Abstract
The tissue organisation of dermal collagen is gaining importance as a contributing factor both in development and ageing, as well as in skin maturation processes. In this work we aim to study different representative parameters of this structural organisation in 45 human skin samples of assorted ages, by means of image analysis. The variation of these parameters on the basis of age was assessed using several regression models (linear, quadratic and cubic). The area occupied by collagen was significantly reduced as a function of age in the papillary dermis (R2 = 0.437, P < 0.0001), as well as the thickness of the collagen bundles (R2 = 0.461, P < 0.0001), following statistical models of cubic and quadratic regression, respectively. The width of the papillary dermis increased in a significant manner over a linear regression model (R2 = 0.26, P < 0.0001). In the reticular dermis, the cubic regression indicated a significant decline (R2 = 0.392, P = 0.002) of the area filled with collagen according to the age. Both collagen thickness and bundle orientation parameters fit a quadratic regression over the age in a significant way (R2 = 0.433 and R2 = 0.334, respectively, both P < 0.0001). The width of the reticular dermis followed also a significant quadratic distribution according to age (R2 = 0.193, P = 0.011). These parameters could partially explain the lifelong functional changes taking place in the skin and propose a baseline providing a useful entry point for future investigation.
Keywords: ageing, collagen, development, image analysis, Masson's trichrome, maturation, morphometry, skin
Introduction
Skin ageing is a widely studied process using a morphological (Moragas et al. 1998), cellular (Dellambra & Dimri, 2009) and molecular approaches (Naylor et al. 2011): the skin is a superficial organ and accounts for one-sixth of a person's total body weight, hence being accessible and highly representative of the ageing processes of the organism (Kohl et al. 2011; Zouboulis & Makrantonaki, 2011). Two basic types of ageing can be distinguished: intrinsic ageing, also known as chronological ageing owing to the passing of time, and extrinsic ageing, also called photoageing, mainly due to the harmful effect of ultraviolet light (UV). However, other factors such as smoking habits or environmental pollution influence this type of ageing (Farage et al. 2009; Chung et al. 2003).
Different agents contribute to intrinsic skin ageing. Cell senescence is a classical conception whereby cells fall into a senescent phenotype at the end of their replicative life span (Passos & von Zglinicki, 2005). As an example, it has been demonstrated that fibroblasts of old individuals' dermis exhibit less replicative capability and present an altered pattern of substance secretion (West et al. 1989; Varani et al. 2006; Quan et al. 2011). Another ageing theory relies on oxidative stress: the levels of reactive oxygen species (ROS) increase and the levels of antioxidants decrease during ageing, thus inducing the expression of several matrix metalloproteinases (MMPs; Callaghan & Wilhelm, 2008), which could partially explain the degradation of collagen that occurs with skin ageing. In turn, the remnants of fragmented collagen conjointly stimulate MMPs and ROS synthesis, closing a vicious cycle which perpetuates the lesions of the extracellular matrix started with intrinsic skin ageing (Fisher et al. 2009).
Another relevant factor is the ageing of the extracellular matrix molecules. Dermal collagen, for example, with a half-life of 15 years is a considerably long-lived molecule, a feature that predisposes it to accumulate lesions such as advanced glycosylation end products (AGE), which have catabolic effects on the molecules they bind (Bailey et al. 1998; Verzijl et al. 2000).
All these factors are steadfastly interrelated and share common pathways and mechanisms leading to ageing. Nonetheless, the structural organisation of the extracellular matrix and its interaction with dermal cells have been the source of concern for some years (Imayama & Braverman, 1989). To the paradigm in which several alterations in a cellular level provoke changes in the extracellular matrix (Chung et al. 2001; Varani et al. 2006) has been added, in the case of skin ageing, the model in which the extracellular matrix itself is able to exert influence over the cells and regulate several parameters related to ageing (Fisher et al. 2008). Specifically, it has been demonstrated that the rests of fragmented collagen inhibit the synthesis of procollagen I and connective tissue growth factor (CTGF) by fibroblast, block their proliferation, induce a senescent fibroblastic morphology, alter the cell cytoskeleton, and stimulate the expression of MMP and synthesis of ROS (Varani et al. 2001; Fisher et al. 2009; Xia et al. 2013).
Morphological changes in the characteristics of the extracellular matrix in the processes of skin development that take place in the first stages of life have also been studied (Gallivan et al. 1997; Sephel et al. 1987; Furth et al. 1997). However, available data are scarce and methodologically limited (Stamatas et al. 2010). Therefore it is still necessary to study in more detail the organisational characteristics of young collagen and find a relationship between these features and the functional data available.
The study of the tissue organisation of collagen bundles and their morphological characteristics is important as these are contributing factors both in intrinsic skin ageing and in the development and maturation processes. We analysed distinct morphological parameters that are representative of the features and structural organisation of collagen during life, in both the papillary dermis and the deeper reticular dermis, to establish an explicative model that relates the morphological and structural changes with the disposable physiological data in the literature.
