |
| V | 157.0 | 141.9 | 149.5 | |
| Abduction | in-vivo/I | 65.7 | 76.4 | 71.1 |
| II | 122.9 | 111.8 | 117.3 | |
| III | 124.5 | 130.5 | 127.5 | |
| IV | 135.0 | 129.7 | 132.4 | |
| V | 148.4 | 145.9 | 147.1 |
Fig. 9.
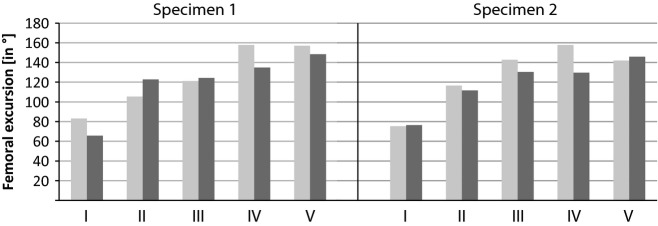
Femoral excursion during in vivo (I) locomotion and cadaver manipulation of different tissue removal steps. II, Intact cadaver; III, integument removed; IV, extrinsic muscles removed; V, intrinsic muscles removed (only ligaments conjoin the femur and pelvis). Light grey: range of retraction. Dark grey: range of abduction/adduction. Note discrepancy between in vivo locomotion and intact cadaver. Excursion mobility increased with step-wise soft tissue removal.
Trajectories of the distal femur revealed a discrepancy between actual in vivo movement during Iguana's locomotion and experimentally derived ex vivo mobility in the hip joint (Fig. 10). Not only did the coxofemoral excursion fields extend with soft tissue removal, the form of trajectories also differed in being more or less triangular during locomotion instead of oval as in the experimental excursion. Except for the excursion into the craniodorsal direction, condition V offers a much larger ROM than is actually used during a steady-state locomotor trial (condition I).
Fig. 10.
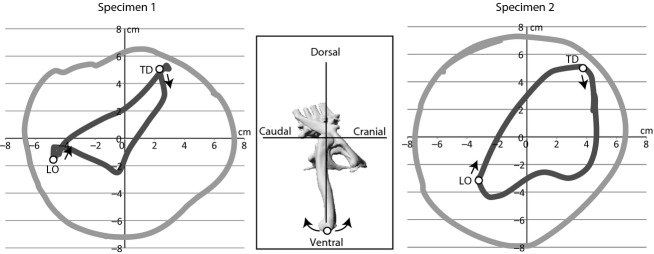
Lateral aspect coxofemoral excursion fields showing the discrepancy between in vivo locomotion (dark grey) and possible mobility of the hip joint conjoined only by ligaments (light grey). Speed of specimen 1: 0.25 m s−1. Speed of specimen 2: 0.53 m s−1. The hip joint is located in the origin of coordinates (see inset). The excursion fields are derived by mapping the displacement of the distal femur (white point in inset) relative to the hip joint. LO, lift off; TD, touch down.
Discussion
Hutson & Hutson (2012, 2013) concluded from their analyses of archosaur elbow and shoulder joints that all structures externally surrounding a joint (i.e. integument, muscles, and ligaments) inhibit its excursion.This holds also true for our study of hip joint mobility of Iguana. Our combination of treadmill locomotion, cadaver manipulation, X-ray videography, and virtual 3D bone models matching with SR allowed a detailed view on the relationship between in vivo motion, ex vivo mobility, and the influence of soft tissue in the hip joint of iguana.
Femoral LAR
The overall increase of LAR with step-wise soft-tissue removal is attributed to the general decrease of soft tissue inhibition with each removal step. Differences in range of LAR between lateral abducted and protracted/retracted thigh orientations are mainly caused by the anatomical arrangement of the rotating intrinsic puboischiofemoralis internus and caudifemoralis longus muscles (Rewcastle, 1983). Due to being maximally stretched, caudifemoralis longus limits rotation at maximum protraction, whereas puboischiofemoralis internus limits rotation at maximum retraction. Additionally, extrinsic muscles (knee flexors, knee extensors, hip adductors) limit rotational mobility (Fig. 6). It is noteworthy that the influence of integument on rotation is considerable. Despite its flexibility between single scales, the squamate integument imposes a high mechanical resistance (Wu et al. 2004). The integument is stretched during rotation, especially with the thigh in a protracted or retracted orientation. Apparently, during our manipulations interscale flexibility was at its maximum.
