Abstract
Brains can perceive or recognize a face even though we are subjectively unaware of the existence of that face. However, the exact neural correlates of such covert face processing remain unknown. Here, we compared the fMRI activities between a prosopagnosic patient and normal controls when they saw famous and unfamiliar faces. When compared with objects, the patient showed greater activation to famous faces in the fusiform face area (FFA) though he could not overtly recognize those faces. In contrast, the controls showed greater activation to both famous and unfamiliar faces in the FFA. Compared with unfamiliar faces, famous faces activated the controls', but not the patient's lateral prefrontal cortex (LPFC) known to be involved in familiar face recognition. In contrast, the patient showed greater activation in the bilateral medial frontal gyrus (MeFG). Functional connectivity analyses revealed that the patient's right middle fusiform gyrus (FG) showed enhanced connectivity to the MeFG, whereas the controls' middle FG showed enhanced connectivity to the LPFC. These findings suggest that the FFA may be involved in both covert and overt face recognition. The patient's impairment in overt face recognition may be due to the absence of the coupling between the right FG and the LPFC.
Keywords: fMRI, face processing, fusiform gyrus, prosopagnosia, covert recognition
Introduction
Faces are one of the most important stimuli in our lives. As a result, the human brain has developed a highly efficient mechanism with which to process faces. Consequently, humans have the ability to not only discriminate among thousands of different faces with ease but also are able to recognize faces that we may not have encountered for decades (for a review, see Pascalis et al. 2011). Further, our brain is able to unconsciously perceive or recognize faces without them even entering our consciousness (for a review, see Barton et al. 2001; Schweinberger and Burton 2003). Evidence for unconscious or covert face processing comes primarily from studies with patients with acquired prosopanosia, wherein the patients exhibited different behaviors (e.g., reaction times: Young et al. 1988; De Haan et al. 1992), physiological responses (e.g., skin conductance: Bauer 1984), or brain electrophysiological activities (e.g., event-related brain potentials: Renault et al. 1989; Bobes et al. 2004) for familiar faces versus unfamiliar faces. The differences occurred despite the fact that they could not overtly recognize the familiar faces. However, little is known about the cortical mechanisms underlying unconscious or covert face recognition.
Recent fMRI studies have suggested that conscious, or overt, face processing is mediated by a distributed neural network (Haxby et al. 2000). This network consists of a “core system” that includes the fusiform face area (FFA, Kanwisher et al. 1997), the occipital face area (OFA, Gauthier et al. 2000), and the superior temporal sulcus (STS; Haxby et al. 2000), in addition to an “extended system” that includes the frontal lobe, the sublobar region, and the limbic system. Within this network, the FFA shows the most specific response to face processing. It is well established that activation of the FFA is correlated with the detection and identification of faces (Grill-Spector et al. 2004; Rotshtein et al. 2005; Kanwisher and Yovel 2006). Activation of the FFA has also been shown to occur when individuals were presented with degraded face images, or even when individuals mistook an ambiguous image of a house for a face, suggesting that this region is hypersensitive to face stimuli (Heekeren et al. 2004; Summerfield et al. 2006).
In addition to overt face recognition, studies have also reported the involvement of the FFA in covert face processing. For example, both Jiang and He (2006) and Morris et al. (2007) demonstrated that the FFA could be activated by face images even when the participants were not aware of them. Additionally, a study by Lehmann et al. (2004) revealed greater activation of the FFA for faces that had been previously presented (i.e., the old faces) than for new faces, regardless of the participants' subjective responses (i.e., whether the face is old or new). Similarly, Kouider et al. (2009) found that repeated presentation of a face resulted in a decrease in FFA activity even when participants did not perceive the initial priming face. Such evidence suggests that the FFA is involved in processing of not only consciously perceived faces but also unconsciously perceived faces.
The existing fMRI studies on covert face processing have used unfamiliar faces, and relatedly, focused almost on neural activity in the FFA (e.g., Lehmann et al. 2004; Morris et al. 2007). Studies of overt familiar face processing have demonstrated that the neural substrates involved in familiar face processing are different from those involved in unfamiliar face processing. The former tends to engage the extended face network to a greater degree (Gobbini and Haxby 2007), most notably, several major regions in the frontal cortex such as the inferior frontal gyrus and the middle frontal gyrus (MFG). Their involvement in overt familiar face processing is perhaps due to the fact that more neural resources are needed to retrieve the identity or autobiographical information associated with a face. It is possible that covert and overt processing of familiar faces may differ from that of unfamiliar faces. Thus, in addition to greater involvement of the core system, familiar face processing, overt and covert alike, likely engages the frontal regions of the extended face network to a greater degree than unfamiliar face processing. However, direct evidence to support these suggestions is largely lacking.