Materials and methods
Study population and skin samples
Forty-five abdominal skin samples of material remaining from autopsy at the Service of Pathology of the University Clinical Hospital in Valencia were included. We discarded all altered samples or those coming from potentially damaged skin (e.g. from accidents, collagenopathies, diabetes mellitus). The age of the individual ranged from a month old to 95 years old (average of 57.02 ± 27.68 years), including 28 men and 17 women.
A sample approximately 2.5 cm long × 2.5 cm wide was taken from the periumbilical region adjacent to the midline in all the cases. The chosen zone is assumed to be protected from sun exposure, allowing the exclusion of the detrimental effects of photoageing or extrinsic skin ageing, and focusing on the intrinsic skin changes. Routinely, haematoxylin-eosin and orcein staining protocols and subsequent examination were followed to confirm the absence of solar elastosis, the most prominent histopathological feature of photoageing (Berneburg et al. 2000; Rabe et al. 2005).
The study was approved by the local research ethics committee. A coded number was assigned to every case to prevent the researchers having access to the age and sex of the individuals by sample processing, image capture, parameter measurement or data analysis, thus ensuring the objectivity of the measurements.
Processing and staining of the samples
The tissue samples were fixed in a 5% formaldehyde solution and underwent paraffin inclusion. Histological sections of 5 μm width were performed with the microtome and were posteriorly stained with a Masson's trichrome protocol that included Harris' haematoxylin, Briebrich Scarlet/acid fuchsin and Aniline Blue as reagents. All types of collagen are stained with this technique, allowing evaluation of collagen as a whole.
Image analysis
The microphotographs were taken with a microscope Leica DMD108 (Leica Microsystems, Wetzlar, Germany) and posteriorly underwent computer study with the software of image analysis image-pro plus 7.0 (Media Cybernetics, Silver Spring, MD, USA). Five photographs were taken for each case and each parameter, following a method of semi-randomisation, which excluded altered zones (e.g. material accumulation, white spaces, and areas containing large structures such as sebaceous glands or pilo-sebaceous follicles) and assured representativeness of the photographs. The magnification of the snapshots was adjusted individually for each parameter. The mean for each image was calculated and, later, the mean of the case from the values of the five images.
Statistical analysis
The statistical analysis was performed with the software spss 17.0 for Windows (SPSS Inc., Chicago, IL, USA). For all the parameters, a Kolmogorov–Smirnov normality test was carried out; after that, the regression analysis was done, including linear regressions [y (x) = a + bx], quadratic regressions [y (x) = a + bx + cx2] and cubic regressions [y (x) = a + bx + cx2 + dx3]. The model with a higher R2 value was selected, and, in case of evenness of the results, the simpler model was chosen. A P-value below 0.05 was considered significant.
Results
Thickness of the papillary dermis
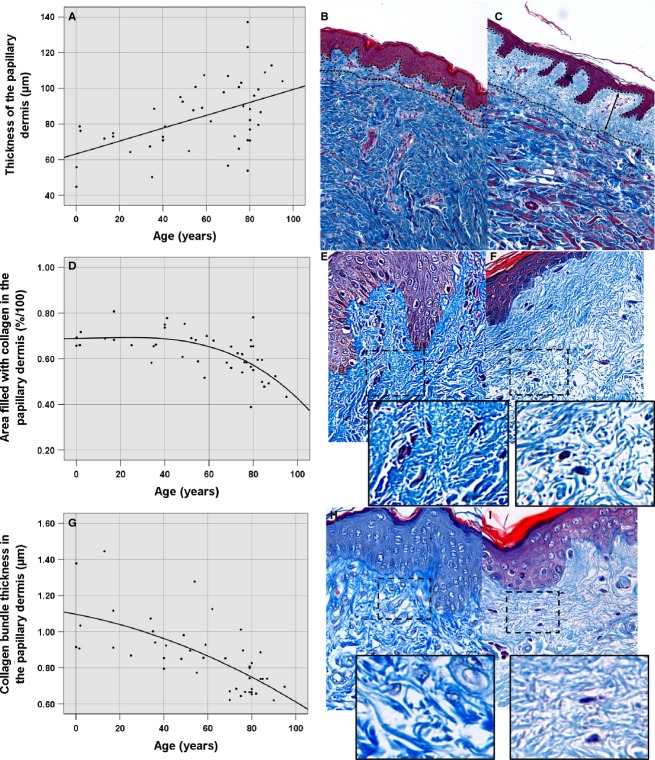
We took 15 representative measurements of the thickness of the papillary dermis in the 100× magnification images, thanks to the contrast produced between the thick collagen bundles integrating the reticular dermis and the thin collagen bundles forming the papillary dermis. A Best Fit filter was applied to improve and maximise that contrast. We found that this parameter was incremented according increasing age following a linear regression model in a statistically significant way (P < 0.0001, R2 = 0.26), from 63.24-μm-thick in the moment of birth until 100 μm, which the regression line predicts for the age of 100 years (Table 1, Fig. 1A–C).
Table 1.
Statistical values of measured parameters in papillary dermis
| Parameter | Regression equation | R2 | F | P | Constant | b1 | b2 | b3 |
|---|---|---|---|---|---|---|---|---|
| Thickness of papillary dermis (μm) | Linear | 0.26 | 15.118 | < 0.0001 | 63.238 | 0.361 | – | – |
| Area fraction occupied by collagen bundles (%) | Quadratic | 0.433 | 16.034 | < 0.0001 | 67.9 | 0.2 | −4.21 × 10−3 | – |
| Thickness of collagen bundles (μm) | Quadratic | 0.461 | 17.953 | < 0.0001 | 1.096 | −0.002 | −2.486 × 10−5 | – |
Fig. 1.