The inhibitory effect of the integument is the reason why hip joints of intact cadavers had only limited rotational mobility. LAR increased in all femoral positions following integument removal. Interestingly, during the step cycle the observed LAR nearly matched the measured ex vivo ROM of condition II (i.e. that of the intact cadaver).
When the relative proportions of internal and external rotation are compared, internal LAR predominates across all ex vivo conditions as well as during in vivo locomotion. Moreover, insertions of the main rotating muscles imply that internal rotation is the main action due to these muscles (Rewcastle, 1983). But even without muscles, external rotation is limited by the pubofemoral ligament. Originating from the pubic pectineal process, it passes on the extensor side of the femur but inserts on the flexor side and inhibits external rotation. Thus, all soft tissues surrounding the hip joint affect the range of LAR but allow a substantial internal rotational mobility. This internal rotation is functionally required for the double-crank mechanism increasing step length (Barclay, 1946; Ashley-Ross, 1994) and maintaining cranially directed thrust by enhancing caudally directed forces (the rotation problem, Rewcastle, 1983). Iguana seems to use the maximum available range of internal LAR during locomotion. Nevertheless, upon maximal dissection (condition V), the joint offers a much greater range of LAR than is actually used during steady-state locomotion. Consequently, ligament preparation and mere bone models are not suitable in assessing LAR of actual locomotion. This is especially the case in the absence of skeletal characteristics that might restrict movement. In the iguana hip joint, we did not find anatomical limitations to rotation, as is the case for the crocodilian hip joint (Pierce et al. 2012), too. Pierce and colleagues concluded that femoral LAR of the extant Nile crocodile was not comparable to that of the early tetrapod Ichthyostega because this fossil possesses a joint anatomy that restricts rotation and differs remarkably from the extant species examined. Our step-wise soft tissue removal suggests that in Iguana the actual femoral LAR during locomotion is very similar to LAR in the intact cadaver. This suggests that future modelling approaches to derive fossil femoral LAR should try to account not only for non-preserved ligaments and muscles but also for the integument.
Femoral excursion
By removing the integument, extrinsic muscles, and intrinsic muscles, the potential retraction and abduction/adduction increased. The excursion field is mainly limited by a strong muscle cuff and integument. Notably, retraction and abduction/adduction during in vivo locomotion are very different from the measured mobility of condition II. Thus, Iguana does not use the maximum range of retraction and abduction/adduction during the step cycle of medium speed locomotion. However, hindlimb kinematics during medium speed steady state locomotion are not representative of all locomotor circumstances, such as high speed or inclined substrate locomotion (Reilly & Delancey, 1997; Fieler & Jayne, 1998; Irschick & Jayne, 1998; Jayne & Irschick, 1999; Higham & Jayne, 2004; Spezzano & Jayne, 2004). Joint mobility without integument and muscles also is very uninformative for assessing ROM in vivo, as revealed by the extended coxofemoral excursion field precluding direct estimation of actual movement from cadaver mobility in Iguana.
Implications for reconstruction of fossil sprawling tetrapods
Pierce et al. (2012, see their supplementary materials) show in their experiments with cadaveric Nile crocodiles that mobility of a ligamentous joint (similar to our condition V) and mobility of their virtual bone model are similar in the shoulder and hip joints, except for LAR in the hip. The authors acknowledge that surrounding soft tissues further restrict mobility, but did not include the integument in their analysis. Based on the similarity of cadaveric ROM and the 3D bones-only model, Pierce and colleagues used virtual manipulations of a 3D bones-only model for their subsequent reconstruction of Ichthyostega's ROM in diverse joints. Interestingly, the range of LAR shows similarities between in vivo locomotion and intact cadaver mobility in the iguanas analysed here. With the exception of a predomination of internal rotation, the range of LAR cannot be deduced from the mobility of condition V in our study. In line with the results of other authors (Hutson & Hutson, 2012, 2013; Cobley et al. 2013), our findings demonstrate clear differences between mobility of intact cadavers and cadavers that have had all soft tissues removed from the joints (see these references for examples of elbow, shoulder, and cervical spine). The range of femoral LAR in locomoting Iguana confirms the importance of this kinematic component in sprawling locomotion and highlights the emphasis on internal rotation. For this specific movement the bare pelvic and femoral bones of Iguana lack any objective limitation to long-axis rotation, and thus LAR during locomotion is not deducible from them. If no obvious osteologic limitations are present, the reconstruction of possible LAR at the hip joint in fossil sprawling tetrapods is therefore problematic. Moreover, the sensitivity analysis of the joint space in the reference pose undertaken in our study suggests a significant influence on the ROM and calls for caution when attempting to reconstruct ROM from bare fossil bones. However, if such an analysis includes comparison to intact cadavers of anatomically similar modern species, it can help to find reasonable boundaries for the potential mobility in joints of a fossil (e.g. Pierce et al. 2012).
