Abstract
Introduction
Brain tumors are inherently difficult to treat in large part due to the cellular blood-brain barriers (BBB) that limit the delivery of therapeutics to the tumor tissue from the systemic circulation. Virtually no large-molecules, including antibody-based proteins, can penetrate the BBB. With antibodies fast becoming attractive ligands for highly specific molecular targeting to tumor antigens, a variety of methods are being investigated to enhance the access of these agents to intracranial tumors for imaging or therapeutic applications.
Areas covered
This review describes the characteristics of the BBB and the vasculature in brain tumors, described as the blood-brain tumor barrier (BBTB). Antibodies targeted to molecular markers of CNS tumors will be highlighted, and current strategies for enhancing the delivery of antibodies across these cellular barriers into the brain parenchyma to the tumor will be discussed. Non-invasive imaging approaches to assess BBB/BBTB permeability and/or antibody targeting will be presented as a means of guiding the optimal delivery of targeted agents to brain tumors.
Expert Opinion
Pre-clinical and clinical studies highlight the potential of several approaches in increasing brain tumor delivery across the blood-brain barrier divide. However, each carries its own risks and challenges. There is tremendous potential in using neuroimaging strategies to assist in understanding and defining the challenges to translating and optimizing molecularly-targeted antibody delivery to CNS tumors to improve clinical outcomes.
Keywords: Immunotargeting, CNS, brain cancer, blood-brain barrier, blood-brain tumor barrier, imaging
1. Overview
Despite the tremendous advances in understanding the molecular and cellular basis of cancer biology, as well as the development of targeted therapies to treat malignancies, there remain critical challenges in treating primary and metastatic disease found within the central nervous system (CNS). An estimated 25,000 new cases of primary malignant brain cancer, and 200,000 secondary metastatic brain tumors are expected to be diagnosed in the USA in 2013 [1,2]. Gliomas broadly refer to all tumors arising from the glial cells of the brain (e.g., astrocytes, oligodendricytes, ependymal cells), and represent 80% of all primary malignant brain tumors [1,2]. In children, brain tumors are the second-leading cancer-related cause of death [3]. In adults, glioblastoma multiforme (GBM), representing ~50% of all gliomas, has a dismal prognosis and is responsible for a large percentage of deaths related to CNS malignancies [4]. Despite aggressive treatment strategies including surgical resection to remove bulk disease, and recent advances in standard-of-care treatment of radiation therapy combined with temozolomide (TMZ) chemotherapy, the overall median survival of GBM patients remains only 14.6 months from diagnosis [5]. Thus, reality demands that we achieve a better understanding of CNS tumors and how best to treat them.
Many investigational agents have demonstrated pre-clinical success, yet none have to-date provided substantial clinical benefit. This could be due to genetic heterogeneity of GBM, complexity of cancer signaling pathways and their redundancy, and inadequate drug delivery to the CNS tumor. The latter factor is accentuated by the fact that primary and metastatic brain cancers reside within the CNS sanctuary formed by the blood-brain barrier (BBB), a cellular barrier found across species that protects the brain from exposure to toxins, both endogenous and exogenous. The BBB serves as a major impediment to effective delivery of many therapies from the bloodstream into the brain parenchyma [6]. Some chemotherapies, including TMZ, can effectively cross the intact BBB, but most drugs cannot pass through the BBB to access the CNS tumor. The conventional wisdom that has evolved in the last decade is that targeting agents to specific vascular determinants in the BBB may boost delivery to the CNS. In recent years, monoclonal antibody (mAb)-based therapy has achieved remarkable success in treating both hematologic and non-CNS solid tumors due to their inherent targeting specificity [7]. Engineered antibodies (Ab), either alone or in combination with other therapeutic agents, represent more than 30% of biopharmaceuticals and biologics currently in clinical trials [8]. Advances in mAb-directed therapy against systemic and extraneural cancer have resulted in ever-increasing number of clinical trials attempting to translate these findings to CNS disease. Understanding and advancing targeted drug delivery approaches to these inaccessible and invasive tumor cells may substantially improve the therapeutic outcomes of patients with CNS tumors.
Here we will review the physiology of the BBB and the pathophysiology of the blood-tumor barrier, and highlight the molecular markers that have reported potential as therapeutic targets against CNS tumors. Examples and strategies for delivery of molecular-targeted agents, namely antibodies as diagnostics and therapeutics, across the BBB will be presented. Finally, noninvasive imaging methods will be presented as a means of guiding the optimal delivery of targeted agents to improve our knowledge and management of brain tumors.
2. The blood-brain barriers for antibody delivery to CNS tumors
2.1 The blood-brain barrier (BBB)
The brain is a highly vascularized organ with an extensive capillary network. Brain capillary endothelial cells (BCECs), pericytes, astrocytes and neurons, are intimately involved in creating the BBB, to control permeability and other functions of the brain microvasculature (Figure 1). BCEC form a tight continuous monolayer lining the microvasculature and serve as the primary interface between blood and the brain parenchyma. The endothelial basal lamina does not generally constitute a diffusion barrier to molecules [9]. Pericytes, which stabilize and monitor the BCECs, send out cellular projections, which penetrate the basal lamina and cover approximately 20-30% of the microvascular circumference [10]. Astrocytes are glial cells that line more that 99% of the abluminal side of the BBB endothelium by astrocytic end-feet through the basal lamina [11]. Neurons contribute to the BBB by innervating the capillary [12]. BCEC is arguably the main diffusion restrictive component of the barrier, protecting the brain from toxic and damaging substances, while selectively permitting delivery of various nutrients, and efflux or removal of toxins and metabolites from the brain.
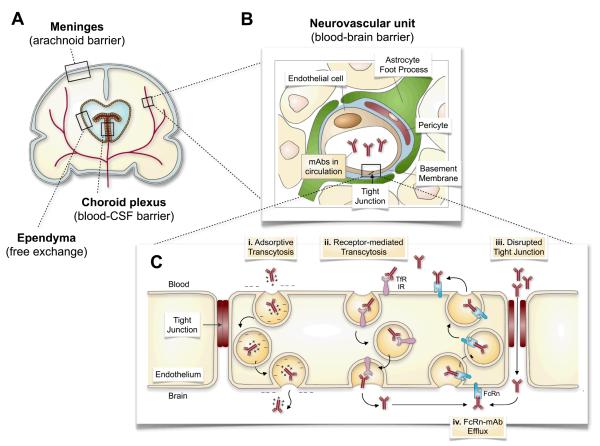
Figure 1.
Routes for antibody delivery across the blood-brain barrier (BBB). (A) The brain is comprised of multiple barriers that control access of administered agents into the brain parenchyma, including the BBB. (B) The BBB is comprised of a neurovascular unit that consists of pericytes, astrocytes, and neurons surrounding brain capillary endothelial cells (and basement membrane). (C) Expansion of the endothelial lining of the BBB shows that intact tight junctions in the normal brain restrict the intercellular diffusion of mAb across the BBB into the brain. (i) Adsorptive transcytosis of cationized mAb can increase transcellular transport across the BBB by their interaction with naturally negatively charged plasma membrane. (ii) MAb engagement of the transferrin receptor (TfR) or the insulin receptor (IR), for example, can also increase mAb transport across the BBB by receptor-mediated transcytosis. (iii) The disruption of tight junctions by pharmacological (osmotic, biochemical) and mechanical (ultrasound, irradiation) can enhance paracellular extravasation of mAbs into the brain parenchyma. (iv) The neonatal Fc receptor (FcRn) serves as an efflux mechanism to remove Fc-containing mAb from the brain and return them to the systemic circulation. Figure 1A and 1B adapted with permission from Elsevier Ltd. (Copyright © 2008): Saunders, N.R. et al. Barriers in the brain: a renaissance? Trends in Neurosciences. 31(6): 279-286. Abbreviations: CSF, cerebrospinal fluid; mAb, monoclonal antibody; TfR, transferrin receptor; IR, insulin receptor; FcRn, neonatal Fc receptor.
The selective permeability properties of the BBB can be attributed to specific characteristics of the BCEC. Tight junctions between these cells form a continuous monolayer to block pericellular transport to the brain [13-15], whereas transcellular passage is also generally ineffective due to lack of caveoli and fenestrations [16-20]. Lastly, the permeability of the BBB is partially under the control of astrocyte end-foot processes via release of chemical factors and signals that modulate the permeability of the brain endothelium [21]. The selective transport across an intact BBB is through transcellular mechanisms such as concentration-driven facilitated and passive diffusion, and active transport, which requires ATP-energy to move molecules across a gradient. However, the BBB hinders drug penetration into the CNS: virtually no large-molecule drugs and fewer than 2% of small-molecule drugs cross the intact BBB [22].
There are additional types of barriers in the brain in addition to the BBB: the blood–cerebrospinal fluid (BCSF) barrier formed by the epithelial cells of the choroid plexus, and the meningeal or arachnoid barrier in the subdural space surrounding the brain (Figure 1) [16]. Although outside the scope of this review, there are surgical-based techniques for drug delivery to CNS tumors that include the use of: 1) Intracerebro-ventricular therapeutic infusion into the choroid plexus, 2) intrathecal administration into the CSF under the arachnoid membrane of the brain or spinal cord, 3) Convection-enhanced delivery directly into tumor, and 4) polymer-drug implant systems (e.g., wafer loaded with bischloroethylnitrosourea (BCNU)) which directly release therapeutics into the CNS tumor. There are several limitations with these delivery approaches; most significantly, the diffusion of drug in the brain parenchyma is very low, thus placement of catheters or implants must be precisely mapped for optimal efficacy, or the tumor must be close to the BCSF or meningeal barriers.
2.2 The blood-brain tumor barrier (BBTB)
The brain tumor endothelium is, distinct from normal brain tissue due to abnormalities in the tumor microenvironment and is thus often described as a “blood-brain tumor barrier” (BBTB) rather than a BBB [23-25]. The tumor vessels in the BBTB of highly angiogenic brain tumors, such as GBM, can have different morphologies ranging from greatly enlarged, thin-walled, and tortuous vessels, to vascular glomeruli that are tangles of microvessels lined with hyperplastic endothelial cells, smooth muscle cells and pericytes. The later are characterized by sluggish and aberrant blood flow, and susceptibility to occlusion which results in diminished perfusion to the tumor tissue. Endothelial cells in BBTB are often hypermeable due to disrupted, leaky tight junctions [26], increased fenestrations and vesicular transcellular transport [27], and an abnormal over expression profile of membrane transporters, such as the drug efflux pumps P-glycoprotein (P-gP), and multi-drug resistance-associated proteins MRP1, MRP3, and the potassium channel KCa and KATP [28]. These changes within the BBTB can enhance passage of low molecular weight compounds, including standard chemotherapeutics, compared with the BBB.
However, these pores can be narrow enough to prevent the effective passage of materials larger than 12 nm (including certain proteins and nanomaterials) [23,29,30]. In addition, drug delivery is impeded by increased interstitial fluid pressure (IFP) in brain tumors, as seen in both patients [31,32] and animal tumor models [33]. Although not fully understood, viable tumor tissue with increased permeability and high resistance to fluid flow in the interstitial space, produces a pressure gradient that moves drugs along with interstitial fluid into necrotic tumor tissue [33], the tumor periphery, or into the surrounding normal brain tissue [31,33-36].