Morphometric parameters in the papillary dermis. (A) Scatter plot of the thickness of the papillary dermis as a function of age. (B) Thickness of the papillary dermis in a 1-month-old individual (mean of 44.84 μm). (C) Thickness of the papillary dermis in a 79-year-old subject (mean of 137.1 μm). (D) Scatter plot of the area occupied by collagen (density of collagen bundles) in the papillary dermis as a function of age. (E) Area occupied by collagen in the papillary dermis in a 40-year-old subject (mean of 73.63% density). (F) Area occupied by collagen in the papillary dermis of a 95-year-old individual (mean of 43.27% density). (G) Scatter plot of the collagen thickness in the papillary dermis on the basis of age. (H) Thickness of collagen in the papillary dermis in a 2-year-old subject (mean of 1.37 μm). (I) Thickness of the collagen of the papillary dermis in a 90-year-old subject (mean of 0.52 μm).
Area occupied by collagen in the papillary dermis
To determine the density of the collagen bundles within the papillary dermis we selected the area occupied by collagen in 400× magnification photographs of the papillary dermis using a semi-automatised procedure. In the images, the area corresponding to the papillary dermis was manually selected, a Hi-Pass filter was applied to optimise individualisation of the bundles, the red colour channel was extracted and, posteriorly, a segmentation of the image that would allow the selection of the area filled with collagen was performed. Over a statistically significant (P < 0.0001, R2 = 0.437) model of quadratic regression with age as independent variable, a diminution in the area occupied by collagen was demonstrated starting from 40 years old onwards, with a value of 69.16% of density until reaching a minimum in the last stage of life, with a value of 45.8% of density (Table 1, Fig. 1D–F).
Thickness of collagen in the papillary dermis
The thickness of 15 collagen bundles was measured in the 400× magnification photographs, enlarging the image so that the limits of the bundles could properly be discriminated. With increasing age the measured parameter in reticular dermis decreased significantly, following a quadratic regression model (P < 0.0001, R2 = 0.461). At the moment of birth, the thickness of collagen bundles was of 1.096 μm, reaching a predicted minimum of 0.6474 μm for 100-year-old individuals (Table 1, Fig. 1G–I).
Thickness of the reticular dermis
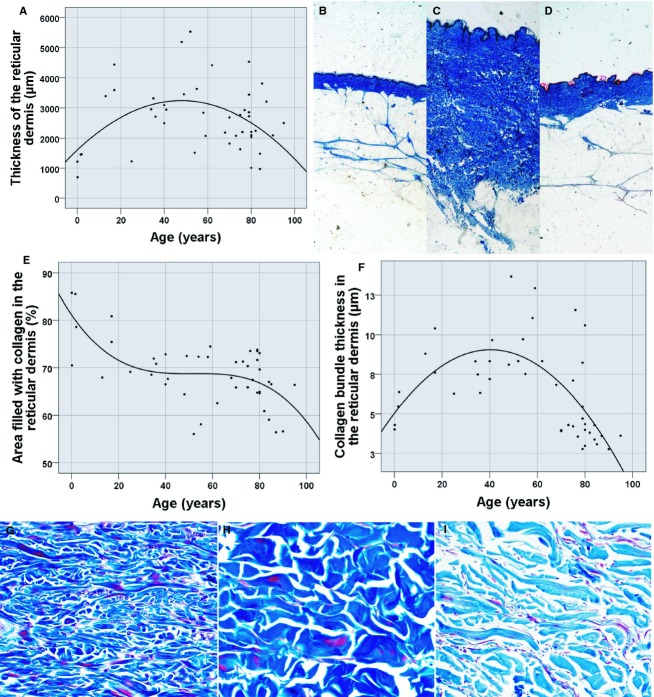
Fifteen representative measurements of the thickness of the reticular dermis were taken either in the 40× magnification detail micrographs or in the macroscopic photograph, according to the case, excluding the papillary dermis of this measure. The thickness fluctuated on the basis of age following a statistically significant quadratic function (P = 0.011, R2 = 0.193). The thickness was minimum in the first and last stages of life, with values of 1603.88 μm at the moment of birth and 1303.48 μm predicted for 100-year-olds, and maximum values in adult skin, reaching a thickness of 3236.18 μm at 50 years of age (Table 2, Fig. 2A–D).
Table 2.
Statistical values of measured parameters in reticular dermis
| Parameter | Regression equation | R2 | F | P | Constant | b1 | b2 | b3 |
|---|---|---|---|---|---|---|---|---|
| Thickness of reticular dermis (μm) | Quadratic | 0.193 | 5.018 | 0.011 | 1603.882 | 68.296 | −0.713 | – |
| Area fraction occupied by collagen bundles (%) | Cubic | 0.392 | 8.803 | < 0.0001 | 81.141 | −0.723 | 0.014 | −9.036 × 10−5 |
| Thickness of collagen bundles (μm) | Quadratic | 0.433 | 16.028 | < 0.0001 | 5.039 | 0.199 | −0.002 | – |
| Collagen orientation | Quadratic | 0.334 | 10.54 | < 0.0001 | 0.757 | 0.004 | −5.578 × 10−5 | – |
Fig. 2.