By including the kinematics of living specimens’ locomotion in our study, we were able to compare it with the data of cadaveric ROM. We documented a discrepancy between actual kinematics, mobility of the hip joint in the intact cadaver, and the mobility of the hip on loose condition V. Additionally we tested for the sensitivity of virtual ROM reconstruction from a digital bone model by varying the basic joint space assumption. Our study presents evidence that the discrepancy between the actual kinematics during locomotion and the possible mobility of a joint of a fossil could be large, as twice the observed ROM was documented in cadaver measurements here for Iguana's hip joint. We propose that reconstruction of locomotion in fossil sprawling tetrapods has to include extensive investigation of locomotor kinematics in combination with the influence of soft tissue morphology on joint mobility in extant taxa, preferably studied within an extant phylogenetic bracket (Witmer, 1995). However, even though the focus of this study was on a sprawling species, this notion is in all likelihood not restricted to sprawling tetrapods, because significant soft tissue influence has been documented in diverse groups using different methodological approaches (e.g. Chan, 2006; Holliday et al. 2010; Mallison, 2010a,b; Hutson & Hutson, 2012, 2013), but has only rarely been considered in fossil reconstructions. Additionally, such an investigation has to be based on a clear methodology regarding conditions of comparison, reproducibility (Hutson & Hutson, 2012), and the basic assumptions of the reconstruction (e.g. joint space, reference pose).
Conclusion
Investigation of the influence of soft tissues on hip joint ROM in Iguana showed that to obtain an approximation of the ROM of joints in fossil sprawling tetrapods, an approach is needed beyond the scope of the bare fossil bone's morphology. Our data suggests that study of a fossil organism's joint ROM can provide an estimate of the boundaries of possible movement for the investigated joint. Comparison with cadaveric ROM of anatomically comparable extant animals is recommended to specify that frame. The large discrepancy between actual movement during locomotion and cadaveric mobility calls for caution when reconstructing joint motion or when interpreting existing ROM data.
Acknowledgments
We thank Matthias Krüger for advice during dissections, Rommy Petersohn for technical support during X-ray experiments, Sabine Bischoff for medical assistance in animal care, and Brandon Kilbourne for improving an earlier version of the manuscript. We also thank two anonymous reviewers for helpful critique. This study was funded by the Volkswagen Foundation (AZ 85857 to M.S.F. and J.A.N.) and the Daimler and Benz Foundation (32-08/12 to J.A.N.).
Conflict of interest
The authors declare no conflict of interest.
Author contributions
P.A. conducted all experiments, analysed and interpreted the data, and drafted the manuscript. J.A.N. designed the study. All authors contributed ideas to the experimental design of the study, the interpretation of the data, and read and approved the final version of the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Video S1. Configuration and reference pose of the digital bone model and the scientific rotoscoping approach.
References
- Ashley-Ross M. Hindlimb kinematics during terrestrial locomotion in a salamander (Dicamptodon tenebrosus. J Exp Biol. 1994;193:255–283. doi: 10.1242/jeb.193.1.255. [DOI] [PubMed] [Google Scholar]
- Barclay OR. The mechanics of amphibian locomotion. J Exp Biol. 1946;23:177–203. doi: 10.1242/jeb.23.2.177. [DOI] [PubMed] [Google Scholar]
- Bennett SC. 1991. Morphology of the Late Cretaceous pterosaur Pteranodon and systematics of the Pterodactyloidea, Volumes I & II. Ph.D. thesis, University of Kansas, Systematics and Ecology.