Clearly, the complex and marked heterogeneity of the CNS tumor microenvironment, and the tumor interaction with the BBB/BBTB can have significant implications for achieving sufficient drug delivery to the tumor (particularly the viable component of the tumor) to effect therapeutic response. The BBTB is generally considered compromised in the situation of large brain lesions (> 2 cm), and invasive and malignant gliomas, favoring drug delivery to the tumor tissue. However, there may well be sites in these and smaller tumors, where the functional integrity of the BBB is intact, especially around the tumor edge or around micrometastases, effectively limiting adequate drug delivery to these tumor cells. A recent animal study of experimental brain metastases from human breast cancer cells found that although most metastases showed some degree of BBTB permeability, the concentration of paclitaxel or doxorubicin accumulated within these brain metastases was <15% of that achieved in extracranial metastases [37]. Consequently, the BBTB impedes the accumulation of therapeutic concentrations of both of these agents thereby limiting their effectiveness for treating intracranial tumors. These remnant tumor cells will continue to grow, develop neovascular structures, and continue to progress to a clinically significant size that would require another surgical/therapeutic intervention.
Thus, to address the challenges of targeted drug delivery to CNS tumors, efforts must be focused on drug-delivery technologies that are capable of penetrating both the intact BBB and the pathologically altered BBB and BBTB to maximize clinical outcomes for patients.
3. Molecular targets for antibody-based therapeutics and diagnostics in CNS tumors
Advances in the understanding of GBM biology and pathogenesis have greatly facilitated the development of antibodies targeting antigens typical of the tumor tissues but absent in normal brain. Since the development of the first FDA-approved therapeutic immunoglobulin (IgG) mAb, muromonab-CD3, this area of research has expanded significantly. In addition to humanizing murine IgG antibodies to make them immune-tolerable, and increasing the production efficiency of therapeutic antibodies, recombinant antibody-based systems have been devised, including antibody fragments (Fab), single-chain variable region fragments (scFv), and bispecific antibodies (Figure 2). With advances in molecular genetics and chemical engineering, it is now possible to design antibody-based reagents that are smaller in size, having multivalent modular domain and conjugated with different cargoes and carriers, for various applications. These agents may be exploited clinically because of their endogenous cytotoxic effects or by rendering them cytotoxic by conjugation to cytotoxic drugs, toxins, or radioisotopes. In a similar vein, antibodies can be functionalized with radioisotopes for non-invasive sensitive imaging using positron emission tomography (PET) or single photon emission computed tomography (SPECT), for monitoring drug delivery and tumor status based on the local level of the biomarker.
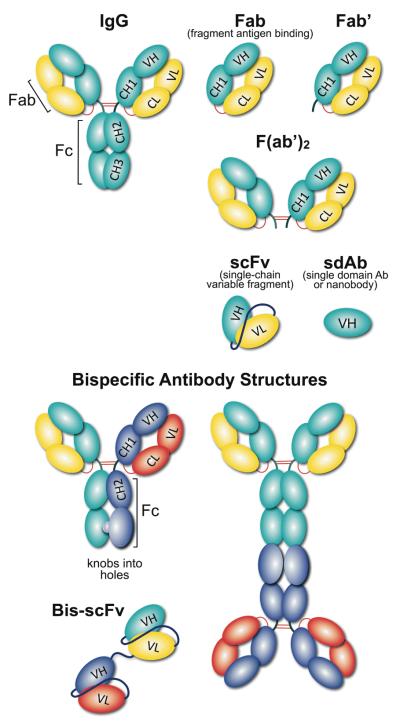
Figure 2.
Schematic representation of different antibody formats. An array of recombinant antibody formats were developed using combinations of antibody domains, joined either with linkers and/or disulfide bonds. Using molecular biology tools, antibody valency and specificity can be tailored into monovalent (sdAb (single domain Ab); Fab and Fab’ (fragment antigen binding), divalent (F(ab’)2) and even multivalent formats. Formats with antibody constant domains are highly stable, however, single chain antibody formats are popular due to their small size, expression and tissue penetration capabilities. Linker length can also influence the antibody behavior in terms of stability, flexibility and aggregation. A bispecific antibody is a recombinant antibody format that recognize two distinct antigens, using two IgG or scFv, linked together either chemically by covalent conjugation; or genetically with peptide linkers; or disulfide bonds.
To date, several clinical trials have evaluated the therapeutic effect of monoclonal antibodies in GBM patients (Table 1). Angiogenesis is a major hallmark of cancer, and is characterized by the formation of new blood vessels required for growth and metastasis of tumors. Brain tumors in particular are highly angiogenic. Therefore, molecularly-targeted interventions designed to normalize tumor endothelium and prevent angiogenesis in systemic cancer may also be effective at controlling brain tumors [38,39]. Vascular endothelial growth factor (VEGF) is a soluble protein (~40 kDa) that serves as the key signaling mediator of angiogenesis and vascular permeability. Bevacizumab, a recombinant human neutralizing VEGF IgG antibody, is the first FDA-approved antiangiogenic agent in systemic cancer treatment. The success of bevacizumab prompted its translation to the treatment of malignant gliomas, Studies released in the past five years reveal favorable radiographic responses and potential survival benefit observed with bevacizumab [39], and resulted in accelerated FDA-approved of bevacizumab in 2009 to treat recurrent GBM. Despite its large molecular size, bevacizumab is effective because it does not rely on disruption of the blood-brain barrier, but rather can work to neutralize VEGF within the lumen of the capillary endothelium. As highlighted in Table 1, anti-VEGF mAb therapy is being extended to newly diagnosed glioblastoma, anaplastic astrocytomas, CNS lymphoma, and secondary cerebral metastases.
Table 1.
Antibody-Based Therapies in Clinical Trials for the Treatment of Malignant Gliomaa
| Antibody | Conjugate | Molecular Target | Other agents | Specific CNS Tumor | Trial Status |
|---|---|---|---|---|---|
| Bevacizumab | VEGF | Recurrent GBM | recruiting | ||
| TRC105 | Endoglin | ||||
| Ramucirumab IMC-3G3 |
VEGFR-2 PDGFR |
Recurrent GBM | active; not recruiting | ||
| TNT-1/B | 131I | Universal, intracellular nucleosomal determinants consisting of histone H1 and DNA |
Progressive or recurrent GBM | completed | |
| TNT-1/B | 131I | Histone H1 and DNA | GBM or AA | completed | |
| Bevacizumab | VEGF | TMZ | GBM or gliosarcoma in older patients | recruiting | |
| TRC105 | Endoglin | Recurrent GBM | recruiting | ||
| chTNT-1/B | 131I | Histone H1 and DNA | Recurrent GBM | completed | |
| Bevacizumab | VEGF | TMZ or irinotecan | Recurrent GBM | active; not recruiting | |
| chTNT-1/B | 131I | VEGF | Recurrent GBM | completed | |
| Bevacizumab | VEGF | TPI287 | Recurrent GBM | recruiting | |
| Bevacizumab | VEGF | Lomustine | Recurrent GBM | recruiting | |
| Bevacizumab | VEGF | Vorinostat | Recurrent GBM | active; not recruiting | |
| Bevacizumab | VEGF | Radiosurgery | GBM | recruiting | |
| Bevacizumab | VEGF | RT | Recurrent GBM | not yet recruiting | |
| Bevacizumab | VEGF | Hypofractionated RT and TMZ |
Recurrent high grade GBM | active; not recruiting | |
| Bevacizumab | VEGF | RT | Newly diagnosed GBM | active; not recruiting | |
| Bevacizumab | VEGF | Trabanaib | Recurrent brain tumor | suspended | |
| TRC105 | Endoglin | Recurrent GBM | recruiting | ||
| Bevacizumab | VEGF | R04929097 | Progressive or recurrent GBM | recruiting | |
| Neuradiab | Tenascin C | Recurrent GBM | unknown | ||
| Bevacizumab | VEGF | ||||
| 3F8 | 131I | Ganglioside GD2 | CNS Cancer or leptomeningeal cancer | recruiting | |
| Bevacizumab | VEGF | Cediranib maleate | Metastasis or unresectable solid tumor, Lymphoma, GBM, gliosarcoma or AA |
active; not recruiting | |
| Bevacizumab | VEGF | Cliengitide | Recurrent GBM | not yet recruiting | |
| 3F8 | 131I | Ganglioside GD2 | Leptomeningeal cancer | completed | |
| 81C6 | 131I | Tenascin C | Recurrent glioma | completed | |
| Me1-14 | Chondroitin proteoglycan sulfate | Metastatic melanoma or brain tumors | completed | ||
| 81C6 | 131I | Tenascin C | Primary brain tumors | completed | |
| 81C6 | 131I | Tenascin C | Primary or metastatic brain tumor | completed | |
| 81C6 | 131I | Tenascin C | RT | Primary brain tumors | completed |
| 81C6 | 211At | Tenascin C | Primary and metastatic brain tumor | completed | |
| MAB-425 | 125I | EGFR | High grade glioma | active; not recruiting | |
| Bevacizumab | VEGF | Sorafenib | Recurrent GBM | completed | |
| Bevacizumab | VEGF | TMZ, external beam RT | GBM and gliosarcoma | active; not recruiting | |
| Daclizumab | CD25 | TMZ | GBM and TMZ-induced lymphopenia | active; not recruiting | |
| Bevacizumab | VEGF | Dasatinib | Recurrent or progressive high-grade glioma or GBM |
recruiting | |
| Bevacizumab | VEGF | Lomustine | GBM in first recurrence | not yet recruiting | |
| Bevacizumab | VEGF | Erlotinib | GBM and gliosarcoma | active; not recruiting | |
| Basiliximab | CD25 | Chemotherapy, RT and vaccine |
GBM that has been surgery resected | active; not recruiting | |
| Nimotuzumab | EGFR | GBM | completed | ||
| Bevacizumab | VEGF | Lenalidomide, sorafenib, temsirolimus, 5-FU, leucovorin, oxaliplatin |
Advanced cancer | recruiting | |
| Bevacizumab | VEGF | Rindopepimut with GM-CSF adjuvant |
EGFR-vIII-positive GBM | recruiting | |
| MGA271 | B7-H3 | Refractory cancer | recruiting | ||
| Bevacizumab | VEGF | Progressive or recurrent glioma | active; not recruiting | ||
| Bevacizumab | VEGF | TMZ | Recurrent glioma | unknown | |
| Bevacizumab | VEGF | BCNU | Relapsed or progressive high-grade glioma | active; not recruiting | |
| Bevacizumab | VEGF | Irinotecan | Recurrent or refractory glioma | completed | |
| Bevacizumab | VEGF | Irinotecan | Malignant glioma | completed | |
| MOC31 | PE | EpCAM | Antigen positive carcinomas | completed | |
| Bevacizumab | VEGF | Colorectal, lung, breast cancer and GBM | completed | ||
| Panitumumab | EGFR | Irinotecan | Malignant glioma | Terminated |
From ClinicalTrials.gov; accessed March 2013.
Abbreviations: VEGF, Vascular endothelial growth factor; VEGFR-2, Vascular endothelial growth factor receptor 2; PDGF, Platelet-derived growth factor receptor; EGFR, Epidermal growth factor receptor; GBM, Glioblasotma multiforme; AA, Anaplastic Astrocytoma; TMZ, Temozolomide; RT, Radiation therapy; B7-H3, B7 homolog 3; EpCAM, epithelial cell adhesion molecule; CD25, α chain of the IL-2 receptor; 5-FU, 5-fluorouracil; GM-CSF, Granulocyte-macrophage colony-stimulating factor; BCNU, bischloroethylnitrosourea; PE, Pseudomonas exotoxin.