Morphometric parameters in the reticular dermis. (A) Scatter plot representing the thickness of the reticular dermis on the basis of age. (B) Thickness of the reticular dermis in a 2-month-old (mean of 698.66 μm), (C) in a 48-year-old (mean of 5192.02 μm), and (D) in an 80-year-old subject (mean of 2032.86 μm). (E) Scatter plot showing the thickness of collagen in the reticular dermis as a function of age. (F) Scatter plot of the area occupied by collagen in the reticular dermis as a function of age. (G–I) Examples in 1-month-old (85.77% of occupied area and 4.02 μm thick), 49-year-old (72.45% of occupied area and 13.68 μm thick) and 90-year-old individuals (56.63% of occupied area and 2.78 μm thick).
Area occupied by collagen in the reticular dermis
In the 200× magnification micrographs we measured the area filled with collagen bundles in the reticular dermis, a parameter which is representative of the density of the bundles. To accomplish that aim, a Best Fit filter was applied to the image; it was transformed to greyscale and manually segmented to select the area occupied by collagen. This parameter adjusted a cubic regression in a statistically significant manner (P < 0.0001, R2 = 0.392), defining a maximal density at the moment of birth (81.14% of density) which is then reduced and kept stable between 40 and 60 years of age (68.7% of density at 50 years of age) and further dwindles with cutaneous ageing (58.48% of density of collagen bundles at 100 years of age; Table 2, Fig. 2E,G–I).
Thickness of the collagen bundles in the reticular dermis
We measured the thickness of 15 collagen bundles in the 200× magnification detail micrographs, manually amplifying the image in pursuance of easy visualisation of the limits between bundles. This parameter was explained by a statistical model of quadratic regression by increasing age in a statistically significant way (P < 0.0001, R2 = 0.433). The maximum thickness of collagen is present in adult age (9.944 μm thick at 45 years old), whereas in the first and last stages of life, this thickness reaches minimum reaching values of 5.039 and 4.939 μm thick at birth and at 100 years of age, respectively (Table 2, Fig. 2F–I).
Orientation of collagen in the reticular dermis
Collagen orientation in the reticular dermis was measured in 200× magnification micrographs following a methodology which has been validated previously (van Zuijlen et al. 2002). This technique applies Fourier transform to the image and, over the resulting 2D power plot, the length and the width of the central figure are measured. The relation between both sets of data is representative of the orientation of the bundles: values which tend to 0 indicate higher orientation, and values tending to 1 indicate a disorientation of the collagen bundles.
The orientation of the bundles in the reticular dermis obeyed a quadratic regression model on the basis of age in a statistically significant way (P < 0.0001, R2 = 0.334), displaying maximal orientation in both first and last stages of life (values of 0.757 at birth and 0.6 at 100 years of age), and a disorganisation of the bundles during adult age, with a 40-year value of 0.8279 (Table 2, Fig. 3).
Fig. 3.
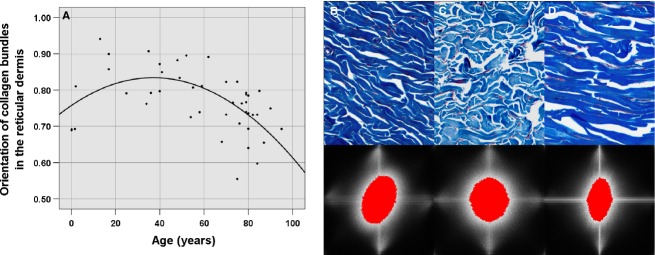
Analysis of the collage orientation in the reticular dermis with the Fourier transform. (A) Scatter plot of the collagen orientation as a function of age. Image of the collagen orientation (B) in a 19-month-old individual, with a mean of 0.6923, (C) in a 35-year-old subject, with a mean of 0.9067 and (D) in a 76-year-old individual, with a mean of 0.7073.
Discussion
In this study we have analysed distinct representative parameters of dermal collagen morphology and organisation, independently for papillary and reticular dermis. Blind research method and semi-automated measurement protocols used for each of the different parameters ensure the maximum objectivity of obtained results.
About skin development and maturation
We found a high density of collagen bundles existing at birth that remains constant until adulthood in the papillary dermis, but not in the reticular dermis, where it diminishes progressively. These results supports the study done by Furth et al. (1997), who reported a high expression of type I and III procollagens in fetal dermis that remained partially elevated during the postnatal period to decrease later with skin maturation. Furthermore, it was shown that the total number of fibroblasts present in human dermis decreases in a progressive way after birth (Gunin et al. 2010). It may be possible that, after birth, remodelling type processes are the dominant ones, inasmuch as collagen synthesis diminishes, eventually leading to the loss of density of these bundles, which is what we quantitatively proved.