Rossion et al. (2003) tested a prosopagnosic patient, known as P.S., who was unable to recognize faces with which she was previously familiar. Nevertheless, she showed normal activation of the right FFA to unfamiliar faces. Further, a recent study with P.S. by Simon et al. (2011) demonstrated that both her FFA and the right MFG showed more activation for famous than for unfamiliar faces. This new finding suggests that covert familiar face processing may indeed be different from covert unfamiliar face processing, with the former engaging both the core and extended face-processing networks to a greater degree. However, due to the absence of a control group in this study, the extent to which the patient's familiar face processing is similar to, or different from, familiar face processing in normal individuals remains unknown. The present study directly addressed this intriguing question by comparing a prosopagnosic patient's processing of familiar and unfamiliar faces with those of normal controls.
To the best of our knowledge, all existing studies on covert face processing and particularly those involving prosopagnosic patients have not explored the functional connectivities among the key regions in the core and extended face networks. Such exploration is necessary because it would shed light on the interactions between the core and extended face-processing networks involved in covert face processing. Further, it would also provide insight into the nature of a patient's prosopagnosia. It is possible that prosopagnosic patients have impairments in any number of key regions in the face-processing network; alternatively, it is possible that patients have intact brain regions but that abnormalities in the functional coupling between these regions is responsible for the impairments in face identification (Fox et al. 2008). To explore these possibilities, the present study also examined the functional connectivities between the patient's face-responsive areas in the middle fusiform gyrus (FG) and the frontal regions in the extended network with the use of psychophysiological interaction (PPI, Friston et al. 1997) and compared it to those of normal controls.
Materials and Methods
Patient
Patient Z.D. was a right-handed male with normal vision (43 years old, at the time of testing), who was suffering from mitochondrial encephalomyopathy. The patient showed great difficulties in recognizing faces with which he was previously familiar, including his family (e.g., his wife and daughter), his doctors, and even his own face in mirrors. However, he was able to accurately recognize the identities of these persons by their voices or their clothes. During testing, Z.D. was asked to name 18 color photos of familiar faces (famous individuals and family members) and 18 daily objects, for example, different kinds of fruit and various kitchen supplies. According to his family members, he had been very familiar with all 36 items before the onset of his disease. After the onset of his disease, he could identify none of the faces, whereas he could accurately discriminate their gender (relying on hairstyle differences) and some non-facial features (e.g., glasses). However, 16 of 18 daily objects were correctly identified, suggesting that Z.D. suffered from a selective impairment in face recognition. Diffusion weighted image (DWI) revealed cytotoxic edema resulting from the onset of his disease in the right temporal lobule, the left temporal lobule, the right occipitotemporal cortex, the right posterior parietal lobule, and the right precuneus (Fig. 1).
Figure 1.
Diffusion weighted image (DWI). The cytotoxic edema (the regions with high intensity) was observed in the right temporal lobule, the left temporal lobule, the right occipitotemporal cortex, the right posterior parietal lobule, and the right precuneus.
Control Participants
In addition to Z.D., 10 normal, right-handed, and age-matched Chinese individuals with normal or corrected-to-normal vision (8 males and 2 females) participated the present study as controls, whose age ranged from 40 to 56 (mean = 46.1). None of these normal participants reported a history of psychiatric disorders or took psychotropic medication. Both Z.D. and all normal participants gave informed consent to participate in the present study. This study was approved by the Human Research Protection Program of Henan Provincial People's Hospital, Zhengzhou, China.
Behavior Experiment
The behavior experiment included 2 tasks. The first was a face/object recognition task, during which the normal participants were presented with 16 famous face photos (face test) and 16 daily objects (object test) and were asked to name the famous faces and the objects, respectively. For the patient Z.D., the same test was performed with 2 additional familiar faces (Z.D.'s wife and daughter) in the face test and 2 additional daily objects in the object test to equalize the number of items between the 2 tests.