The identification of non-responders to bencizumab from the onset of therapy suggests that tumors may be driven by angiogenic factors other than VEGF. Another widely explored angiogenic targets in GBM patients is the cell-surface tyrosine kinase receptor epidermal growth factor receptor (EGFR). Overexpression of EGFR is found in approximately 50–60% of GBM tumor cells. EGFRvIII, a mutant EGFR with deletion of exon 2-7, rendering the receptor constitutively active, is the most common EGFR mutant in GBM and is present in 24–67% of GBM [40,41]. EGFR is generally considered a favorable candidate for antibody targeting due to high accessibility. Cetuximab is a chimeric IgG1 mAb that can block EGFR activation by binding to the ligand-binding domain, which induces internalization of EGFR thereby preventing downstream signaling [42]. PET imaging of 89Zr-labeled cetuximab has been investigated in several pre-clinical studies, and has potential as a scouting procedure before radioimmunotherapy with 90Y-cetuximab or 177Lu-cetuximab to confirm tumor targeting and allow estimation of radiation dose delivery to tumors and normal tissues [43,44]. Similarly, imaging of EGFR mutant expression might be more useful in selecting the optimal patient population for personalized treatment as well as predicting therapeutic response. This image-based scouting approach for treating brain tumors in patients is currently under-explored. Other candidates under clinical investigation for antibody-based therapy include the growth factor receptor tenascin [45-47], and MRP3 [48]. The initial response of some patients to anti-VEGF therapy soon follows to disease progression with a nonangiogenic and invasive phenotype, suggesting it is necessary to combine anti-angiogenic approaches with anti-invasive therapy.
Currently, 165 anti-tumor mAbs are in malignant glioma clinical studies, including 89 in Phase I; 64 (39%) and 12 (7%) have advanced to Phase II and Phase III, respectively. While nearly 50% of the mAbs used in these studies are intact IgG antibodies, the remainder are non-canonical antibodies that have the capacity of further modification with specific therapeutic or imaging modularity [49]. Antibodies can bind to two adjacent epitopes, which increases the affinity, and induce natural host-mediated cytotoxic effects via the Fc-fragment. Non-canonical antibodies include bispecific antibodies, antibody–cytokine fusion proteins, and immunotoxins.
Bispecific antibodies can bind to two different antigens and can therefore be more specific in therapeutic targeting. Their molecular framework can vary in terms of the type of IgG used and antigen-binding fragment combinations (Figure 2). Although most bispecific antibodies are currently in early phase pre-clinical development, ten different bispecific mAbs are being evaluated in Phase I and Phase II trials. Catumaxomab, a bispecific IgG targeting CD3 and epithelial cell adhesion molecule (EpCAM), has been approved for clinical use in patients with malignant ascites in Europe [50].
Cytokines are natural immunomodulatory agents that induce pro-inflammatory and potentially cytotoxic signaling cascades. Immunotherapy with cytokines, such as interferin (IFN)-α, interleukin (IL)-7, IL-10, IL-12, IL-15, and IL-21, is being used in clinic or is currently in clinical trials for cancer treatment [51,52]. However, systemic cytokine treatment often results in low concentrations of therapeutic cytokines at the target site with severe off-target toxicities. To improve the therapeutic window of cytokines, antibody-cytokine fusion proteins have been developed [53]. The antibody module of an antibody-cytokine fusion protein is to specifically localize the cytokines to the target site, i.e. a tumor. The fusion protein design often involves fusing a therapeutic cytokine with whole IgG, or an antibody fragment such as Fab, scFv, scFv-Fc, or divalent derivatives. Several antibody-cytokine fusion proteins are under clinical evaluation, and some demonstrate encouraging results in terms of clinical benefit and limited off-target toxicity. Challenges in using these agents in clinical settings include the need to prolong cytokine stability, maintain therapeutic efficacy, reduce immunogenicity, and increase tissue penetration.
An immunotoxin is a conjugate comprised of a targeting moiety (either a natural ligand or an antibody) and a toxin that can kill the target cells once internalized. Several immunotoxins have been evaluated in pre-clinical and clinical trials. For example, Pseudomonas exotoxin mutant (PE) conjugated to the protein cytokine interleukin-4 (IL-4-PE) for targeting the IL-4 receptor on T-cells, is in clinical trials for GBM. The immunotoxin conjugates of anti-TfR (transferrin receptor) mAb with the saporin toxin (anti-TfR-SAP), PE conjugated with mAb DTAT13 directed to urokinase plasminogen activator receptor (uPAR-PE), and antibodies to the receptor-type protein tyrosine phosphatase modified with PE (RPTPβ-PE), are under pre-clinical evaluation [54].
The majority of studies involving these targeted agents involve peripheral and hematologic malignancies. In order to effectively use them in patients with initial or recurrent CNS tumors, the challenging problem of limited CNS delivery needs to be resolved.
4. Approaches to targeted delivery to the CNS
One of the challenges of brain tumor chemotherapy is that, due to specific local mechanisms limiting extravasation described above, carriers improving the pharmacokinetics and local delivery of anti-tumor agents (e.g., antibodies, liposomes, nanocarriers) have no or limited access to the target [55]. Below, we briefly outline current strategies for resolving this problem.
4.1 Antibody delivery across intact BBB
4.1.1 Adsorptive-mediated transcytosis of cationized antibodies
Adsorptive-mediated transcytosis is a non-specific vesicular transport mechanism that can enhance the ability of cationic macromolecules, such as peptides, proteins, and antibodies, to enter the CNS (Figure 1). Covalent modification of the molecule with cationic polyamines, such as hexamethylenediamine or tetramethylenediamine [56,57], raises the isoelectric point (pI) of proteins from pI 4 to greater than 10. Like most cell membranes, the luminal plasma membrane of BCECs is slightly anionic; hence cationic carriers bind to and have a chance to undergo transcytosis across the BBB [58,59]. This mechanism is not mediated by specific recognition of target determinants. Cationic carriers bind to blood elements and endothelium in systemic vasculature- first of all, in the lung after intravenous (IV) injection [60]. Local administration via the cerebral arteries helps to enrich binding in the downstream CNS microvasculature [60].
The enhanced CNS delivery of cationized IgG by absorptive-mediated transcytosis across the BBB was first explored in the late 1980s [58]. Recent studies showed that as compared to native protein, a polyamine-modified F(ab’)2 fragment targeted to Aβ plaques has increased uptake in the mouse Alzheimer’s brain relative to wild type mouse brain [61]. However, clinically meaningful therapeutic benefits from using cationized antibodies remain to be validated in animal models of CNS cancers. Chemical modification may aggregate carrier proteins and yield heterogeneous molecular species. Attention should be paid to modification of critical amino acids required for antibody-antigen binding which can result in diminished immunoreactivity and consequent therapeutic efficacy. Functionalization of site-specific tags introduced chemically or using recombinant methods to introduce modifiable amino acids in the carrier protein helps to solve some of these technical issues [62].
4.1.2 Receptor-mediated transcytosis of antibody constructs
Endogenous macromolecules necessary for normal brain function are delivered to the brain by specific receptors expressed on the luminal side of the endothelial cells forming the BBB (receptor-mediated transport, RMT) (Figure 1). Ligand binding and clustering of receptor molecules induces endothelial endocytosis of the complex, which, in some cases traffics via series of intracellular vesicles across the cell. Dissociation of the ligand and receptor presumably occurs during cellular transit or during the exocytotic event. The receptors most often studied for RMT at the BBB are TfR, and insulin receptor (IR) [63]. BCEC also express other receptors capable of RMT: insulin-like growth factor (IGF) receptor [64]; leptin receptor (LEPR) [65]; and scavenger receptors (scavenger receptor, class B, member 1 [SR-B1]), also commonly referred to as the acetylated low-density lipoprotein (LDL) receptor [66].
RMT can occur by different entry points on the plasma membrane depending on the engagement of receptors with specific cytoplasmic domains (e.g., clathrin versus caveolae-coated vesicles) [67]. Each route offers the interesting possibility that the point of entry determines the subsequent fate of a particular internalized cargo molecule. Interestingly, BCEC have the lowest frequency of caveolae, and while there is general agreement that RMT with TfR is clathrin-mediated, the exact mechanism for RMT of other receptors, including IR and LDL remain unresolved [67].
Conjugating therapeutics and drug cargos to ligands (antibodies or peptides) targeted to BBB targets for RMT have long been proposed. However, considerations must be made to how the entities need to be linked to each other. Certain cargos (antibodies or drugs) may not be pharmacologically active following attachment to a BBB-directed ligand for RMT either due to inactivation due to chemical modification or steric hindrance. Thus, it may be necessary to attach the cargoes to the BBB transport ligand either through a cleavable linker (e.g., disulfide bond or acid-sensitive linker) or through a long spacer arm (e.g., polyethylene glycol (PEG) linker), respectively. The choice of a cleavable linker is highly dependent on which chemistry ensure an active cargo molecule following release from the transport vector, and in what intracellular compartment the cargo needs to be released to ensure access to the brain parenchyma on the abluminal side. Acid-sensitive linkers could be cleaved at the lower pH present in late endosomes or lysosomes. Thioredoxin proteins within the compartments endocytic pathway can also create a reducing environment to facilitate cleavage of disulfide bonds. Notably, premature cleavage of the antibody construct from the RMT-directing vector within these endocytic compartments could lead to trafficking of the therapeutic cargoes to lysosomes for degradation rather than transport across the endothelial cell to the target brain cell. Alternatively, a site-directed PEGylation approach could serve to minimize inactivation due to chemical modification or to limit any steric hindrance imparted by the close proximity of the cargo with the BBB-transport vector. These factors illustrate the various approaches for conjugating CNS tumor targeting cargoes to RMT vectors depending on the specific functional needs of the cargo under consideration.
Proof-of-concept studies of RMT-mediated CNS delivery have been conducted in animal models [22,63,68]. For example, conjugation of the murine TfR-specific mAb OX26 to 111In-labeled EFG-peptide separated by a PEG linker permitted successful ex vivo imaging of a conjugate targeting to intracranial GBM tumors expressing EGFR in rats, whereas EGF peptide alone did not bind to the tumor due to its inability to cross the BBB (Figure 3) [69]. However, widespread expression of these receptors on peripheral organs can limit the capability of RMT for specific brain delivery and, especially for anti-IR antibodies, may present additional toxicity.
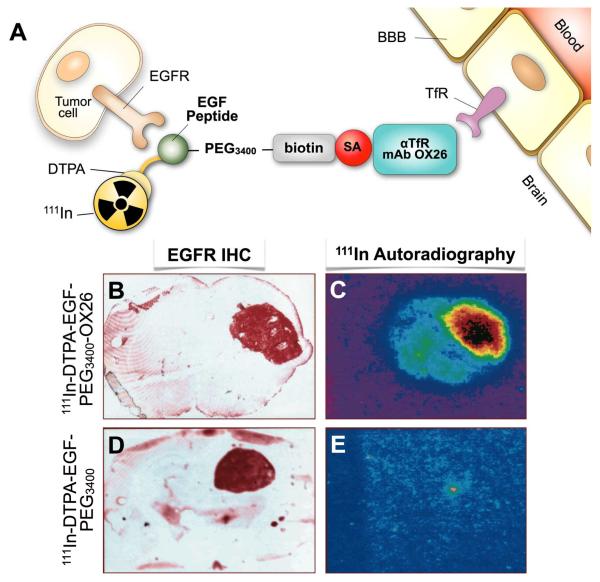
Figure 3.
