Abstract
To date, four subtypes of adenosine receptors have been cloned (A1R, A2AR, A2BR, and A3R). In a previous study we used confocal immunocytochemistry to identify A1R and A2AR receptors at mouse neuromuscular junctions (NMJs). The data shows that these receptors are localized differently in the three cells (muscle, nerve and glia) that configure the NMJs. A1R localizes in the terminal teloglial Schwann cell and nerve terminal, whereas A2AR localizes in the postsynaptic muscle and in the axon and nerve terminal. Here, we use Western blotting to investigate the presence of A2BR and A3R receptors in striated muscle and immunohistochemistry to localize them in the three cells of the adult neuromuscular synapse. The data show that A2BR and A3R receptors are present in the nerve terminal and muscle cells at the NMJs. Neither A2BR nor A3R receptors are localized in the Schwann cells. Thus, the four subtypes of adenosine receptors are present in the motor endings. The presence of these receptors in the neuromuscular synapse allows the receptors to be involved in the modulation of transmitter release.
Keywords: adenosine receptors, cholinergic synapses, immunofluorescence, motor end-plate, motor nerve terminal
Introduction
In neuronal contacts, neurotransmitter release is ultimately controlled by the functional confluence of several metabotropic receptor-mediated signaling pathways modulated in an activity-dependent manner. In the neuromuscular synapse, presynaptic muscarinic (mAChRs, Santafe et al. 2003, 2004) and nicotinic (nAChRs, Salgado et al. 2000) ACh autoreceptors directly couple ACh secretion to the regulation of the release mechanism itself. Presynaptic neurotrophin and cytokine receptors (Bibel & Barde, 2000; Roux & Barker, 2002; Pitts et al. 2006), responding to target-derived mediators, also cooperate. In addition, nerve terminal and muscular activity contributes to the build up of extracellular adenosine in the neuromuscular junction (NMJ; Cunha & Sebastiao, 1993; Ribeiro et al. 1996), which also seems to modulate presynaptic ACh release through adenosine purinergic receptors (P1Rs; Correia-de-Sa et al. 1991; Salgado et al. 2000; Garcia et al. 2013). To date, four subtypes of P1R receptors have been cloned (A1R, A2AR, A2BR, and A3R; Ralevic & Burnstock, 1998).
It has been reported that micromolar concentrations of the P1R endogenous agonist adenosine reduced evoked quantal content and/or spontaneous ACh release in frog (Searl & Silinsky, 2005; Shakirzyanova et al. 2006; Adamek et al. 2010) and rat NMJs (De Lorenzo et al. 2006; Pousinha et al. 2010). In rats, however, submicromolar adenosine can have the opposite effect (Pousinha et al. 2010). It has been suggested that a complex balance of A1R/A2AR may determine the inhibition/potentiation of ACh release (Pousinha et al. 2010). However, most functional experiments have been carried out in recording conditions that interfere with the synapse physiology (high Mg2+ or curare for preventing muscle contraction). We performed experiments with μ-CgTx-GIIIB in which only the voltage-dependent sodium channel of the muscle cells was shut down, thus resulting in non-contractile muscles that have preserved the NMJ physiology (Garcia et al. 2013). In these conditions, we observed A1R and A2AR immunolabeling in the motor nerve terminals (see also Baxter et al. 2005) and both A1R and A2AR may protect synaptic function by reducing depression during repetitive activity (Garcia et al. 2013). Our observation that the P1R generic agonist adenosine and the non-selective antagonist 8-SPT modify the frequency-dependent depression of transmission, whereas the A1R and A2AR selective receptor agonist and antagonist ligands (for A1R: CCPA and DPCPX; for A2AR subtype CGS-21680 and SCH-58261) have no effect (Garcia et al. 2013), strongly suggests that some effects might be caused by other adenosine receptors (A2BR and A3R). Therefore, we used immunohistochemistry to investigate the presence of A2BR and A3R receptors in the three cells of the adult NMJ (neuron, glia and muscle). The data show that A2BR and A3R receptors are present in the nerve terminal and muscle cells on the NMJs. Thus, the four subtypes of adenosine receptors are present in the motor endings.
Materials and methods
Animals
Experiments were performed on the Levator auris longus (LAL) muscle of adult male Swiss mice (30–40 days postnatal; Criffa, Barcelona, Spain). The mice were cared for in accordance with the guidelines of the European Community's Council Directive of 24 November 1986 (86/609/EEC) for the humane treatment of laboratory animals. The animals were anesthetized with 2% tribromoethanol (0.15 mL 10 g–1 body weight, i.p.). The study was approved by the Ethics Committee of the Rovira i Virgili University (Ref. number 233).