The second test was a face/object discrimination task that also included face and object photos. For each face test trial, a face image was first presented for 1 s, followed by a 1-s fixation, and then a second image was presented which consisted of 2 simultaneously presented faces appearing side by side. All paired faces had similar haircuts and face contours, but one of the faces in the pair was the same as the face in the first image. During the presentation of the second image, participants were instructed to indicate which one of them was the same as the first-presented image using their index or middle finger to press the corresponding key as quickly as possible. The second image was presented until participants responded. However, a response occurring after 10 s was considered a missing response. For the object test, the trial paradigm was similar to that of the face test except that nonface objects replaced the face images. Further, in the second image, the side-by-side arrangements of paired objects were from the same object class (e.g., 2 houses or 2 watches). The spanned visual angles were about 10.3° by 6.3° and 24.8° by 6.3° for the single-presented picture and side-by-side-presented 2 pictures, respectively.
fMRI Experiment
Procedure
fMRI scanning consisted of 2 passive viewing sessions, the famous face session and the unfamiliar face session. The famous face session included three famous face epochs and three common object epochs with 12-s fixation intervals between 2 adjacent epochs. Each epochs was presented for 18 s. Nine famous faces were used, and they were selected based on a prescanning questionnaire answered by each participant, or in Z.D.'s case by his family members, and consisted of celebrities such as the national leaders of China and various television stars originally highly familiar to Z.D. Nine common objects were used and they consisted of typical daily objects such as a bag, a flower, and a chair. The spanned visual angle of each stimuli picture was about 15.1° by 11.2°. Each epoch contains 7 gray and 2 color images, each of which was presented for 1200-ms and followed by an 800-ms fixation. The color images were presented in random order. During each epoch, the participants were instructed to passively view the image and press a key when they saw a color image (this procedure ensured that the participants were paying attention during the passive viewing task). The unfamiliar face session was the same as the famous face session except that faces that the participants had not seen before were presented in place of the famous faces. Additionally, the common objects used in the unfamiliar face session were completely different from those used in the famous face session. Each session began with a 6-s fixation to acclimatize the participants and ended with a 12-s fixation to compensate for the delay of the hemodynamic response.
fMRI Data Acquisition
Structural and functional MRI data were collected using a 3.0-T MR imaging system (Siemens Trio, Germany). The functional MRI series were collected using a single shot, T2*-weighted gradient-echo planar imaging sequence (TR/TE = 2000/30 ms; 31slices; 4 mm thickness; matrix = 64 × 64) covering the whole brain with a resolution of 3.75 × 3.75 mm. High-resolution anatomical scans were acquired with a T1-weighted 3D enhanced fast gradient-echo sequence (voxel size: 1 × 1 × 1 mm3, matrix: 256 × 256 × 176).
fMRI Data Analysis
Spatial preprocessing and statistical mapping were performed with SPM8 software (www.fil.ion.ucl.ac.uk/spm, Friston et al. 1994). After slice-timing correction, spatial realignment and normalization to the MNI152 template (Montreal Neurological Institute), the scans of each session were re-sampled into 2 × 2 × 2 mm3 voxels, and then spatially smoothed with an isotropic 6-mm full-width-half-maximal (FWHM) smoothing function. The time series of each session was high-pass filtered (high-pass filter = 128-s) to remove low-frequency noise possibly containing scanner drift (Friston et al. 1994).
After preprocessing, a general linear model (GLM) was constructed by including 4 condition regressors (i.e., famous face and common object for famous face session; unfamiliar face and common object for unfamiliar face session). Each condition regressor was obtained by convolving a canonical hemodynamic response function with a box function corresponding to the onset time series of each stimulus category. Movement parameters were used in the GLM as additional regressors to account for movement related artifacts. After participant-specific parameters were estimated, a conventional whole-brain analysis was performed at the individual level using contrasts of famous face minus common object, unfamiliar face minus common object, and familiar face minus unfamiliar face. Then, the group result for each of the contrasts was obtained by averaging corresponding individual contrast maps using a random effect analysis with a statistical threshold of P < 0.001 (uncorrected) and cluster threshold of k ≥ 12.
Results
Behavioral Results
For the face/object recognition task which had no time limit, the patient Z.D. could not identify any of the 18 famous faces, but accurately recognized 16 of 18 daily objects. In contrast, on average, the normal participants successfully recognized 13.8 of the 16 famous faces (mean accuracy ratio = 86%, standard deviation [SD] = 10%) and 15.6 of the 16 daily objects (mean accuracy ratio = 98%, SD = 3%) (Fig. 2, top).
Figure 2.

The behavior results (mean ± SD) for the patient Z.D. and the normal participants. The accuracy ratio for the face/object recognition task (top), the accuracy ratio and missing ratio for the face test in the face/object discrimination task (middle), and the accuracy ratio and missing ratio for the object test in the face/object discrimination task (bottom). NP, normal participant.