A, Structure of dual targeted epidermal growth factor (EGF) chimeric peptide conjugated to rat transferrin receptor (TfR) targeted mAb OX26. The EGF is modified with diethylenetriamine pentaacetic acid (DTPA) to chelate the radioisotope 111In. The EGF is attached to a 3400-Da polyethylene glycol linker (PEG3400) terminated with a biotin moiety for conjugation to streptavidin (SA)-OX26 MAb. B, D. Brain sections of U87 human glioma tumor-bearing rats were stained immunocytochemically using an MAb to the human EGF receptor (EGF-R), which demonstrates expression of the EGF-R in brain tumor specimens. C, E. Ex vivo 111In-autoradiography demonstrates that the brain tumor can be imaged with the EGF chimeric peptide that can undergo transport across the BBB in vivo via antiTfR mAb OX26. (C) There is no imaging of the brain tumor when the non TfR-targeted EGF peptide radiopharmaceutical is administered, because the EGF does not cross the BBB alone (E). Adapted with permission from the American Association for Cancer Research (Copyright © 1999): Kurihara A and Pardridge WM. Imaging brain tumors by targeting peptide radiopharmaceuticals through the blood-brain barrier. Cancer Res. 1999; 59 (24), 6159-63.
More recent work highlights the role of optimal, not necessarily the highest, affinity of targeting antibody [70]. High affinity TfR antibodies do not dissociate from the receptor and thus are retained in brain capillary endothelial cells, whereas a low-affinity counterpart offers more effective delivery into the brain parenchyma [70]. The therapeutic potential of this approach was demonstrated in vivo by targeting BACE 1 (β-amyloid precursor protein cleaving enzyme 1), the key contributor to the production of amyloid-β (Aβ) peptide and eventual Aβ plaque deposition, the latter being a hallmark of Alzheimer’s disease (AD). Wild-type mice injected with a bispecific antibody (anti-TfR/BACE1) that binds both TfR and BACE1 with >25-fold lower TfR affinity than the parent TfR mAb, resulted in ~50% reduction of endogenous Aβ peptide brain levels due to more effective inhibition of BACE1 enzyme activity compared to anti-BACE1 mAb alone [70]. These results are suggestive of the potential of RMT in enhancing therapeutic antibody delivery to the brain to reducing Aβ plaque burden in AD.
The potential for antibody efflux in the brain to blood direction must also be taken into consideration when developing antibody-based diagnostic and therapeutic strategies for CNS cancers. Endothelial cells at the BBB express the neonatal Fc receptor (FcRn), which recognizes the constant region (Fc) of IgG antibodies, and mediates its efflux from the abluminal side to the luminal side of the BBB by RMT (Figure 1C) [71]. This efflux mechanism can be avoided when using antibody fragments devoid of Fc regions, including F(ab’)2, and scFv though the absence of Fc will also preclude induction of cytotoxicity.
4.2 Enhancing antibody delivery through blood-brain barrier disruption (BBBD)
The transient disruption of the physical barrier presented by BBB and/or BBTB is another approach to improving CNS delivery of agents that would typically be impermeable. The subsequent section reviews current BBBD approaches that may help increase extravasation of macromolecular drugs into the brain parenchyma and facilitate access to CNS tumors. The advantage of this strategy is that it may be applied to diverse drugs and carriers, whereas the safety and practical utility represent its common challenge.
4.2.1. Osmotic BBBD
Transient disruption of the BBB is achievable via intra-arterial (IA) infusion of concentrated hyperosmotic solutions [72]. Early work demonstrated that osmotic disruption of BBB in patients with CNS melanoma metastasis can increase transient brain uptake of 131I-labeled Fab targeted to melanoma antigens, as confirmed by SPECT imaging [73]. To date, a number of substances have been studied as osmotic BBBD agents, with IA infusion of hypertonic mannitol solution via the internal-carotid artery being the most commonly reported method in pre-clinical and clinical studies [74-76]. These treatments result in osmotic shrinkage of the brain capillary endothelial cells, followed by disruption of tight junctions, and increased permeability for several hours through intercellular pores [77]. Animal studies suggest that this method is able to increase concentrations of various small molecule chemotherapeutic agents in the brain 4 to 90-fold [78]. In the last decade there have been an increasing number of clinical trials evaluating the efficacy of osmotic BBBD and improved drug delivery in primary CNS lymphoma, malignant gliomas and brain metastases [79-81]. Included in this group of trials are those evaluating concomitant delivery of targeted agents, such as bevacizumab [81-83].
The clinical impact, safety and tolerability of this approach needs to be evaluated in larger Phase II and III trials that are currently ongoing at multiple centers. Overall the concerns are regarding the safety and efficacy of clinical osmotic BBBD [75,84]. First, the technique is invasive and its clinical use requires considerable expertise [84]. Second, endothelial tight junction disruption is non-selective, and these transient changes in microvasculature permeability can enhance entry of potentially toxic blood-borne substances throughout the CNS [85]. Finally, increased blood-brain barrier permeability may be limited spatially, thus complicating drug delivery to the whole organ or specific loci [86]. The effectiveness of the procedure can be influenced by different factors, including hemodynamic variables, type of anesthesia and rate of hyperosmolar solution infusion [87,88]. It is thus paramount to monitor the degree of the barrier permeabilization obtained after a procedure, as it can be highly variable from patient to patient, and even with repeated procedures in the same subject. However, applications of IA delivery of chemotherapeutics via the internal carotid approach was introduced into clinical practice decades ago to treat GBM [89]. And, as recently reported, in experienced hands, these procedures can be regarded as clinically safe and effective [81,90].
4.2.2. Biochemical BBBD
There are a variety of biochemical agents that increase the permeability of the brain capillary network by pharmacologic effects on BCECs. This class of vascular disrupting agents requires IA infusion, and can thus present similar challenges as indicated with osmotic BBBD strategies. Bradykinin, a peripheral vasodilator peptide, and the synthetic bradykinin analog RMP-7, can increase tight junction permeability by activating B2 receptors on BCECs through a calcium-mediated mechanism [91,92]. In pre-clinical tumor models, RMP-7 has been found to increase the brain concentration of systemically administered (IA or IV) carboplatin chemotherapy [93]. However, improved efficacy of carboplatin following RMP-7 mediated BBBD could not be demonstrated in Phase II and III clinical trials in pediatric as well as recurrent adult primary CNS malignancies [94,95].
Nitric oxide (NO) donors, leukotrienes, soluble guanylate cyclase activators, and calcium-dependent potassium channels (KCa) agonists have all been reported to increase BBB permeability to enhance drug delivery to brain tumors in pre-clinical models [28,96-99]. However, current efforts are largely focused towards disrupting the BBB in a more tumor-specific manner to limit neurotoxicity by allowing the BBB to exert its neural protective effects, and to achieve more selective delivery to the tumor by controlling the duration of BBBD. Dysregulation of KCa channels has been implicated in a wide spectrum of diseases, including epilepsy [100] and cancer [101]. In pre-clinical models, brain tumors and brain tumor capillaries express significantly higher KCa levels than normal brain tissue or capillaries [96,102], with expression of KCa correlated with malignancy and aggressive growth phenotypes [102-104]. The KCa receptor agonist NS-1619 causes depolarization selectively in murine brain tumor vasculature to enhance BBTB permeability. In a murine brain tumor model, co-administration of NS-1619 with the HER2-targeted mAb trastuzumab significantly improved distribution of the fluorescently-tagged antibody, and mice that received this combination also showed improved median survival [102]. Thus, these selective reagents may represent a safer biochemical mechanism for BBB disruption. However, the effects of potassium channel agonists on BBTB permeability vary between syngeneic and allogeneic animal models due to different expression levels of KCa. This mirrors observations that the expression of potassium channels in human brain tumors is variable, which may be associated with different tumor permeability to therapeutic agents among patients [105]. Further, while well-established tumors show such neovascularization, invasive cells and nascent tumors, which are the primary cause of CNS tumor recurrence, have not yet formed a tumor vasculature with differential KCa receptor expression. This could very well limit access of therapeutics to these sites by BBTB-specific disrupting strategies.
4.2.3. Focused ultrasound (FUS) for BBBD
Focused ultrasound (FUS) has been used in pre-clinical investigations as a method for increasing drug delivery to the brain by inducing temporary BBB disruption [106,107]. Development of FUS technology has enabled ultrasound to function not only as a diagnostic tool but also as a therapeutic modality when using gas-filled microbubbles as ultrasound contrast agents. Acoustic waves can be focused deeply into soft tissue without the need for surgical intervention. The mechanical interaction between the ultrasonic wave, the microbubbles, and the vasculature causes a transient disassembly of BBB tight junction proteins [108,109], and can stimulate active transport [110] to create a transient window for drug delivery. This technique has several advantages over other approaches in that it is noninvasive, readily repeatable, and targeted only to desired regions in the brain to where FUS is applied. Animal studies have shown that tight junction disruption increases the permeability of the BBTB [111,112], with the barrier restored within a few hours [106,108,113]. FUS as a BBBD is reportedly is not associated with significant tissue damage [114-116]. Recent work demonstrates that FUS following microbubble administration is capable of enhancing brain tumor delivery of BCNU chemotherapy [117] or trastuzumab [118], improving survival in a rat glioma model [117] and HER2-positive breast cancer brain metastases model, respectively [119].
4.2.4. Radiation-induced BBBD
Radiation therapy (RT), the use of ionizing radiation to damage DNA and instruct cell death, has become a mainstay of treatment for brain tumors. The safety record of RT now spans many decades. RT has become increasingly precise in recent years with innovations such as tumor definition with imaging, and conforming radiation delivery to the tumor by beam shaping, permits focused targeting of RT to tumors while minimizing radiation dose to normal tissue. In addition to having a direct anti-tumor effect, RT may also enhance drug delivery to brain tumors. Pre-clinical and clinical studies support the premise that RT results in focal disruption of the BBTB while minimizing disruption of the adjacent BBB [120-127]. Early studies with irradiated rat brain found diffuse changes in rat brain when using more therapeutically relevant dosages of 20 to 25 Gy (a dose employed in clinical stereotactic hypofractionated RT), including dilation of blood vessel lumen, thickening of the blood vessel wall, enlargement of endothelial cell nuclei, and hypertrophy of adjacent astrocytes [128]. Others report reduced expression of drug efflux pump P-gP on BCECs by almost 60% [129].
New technologies such as the Small Animal Radiation Research Platform (SARRP) micro-irradiator, enables image-guided focal RT of murine brain tumors for translational investigations of RT-induced BBBD [130,131]. Large molecular weight fluorescent dye (60 kDa) that is impermeable to an intact BBB, accumulates significantly in a mouse brain tumor model following focused RT with SARRP [130]. SARRP irradiation focused on either control mouse brain or a brain tumor, as shown in Figure 4, also results in extravasation of the typically BBB impermeable Evans Blue dye, and systemic IgG [131]. IgG extravasation is considerable higher in irradiated brain tumor relative to irradiated normal brain, suggesting that the BBTB is more sensitive to BBBD relative to an intact BBB. These results together indicate that targeted RT can potentially serve to modulate BBTB permeability to enhance therapeutic drug delivery to CNS tumors.
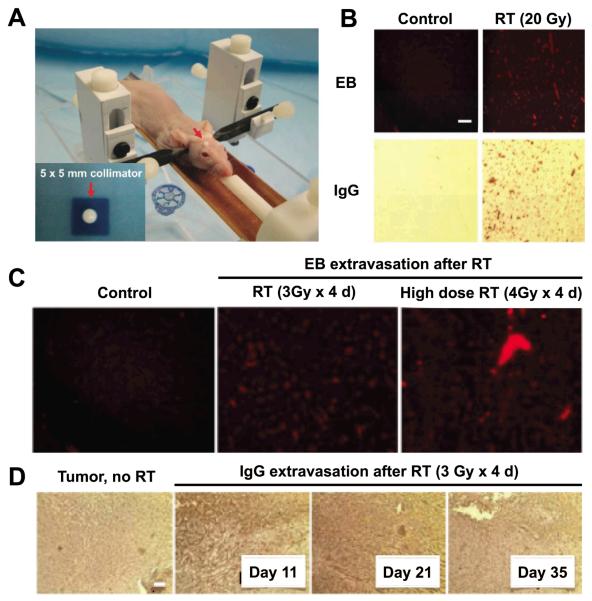
Figure 4. Targeted radiation therapy disrupts brain tumor blood-brain barrier and permits greater penetration of Evans Blue dye and IgG.