- Bennett SC. The arboreal leaping theory of the origin of pterosaur flight. Hist Biol. 1997a;12:265–290. [Google Scholar]
- Bennett SC. Terrestrial locomotion of pterosaurs: a reconstruction based on Pteraichnus trackways. J Vertebr Paleontol. 1997b;17:104–113. [Google Scholar]
- Bonnan MF, Sandrik JL, Nishiwaki T, et al. Calcified cartilage shape in archosaur long bones reflects overlying joint shape in stress-bearing elements: implications for nonavian dinosaur locomotion. Anat Rec. 2010;293:2044–2055. doi: 10.1002/ar.21266. [DOI] [PubMed] [Google Scholar]
- Brainerd EL, Baier DB, Gatesy SM, et al. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J Exp Zool A Ecol Genet Physiol. 2010;313:262–279. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- Bramwell CD, Whitfield G. Biomechanics of Pteranodon. Philos Trans R Soc Lond B. 1974;267:503–581. [Google Scholar]
- Brinkman D. The hind limb step cycle of Iguana and primitive reptiles. J Zool. 1981;181:91–103. [Google Scholar]
- Canillas F, Delgado-Martos M, Touza A, et al. An approach to comparative anatomy of the acetabulum from amphibians to primates. Anat Histol Embryol. 2011;40:466–473. doi: 10.1111/j.1439-0264.2011.01095.x. [DOI] [PubMed] [Google Scholar]
- Chan LK. Glenohumeral mobility in primates. Folia Primatol. 2006;78:1–18. doi: 10.1159/000095682. [DOI] [PubMed] [Google Scholar]
- Chan LK. The range of passive arm circumduction in primates: do hominoids really have more mobile shoulders? Am J Phys Anthropol. 2008;136:265–277. doi: 10.1002/ajpa.20800. [DOI] [PubMed] [Google Scholar]
- Chapman R, Andersen A, Jabo S. Construction of the virtual Triceratops: procedures, results, and potentials. J Vertebr Paleontol. 1999;19:37A. [Google Scholar]
- Cobley MJ, Rayfield EJ, Barrett PM. Inter-vertebral flexibility of the ostrich neck: implications for estimating sauropod neck flexibility. PLoS ONE. 2013;8:e72187. doi: 10.1371/journal.pone.0072187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzemski G, Christian A. Flexibility along the neck of the ostrich (Struthio camelus) and consequences for the reconstruction of dinosaurs with extreme neck length. J Morphol. 2007;268:701–714. doi: 10.1002/jmor.10542. [DOI] [PubMed] [Google Scholar]
- Edwards JL. The evolution of terrestrial locomotion. In: Hecht MK, Goody PC, Hecht BM, editors. Major Patterns in Vertebrate Evolution. London: NATO Advanced Study Institutes Series; 1977. pp. 553–577. [Google Scholar]
- Fieler C, Jayne BC. Effects of speed on the hindlimb kinematics of the lizard Dipsosaurus dorsalis. J Exp Biol. 1998;201:609–622. doi: 10.1242/jeb.201.4.609. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Taru H, Suzuki D. Shape of articular surface of crocodilian (Archosauria) elbow joints and its relevance to sauropsids. J Morphol. 2010;271:883–896. doi: 10.1002/jmor.10846. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Bäker M, Hutchinson JR. Constraint-based exclusion of limb poses for reconstructing theropod dinosaur locomotion. J Vertebr Paleontol. 2009;29:535–544. [Google Scholar]
- Gatesy SM, Baier DB, Jenkins FA, et al. Scientific rotoscoping: a morphology-based method of 3-D motion analysis and visualization. J Exp Zool A: Ecol Genet Physiol. 2010;313:244–261. doi: 10.1002/jez.588. [DOI] [PubMed] [Google Scholar]