Antibodies
To study the presence and locate the A2B and A3R adenosine receptors, we used two different antibodies for each receptor. For the A2BR adenosine receptor we tested rabbit anti-A2BR adenosine receptor (AB1589P; Millipore Corporation, Temecula, CA, USA) and goat polyclonal anti-A2BR adenosine receptor (N-19) (sc-7506; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The anti-A2BR adenosine receptor (AB1589P; Millipore) recognizes not only a band of approximately 50–52 kDa in several rat, mouse and human tissues (thymus, placenta, colon and small intestine) but also a 35-kDa band in small intestine (data sheet). It stained human skeletal muscle cell membrane (Lynge & Hellsten, 2000). The goat anti-A2BR adenosine receptor (N-19) (sc-7506; Santa Cruz Biotechnology, Inc.) can detect a single band at 45 kDa (data sheet). This antibody was used by Carreira et al. (2006) to detect this band in growth hormone secretagogue receptor type-1a cells (GHS-R1a) and also to detect immunoreactivity in brain (Rosi et al. 2003). For the A3R adenosine receptor we used two polyclonal anti-rabbit antibodies (anti-A3R adenosine receptor: AB1590P; Millipore Corporation), and anti-A3R adenosine receptor (H80) (sc-13938; Santa Cruz Biotechnology, Inc.). The anti-A3R adenosine receptor (AB1590P, Millipore) from rat brain membranes runs as a 40-kDa band and a 52-kDa band in rabbit uroepithelium membranes (Yu et al. 2006). With this antibody, Rebola et al. (2005) located A3R in cortical neurons and Lopes et al. (2003) in hippocampus. The anti-A3R adenosine receptor (H80) (sc-13938; Santa Cruz) detected three different bands at 44, 52 and 66 kDa (data sheet). These antibodies provided a strong and specific mark and detected A2BR and A3R at the same localization at the neuromuscular junction.
Specificity of antibodies
As a negative control, the primary antibodies were omitted from some muscles during the immunohistochemical and Western blot procedures to determine background staining for secondary antibodies. These control muscles did not exhibit positive staining or reveal bands of the appropriate molecular weight with the respective procedures. Moreover, as a negative control, in Western blot experiments, antigenic peptides for A2BR and A3R were preabsorbed with their respective primary antibodies overnight [5 : 1 μg of control peptide: primary antibody; A2BR peptide AG292 (Millipore Corporation), A2BR peptide sc-7506P (Santa Cruz Biotechnology, Inc.), and A3R AG294 (Millipore Corporation)]. As a positive control for A3R from Santa Cruz we used a mouse brain extract (sc-2253; Santa Cruz, Biotechnology, Inc., not shown).
Various different types of negative control were carried out in immunohistochemistry protocols: in the first, the primary antibody was omitted (not shown); in the second, the primary antibody was preincubated with a control peptide (5 : 1 μg of control peptide: primary antibody) for 4 h prior to use (see Figs 2D,I and 3D), and in the third, we demonstrated a possible cross-linking between the primary antibodies that joined the secondary antibodies. We omitted the primary antibody for adenosine A2BR and A3R but not the corresponding secondary antibody Alexa-Fluor® 488-donkey anti-rabbit or Alexa-Fluor 488®-donkey anti-goat. We also incubated with the appropriate primary antibody to detect the SYN or S-100 protein and with the specific secondary antibody Alexa-Fluor 647-donkey anti-mouse (see Fig. 3H for the A3R example from Santa Cruz). nAChRs were stained with TRITC-α-BTX. In all cases the immunostaining was completely abolished.
Fig. 3.
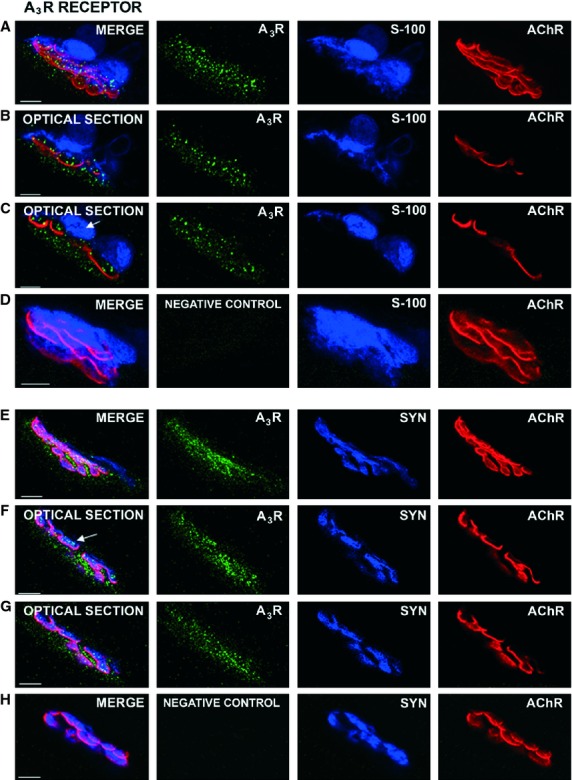
Localization of the A3R receptors by immunohistochemistry at the neuromuscular junction. In these adult muscles, A3R receptors are strongly immunolabeled in the neuromuscular junction. (A–D) images were obtained using the rabbit polyclonal antibody AB1590P (Millipore Corporation) and (E–H) images using the rabbit polyclonal antibody (H80) (sc-13938, Santa Cruz Biotechnology, Inc.). The merge images (from at least 10 confocal Z planes) of the synapses in (A,E) show an abundant granular A3R labeling in the synaptic area. The optical sections (from the synapse in A) in (B,C) show immunolabeled spots in the nerve terminal position (blue in the space between glial cells and red in the space between muscle cells). Syntaxin-labeled terminal axons are also positive to the A3R (arrow in F is an individual optical section of the synapse in E). Fine granular marks are observed in the muscle cell below the postsynaptic gutters (for instance in F,G). The A3R label does not colocalize with S-100 (arrow in C). (D,H) Examples of negative control for A3R proteins (see Results). Scale bar: 10 μm.