Nevertheless, in spite of the loss of density of the bundles observed in our study, collagen bundles in reticular dermis show an increased thickness during adulthood compared with at the moment of birth, or during infancy and adolescence (first stage of life). This could be explained as owing to intermolecular cross-linking of the collagen molecules that occurs during development in a very precise enzymatic process carried on by lysyl oxidase. Initially divalent and immature, these cross-links become trivalent and more stable in a spontaneous process through well-established biochemical pathways (Bailey et al. 1998). Hence, we postulate that, at the beginning of this development stage, there is a high density of small diameter collagen bundles which, as the individual ages, are involved in this process of enzymatic cross-links formation and maturation of the existing ones from divalent to trivalent, leading to the presence of thicker and more functional collagen bundles in adulthood.
The fact that the thickness of collagen bundles in papillary dermis shows no increase in adulthood could be due to exclusive morphologic and biochemical characteristics of this papillary collagen. Those differences are masked in studies which do not differentiate between papillary and reticular dermis. Morphologic and functional differences between papillary and reticular fibroblasts have been proven, which also supposes differential morphologic and functional characteristics in the type of collagen secreted by these cells (Mine et al. 2008; Pageon et al. 2012; Janson et al. 2013). Subsequent studies about collagen in papillary dermis will clarify these differences.
Changes in the thickness of reticular dermis during skin development and maturation can be interpreted with the obtained data. During the postnatal period, the thickness of reticular dermis is minimal as, despite the high density of collagen bundles, these are thin and display parallel arrangement, as found in our study. However, as reticular dermis develops and matures, it progressively become thicker, until reaching the maximum thickness during adulthood, approximately in the fourth decade of life. This could be explained as follows: in spite of the reduction in synthesis and density of collagen bundles, these bundles progressively become thicker and spatially disorganised (i.e. in any of the three spatial directions) in the tissue, i.e. they acquire the characteristics of disorganised dense connective tissue. This increase in the thickness of reticular dermis is supported by previously published studies (Moragas et al. 1998; Kakasheva-Mazhenkovska et al. 2011).
One might expect that, during these development processes, synthetic and cellular changes carried out by fibroblasts take precedence over changes in the extracellular matrix (cells→matrix) and not the other way around, considering that, due to the relative lack of stimuli damaging the extracellular matrix during these life stages, collagen bundles are thicker, arranged in all spatial directions and show no signs of degradation, inducing a higher mechanical stimulation of fibroblasts. This would result in a phenotype characterised by the synthesis of extracellular matrix components (Sarasa-Renedo & Chiquet, 2005; Chiquet et al. 2009). Consequently, we postulate that a maximal homeostatic point would be reached during the adult stage: the decrease in the number of fibroblasts and their intrinsic collagen synthesis and, on the other hand, the integrity and three-dimensional spatial orientation of collagen bundles would establish a equilibrium, thereby maintaining, thanks to mechanical stimulation of fibroblasts, the homeostasis of collagen and catabolic enzymes of the extracellular matrix (Quan et al. 2013).
About skin ageing
In our study we perceived a loss of density of collagen bundles, occurring since birth in the case of reticular dermis but from the fourth or fifth decade of life in the papillary layer, which concurs with findings of other studies (Moragas et al. 1998; Kakasheva-Mazhenkovska et al. 2011). This could be explained by the progressive decrease of type I and III collagen secretion by dermal fibroblasts after the second decade of life (Dumas et al. 1994), and the already proved reduction in type I procollagen synthesis and fibroblast proliferation that take place during intrinsic skin ageing (Varani et al. 2006), due to the decreased expression of transforming growth factor (TGF)-β and CTGF by these cells (Quan et al. 2010). In another study the density of collagen network was observed to increase during ageing; however, no objective measurements were provided to support this (Lavker et al. 1987).
Together with collagen synthesis reduction by dermal fibroblasts in intrinsic skin ageing, it has also been proved that there is an increase in MMP-1, MMP-2 and MMP-9 expression (Varani et al. 2000; Quan et al. 2011). This abnormal pattern of molecular secretion was designated as ‘age-associated secretory phenotype’ (Quan et al. 2011). MMPs are degrading enzymes that act on extracellular matrix molecules by inducing the advent of fragmented collagen remainders in the tissue (Hernández-Pérez & Mahalingam, 2012). This is congruous with the thickness reduction in collagen bundles that we observed in papillary and reticular dermis according to age, and supports the observation of thinner and more fragmented collagen bundles in aged dermis reported by other studies (Varani et al. 2001, 2006; Baroni Edo et al. 2012; El-Aal et al. 2012).
This reduction in the thickness of dermal collagen could also be explained by molecular mechanisms causing ageing of these molecules, which include the cross-linking between collagen bundles that occurs in an indirect and non-enzymatic way, basically by glycosylation of glucose and its indirect products. This leads to accumulation of advanced AGEs, which affect the aggregation of collagen monomers, with catabolic effects on the molecules where they settle (Bailey et al. 1998). Skin collagen is a long-lasting molecule, showing a half-life of about 15 years, which predisposes it to these molecular ageing processes (Verzijl et al. 2000).