A, Cranial RT is delivered using a novel mouse restrainer housed within the Small Animal Radiation Research Platform (SARRP). RT is focused using a 5 × 5 mm collimator (inset). B, Healthy nude mice, mock-irradiated (“Control”, left column) or irradiated with 20 Gy in a single fraction to the right cerebral hemisphere (“RT”, right column), show BBB disruption (BBBD) as visualized with Evans Blue dye (EB) extravasation 24 h post RT (top row), or via staining for IgG extravasation out of the systemic circulation (bottom row). C, Dose-response of BBBD by focused RT with normal mice that are mock-irradiated (left panel), irradiated with 3 Gy daily x 4 days (middle panel) or 4 Gy daily x 4 days (right panel). EB is injected and mice sacrificed 24 h after the final RT fraction, and dye uptake visualized with fluorescence imaging. D, RT induces Tumor-BBBD in GBM intracranial orthografts compared to mock-irradiated tumor-bearing controls. Mice with intracranial orthotopic GBM tumors are either mock-irradiated or irradiated with 3 Gy daily x 4 days. IgG extravasation out of the systemic circulation is evident out to 35 days after RT. Reprinted with permission from Baumann, BC et al. Oncotarget. Copyright © 2012 Impact Journals.
In summary, improving brain delivery via BBBD is a double-edged sword that requires precise control of extent, location and duration of BBBD avoiding potentially dangerous side effects including the enhanced passage of unwanted substances through the protective barrier.
5. Noninvasive imaging approaches to guide success of molecular CNS tumor therapies
Despite the many candidate molecular markers of CNS cancers, it is increasingly evident that the delivery of immunotargeted therapeutics to these markers in brain tumors is limited in large part due to the cellular barriers of the BBB and BBTB. Noninvasive neuroimaging technologies used clinically, such as MRI, CT, PET, and SPECT, have greatly improved the accuracy of brain cancer diagnosis, are indispensible in pre-surgical planning, and also serve as a means for tumor evaluation during or after therapy. These same imaging techniques, as well as the optical imaging methods more often used in pre-clinical research, can also be used to explore important questions for any targeted drug delivery strategy to the brain. These questions include whether the molecular target is expressed and accessible, how much drug is delivered and to which area of the tumor. Undoubtedly, the development of imaging surrogates (functional and molecular) will allow appropriate patient and treatment selection, define optimum biological dose, predict treatment response, and identify drug resistance. Once validated, these surrogate imaging markers would be of great benefit in facilitating translational research.
5.1 Imaging BBTB permeability
Functional imaging of BBTB permeability in brain tumor patients using high-resolution, noninvasive and quantitative in vivo methods is gaining renewed interest for several reasons. First, BBTB permeability may serve as a surrogate marker for brain tumor angiogenesis, and would be useful for studying the effectiveness of anti-angiogenic drugs on decreasing vessel permeability. Second, understanding the mechanisms of BBTB permeability can identify which therapeutic agents can enter the brain parenchyma and target to tumor tissue. Third, as described in Section 4.2, understanding how increasing permeability results from BBBD methods can help in the optimizing selective permeability of the BBTB to enhance drug delivery. Additionally, the detection of functional changes in BBB/BBTB would serve to limit brain injury, and could permit a more objective assessment of therapeutic benefit on a shorter time scale than is possible with anatomical techniques. Magnetic resonance imaging (MRI) [122], computed tomography (CT) [132], SPECT [133], and PET [134] are translational imaging modalities available to quantify BBB/BBTB permeability noninvasively. However, the measure of BBB permeability in fact depends on the size, charge, and composition of whatever contrast agent is being used to measure BBB function, as defined by status of BCEC tight junctions, transporters, and channels.
In CT and MRI applications, BBB vessel permeability is assessed using intravenous contrast materials, such as iodine-based or gadolinium (Gd)-based agents, respectively [135]. MRI is a practical modality for assessing BBB permeability since it is already widely used clinically to assess vascular changes in tumor growth. MRI images are derived from the detected changes in relaxation properties (T 1 1 and T2) of excited hydrogen ( H) nuclei in water and lipids of soft tissue generated from an electro-magnetic field. MRI contrast agents containing paramagnetic nuclei, such as Gd, change the chemical bonding of water to increase the rates of 1H relaxivity, thereby altering the signal strength. Most studies for assessing leakiness of the blood–brain barrier in human brain tumors have used T1-weighted dynamic contrast-enhanced MRI (DCE-MRI) using Gd chelated to diethylene triamine pentaacetic acid (DTPA). It should be noted that changes in MRI signal strength are not linear over the range of concentrations of contrast found in tissue thus measurements are typically made relative to a reference region. The disadvantage with MRI, however, is that MRI protocols and instrument performance have substantial effects on signal strength, making it difficult to compare data obtained with different instruments. With Gd-DTPA having low toxicity and MRI involving non-ionizing radiation, repeat imaging is possible and is limited mainly by instrument availability and expense. Perfusion CT images of BBB permeability are obtained from the x-ray attenuation of tissues containing an iodinated contrast agent, most often iohexol (MW 821 kDa). Data from CT imaging may be more quantitative than that obtained by MRI because the intensity of signal attenuation is directly proportional to concentration of contrast in a given region of interest. In addition, CT has the highest spatial resolution of all imaging modalities. However, the sensitivity of CT is limited since the high concentrations of CT contrast agent required for optimal signal attenuation can be toxic. Together with the relatively high radiation dose imparted to patients, the use of repeated CT scanning is limited.
Many studies have investigated the correlation between the presence of enhancement and the glioma grade [136,137]. These studies have shown that enhancement alone does not clearly distinguish varying grades of tumor malignancy. For example, approximately 30% of grade III anaplastic astrocytomas (AA) have functional BBB/BBTB as the tumors do not display contrast enhancement [138], whereras some higher grade IV GBM do not show enhancement at all. Currently, CT or MR imaging contrast agents used in the clinic are of low molecular weight (~0.5 to 0.9 kDa) and have an average hydrodynamic diameter of 1 nm. Since permeability measurements depend on the leakage of materials from the blood to the interstitial space, it is conceivable that low-molecular-weight contrast agents can leak relatively easily from the blood into the brain parenchyma, which might over-estimate vascular permeability. In a recent pre-clinical MRI study, it was noted that when comparing low molecular weight (0.5 kDa) and macromolecular (3.5 kDa) gadolinium contrast agents, there was differential enhancement in intracranial tumors after RT and anti-angiogenic therapies [121]. This was presumably because of different sizes pores created within the BBTB following RT. Generally, CT and MR imaging contrast agents do not differ much in their molecular weight. However, the disparity in permeability measures between these two modalities could be due to the difference in surface charge of nonionic CT contrast agents as compared with ionic MRI contrast agents.
Imaging of BBB status in patients can also be performed with SPECT, using 99mTc-DTPA and 99mTc-glucoheptonate (99mTc-GH) radiopharmaceuticals. In patients with malignant brain tumors, 99mTc-GH (physical half-life (t1/2) of 6 h) imaging showed that the permeability of the BBB in and around the tumor increases from approximately 20% to 75% following 30 Gy irradiation [120]. However, obtaining quantitative data from SPECT remains challenging. In principle, PET is uniquely capable of quantifying total tracer uptake and determining the kinetics of tracer transport across the BBB with dynamic studies. Both 68Ga-ethylenediamino tetraacetic acid (68Ga-EDTA, t1/2 68 min) and 82RbCl (t1/2 75 sec) have been successfully used to image permeability in a variety of malignancies in the human brain [134]. The permeability values measured with these two tracers are not the same, because of their large differences in chemical properties and molecular size. 68Ga-EDTA measures the passive physical permeability of the BBB as seen in dynamic contrast-enhanced MRI. In patients with primary CNS tumors, 68Ga-EDTA-PET can detect significant changes in tumor permeability; following chemotherapy and reported normalization of tumor vasculature, tumor permeability measurements by PET are comparable to that of the normal brain [139]. 82Rb+ is a potassium analogue that also crosses the intact BBB, albeit slowly due to its positive charge and significant hydration. Despite its very short half-life, 82Rb-PET can be used to rapidly quantify changes in BBB permeability, for example in patients with intracranial tumors [140], and after mannitol-induced BBBD [141].
Imaging BBB/BBTB permeability with currently available contrast agents is very helpful in identifying areas of leaky vasculature, and determining how and on what time scale BBBD strategies can further increase permeability. Using these techniques, some authors have reported an association between the degree of the BBBD as a surrogate of drug delivery and survival in patients bearing primary central nervous system lymphomas treated with a BBBD procedure [142]. However, these contrast agents with sizes much smaller than Abs, would only report on the delivery potential of therapeutics of comparable size to enter the brain tumor, and could be a false predictor of mAb delivery and improved clinical benefit. Thus, the arsenal of imaging probes for brain permeability must include CNS-deliverable reporters that should inform drug accessibility and targeting of larger immunotargeted agents across the BBB/BBTB.
5.2 Imaging immunotargeting
Molecular imaging is used to detect characteristic biomolecules, whose concentration is often changed by disease. Detection sensitivity of probes for CT imaging systems is in mmol/L whereas for MRI it is ~10 μmol/L. PET and SPECT are the most sensitive clinical imaging techniques where pmol/L sensitivity is achievable (note that PET is two to three order of magnitude more sensitive than SPECT) [143]. Many drugs and therapeutics can be labeled and thus detected directly by imaging techniques. To date, the FDA has approved four antibodies for the detection of cancer (anti-TAG-72 [tumor-associated glycoprotein 72] mAb 111In-satumomab pendetide, anti-EGP40 [epithelial glycoprotein 40] Fab 99mTc-nofetumomab merpentan, anti-CEA [carcinoembryonic antigen] Fab’ 99mTc-arcitumomab, anti-PSMA [prostate specific membrane antigen] mAb 111In-capromab pendetide), all of which are radioactively tagged for SPECT imaging [144]. Immuno-PET, which combines positron-emitting isotopes with mAbs, is an attractive approach to detecting mAb targeting due the very high sensitivity and spatial resolution of PET, as compared to SPECT. Hybrid scanners such as PET/CT and PET/MRI add the additional benefit of allowing co-registered anatomical and functional imaging. Further, PET imaging allows repeated measurements over time to obtain pharmacokinetic data on uptake, distribution, and clearance, and is equally applicable in animals and humans. For example, in vivo data can show whether the candidate immunotherapeutic reaches the brain tumor in appreciable concentrations or whether it accumulates excessively in another organ, alluding to drug efficacy and/or toxicity. The potential to quantify molecular interactions makes immuno-PET especially attractive as a scouting procedure prior to therapy.
Imaging of antibodies and their derivatives is a key application in their clinical translation seeing as size is a determinant for biodistribution and clearance kinetics. Typical examples of biological t1/2 decreasing with decreasing antibody size include IgG (150 kDa, t1/2 ~21 d), minibodies (80 kDa, t1/2 6–11 h), diabodies (55 kDa, t1/2 3–7 h), and, scFv (25 kDa, t1/2 0.5–2.0 h). Antigen affinity, epitope accessibility, and the fate of the probe upon cell binding are highly dependent on antibody design, and imaging can thus inform re-engineering of construct design to optimize antibody-tumor targeting and off-target profiles.