Western immunoblotting
Western blot analysis was performed on adult and newborn P6 LAL muscles and adult brain and spinal cord in homogenized samples (1/10 w/v) with buffer containing 150 mm NaCl, 20 mm Tris-HCl, pH 7.5, 2 mm EGTA, and 5 mm EDTA supplemented with 1% Triton X-100, 10 mm β-mercaptoethanol, and a protease inhibitor mixture [1.4 mm aminoethyl benzenesulfonyl fluoride hydrochloride (AEBSF, serine protease inhibitor), 0.8 μm aprotinin, 0.02 μm leupeptin, 0.04 μm bestain, 0.015 μm pepstatin A, 0.015 μm E-64, Sigma, St. Louis, MO, USA)]. After the insoluble material had been extracted by centrifugation at 4000 × g for 5 min, the samples were centrifuged at 15 000 × g for 15 min. Finally, the resulting supernatants (total protein lysates) were collected. Protein concentrations were determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA, USA). Each sample was diluted with one volume of SDS-PAGE buffer containing Tris 0.5 m, pH = 6.8, 10 mm β mercaptoethanol, 10% sodium dodecylsulfate (SDS), 20% glycerol and 0.006% bromophenol blue. The proteins (100 μg) were separated according to their molecular weight by 10% SDS (Laemmli, 1970). Immunoblotting was conducted using polyvinylidene difluoride (PVDF) membranes (HybondTM-P; GE Healthcare, Amersham, UK) and the transfer was carried out for 1 h at 225 mA per membrane. The membranes were blocked with 5% nonfat milk in Tris-buffered saline (50 mm Tris pH 7.4, 200 mm NaCl, 0.1% Triton X-100, 0.2% Tween 20 for 1 h) and then incubated overnight at +4 °C with polyclonal antibodies against the adenosine receptors (see Antibodies) (A2BR: rabbit polyclonal antibody anti-A2BR adenosine receptor (1/500), AB1589P, Millipore; and goat polyclonal antibody anti- A2BR adenosine receptor 1/500, N-19: sc-7506, Santa Cruz); for A3R, rabbit polyclonal antibody anti-A3R adenosine receptor (1/500), AB1590P (Millipore), and rabbit polyclonal antibody anti-A3R adenosine receptor (1/500) were used (H80: sc-13938; Santa Cruz). After washing, the membranes were incubated with a secondary antibody coupled to horseradish peroxidase (1 : 10 000 dilution) from Jackson Immunoresearch (West Grove, PA, USA) and revealed by enhanced chemiluminescence with the ECL kit (Amersham Live Science). The blots were visualized with a VersaDoc 3000 (Bio-Rad). The densitometry program metamorph and microscopy automation & image analysis software (Molecular Devices, LLC, US) was used to analyse the density of the various bands. Data were taken from densitometry measurements made in at least five separate experiments, and normalized by actin (rabbit polyclonal antibody; 4969, Cell Signalling Technology, Danvers, MA, USA; not shown). The integrated optical density of the bands was normalized to the background values. As a loading control, we tested two different concentrations of whole lysates that were normalized to actin. We did not observe any saturation signaling in ECL detection and similar quantitative results have been obtained with these different dilutions. Finally, we used a total protein analysis (Sypro Ruby protein blot stain, Bio-Rad) to measure the total protein concentration on polyvinylidene difluoride (PVDF) membranes. Each sample contained the same amount of total protein and ratios between different lines were always lower than 1.12 ± 0.1. Using this methodology we obtained the same results when normalization was carried out with respect to actin or to total protein concentration, ensuring the accuracy of the results. The relative variations between the bands in P6 and P30 muscles were calculated in the same image. Data are mean values ± SD.
Immunohistochemistry
To confirm the presence of adenosine A2BR and A3R receptors in the nerve terminals, we used whole mounts of LAL muscles which were removed and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 45 min at room temperature (∼ 22 °C). Muscles were incubated in mixtures of primary antibodies raised in different species (anti-A2BR or A3R antibody and anti-syntaxin or anti-S100) and then rinsed and incubated with appropriate secondary antibodies. Muscles were incubated overnight at 4 °C with the rabbit polyclonal antibody against the adenosine A2BR (1/50), AB1589P (Millipore), goat polyclonal antibody anti-A2BR adenosine receptor (1/100), N-19: sc-7506 (Santa Cruz); rabbit polyclonal antibody anti-A3R adenosine receptor (1/200), AB1590P (Millipore); and rabbit polyclonal antibody anti-A3R adenosine receptor (1/500) (H80) (sc-13938; Santa Cruz). For the immunohistochemistry we also used antibodies that are commonly used as markers to detect the different parts of the NMJ (syntaxin and S100): the mouse monoclonal antibody (Sigma) and the mouse anti-S100 antibody (Acris, Germany). The AChRs were detected with α-BTX conjugated with TRITC from Molecular Probes (Eugene, OR, USA). The muscles were then incubated for 4 h at room temperature in a mixture of secondary antibodies conjugated with Alexa-Fluor 488® or Alexa-Fluor 647®. Muscle fibers were mounted in Mowiol with p-phenylenediamide (Sigma).
Labeled NMJs were viewed with a laser scanning confocal microscope (Nikon TE2000-E). Special consideration was given to the possible contamination of one channel by another. In experiments involving negative controls, the photomultiplier tube gains and black levels were identical to those used for a labeled preparation made in parallel with the control preparations. In the immunostaining experiments, at least six muscles and 150 NMJs were observed for each receptor and age. Five additional muscles were used as negative controls. Images were assembled using Adobe photoshop software, and the contrast and brightness were not modified.