Supplementary to ageing processes centered on the role of cells in the extracellular matrix, the influence of the matrix itself on the cellular component of the tissue has been studied recently. The presence of fragmented collagen remainders in aged dermis inhibits both fibroblast proliferation and type I procollagen synthesis by these cells (Varani et al. 2001), induces an increase in MMP-1 expression and its AP-1/α-2-β-1-integrin signalling pathway, and increments ROS (Fisher et al. 2009). At the same time, MMP-1 fragments dermal collagen, hence configuring a vicious circle that could have a role in skin ageing.
Therefore, it is postulated that collagen tissue organisation may also play a role in mechanic stimulation of fibroblasts (Fisher et al. 2008). In aged skin, it has been proven that fibroblasts partially lose their characteristic elongated shape, presenting less contact surface with collagen bundles (Varani et al. 2006). In addition, cell cultures of dermal fibroblasts that have been transfected to express a mutant MMP-1 (MMP-1 V94G) experience fragmentation of the synthesised collagen and, what is more relevant, morphologic and functional alterations similar to the ones occurring with ageing: a rounded aspect and decreased contact surface with collagen, decreased procollagen and CTFG production due to alterations in TGF-β pathway and actin skeleton disassembly, which participates in the transmission of mechanical forces among extracellular matrix and fibroblasts (Oender et al. 2008; Xia et al. 2013).
The parallel arrangement of skin collagen during senescence that was demonstrated in our study could at least partly explain this lack of fibroblast mechanical stress transduction, as a result of progressing from an adult dermis with disorganised collagen bundles and arranged in the three spatial directions (and with important tridimensional stress forces transmitted to fibroblasts), to an aged dermis with a parallel arrangement of collagen (and with a minor capability to transmit stress forces to fibroblasts). This superior parallel arrangement of dermal collagen has been only proved in one previous study (Moragas et al. 1998). The loss of thickness of dermal collagen during ageing would also have a role in this defective fibroblast mechanical stress transduction (Varani et al. 2006). All these findings highlight the importance of the extracellular matrix in the skin ageing process.
Especially noteworthy is the fact that, with age, we have found an increase in the thickness of papillary dermis, in spite of the decrease in the density of collagen bundles and the loss of thickness of these bundles that takes place. The increase of the thickness of papillary dermis with ageing has already been demonstrated in a previous study (Branchet et al. 1990). It might be possible that the thickness of this dermal layer increases at the expense of dermatan sulphate, because the quantity of this molecule in papillary dermis has been proved to increase since the fifth decade of life (Longas et al. 1987; Willen et al. 1991). Nevertheless, the exact cause of this observation remains to be elucidated and should be researched in depth. Besides, ecography-based studies invariably show the presence of a subepidermal low echogenic band (SLEB or SENEB) in aged skin (Gniadecka, 2001). This band, located in papillary dermis, increases its thickness according to age and is correlated with changes in collagen structure and water accumulation (Waller & Maibach, 2005). Accordingly, this increase in the amount of water, possibly related to the previously mentioned increase in dermatan sulphate, could explain the increase in the thickness of papillary dermis with age that we have shown.
Different parameters determined in the present study have relevance for dermal tissue functionality. On the one hand, mechanical force and stress resistance depend not only on the number of collagen bundles but also on their tissue orientation. Bundle diameter is important as well (Ottani et al. 2001), as a loss of collagen thickness in different tissues, with small and rigid molecules that are not very break-resistant, has been described during senescence, and big bundles are the ones that support high stress levels (Parry, 1988).
Concluding remarks
The arrangement of collagen bundles an exceptional importance: it is important to delve into tissue structure and the disposition adopted by collagen bundles, not just into the synthesis and degradation of these molecules. The present study provides a detailed description of dermal collagen bundles and their arrangement in the tissue, and discusses the possible relationship with functional parameters that have shown their involvement in skin development and ageing, emphasising the fundamental role played by connective tissue microenvironment in these processes.
Using data obtained from our work we have developed an evolutionary morphological model of different histopathological parameters and their modification according to age (Fig. 4).
Fig. 4.
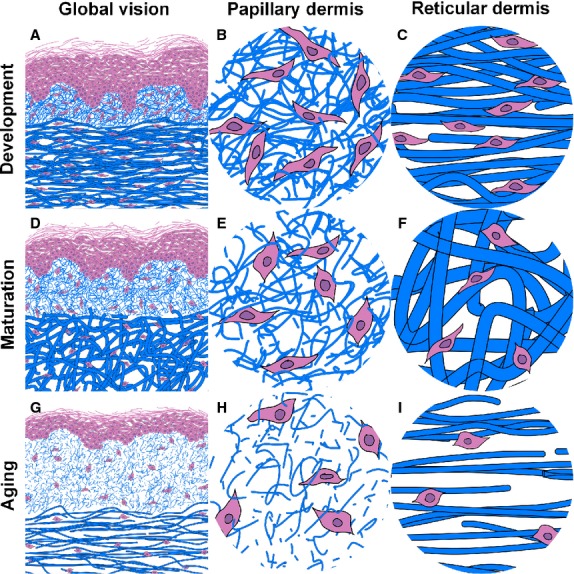
Proposed evolutional model from the histopathological parameters throughout life. After birth, the skin has a high density of fine bundles of collagen, in a parallel disposition in the reticular dermis. Progressive development and remodelling of the tissue cause several observable changes during adult life: the density of the bundles is reduced in the reticular dermis, but these bundles reach their maximal thickness and disposition in the three directions of the space, which conditions an increase in the thickness of the reticular dermis; in the papillary dermis, on the other hand, the density is kept stable but the bundles lose thickness and the papillary dermis increases its width. Finally, in the last stage of life during the intrinsic cutaneous ageing the collagen bundles lose density and thickness both in the papillary and reticular dermis, and, in this last localisation, the collagen is again disposed in a parallel manner. The reticular dermis becomes thinner but the papillary dermis continues to increase in thickness.