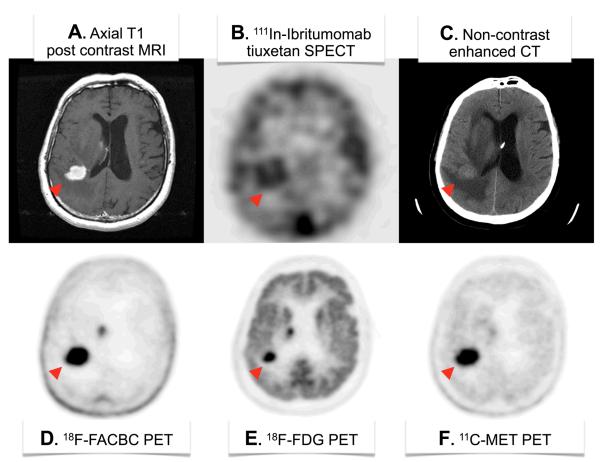
Currently, the information obtained in most neuro-oncological imaging studies in brain tumor patients is limited to anatomic features in addition to a small subset of functional biological processes, such as enhancement, glucose metabolism, and amino acid metabolism (Figure 5). Remarkably, very few studies have evaluated drug concentrations in brain tissue following administration. PET has been used in early clinical drug development trials, as was done in a Phase II trial for 11C-TMZ to assess tracer pharmacokinetics in gliomas and normal brains [145]. However, nuclear imaging of therapeutic mAb delivery to brain tumors following systemic administration is limited to SPECT examples (Figure 5B). The information obtained from immune-PET imaging studies can be very helpful in guiding the translation of investigational antibody agents from pre-clinical studies to early-phase clinical trials through to late-phase studies.
Figure 5.
Various brain scans in a patient with primary CNS lymphoma (red arrowhead). (A) Axial post-contrast MRI shows brisk enhancement indicative of BBBD. (B) 111In-Ibritumomab tiuxetan SPECT shows binding of this anti-CD20 IgG mAb to the malignancy. (C) Non-contrast head CT reveals the anatomic mass and surrounding mass effect and edema. (D) 18F-FACBC is a synthetic L-amino acid analog showing high amino acid metabolism in the disease and very low background utilization in normal brain parenchyma. (E) 18F-FDG shows high glucose metabolism throughout the gray matter with focally increased uptake in sites of disease. (F) The positron-emitting natural amino acid 11C-methionine also shows the high amino acid metabolism in the disease by PET, but with more uptake in normal brain parenchyma as compared to 18F-FACBC. Images courtesy of Department of Radiology, Memorial Sloan-Kettering Cancer Center. Abbreviations: FDG, fluorodeoxyglucose; FACBC, 1-amino-3-fluorocyclobutane-1-carboxylic acid; MET, L-methionine.
6. Conclusions
Ultimately, the adequate delivery of therapeutics to brain tumors is critical to kill all residual cancer cells for optimal patient outcomes. As described in this review, the BBB and BBTB presents a significant physical barrier that muse be overcome to achieving adequate therapeutic concentrations in the brain from the systemic circulation. Various approaches have been designed to improve brain delivery of immunotargeted agents. And, while each have the potential to play a significant role in the treatment of CNS disease, in vivo pre-clinical and clinical studies systematically quantifying accessibility and delivery of immunotargeted agents to the brain, and measuring therapeutic response and safety in CNS tumors is significantly lacking. Evaluating the efficacy of new treatment paradigms is extremely time-consuming and expensive owing to the standard clinical end points of radiographic response and survival outcomes. Noninvasive imaging with MRI, CT, PET and SPECT can serve to streamline the process by measuring surrogates of treatment response, including biomarkers that are themselves the target of therapy, that are more responsive to phenotypic changes in the tumor status. Patients, providers and payers alike would eagerly await the outcomes of such clinical trials to test the safety and efficacy of these exciting new approaches as definitive brain tumor therapies.
7. Expert Opinion
CNS malignancy remains a challenge in management, particularly in GBM where no currently available treatment is curative and prognosis is poor. Parallel to the search for a definitive treatment for CNS tumors, there is a need to improve the delivery of currently available drugs and those in development to adequately evaluate their therapeutic potential. As reviewed here, antibody-based devices, which are inherently targeted, still suffer from poor delivery across the blood-brain barrier into CNS tumors. Many approaches are being explored to enhance the delivery of these agents across the intact BBB by receptor-mediated transcytosis, or following osmotic or radiation-induced BBBD, for example. While some pre-clinical studies highlight the significant potential of these approaches in achieving improved brain tumor delivery of immunotargeted agents, the ultimate goal is the definitive assessment if the therapeutic value of such strategies and the subsequent translation of these approaches to clinical trials. Thus, current efforts should also be focused on evaluating the efficacy of these approaches to develop newer generations of potent and selective agents for optimal in vivo benefit. It should also be kept in mind that those strategies that increase normal brain exposure to currently approved drugs as compared to tumor exposure may alter the therapeutic index and safety profile of many drugs.
Imaging procedures have made important contributions to drug discovery and drug targeting, particularly in determining drug pharmacokinetics of directly labeled therapeutics, including those that are immunotargeted. Also valuable in the setting of CNS tumor imaging is the assessment of BBB/BBTB permeability, especially in the context of evaluating BBB disrupting strategies that are being explored clinically. While helpful in delineating the general status of the cellular barriers that can act to restrict access of contrast agents to the brain, one important caveat is that these measures are typically obtained from contrast agents that are less that 3.5 kDa in size. Thus, permeability measures can only be extrapolated for drug delivery of therapeutics of that size, and may not serve as a surrogate measure for delivery potential of antibodies and larger immunotargeted carriers (>25 kDa). Nonetheless, the various imaging techniques are complementary, and multimodal neuroimaging strategies will help to accelerate drug development by assisting in understanding and defining the challenges to translating molecularly targeted agents to the brain and tumors contained within this organ.
Undoubtedly, bevacizumab has had remarkable clinical benefit in brain tumor shrinkage and an increase in 6-month progression free survival. However, antiVEGF-therapy is not curative and can transform tumor growth pattern toward a more invasive phenotype, suggesting that targeting angiogenesis alone is not sufficient for effective tumor control. Given the relative success of bevacizumab, along with the low rate of anti-cancer mAbs that have advanced into Phase III trials, the questions remains as to why numerous other immunotargeted agents developed in the past decade were effective in preclinical laboratory models, but have been “lost in translation”. It could be that current heterotropic and orthotopic xenograft models of CNS cancer fail to recapitulate the complex tumor microenvironment found in human disease. More recent alternative models include genetically enginereed mouse models (GEMMs) [146], and cancer stem cell models [147]. Spontaneous canine GBM, which is highly invasive and exhibits the classical patterns of human GBM invasion, also represents a very valuable preclinical model to test not only the efficacy of novel therapies, but also their toxicity to the normal brain.[148]
While newer immuotargeted agents are designed to be more specific and potent with ideal pharmacokinetics to target disease, the first trials were in unselected patient populations and favorable responses to certain therapeutics were diluted since not all patients were positive for the specific molecular abnormality that was targeted. Using noninvasive imaging technologies like PET and SPECT is an ideal approach to identifying the molecular phenotype of patient’s tumors in real time for subsequent treatment stratification with an individualized therapeutic regimen based on their molecular signature. Perhaps under these conditions it will be easier to identify improved overall survival outcomes.
A final barrier to consider for the clinical translation of these new discoveries could potentially be linked to the high cost associated with developing biologics relative to small molecule inhibitors. Alternatives to these non-trivial issues would include the development of smaller antibody fragments and peptides that have comparable targeting specificity and potency but are easier and cheaper to produce as compared to whole IgG antibody-based agents. Ideally, the reduction in development costs would decrease the barrier to patient access to these innovative treatments without incurring costs that can be in excess of $100,000 per year for many biologics.
While the current landscape for developing effective immunotargeted agents with optimal delivery to CNS tumors may seem daunting given the challenges presented, there is tremendous opportunity for the drug delivery field to make important contributions in identifying definitive brain tumor therapies and make them more accessible to patients to change the course of their disease and improve their overall survival.