Statistical procedure
Western immunoblotting
The statistical software spss© v20 was used to analyse the results. The medians between two groups were compared using the nonparametric Mann–Whitney test for independent samples.
Results
Presence of A2BR and A3R proteins in the LAL muscle
Western blot analysis was performed to determine the presence of the A2BR and A3R receptors in the newborn (P6) and adult (P30) skeletal muscle as compared with the adult brain and spinal cord (Fig. 1). We used various antibodies to determine the presence of these proteins and the experiments revealed significant amounts of A2BR and A3R adenosine receptors in the muscle tissue (Fig. 1). All antibodies reacted with bands consistent with the predicted molecular weight. The antibodies used only recognized the corresponding protein and no cross-reactivity between adenosine receptors was detected. A2BR and A3R were detected in whole lysates of muscle and nerve tissues. Using the A2BR antibody from Millipore (AB1589P) we detected a band at 51 kDa (brain and spinal cord samples) and a band at 52 kDa (P6 and P30 muscle samples). With the Santa Cruz antibody (sc-7506) bands appeared at approximately 47 kDa for muscle and 45 kDa for adult brain and spinal cord (Fig. 1A, top). A3R Millipore antibody (AB1590P) and A3R Santa Cruz antibody (sc-13938) detected a single band at 44 kDa similar to the expected molecular weight (Fig. 1A, bottom). We performed a quantitative study (densitometry program: metamorph and microscopy automation & image analysis software; Molecular Devices, LLC, Sunnyvale, CA, US) to analyse the density of the bands and evaluate the relative amount of the two adenosine receptors in both the newborn and adult muscle (Fig. 1B). The results show that the A2BR and A3R proteins are expressed slightly, although significantly (P < 0.05), more in the adult muscle than in the newborn. The results were the same (P > 0.05) when both antibodies were used for each receptor (for A2BR: adult/newborn ratio = 1.34 ± 0.2 and 1.41 ± 0.1 (n = 5) and for A3R: adult/newborn ratio = 1.7 ± 0.15 and 1.6 ± 0.07 n = 5) for Santa Cruz and Millipore antibodies, respectively. In the adult (P30), A2BR in brain is higher than in muscle (∼ 2.5 times higher with both antibodies). A2BR in the adult spinal cord was 1.92 and 1.78 times greater than in muscle when the Santa Cruz and the Millipore antibodies were used, respectively (P < 0.05 vs. P = 0.029). In addition, A3R protein is slightly more abundant in P30 muscle than in brain and spinal cord: muscle/brain ratio = 1.43 ± 0.13 and 1.6 ± 0.1 (n = 5) and muscle/spinal cord ratio = 1.7 ± 0.1 and 1.73 ± 0.03 for Santa Cruz and Millipore antibodies, respectively; P < 0.05 vs. P = 0.029.
Fig. 1.
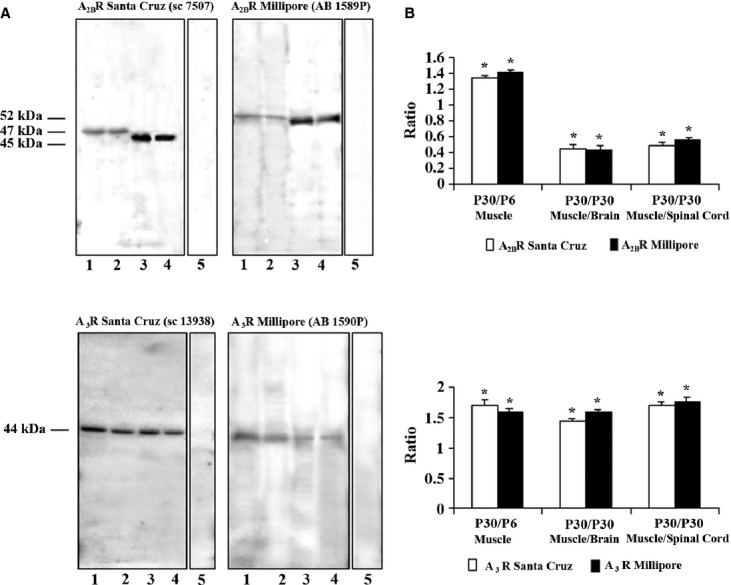
Presence of A2BR and A3R in P6 and P30 LAL muscle. (A) Representative Western blotting analysis of A2BR (top) and A3R (bottom) (with antibodies from Millipore and Santa Cruz) protein presence in lysates of adult muscle (P30, line 1), neonatal muscle (P6, line 2), adult brain (line 3) and adult spinal cord (line 4). Samples are 100 μg of protein. Line 5 (for A2BR and A3R from Millipore) is the negative control, incubated with blocked peptide. Line 5 (for A3R from Santa Cruz) is the negative control incubated without primary antibody). As a positive control, we used adult brain and spinal cord. Actin immunoblots were used for protein loading controls (not shown). (B) Quantitative analysis of several A2BR (top) and A3R (bottom) Western blots normalized by actin staining. The data show that the presence of A2BR and A3R protein is more abundant in the adult muscle than in the newborn muscle when both antibodies are used. Also, A2BR is more abundant in brain and spinal cord than in muscle whereas A3R protein is slightly more abundant in muscle than in brain and spinal cord. Results are expressed as mean ± SEM of five independent experiments, *P < 0.05.