As a conclusion, the present paper compiles and confirms in one study population different histopathological parameters for intrinsic skin ageing and skin development. The model that we present provides a homogeniser baseline data that could be useful in future studies trying to delve into the effects that different drugs, cutaneous or systemic diseases or extrinsic conditions (e.g. environmental pollutants, solar radiation) produce on the macrostructural arrangement of dermal collagen, but also in the existing relationship between morphologic characteristics of the skin and functional factors involved in development and ageing biology. Further studies should confirm whether these findings are also applicable to other anatomical sun-protected skin sites, and objective comparison with photoageing should be done to clarify the extent to which the histopathological and morphometric changes in intrinsic ageing and photoageing differ.
Acknowledgments
We are grateful to Juan Bellido Blasco for his support in designing and managing the statistics found on this research paper.
Author contributions
Víctor Marcos Garcés carried out the morphometric analysis, interpreted the data, designed the tables and graphs and wrote the article. Pilar Molina Aguilar reviewed the work and contributed to the data collection. Antonio Ferrández Izquierdo and José Benavent Seguí took the samples and processed them for its histopathological analysis. Víctor García Bustos and Carlos Bea Serrano contributed to the morphometric analysis and data interpretation. Amparo Ruiz Saurí designed the study, interpreted the data and supervised the work. All authors reviewed the final version of the manuscript and provided their consent before its submission.
References
- Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Baroni Edo R, Biondo-Simões M de LP, Auersvald A, et al. Influence of aging on the quality of the skin of white women: the role of collagen. Acta Cir Bras. 2012;27:736–740. doi: 10.1590/s0102-86502012001000012. [DOI] [PubMed] [Google Scholar]
- Berneburg M, Plettenberg H, Krutmann J. Photoaging of human skin. Photodermatol Photoimmunol Photomed. 2000;16:239–244. doi: 10.1034/j.1600-0781.2000.160601.x. [DOI] [PubMed] [Google Scholar]
- Branchet MC, Boisnic S, Frances C, et al. Skin thickness changes in normal aging skin. Gerontology. 1990;36:28–35. doi: 10.1159/000213172. [DOI] [PubMed] [Google Scholar]
- Callaghan TM, Wilhelm K-P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: cellular and molecular perspectives of skin ageing. Int J Cosmet Sci. 2008;30:313–322. doi: 10.1111/j.1468-2494.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Gelman L, Lutz R, et al. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Chung JH, Seo JY, Choi HR, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001;117:1218–1224. doi: 10.1046/j.0022-202x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- Chung JH, Hanft VN, Kang S. Aging and photoaging. J Am Acad Dermatol. 2003;49:690–697. doi: 10.1067/s0190-9622(03)02127-3. [DOI] [PubMed] [Google Scholar]
- Dellambra E, Dimri GP. Chapter 7 – Cellular senescence and skin aging. In: Dayan N, editor. Skin Aging Handbook. Norwich, NY: William Andrew Publishing; 2009. pp. 129–148. Personal Care & Cosmetic Technology. Available at: http://www.sciencedirect.com/science/article/pii/B9780815515845500119 [accessed 11 October 2013] [Google Scholar]
- Dumas M, Chaudagne C, Bonté F, et al. In vitro biosynthesis of type I and III collagens by human dermal fibroblasts from donors of increasing age. Mech Ageing Dev. 1994;73:179–187. doi: 10.1016/0047-6374(94)90050-7. [DOI] [PubMed] [Google Scholar]
- El-Aal NHA, El-Wadood FAA, Moftah NH, et al. Morphometry and epidermal fas expression of unexposed aged versus young skin. Indian J Dermatol. 2012;57:181–186. doi: 10.4103/0019-5154.96188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage MA, Miller KW, Berardesca E, et al. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10:73–86. doi: 10.2165/00128071-200910020-00001. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth JJ, Allen RG, Tresini M, et al. Abundance of α1(I) and α1(III) procollagen and p21 mRNAs in fibroblasts cultured from fetal and postnatal dermis. Mech Ageing Dev. 1997;97:131–142. doi: 10.1016/s0047-6374(97)00051-1. [DOI] [PubMed] [Google Scholar]
- Gallivan K, Alman BA, Moriarty KP, et al. Differential collagen I gene expression in fetal fibroblasts. J Pediatr Surg. 1997;32:1033–1036. doi: 10.1016/s0022-3468(97)90393-6. [DOI] [PubMed] [Google Scholar]
- Gniadecka M. Effects of ageing on dermal echogenicity. Skin Res Technol. 2001;7:204–207. doi: 10.1034/j.1600-0846.2001.70310.x. [DOI] [PubMed] [Google Scholar]
- Gunin AG, Kornilova NK, Vasilieva OV, et al. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol A Biol Sci Med Sci. 2010;66A:385–392. doi: 10.1093/gerona/glq205. [DOI] [PubMed] [Google Scholar]
- Hernández-Pérez M, Mahalingam M. Matrix metalloproteinases in health and disease: insights from dermatopathology. Am J Dermatopathol. 2012;34:565–579. doi: 10.1097/DAD.0b013e31821e8744. [DOI] [PubMed] [Google Scholar]