- Haines RW. The tetrapod knee joint. J Anat. 1942;76:270. [PMC free article] [PubMed] [Google Scholar]
- Haines R. Epiphyses and sesamoids. In: Gans C, editor. Biology of the Reptilia, Volume 1, Morphology A. London: Academic Press; 1969. pp. 81–115. [Google Scholar]
- Hankin E, Watson DMS. On the flight of pterodactyls. Aeronaut J. 1914;18:324–335. [Google Scholar]
- Higham TE, Jayne BC. Locomotion of lizards on inclines and perches: hindlimb kinematics of an arboreal specialist and a terrestrial generalist. J Exp Biol. 2004;207:233–248. doi: 10.1242/jeb.00763. [DOI] [PubMed] [Google Scholar]
- Holliday CM, Ridgely RC, Sedlmayr JC, et al. Cartilaginous epiphyses in extant archosaurs and their implications for reconstructing limb function in dinosaurs. PLoS ONE. 2010;5:e13120. doi: 10.1371/journal.pone.0013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson JD, Hutson KN. A test of the validity of range of motion studies of fossil archosaur elbow mobility using repeated-measures analysis and the extant phylogenetic bracket. J Exp Biol. 2012;215:2030–2038. doi: 10.1242/jeb.069567. [DOI] [PubMed] [Google Scholar]
- Hutson JD, Hutson KN. Using the American alligator and a repeated-measures design to place constraints on in vivo shoulder joint range of motion in dinosaurs and other fossil archosaurs. J Exp Biol. 2013;216:275–284. doi: 10.1242/jeb.074229. [DOI] [PubMed] [Google Scholar]
- Irschick DJ, Jayne BC. Effects of incline on speed, acceleration, body posture and hindlimb kinematics in two species of lizard Callisaurus draconoides and Uma scoparia. J Exp Biol. 1998;201:273–287. doi: 10.1242/jeb.201.2.273. [DOI] [PubMed] [Google Scholar]
- Jayne BC, Irschick DJ. Effects of incline and speed on the three-dimensional hindlimb kinematics of a generalized iguanian lizard (Dipsosaurus dorsalis. J Exp Biol. 1999;202:143–159. doi: 10.1242/jeb.202.2.143. [DOI] [PubMed] [Google Scholar]
- Karakasiliotis K, Schilling N, Cabelguen J-M, et al. Where are we in understanding salamander locomotion: biological and robotic perspectives on kinematics. Biol Cybern. 2012;107:529–544. doi: 10.1007/s00422-012-0540-4. [DOI] [PubMed] [Google Scholar]
- Kemp T. Mammal-like Reptiles and the Origin of Mammals. London: Academic Press; 1982. [Google Scholar]
- Lipkin C, Carpenter K. Looking Again at the Forelimb of Tyrannosaurus rex. In: Larson P, Carpenter K, editors. Tyrannosaurus rex: The Tyrant King. Bloomington: Indiana University Press; 2008. pp. 166–190. [Google Scholar]
- Mallison H. CAD assessment of the posture and range of motion of Kentrosaurus aethiopicus Hennig 1915. Swiss J Geosci. 2010a;103:211–233. [Google Scholar]
- Mallison H. The digital Plateosaurus II: an assessment of the range of motion of the limbs and vertebral column and of previous reconstructions using a digital skeletal mount. Acta Palaeontol Pol. 2010b;55:433–458. [Google Scholar]
- Mallison H. 2012. p. 137. Digital range of motion analysis in vertebrates – capabilities, limitations, and future developments. In Royo-Torres, R., Gascó, F. and Alcalá, L., coord. (2012). 10th Annual Meeting of the European Association of Vertebrate Palaeontologists. 20: 1–290.