In conclusion, A2BR and A3R receptors are expressed in whole skeletal muscle. An immunohistochemical analysis was performed to identify the cellular localization in the NMJ (i.e. muscle cells, Schwann cells or nerve terminals).
Immunohistochemical localization of the A2BR and A3R receptors at the neuromuscular junction
We performed immunofluorescence staining coupled with confocal microscopy analysis to determine the possible presence and localization of A2BR and A3R receptors at P30 NMJs (Figs 2 and 3).
Fig. 2.
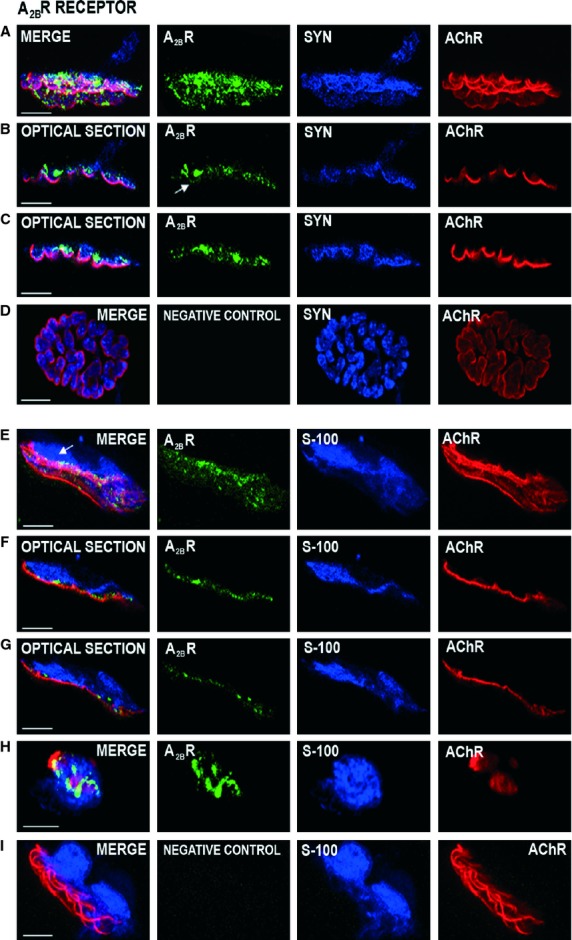
Localization of the A2BR receptors by immunohistochemistry at the neuromuscular junction. Immunofluorescence staining and confocal microscopy analysis. Triple labeling of A2BR protein (green fluorescence) with syntaxin or S-100 (blue fluorescence) and nAChR-alpha-bungarotoxin (red fluorescence). Images (A–C) were obtained using the rabbit polyclonal antibody against the adenosine A2BR (AB1589P, Millipore Corporation) and (E–H) images using the goat polyclonal antibody anti-A2BR-adenosine receptor, N-19 (sc-7506, Santa Cruz Biotechnology Inc.). A2BR immunoreactivity shows that the receptor is present in the nerve terminal. In (A), the A2BR label is observed in a wide area on the syntaxin positive nerve ending. In (B,C), the side view of the two individual optical sections of the synapse in (A) shows the A2BR spots colocalized with the blue syntaxin label in some synaptic gutters and in the postsynaptic membrane (arrow in B, green image). In addition, a fine line granular labeling occurs in the space of the nerve terminals between the blue Schwann cell and the red postsynaptic line (F and G are two individual optical sections of the synapse in E). The A2BR is not labeled in the area that corresponds to S-100 antibody (arrow in E). (H) shows a detail of A2BR positive synaptic boutons on a nerve terminal (in green) seen en face. (D,I) An example of negative control for A2BR proteins (see Results). Scale bar: 10 μm.
A2BR
The A2BR receptor was present at the neuromuscular junction (Fig. 2). The conventional immunohistochemistry (A) with syntaxin as a co-marker and (E) with S-100 as a co-marker shows a green label in the neuromuscular area (Fig. 2). These figures are images that have been merged from at least 10 confocal Z planes obtained every 0.5 μm in the Z-stacks from two NMJs obtained from the whole-muscle preparation. In several individual confocal optical sections [for instance, (B) and (C) are two sections of the synapse in (A)] clear side views of these synapses show a good colocalization of the blue syntaxin label and A2BR receptor in some synaptic gutters. However, it seems that A2BR receptor immunolabeling is not present in the teloglial, preterminal Schwann cells (arrow in Fig. 2E ). The absence of the A2BR mark in the glia can be best observed in Fig. 2(F,G), which are two single confocal Z planes of the total images from the NMJ in (E). Figure 2(E-G) also shows a fine granulated line in the area between the red nAChR clusters and the S-100-positive blue Schwann cell which corresponds to the nerve terminal region. Figure 2(H) shows an en face NMJ with the nerve terminal synaptic boutons in green. Interestingly, in many optical sections, a very fine granulate immunoreactivity can be observed in the postsynaptic membrane that coincides perfectly with the red AChR cluster line (see arrows in Fig. 2B). Figure 2(D,I) shows examples of negative control for the A2BR antibody from Millipore and Santa Cruz, respectively, using preteatment with an excess of the appropriate blocking peptide. The control muscles did not exhibit positive staining.
In summary, the A2BR receptor seems to be localized in the nerve terminals and postsynaptic membrane of the NMJ.