- Imayama S, Braverman IM. A hypothetical explanation for the aging of skin. Chronologic alteration of the three-dimensional arrangement of collagen and elastic fibres in connective tissue. Am J Pathol. 1989;134:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- Janson D, Saintigny G, Mahé C, et al. Papillary fibroblasts differentiate into reticular fibroblasts after prolonged in vitro culture. Exp Dermatol. 2013;22:48–53. doi: 10.1111/exd.12069. [DOI] [PubMed] [Google Scholar]
- Kakasheva-Mazhenkovska L, Milenkova L, Kostovska N, et al. Histomorphometrical characteristics of human skin from capillitium in subjects of different age. Prilozi. 2011;32:105–118. [PubMed] [Google Scholar]
- Kohl E, Steinbauer J, Landthaler M, et al. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25:873–884. doi: 10.1111/j.1468-3083.2010.03963.x. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Zheng PS, Dong G. Aged skin: a study by light, transmission electron, and scanning electron microscopy. J Invest Dermatol. 1987;88:44s–51s. doi: 10.1111/1523-1747.ep12468934. [DOI] [PubMed] [Google Scholar]
- Longas MO, Russell CS, He XY. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydr Res. 1987;159:127–136. doi: 10.1016/s0008-6215(00)90010-7. [DOI] [PubMed] [Google Scholar]
- Mine S, Fortunel NO, Pageon H, et al. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS ONE. 2008;3:e4066. doi: 10.1371/journal.pone.0004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moragas A, García-Bonafé M, Sans M, et al. Image analysis of dermal collagen changes during skin aging. Anal Quant Cytol Histol. 1998;20:493–499. [PubMed] [Google Scholar]
- Naylor EC, Watson REB, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69:249–256. doi: 10.1016/j.maturitas.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Oender K, Trost A, Lanschuetzer C, et al. Cytokeratin-related loss of cellular integrity is not a major driving force of human intrinsic skin aging. Mech Ageing Dev. 2008;129:563–571. doi: 10.1016/j.mad.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Ottani V, Raspanti M, Ruggeri A. Collagen structure and functional implications. Micron. 2001;32:251–260. doi: 10.1016/s0968-4328(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Pageon H, Zucchi H, Asselineau D. Distinct and complementary roles of papillary and reticular fibroblasts in skin morphogenesis and homeostasis. Eur J Dermatol. 2012;22:324–332. doi: 10.1684/ejd.2012.1693. [DOI] [PubMed] [Google Scholar]
- Parry DA. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys Chem. 1988;29:195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40:466–472. doi: 10.1016/j.exger.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Quan T, Shao Y, He T, et al. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Invest Dermatol. 2010;130:415–424. doi: 10.1038/jid.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Robichaud P, et al. CCN1 contributes to skin connective tissue aging by inducing age-associated secretory phenotype in human skin dermal fibroblasts. J Cell Commun Signal. 2011;5:201–207. doi: 10.1007/s12079-011-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Wang F, Shao Y, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133:658–667. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe JH, Mamelak AJ, McElgunn PJS, et al. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2005;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand J Med Sci Sports. 2005;15:223–230. doi: 10.1111/j.1600-0838.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Sephel GC, Buckley A, Davidson JM. Developmental initiation of elastin gene expression by human fetal skin fibroblasts. J Invest Dermatol. 1987;88:732–735. doi: 10.1111/1523-1747.ep12470403. [DOI] [PubMed] [Google Scholar]
- Stamatas GN, Nikolovski J, Luedtke MA, et al. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol. 2010;27:125–131. doi: 10.1111/j.1525-1470.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Spearman D, Perone P, et al. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11:221–235. doi: 10.1111/j.0909-725X.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- West MD, Pereira-Smith OM, Smith JR. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989;184:138–147. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- Willen MD, Sorrell JM, Lekan CC, et al. Patterns of glycosaminoglycan/proteoglycan immunostaining in human skin during aging. J Invest Dermatol. 1991;96:968–974. doi: 10.1111/1523-1747.ep12476335. [DOI] [PubMed] [Google Scholar]
- Xia W, Hammerberg C, Li Y, et al. Expression of catalytically active matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell. 2013;12:661–671. doi: 10.1111/acel.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- van Zuijlen PPM, de Vries HJC, Lamme EN, et al. Morphometry of dermal collagen orientation by Fourier analysis is superior to multi-observer assessment. J Pathol. 2002;198:284–291. doi: 10.1002/path.1219. [DOI] [PubMed] [Google Scholar]