Acknowledgements and disclosures
This work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000139 (AMC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflict of interest.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.CBTRUS . CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2008. Central Brain Tumor Registry of the United States; Hinsdale, IL: 2012. [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2005-2009. Neuro-Oncology. 2012;14:v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurney JG, Smith MA, Bunin GR. Chapter III: CNS and miscellaneous intracranial and intraspinal neoplasms. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program; Bethesda, MD: 1999. [Google Scholar]
- 4.Bernstein M, Berger MS. Neuro-oncology: the essentials. 2nd ed. Thieme Medical Publishers, Inc.; New York: 2008. [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. Drug targeting, drug discovery, and brain drug development. Cambridge University Press; Cambridge: 2001. [Google Scholar]
- 7.Bluml S, McKeever K, Ettinger R, et al. B-cell targeted therapeutics in clinical development. Arthritis Res Ther. 2013;15(Suppl 1):S4. doi: 10.1186/ar3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson PJ, Souriau C. Engineered antibodies. Nat Med. 2003;9:129–34. doi: 10.1038/nm0103-129. [DOI] [PubMed] [Google Scholar]
- 9.Pardridge WM. Vector-mediated peptide drug-delivery to the brain. Adv Drug Delivery Rev. 1995;15:109–46. [PubMed] [Google Scholar]
- 10.Frank RN, Dutta S, Mancini MA. Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest Ophthalmol Vis Sci. 1987;28:1086–91. [PubMed] [Google Scholar]
- 11.Johanson CE. Permeability and vascularity of the developing brain: cerebellum vs cerebral cortex. Brain Res. 1980;190:3–16. doi: 10.1016/0006-8993(80)91155-5. [DOI] [PubMed] [Google Scholar]
- 12.Kapadia SE, de Lanerolle NC. Immunohistochemical and electron microscopic demonstration of vascular innervation in the mammalian brainstem. Brain Res. 1984;292:33–9. doi: 10.1016/0006-8993(84)90887-4. [DOI] [PubMed] [Google Scholar]
- 13.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–37. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 14.Miller FN, Sims DE. Contractile elements in the regulation of macromolecular permeability. Fed Proc. 1986;45:84–8. [PubMed] [Google Scholar]
- 15.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders NR, Ek CJ, Habgood MD, et al. Barriers in the brain: a renaissance? Trends Neurosci. 2008;31:279–86. doi: 10.1016/j.tins.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Saunders NR, Knott GW, Dziegielewska KM. Barriers in the immature brain. Cell Mol Neurobiol. 2000;20:29–40. doi: 10.1023/A:1006991809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin Exp Pharmacol Physiol. 1999;26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- 19.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, I. Adult brain. Clin Exp Pharmacol Physiol. 1999;26:11–9. doi: 10.1046/j.1440-1681.1999.02986.x. [DOI] [PubMed] [Google Scholar]
- 20.Stewart PA. Endothelial vesicles in the blood-brain barrier: are they related to permeability? Cell Mol Neurobiol. 2000;20:149–63. doi: 10.1023/A:1007026504843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 22.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3:90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 23.Schlageter KE, Molnar P, Lapin GD, et al. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58:312–28. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 24.Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal S, Sane R, Oberoi R, et al. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. doi: 10.1017/S1462399411001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebner S, Fischmann A, Rascher G, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–31. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 27.Shibata S. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathol. 1989;78:561–71. doi: 10.1007/BF00691283. [DOI] [PubMed] [Google Scholar]
- 28.Ningaraj NS. Drug delivery to brain tumours: challenges and progress. Expert Opin Drug Deliv. 2006;3:499–509. doi: 10.1517/17425247.3.4.499. [DOI] [PubMed] [Google Scholar]
- 29.Sarin H, Kanevsky AS, Wu H, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6:80. doi: 10.1186/1479-5876-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squire JM, Chew M, Nneji G, et al. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001;136:239–55. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 31.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478–84. [PubMed] [Google Scholar]
- 32.Boucher Y, Salehi H, Witwer B, et al. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br J Cancer. 1997;75:829–36. doi: 10.1038/bjc.1997.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navalitloha Y, Schwartz ES, Groothuis EN, et al. Therapeutic implications of tumor interstitial fluid pressure in subcutaneous RG-2 tumors. Neuro Oncol. 2006;8:227–33. doi: 10.1215/15228517-2006-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gumerlock MK, Neuwelt EA. Therapeutic opening of the blood-brain barrier in man. In: Bradbury MWB, editor. Physiology and Pharmacology of the Blood-Brain Barrier. Spinger; Berlin: 1992. pp. 525–42. [Google Scholar]
- 35.Vavra M, Ali MJ, Kang EW, et al. Comparative pharmacokinetics of 14C-sucrose in RG-2 rat gliomas after intravenous and convection-enhanced delivery. Neuro Oncol. 2004;6:104–12. doi: 10.1215/S1152851703000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netti PA, Baxter LT, Boucher Y, et al. Time-dependent behavior of interstitial fluid pressure in solid tumors: implications for drug delivery. Cancer Res. 1995;55:5451–8. [PubMed] [Google Scholar]
- 37.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–78. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong ET, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw. 2008;6:515–22. doi: 10.6004/jnccn.2008.0039. [DOI] [PubMed] [Google Scholar]
- 39.Brem S, Wong E. Angiogenesis and brain tumors: Molecular targets and molecular scalpels. In: Winn HR, editor. Youmans Neurological Surgery. 6th ed. Elsevier; Philadelphia: 2011. pp. 1151–117.pp. e271–277. •• This book chapter offers an in-depth perspective to the role of angiogenesis and brain tumors, and a comprehensive summary of clinical trials with anti-angiogenesis therapy with bevacizumab
- 40.Heimberger AB, Suki D, Yang D, et al. The natural history of EGFR and EGFRvIII in glioblastoma patients. J Transl Med. 2005;3:38. doi: 10.1186/1479-5876-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuan CT, Wikstrand CJ, Bigner DD. EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer. 2001;8:83–96. doi: 10.1677/erc.0.0080083. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein NI, Prewett M, Zuklys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–8. [PubMed] [Google Scholar]
- 43.Perk LR, Visser GW, Vosjan MJ, et al. 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med. 2005;46:1898–906. [PubMed] [Google Scholar]
- 44.Aerts HJ, Dubois L, Perk L, et al. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50:123–31. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- 45.Sivasankaran B, Degen M, Ghaffari A, et al. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res. 2009;69:458–65. doi: 10.1158/0008-5472.CAN-08-2610. [DOI] [PubMed] [Google Scholar]
- 46.Zalutsky MR, Reardon DA, Akabani G, et al. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2008;49:30–8. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reardon DA, Zalutsky MR, Akabani G, et al. A pilot study: 131I-antitenascin monoclonal antibody 81c6 to deliver a 44-Gy resection cavity boost. Neuro Oncol. 2008;10:182–9. doi: 10.1215/15228517-2007-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuan CT, Wakiya K, Herndon JE, 2nd, et al. MRP3: a molecular target for human glioblastoma multiforme immunotherapy. BMC Cancer. 2010;10:468. doi: 10.1186/1471-2407-10-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichert JM, Dhimolea E. The future of antibodies as cancer drugs. Drug Discov Today. 2012;17:954–63. doi: 10.1016/j.drudis.2012.04.006. •• This review describes current trends in antibody-based drug development
- 50.Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs. 2010;2:129–36. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baxevanis CN, Perez SA, Papamichail M. Cancer immunotherapy. Crit Rev Clin Lab Sci. 2009;46:167–89. doi: 10.1080/10408360902937809. [DOI] [PubMed] [Google Scholar]
- 52.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 53.Kontermann RE. Antibody-cytokine fusion proteins. Arch Biochem Biophys. 2012;526:194–205. doi: 10.1016/j.abb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Madhumathi J, Verma RS. Therapeutic targets and recent advances in protein immunotoxins. Curr Opin Microbiol. 2012;15:300–9. doi: 10.1016/j.mib.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 56.Poduslo JF, Curran GL. Increased permeability of superoxide dismutase at the blood-nerve and blood-brain barriers with retained enzymatic activity after covalent modification with the naturally occurring polyamine, putrescine. J Neurochem. 1996;67:734–41. doi: 10.1046/j.1471-4159.1996.67020734.x. [DOI] [PubMed] [Google Scholar]
- 57.Poduslo JF, Curran GL. Polyamine modification increases the permeability of proteins at the blood-nerve and blood-brain barriers. J Neurochem. 1996;66:1599–609. doi: 10.1046/j.1471-4159.1996.66041599.x. [DOI] [PubMed] [Google Scholar]
- 58.Triguero D, Buciak JB, Yang J, et al. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci U S A. 1989;86:4761–5. doi: 10.1073/pnas.86.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vorbrodt AW, Lossinsky AS, Dobrogowska DH, et al. Distribution of anionic sites and glycoconjugates on the endothelial surfaces of the developing blood-brain barrier. Brain Res. 1986;394:69–79. doi: 10.1016/0165-3806(86)90083-0. [DOI] [PubMed] [Google Scholar]
- 60.Danielyan K, Ding BS, Gottstein C, et al. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J Pharmacol Exp Ther. 2007;321:947–52. doi: 10.1124/jpet.107.120535. [DOI] [PubMed] [Google Scholar]
- 61.Ramakrishnan M, Wengenack TM, Kandimalla KK, et al. Selective contrast enhancement of individual Alzheimer’s disease amyloid plaques using a polyamine and Gd-DOTA conjugated antibody fragment against fibrillar Abeta42 for magnetic resonance molecular imaging. Pharm Res. 2008;25:1861–72. doi: 10.1007/s11095-008-9600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foley TL, Burkart MD. Site-specific protein modification: advances and applications. Curr Opin Chem Biol. 2007;11:12–9. doi: 10.1016/j.cbpa.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 63.Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem. 2008;19:1327–38. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- 64.Holly J, Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83:154–60. doi: 10.1159/000095523. [DOI] [PubMed] [Google Scholar]
- 65.Uotani S, Bjorbaek C, Tornoe J, et al. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 1999;48:279–86. doi: 10.2337/diabetes.48.2.279. [DOI] [PubMed] [Google Scholar]
- 66.Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54:1882–8. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- 67.Tuma P, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Pardridge WM. Delivery of beta-galactosidase to mouse brain via the blood-brain barrier transferrin receptor. J Pharmacol Exp Ther. 2005;313:1075–81. doi: 10.1124/jpet.104.082974. [DOI] [PubMed] [Google Scholar]
- 69.Kurihara A, Pardridge WM. Imaging brain tumors by targeting peptide radiopharmaceuticals through the blood-brain barrier. Cancer Res. 1999;59:6159–63. [PubMed] [Google Scholar]
- 70.Yu YJ, Zhang Y, Kenrick M, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. •This research article describes the role of antibody affinity in optimizing the delivery antibody constructs to the brain by RMT. This article also described a novel bispecific antibody with enhanced brain uptake capacity for targeted therapy against Alzheimer’s Disease.
- 71.Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114:168–72. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 72.Rapoport SI. Effect of concentrated solutions on blood-brain barrier. Am J Physiol. 1970;219:270–4. doi: 10.1152/ajplegacy.1970.219.1.270. [DOI] [PubMed] [Google Scholar]
- 73.Neuwelt EA, Specht HD, Barnett PA, et al. Increased delivery of tumor-specific monoclonal antibodies to brain after osmotic blood-brain barrier modification in patients with melanoma metastatic to the central nervous system. Neurosurgery. 1987;20:885–95. doi: 10.1227/00006123-198706000-00011. [DOI] [PubMed] [Google Scholar]
- 74.Blanchette M, Pellerin M, Tremblay L, et al. Real-time monitoring of gadolinium diethylenetriamine penta-acetic acid during osmotic blood-brain barrier disruption using magnetic resonance imaging in normal wistar rats. Neurosurgery. 2009;65:344–50. doi: 10.1227/01.NEU.0000349762.17256.9E. [DOI] [PubMed] [Google Scholar]
- 75.Bellavance MA, Blanchette M, Fortin D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10:166–77. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbott NJ, Revest PA. Control of brain endothelial permeability. Cerebrovasc Brain Metab Rev. 1991;3:39–72. [PubMed] [Google Scholar]
- 77.Rapoport SI, Robinson PJ. Tight-junctional modification as the basis of osmotic opening of the blood-brain barrier. Ann N Y Acad Sci. 1986;481:250–67. doi: 10.1111/j.1749-6632.1986.tb27155.x. [DOI] [PubMed] [Google Scholar]
- 78.Williams PC, Henner WD, Roman-Goldstein S, et al. Toxicity and efficacy of carboplatin and etoposide in conjunction with disruption of the blood-brain tumor barrier in the treatment of intracranial neoplasms. Neurosurgery. 1995;37:17–27. doi: 10.1227/00006123-199507000-00003. discussion 27-8. [DOI] [PubMed] [Google Scholar]
- 79.Fortin D, Desjardins A, Benko A, et al. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the Sherbrooke experience. Cancer. 2005;103:2606–15. doi: 10.1002/cncr.21112. [DOI] [PubMed] [Google Scholar]