A3R
The A3R receptor is present in muscle cells and the nerve terminal (Fig. 3). The conventional immunohistochemistry (A) with S-100 as a co-marker and (E) with syntaxin as a co-marker shows an A3R-positive green fine granulation in the neuromuscular area (Fig. 3). In greater detail, the optical sections (from the synapse in 3A) in Fig. 3(B,C) show spot-like immunoreactive deposits in the locality of the nerve terminals, in the space between the glial cell (blue) and the muscle cell (red). The lateral view of the NMJ in Fig. 3(E) was subject to detailed analysis in two optical sections (Fig. 3F,G) and it shows a coincidence between A3R and the blue-labeled syntaxin mark (arrow in Fig. 3F). The teloglial Schwann cell does not show any immunofluorescence (arrow in Fig. 3C). The A3R receptor is also present below the red postsynaptic receptors (see Fig. 3F,G). Figure 3(D) shows an example of a negative control for the A3R antibody from Millipore, using preteatment with an excess of appropriate blocking peptide and Fig. 3(H) shows an example of negative control for the A3R antibody from Santa Cruz. In this case, we omitted the primary antibody for adenosine A3R and incubated with the appropriate primary antibody to detect the SYN protein. We used two specific secondary antibodies (Alexa-Fluor 488®-donkey anti-rabbit and Alexa-Fluor 647®-donkey anti-mouse). These control muscles did not exhibit positive staining.
In summary, the A3R receptor is also localized in the pre- and postsynaptic components of the NMJ.
Discussion
Since the pioneering studies by Ribeiro et al. in the early 1970s (Ribeiro & Walker, 1973), it is now known that adenosine and adenosine triphosphate (ATP) released by nerve endings help to modulate the presynaptic function through purinergic autoreceptors (adenosine P1Rs and ATP P2Rs; Correia-de-Sa et al. 1991; Salgado et al. 2000). Recently, we observed in adult mouse NMJs that the involvement of adenosine receptors mainly reduces spontaneous quantal leak of ACh (an A1R effect) and protects the synaptic function by reducing depression during repetitive activity (an A1R and A2AR effect; Garcia et al. 2013). A non-selective antagonist of the adenosine receptors (8-SPT) increases the frequency-dependent depression of transmission, whereas A1R and A2AR selective antagonists (DPCPX and SCH-58261, respectively) have no effect (Garcia et al. 2013), which strongly suggests that some effects might be caused by other adenosine receptors (A2BR and A3R). Thus, here, we have performed experiments to identify A2BR and A3R receptors in striated muscle and localize them in the NMJ.
A2BR and A3R receptors in striated muscle
Our previous Western blot analysis shows that A1R and A2AR are highly expressed in muscle. A1R is more abundant in the adult and A2AR is more abundant in the newborn (Garcia et al. 2013). No previous studies have been carried out to determine specifically the molecular form of the A2BR and A3R in striated skeletal muscle, although different molecular sizes have been reported in different species and tissues when different antibodies have been used. Therefore, we decided to compare two different commercial antibodies for each receptor protein.
A2BR
Using A2BR antibody from Millipore (AB1589P) we detected a band at 51 kDa (brain and spinal cord samples) and a band at 52 kDa (P6 and P30 muscle samples). Peyot et al. (2000) and Jackson et al. (2002) detected a protein band at 52 kDa in arterial smooth muscle cells and kidney, respectively. However, Lynge & Hellsten (2000) found a single band at 28 kDa in human skeletal muscle. This band may correspond to a part of the molecule. Using the Santa Cruz antibody (sc-7506), we detected a 47-kDa band in muscle and a 45-kDa band in adult brain and spinal cord. Similar results have been obtained by other groups. Using the same antibody, Carreira et al. (2006) detected a 45-kDa band in cell line cultures expressing the growth hormone secretagogue receptor type-1a (GHS-R1a), and Grube et al. (2011) detected a single band near 40 kDa in isolated rat cardiomyocytes. Likewise Puffinbarger et al. (1995) detected human A2BR of differing sizes, 35 kDa in small intestine, and 50–52 kDa in thymus, colon and placenta. Jackson et al. (2002) presented evidence for a 52-kDa A2BR in rat aorta and preglomerular microvasculature.
A3R
In our Western blots of A3R protein using the Millipore antibody (AB1590P) we identified a single protein band of 44 kDa in newborn and adult skeletal muscle, adult brain and spinal cord that had the molecular mass expected for this receptor (40–52 kDa). A 52-kDa band was detected by Jackson et al. (2002) in rat preglomerular microvessels and by Zou et al. (1999) in kidney. Lopes et al. (2003) detected a 44-kDa band in hippocampus and Yu et al. (2006) observed a 40-kDa band in rat brain membranes. The adenosine A3R antibody (sc-13938) from Santa Cruz detects three different bands at 44, 52 and 66 kDa (data sheet). Western blot analysis with this antibody revealed three prominent immunogenic bands at 44, 52 and 64 kDa in human glioblastoma cells, enterochromaffin cells, jejunum cells, testis and colon (Christofi et al. 2001). Carreira et al. (2006) detected a 52-kDa protein in GHS-R1a-expressing cells. In our samples we identified a single 44-kDa protein (similarly to the Millipore antibody).
In summary, our Western blots (Fig. 1B) are in good agreement with those previously published. In addition, our results indicate that both A2BR and A3R receptor proteins are more highly expressed in adult muscle than in newborn muscle. However, whereas A2BR is much more abundant in the mature central nervous tissues than in muscle, the level of A3R protein is slightly higher in muscle than in brain and spinal cord. These developmental and tissue-specific differences may be related to neurotransmission and we analysed the presence of the receptors in the adult NMJ. However, it can be stated that total levels of adenosine receptors in whole tissue may not be an accurate measure of pre- and postsynaptic receptors, as large amounts are present on other cell types and blood vessels, which may swamp the neuronal signal, making comparisons less valid.