- Morbeck M. Problems in reconstruction of fossil anatomy and locomotor behaviour: the Dryopithecus elbow complex. J Hum Evol. 1976;5:223–233. [Google Scholar]
- Nicholls EL, Russell AP. Structure and function of the pectoral girdle and forelimb of Struthiomimus altus (Theropoda: Ornithomimidae) Palaeontology. 1985;28:643–677. [Google Scholar]
- Nyakatura JA, Petrovitch A, Fischer MS. Limb kinematics during locomotion in the two-toed sloth (Choloepus didactylus, Xenarthra) and its implications for the evolution of the sloth locomotor apparatus. Zoology. 2010;113:221–234. doi: 10.1016/j.zool.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Nyakatura JA, Andrada E, Curth S, et al. Bridging ‘Romer's Gap’: Locomotor mechanics of an extant belly-dragging lizard model inform debate on crown-group node tetrapod locomotion. J Evol Biol. 2013 DOI: 10.1007/s11692-013-9266-z. [Google Scholar]
- Pierce SE, Clack JA, Hutchinson JR. Three-dimensional limb joint mobility in the early tetrapod Ichthyostega. Nature. 2012;486:523–526. doi: 10.1038/nature11124. [DOI] [PubMed] [Google Scholar]
- Reilly S, Delancey M. Sprawling locomotion in the lizard Sceloporus clarkii: the effects of speed on gait, hindlimb kinematics, and axial bending during walking. J Zool. 1997;243:417–433. [Google Scholar]
- Rewcastle SC. Stance and gait in tetrapods: an evolutionary scenario. Symp Zool Soc Lond. 1981;48:239–267. [Google Scholar]
- Rewcastle SC. Fundamental adaptations in the lacertilian hind limb: a partial analysis of the sprawling limb posture and gait. Copeia. 1983;1983:476–487. [Google Scholar]
- Roos H, Brugger S, Rauscher T. Über die biologische Wertigkeit der Bewegungen in den Radioulnargelenken bei Katze und Hund. Anat Histol Embryol. 1992;21:199–205. doi: 10.1111/j.1439-0264.1992.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Russell AP. A contribution to the functional analysis of the foot of the Tokay, Gekko gecko (Reptilia: Gekkonidae) J Zool. 1975;176:437–476. [Google Scholar]
- Russell A, Bels V. Biomechanics and kinematics of limb-based locomotion in lizards: review, synthesis and prospectus. Comp Biochem Physiol A Mol Integr Physiol. 2001;131:89–112. doi: 10.1016/s1095-6433(01)00469-x. [DOI] [PubMed] [Google Scholar]
- Schwarz D, Wings O, Meyer CA. Super sizing the giants: first cartilage preservation at a sauropod dinosaur limb joint. J Geol Soc. 2007;164:61–65. [Google Scholar]
- Sellers W, Manning P, Lyson T, et al. Virtual palaeontology: gait reconstruction of extinct vertebrates using high performance computing. Palaeontol Electronica. 2009;12:11–26. [Google Scholar]
- Senter P. Function in the stunted forelimbs of Mononykus olecranus (Theropoda), a dinosaurian anteater. Paleobiology. 2005;31:373–381. [Google Scholar]
- Senter P. Analysis of forelimb function in basal ceratopsians. J Zool. 2007;273:305–314. [Google Scholar]
- Senter P, Robins JH. Range of motion in the forelimb of the theropod dinosaur Acrocanthosaurus atokensis, and implications for predatory behaviour. J Zool. 2005;266:307–318. [Google Scholar]
- Snyder RC. Quadrupedal and bipedal locomotion of lizards. Copeia. 1952;1952:64–70. [Google Scholar]
- Snyder RC. The anatomy and function of the pelvic girdle and hindlimb in lizard locomotion. Am J Anat. 1954;95:1–36. doi: 10.1002/aja.1000950102. [DOI] [PubMed] [Google Scholar]
- Spezzano LC, Jayne BC. The effects of surface diameter and incline on the hindlimb kinematics of an arboreal lizard (Anolis sagrei. J Exp Biol. 2004;207:2115–2131. doi: 10.1242/jeb.00995. [DOI] [PubMed] [Google Scholar]
- Sukhanov VB. General System of Symmetrical Locomotion of Terrestrial Vertebrates and Some Features of Movement of Lower Tetrapods [Obshchaya Sistema Simmetricheskoi Lokomotsii Nazemnykh Pozvonochynkh i Osobennosti Peredvizheniya Nizshikh Tetrapod] Washington, DC: Smithsonian Institution and the National Science Foundation; 1974. [Google Scholar]
- Sullivan CS. 2007. Function and evolution of the hind limb in Triassic archosaurian reptiles. Ph.D. thesis, Harvard University.
- Tsai HT, Holliday CM. 2013. Anatomical reconstruction of archosaur hip joint soft tissues and its significance for interpreting hindlimb function. In 10th International Congress of Vertebrate Morphology). Barcelona.
- Witmer LM. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason J, editor. Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press; 1995. pp. 19–33. [Google Scholar]
- Wu P, Hou L, Plikus M, et al. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Configuration and reference pose of the digital bone model and the scientific rotoscoping approach.