- 80.Fortin D, Gendron C, Boudrias M, et al. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in the treatment of cerebral metastasis. Cancer. 2007;109:751–60. doi: 10.1002/cncr.22450. [DOI] [PubMed] [Google Scholar]
- 81.Boockvar JA, Tsiouris AJ, Hofstetter CP, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J Neurosurg. 2011;114:624–32. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin BJ, Burkhardt JK, Riina HA, et al. Superselective intra-arterial cerebral infusion of novel agents after blood-brain disruption for the treatment of recurrent glioblastoma multiforme: a technical case series. Neurosurg Clin N Am. 2012;23:323–9. ix–x. doi: 10.1016/j.nec.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 83.Burkhardt JK, Riina H, Shin BJ, et al. Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: progression-free survival and overall survival. World Neurosurg. 2012;77:130–4. doi: 10.1016/j.wneu.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller G. Drug targeting. Breaking down barriers. Science. 2002;297:1116–8. doi: 10.1126/science.297.5584.1116. [DOI] [PubMed] [Google Scholar]
- 85.Kemper EM, Boogerd W, Thuis I, et al. Modulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30:415–23. doi: 10.1016/j.ctrv.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 86.Brown RC, Egleton RD, Davis TP. Mannitol opening of the blood-brain barrier: regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 2004;1014:221–7. doi: 10.1016/j.brainres.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 87.Fortin D, Salame JA, Desjardins A, et al. Technical modification in the intracarotid chemotherapy and osmotic blood-brain barrier disruption procedure to prevent the relapse of carboplatin-induced orbital pseudotumor. AJNR Am J Neuroradiol. 2004;25:830–4. [PMC free article] [PubMed] [Google Scholar]
- 88.Remsen LG, Pagel MA, McCormick CI, et al. The influence of anesthetic choice, PaCO2, and other factors on osmotic blood-brain barrier disruption in rats with brain tumor xenografts. Anesth Analg. 1999;88:559–67. doi: 10.1097/00000539-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 89.Hochberg FH, Pruitt AA, Beck DO, et al. The rationale and methodology for intra-arterial chemotherapy with BCNU as treatment for glioblastoma. J Neurosurg. 1985;63:876–80. doi: 10.3171/jns.1985.63.6.0876. [DOI] [PubMed] [Google Scholar]
- 90.Doolittle ND, Miner ME, Hall WA, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–47. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 91.Doctrow SR, Abelleira SM, Curry LA, et al. The bradykinin analog RMP-7 increases intracellular free calcium levels in rat brain microvascular endothelial cells. J Pharmacol Exp Ther. 1994;271:229–37. [PubMed] [Google Scholar]
- 92.Sanovich E, Bartus RT, Friden PM, et al. Pathway across blood-brain barrier opened by the bradykinin agonist, RMP-7. Brain Res. 1995;705:125–35. doi: 10.1016/0006-8993(95)01143-9. [DOI] [PubMed] [Google Scholar]
- 93.Emerich DF, Snodgrass P, Dean R, et al. Enhanced delivery of carboplatin into brain tumours with intravenous Cereport (RMP-7): dramatic differences and insight gained from dosing parameters. Br J Cancer. 1999;80:964–70. doi: 10.1038/sj.bjc.6690450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Warren K, Jakacki R, Widemann B, et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children’s Oncology Group. Cancer Chemother Pharmacol. 2006;58:343–7. doi: 10.1007/s00280-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 95.Prados MD, Schold SJS, Fine HA, et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003;5:96–103. doi: 10.1093/neuonc/5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ningaraj NS, Rao M, Hashizume K, et al. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;301:838–51. doi: 10.1124/jpet.301.3.838. [DOI] [PubMed] [Google Scholar]
- 97.Baba T, Chio CC, Black KL. The effect of 5-lipoxygenase inhibition on blood-brain barrier permeability in experimental brain tumors. J Neurosurg. 1992;77:403–6. doi: 10.3171/jns.1992.77.3.0403. [DOI] [PubMed] [Google Scholar]
- 98.Chio CC, Baba T, Black KL. Selective blood-tumor barrier disruption by leukotrienes. J Neurosurg. 1992;77:407–10. doi: 10.3171/jns.1992.77.3.0407. [DOI] [PubMed] [Google Scholar]
- 99.Walker WL, Cook J. Drug delivery to brain tumors. Bull Math Biol. 1996;58:1047–74. doi: 10.1007/BF02458383. [DOI] [PubMed] [Google Scholar]
- 100.Salkoff L, Butler A, Ferreira G, et al. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen SF, Stock C. Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res. 2013;73:1658–61. doi: 10.1158/0008-5472.CAN-12-4188. [DOI] [PubMed] [Google Scholar]
- 102.Ningaraj NS, Sankpal UT, Khaitan D, et al. Modulation of KCa channels increases anticancer drug delivery to brain tumors and prolongs survival in xenograft model. Cancer Biol Ther. 2009;8:1924–33. doi: 10.4161/cbt.8.20.9490. [DOI] [PubMed] [Google Scholar]
- 103.Ransom CB, Sontheimer H. BK channels in human glioma cells. J Neurophysiol. 2001;85:790–803. doi: 10.1152/jn.2001.85.2.790. [DOI] [PubMed] [Google Scholar]
- 104.Sontheimer H, Black JA, Waxman SG. Voltage-gated Na+ channels in glia: properties and possible functions. Trends Neurosci. 1996;19:325–31. doi: 10.1016/0166-2236(96)10039-4. [DOI] [PubMed] [Google Scholar]
- 105.Black KL, Yin D, Konda BM, et al. Different effects of KCa and KATP agonists on brain tumor permeability between syngeneic and allogeneic rat models. Brain Res. 2008;1227:198–206. doi: 10.1016/j.brainres.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hynynen K, McDannold N, Vykhodtseva N, et al. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–6. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 107.Hynynen K. Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev. 2008;60:1209–17. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sheikov N, McDannold N, Sharma S, et al. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093–104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shang X, Wang P, Liu Y, et al. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43:364–9. doi: 10.1007/s12031-010-9451-9. [DOI] [PubMed] [Google Scholar]
- 110.Sheikov N, McDannold N, Jolesz F, et al. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32:1399–409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 111.Yang FY, Lin GL, Horng SC, et al. Pulsed high-intensity focused ultrasound enhances the relative permeability of the blood-tumor barrier in a glioma-bearing rat model. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:964–70. doi: 10.1109/TUFFC.2011.1897. [DOI] [PubMed] [Google Scholar]
- 112.Yang FY, Teng MC, Lu M, et al. Treating glioblastoma multiforme with selective high-dose liposomal doxorubicin chemotherapy induced by repeated focused ultrasound. Int J Nanomedicine. 2012;7:965–74. doi: 10.2147/IJN.S29229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hynynen K, McDannold N, Sheikov NA, et al. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 114.McDannold N, Vykhodtseva N, Raymond S, et al. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–37. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 115.Hynynen K, McDannold N, Vykhodtseva N, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–54. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 116.Baseri B, Choi JJ, Tung YS, et al. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol. 2010;36:1445–59. doi: 10.1016/j.ultrasmedbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu HL, Hua MY, Chen PY, et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415–25. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 118.Kinoshita M, McDannold N, Jolesz FA, et al. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park EJ, Zhang YZ, Vykhodtseva N, et al. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163:277–84. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin DX, Zheng R, Tang J, et al. Influence of radiation on the blood-brain barrier and optimum time of chemotherapy. Int J Radiat Oncol Biol Phys. 1990;19:1507–10. doi: 10.1016/0360-3016(90)90364-p. [DOI] [PubMed] [Google Scholar]
- 121.Lemasson B, Serduc R, Maisin C, et al. Monitoring blood-brain barrier status in a rat model of glioma receiving therapy: dual injection of low-molecular-weight and macromolecular MR contrast media. Radiology. 2010;257:342–52. doi: 10.1148/radiol.10092343. [DOI] [PubMed] [Google Scholar]
- 122.Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23:4127–36. doi: 10.1200/JCO.2005.07.144. •The authors in this paper describe the differential measurements of BBB permeability by MRI as defined by the molecular size of the gadolinium contrast agent used to measure vascular disruption following radiotherapy.
- 123.Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res. 2007;13:1675–83. doi: 10.1158/1078-0432.CCR-06-2489. [DOI] [PubMed] [Google Scholar]
- 124.Li YQ, Chen P, Haimovitz-Friedman A, et al. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003;63:5950–6. [PubMed] [Google Scholar]
- 125.Yuan H, Gaber MW, Boyd K, et al. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys. 2006;66:860–6. doi: 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 126.van Vulpen M, Kal HB, Taphoorn MJ, et al. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (Review). Oncol Rep. 2002;9:683–8. [PubMed] [Google Scholar]
- 127.Diserbo M, Agin A, Lamproglou I, et al. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can J Physiol Pharmacol. 2002;80:670–8. doi: 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- 128.Reinhold HS, Calvo W, Hopewell JW, et al. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol Biol Phys. 1990;18:37–42. doi: 10.1016/0360-3016(90)90264-k. [DOI] [PubMed] [Google Scholar]
- 129.Mima T, Toyonaga S, Mori K, et al. Early decrease of P-glycoprotein in the endothelium of the rat brain capillaries after moderate dose of irradiation. Neurol Res. 1999;21:209–15. doi: 10.1080/01616412.1999.11740920. [DOI] [PubMed] [Google Scholar]
- 130.Baumann BC, Benci JL, Santoiemma PP, et al. An integrated method for reproducible and accurate image-guided stereotactic cranial irradiation of brain tumors using the small animal radiation research platform. Transl Oncol. 2012;5:230–7. doi: 10.1593/tlo.12136. ••Recent article describing a small animal research radiation platform (SARRP) that selectively disrupts the BBB in mice bearing intracranial tumors, and facilitates the entry of large molecular weight macromolecules.
- 131.Baumann BC, Kao GD, Mahmud A, et al. Enhancing the Efficacy of Drug-loaded Nanocarriers against Brain Tumors by Targeted Radiation Therapy. Oncotarget. 2012 doi: 10.18632/oncotarget.777. •Article highlighting the enhanced delivery of drug-loaded nanocarriers to tumors following selective irradiation of mouse intracranial tumors. Also included are the survival curves demonstrating the improved therapeutic efficacy in combining irradiation with drug delivery.
- 132.Jain R, Ellika SK, Scarpace L, et al. Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. AJNR Am J Neuroradiol. 2008;29:694–700. doi: 10.3174/ajnr.A0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saha GB, MacIntyre WJ, Go RT. Radiopharmaceuticals for brain imaging. Semin Nucl Med. 1994;24:324–49. doi: 10.1016/s0001-2998(05)80022-4. [DOI] [PubMed] [Google Scholar]
- 134.Webb S, Ott RJ, Cherry SR. Quantitation of blood-brain barrier permeability by positron emission tomography. Phys Med Biol. 1989;34:1767–71. doi: 10.1088/0031-9155/34/12/001. [DOI] [PubMed] [Google Scholar]
- 135.Miller JC, Pien HH, Sahani D, et al. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst. 2005;97:172–87. doi: 10.1093/jnci/dji023. [DOI] [PubMed] [Google Scholar]
- 136.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 137.Dean BL, Drayer BP, Bird CR, et al. Gliomas: classification with MR imaging. Radiology. 1990;174:411–5. doi: 10.1148/radiology.174.2.2153310. [DOI] [PubMed] [Google Scholar]
- 138.Chamberlain MC, Murovic JA, Levin VA. Absence of contrast enhancement on CT brain scans of patients with supratentorial malignant gliomas. Neurology. 1988;38:1371–4. doi: 10.1212/wnl.38.9.1371. [DOI] [PubMed] [Google Scholar]
- 139.Ott RJ, Brada M, Flower MA, et al. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer. 1991;27:1356–61. doi: 10.1016/0277-5379(91)90009-3. [DOI] [PubMed] [Google Scholar]
- 140.Dhawan V, Jarden JO, Moeller JR, et al. Positron emission tomographic measurement of blood-to-brain and blood-to-tumour transport of 82Rb. II: Clinical data and validation of technique. Phys Med Biol. 1989;34:1785–94. doi: 10.1088/0031-9155/34/12/003. [DOI] [PubMed] [Google Scholar]
- 141.Zunkeler B, Carson RE, Olson J, et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J Neurosurg. 1996;85:1056–65. doi: 10.3171/jns.1996.85.6.1056. [DOI] [PubMed] [Google Scholar]
- 142.Kraemer DF, Fortin D, Doolittle ND, et al. Association of total dose intensity of chemotherapy in primary central nervous system lymphoma (human non-acquired immunodeficiency syndrome) and survival. Neurosurgery. 2001;48:1033–40. doi: 10.1097/00006123-200105000-00013. discussion 40-1. [DOI] [PubMed] [Google Scholar]
- 143.Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 144.Zuckier LS, DeNardo GL. Trials and tribulations: oncological antibody imaging comes to the fore. Semin Nucl Med. 1997;27:10–29. doi: 10.1016/s0001-2998(97)80033-5. [DOI] [PubMed] [Google Scholar]
- 145.Saleem A, Brown GD, Brady F, et al. Metabolic activation of temozolomide measured in vivo using positron emission tomography. Cancer Res. 2003;63:2409–15. [PubMed] [Google Scholar]
- 146.Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–97. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- 147.Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–52. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen L, Zhang Y, Yang J, et al. Vertebrate animal models of glioma: Understanding the mechanisms and developing new therapies. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbcan.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]