Localization of A2BR and A3R receptors in the NMJ
In addition to the Western blot experiments that determine the presence of A2BR and A3R receptor proteins in muscle, mRNA levels for the adenosine receptor types have been determined in various tissues, including skeletal muscle (Dixon et al. 1996). However, A3R mRNA has not been detected in skeletal muscle (Dixon et al. 1996) although the protein may arrive by axonal transport. Our study provides evidence for the specific cellular localization of A2BR and A3R receptor proteins in the neuromuscular junction.
A2BR
A2BR protein has been observed by immunohistochemistry in the plasma membrane and cytosol of skeletal muscle cells (Lynge & Hellsten, 2000; Lynge et al. 2003; Ralevic & Burnstock, 1998). In adult mouse LAL muscles, we used two different antibodies to determine that A2BR immunoreactivity is well localized on the NMJ. The label is found in the position of the nerve terminals between the Schwann cell (labeled with S-100) and the AChR-enriched postsynaptic membrane. The fine labeling also colocalizes very well with the postsynaptic membrane itself and with the nerve terminal marker syntaxin.
A3R
A3R receptors have been immunocytochemically identified in cultured cortical neurons (Rebola et al. 2005) and in NMJ (Cinalli et al. 2013). Here, we show that these receptors are also localized in the nerve terminals and muscle cells, although in this case the postsynaptic localization also includes the sarcoplasm under the AChRs.
In a previous study we observed that A1R localizes in the terminal Schwann cell and nerve terminal, whereas A2AR localizes in the postsynaptic muscle and in the axon and nerve terminal (Garcia et al. 2013). The present report is the first to provide high resolution data on A2BR and A3R immunoreactivity at the NMJ. Both receptors are localized in the pre- and postsynaptic components. Neither A2BR nor A3R is localized in the teloglial Schwann cells. They must at least be present in the motor endings if they are directly involved in transmitter release modulation. The suggestion may be made that a complex balance between all adenosine receptor subtypes (in response to variable amounts of adenosine produced in the synaptic cleft in several activity conditions) may determine their involvement in the control of synaptic function. In addition, A3R receptors seems to be influenced by metabolites of adenosine (Cinalli et al. 2013).
Interestingly, astrocytes and other glial cells are endowed with all the known subtypes of adenosine receptors. A2BR receptors in the central nervous system have been mostly ascribed to astrocyte function rather than to neurons (Daré et al. 2007). Our results show an absence of A2BR and A3R receptors in Schwann cells and, although teloglial Schwann cells are not typical astrocytes, this discrepancy deserve further study.
Concluding remarks
A2BR and A3R receptors are expressed in the skeletal muscle. A2BR and A3R receptors are present in the nerve terminal and muscle cells on the neuromuscular junctions. The results fit well with published physiological data which suggested the presence of these receptor subtypes at the neuromuscular synapse.
Acknowledgments
This work was supported by a grant from MEC (SAF2011-23711) and a grant from the Catalan Government (Generalitat) (2009SGR01248) (J.T.). The authors declare no conflicting financial interests.
Author contributions
N.G.: Concept, literature search, data interpretation, manuscript preparation, confocal, microscopy, quantitative analysis, statistics. M.P. and E.H.: Western blotting techniques, immunohistochemical techniques, data collection, quantitative analysis. T.O.: Western blotting techniques, data collection, quantitative analysis. M.M.S.: Concept, literature search, data interpretation, quantitative analysis. M.T.: data collection, quantitative analysis, immunohistochemical techniques. M.A.L.: Confocal microscopy, concept, literature search, data interpretation. J.T.: Concept, literature search, data interpretation, manuscript preparation.
References
- Adamek S, Shakirzyanova AV, Malomouzh AI, et al. Interaction of glutamate- and adenosine-induced decrease of acetylcholine quantal release at frog neuromuscular junction. Physiol Res. 2010;59:803–810. doi: 10.33549/physiolres.932024. [DOI] [PubMed] [Google Scholar]
- Baxter RL, Vega-Riveroll LJ, Deuchars J, et al. A2A adenosine receptors are located on presynaptic motor nerve terminals in the mouse. Synapse. 2005;57:229–234. doi: 10.1002/syn.20173. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Carreira MC, Camiña JP, Díaz-Rodríguez E, et al. Adenosine does not bind to the growth hormone secretagogue receptor type-1a (GHS-R1a) J Endocrinol. 2006;191:147–157. doi: 10.1677/joe.1.06714. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Zhang H, Yu JG, et al. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol. 2001;439:46–64. doi: 10.1002/cne.1334. [DOI] [PubMed] [Google Scholar]
- Cinalli AR, Guarracino JF, Fernandez V, et al. Inosine induces presynaptic inhibition of acetylcholine release by activation of A3 adenosine receptors at the mouse neuromuscular junction. Br J Pharmacol. 2013;169:1810–1823. doi: 10.1111/bph.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-de-Sa P, Sebastiao AM, Ribeiro JA. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve ending of the rat. Br J Pharmacol. 1991;103:1614–1620. doi: 10.1111/j.1476-5381.1991.tb09836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Sebastiao AM. Adenosine and adenine nucleotides are independently released from both the nerve terminals and the muscle fibres upon electrical stimulation of the inner. Pflugers Arch. 1993;424:503–510. doi: 10.1007/BF00374914. [DOI] [PubMed] [Google Scholar]
- Daré E, Schulte G, Karovic O, et al. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- De Lorenzo S, Veggetti M, Muchnik S, et al. Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction. Neuroscience. 2006;142:71–85. doi: 10.1016/j.neuroscience.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, et al. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:461–468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N, Priego M, Obis T, et al. Adenosine A1 and A2A receptor-mediated modulation of acetylcholine release in the mice neuromuscular junction. Eur J Neurosci. 2013;38:2229–2241. doi: 10.1111/ejn.12220. [DOI] [PubMed] [Google Scholar]
- Grube K, Rüdebusch J, Xu Z, et al. Evidence for an intracellular localization of the adenosine A2B receptor in rat cardiomyocytes. Basic Res Cardiol. 2011;106:385–396. doi: 10.1007/s00395-011-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol. 2002;283:F41–F51. doi: 10.1152/ajprenal.00232.2001. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Rebola N, Pinheiro PC, et al. Adenosine A3 receptors are located in neurons of the rat hippocampus. NeuroReport. 2003;14:1645–1648. doi: 10.1097/00001756-200308260-00021. [DOI] [PubMed] [Google Scholar]
- Lynge J, Hellsten Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta Physiol Scand. 2000;169:283–290. doi: 10.1046/j.1365-201x.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- Lynge J, Schulte G, Nordsborg N, et al. Adenosine A2B receptors modulate cAMP levels and induce CREB but not ERK1/2 and p38 phosphorylation in rat skeletal muscle cells. Biochem Biophys Res Commun. 2003;307:180–187. doi: 10.1016/s0006-291x(03)01125-2. [DOI] [PubMed] [Google Scholar]
- Peyot ML, Gadeau AP, Dandré F, et al. Extracellular adenosine induces apoptosis of human arterial smooth muscle cells via A(2b)-purinoceptor. Circ Res. 2000;86:76–85. doi: 10.1161/01.res.86.1.76. [DOI] [PubMed] [Google Scholar]
- Pitts EV, Potluri S, Hess DM, et al. Neurotrophin and Trk-mediated signaling in the neuromuscular system. Int Anesthesiol Clin. 2006;44:21–76. doi: 10.1097/00004311-200604420-00004. [DOI] [PubMed] [Google Scholar]
- Pousinha PA, Correia AM, Sebastiao AM, et al. Predominance of adenosine excitatory over inhibitory effects on transmission at the neuromuscular junction of infant rats. J Pharmacol Exp Ther. 2010;332:153–163. doi: 10.1124/jpet.109.157255. [DOI] [PubMed] [Google Scholar]
- Puffinbarger NK, Hansen KR, Resta R, et al. Production and characterization of multiple antigenic peptide antibodies to the adenosine A2b receptor. Mol Pharmacol. 1995;47:1126–1132. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, et al. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Walker J. Action of adenosine triphosphate on endplate potentials recorded from muscle fibres of the rat-diaphragm and frog sartorius. Br J Pharmacol. 1973;49:724–725. doi: 10.1111/j.1476-5381.1973.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA, Cunha RA, Correia-de-Sa P, et al. Purinergic regulation of acetylcholine release. Prog Brain Res. 1996;109:231–241. doi: 10.1016/s0079-6123(08)62107-x. [DOI] [PubMed] [Google Scholar]
- Rosi S, McGann K, Hauss-Wegrzyniak B, et al. The influence of brain inflammation upon neuronal adenosine A2B receptors. J Neurochem. 2003;86:220–227. doi: 10.1046/j.1471-4159.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Salgado AI, Cunha RA, Ribeiro JA. Facilitation by P(2) receptor activation of acetylcholine release from rat motor nerve terminals: interaction with presynaptic nicotinic receptors. Brain Res. 2000;877:245–250. doi: 10.1016/s0006-8993(00)02679-2. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Salon I, Garcia N, et al. Modulation of ACh release by presynaptic muscarinic autoreceptors in the neuromuscular junction of the newborn and adult rat. Eur J Neurosci. 2003;17:119–127. doi: 10.1046/j.1460-9568.2003.02428.x. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Salon I, Garcia N, et al. Muscarinic autoreceptors related with calcium channels in the strong and weak inputs at polyinnervated developing rat neuromuscular junctions. Neuroscience. 2004;123:61–73. doi: 10.1016/j.neuroscience.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Searl TJ, Silinsky EM. Modulation of Ca2+-dependent and Ca2+-independent miniature endplate potentials by phorbol ester and adenosine in frog. Br J Pharmacol. 2005;145:954–962. doi: 10.1038/sj.bjp.0706248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakirzyanova AV, Bukharaeva EA, Nikolsky EE, et al. Negative cross-talk between presynaptic adenosine and acetylcholine receptors. Eur J Neurosci. 2006;24:105–115. doi: 10.1111/j.1460-9568.2006.04884.x. [DOI] [PubMed] [Google Scholar]
- Yu W, Zacharia LC, Jackson EK, et al. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol. 2006;291:C254–C265. doi: 10.1152/ajpcell.00025.2006. [DOI] [PubMed] [Google Scholar]
- Zou AP, Wu F, Li PL, et al. Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension. 1999;33(1 Pt 2):511–516. doi: 10.1161/01.hyp.33.1.511. [DOI] [PubMed] [Google Scholar]