For the face/object discrimination task that had time limits, Z.D. had a correct discrimination rate of 25% (missing 50%) for the face test, and 50% (missing 22%) for the object test (Table 1). In contrast, the normal participants had a mean correct discrimination rate of 93% (SD = 11%, missing 2%) for the face test, and 94% (SD = 11%, missing 3%) for the object test (Fig. 2, middle and bottom). Figure 2 shows the behavior performance for Z.D. and normal participants. In order to compare the behavior performance of Z.D. to that of the normal participants, we first obtained the means and SDs of behavior performance scores of the normal participants (e.g., the accuracy of the face test). Then, based on such results, we calculated the Z-score of the behavior performance of Z.D. (e.g., the accuracy of the face test) relative to the performances of the normal participants. As revealed by the comparison, for both the face and object tests, Z.D. performed significantly worse than normal participants (face test Z = −6.39, and object test Z = −4.00).
Table 1.
The results in the face/object discrimination task
| Face test |
Object test |
|||
|---|---|---|---|---|
| Accuracy | Missing | Accuracy | Missing | |
| Patient Z.D. | ||||
| Mean | 0.25 | 0.50 | 0.50 | 0.22 |
| Normal participant | ||||
| Mean | 0.93 | 0.02 | 0.94 | 0.03 |
| SD | 0.11 | 0.06 | 0.11 | 0.06 |
Note: SD, standard deviation.
In sum, these behavioral results indicated that the patient Z.D., compared with the normal participants, showed a severe impairment in face recognition and a relatively less severe impairment in object recognition.
fMRI Results
Results of GLM Analysis
One of the primary aims of the present study was to examine whether a patient with prosopagnosia will show a face-selective response in the ventral occipitotemporal cortex, similar to that of a normal participant. To determine this, we compared the activation for famous faces and unfamiliar faces to that for common objects. As shown in Figure 3, greater activation was observed in Z.D.'s bilateral middle FG for famous faces than for common objects. However, there was no difference in activation between unfamiliar faces and common objects in Z.D.'s middle FG. On the other hand, for most normal participants, when comparing famous faces to common objects, greater activation was observed in the middle FG (right hemisphere: n = 9; left hemisphere: n = 7). In the same way, greater activation was observed in the middle FG when comparing unfamiliar faces to common objects (right hemisphere: n = 7; left hemisphere: n = 5). Table 2 summarizes the locus, extent, and activation intensity of the face-selective region for Z.D. and each normal participant. In line with a large body of research (e.g., Rossion et al. 2003; Kanwisher and Yovel 2006), the loci of these regions were consistent with that of the FFA (Kanwisher, et al. 1997), and we will henceforth refer to these regions as the FFA.
Figure 3.
Activation within the right middle fusiform gyrus induced by famous faces relative to common objects for patient Z.D. and 3 examples of the normal participants (P < 0.001, uncorrected).
Table 2.
The loci and peak activations of the bilateral middle fusiform gyrus identified by the contrast of famous faces minus common objects and that of unfamiliar faces minus common objects (P < 0.001 uncorrected)
| RFFA |
LFFA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Talairach |
T | Cluster voxels | Talairach |
T | Cluster voxels | |||||
| x | y | z | x | y | z | |||||
| Famous face minus common objects | ||||||||||
| Patient Z.D. | ||||||||||
| 38 | −56 | −22 | 4.56 | 75 | −42 | −52 | −21 | 4.85 | 54 | |
| Normal participants | ||||||||||
| S01 | 42 | −35 | −20 | 7.49 | 500 | −40 | −31 | −19 | 5.79 | 171 |
| S02 | 43 | −62 | −12 | 5.72 | 370 | −40 | −41 | −18 | 7.58 | 518 |
| S03 | 44 | −41 | −19 | 4.00 | 31 | |||||
| S04 | 40 | −47 | −19 | 6.48 | 295 | −42 | −45 | −17 | 5.50 | 130 |
| S05 | 34 | −41 | −19 | 7.18 | 283 | −34 | −35 | −23 | 4.68 | 35 |
| S06 | ||||||||||
| S07 | 42 | −62 | −14 | 4.13 | 522 | −43 | −56 | −22 | 3.59 | 30 |
| S08 | 40 | −45 | −17 | 5.96 | 337 | |||||
| S09 | 34 | −39 | −19 | 8.21 | 192** | −40 | −41 | −18 | 6.15 | 373** |
| S10 | 40 | −42 | −13 | 6.27 | 353 | −43 | −45 | −19 | 5.69 | 445 |
| Mean | 40 | −46 | −17 | 6.16 | 320 | −40 | −42 | −19 | 5.57 | 243 |
| SD | 3 | 10 | 3 | 1.42 | 150 | 3 | 8 | 2 | 1.24 | 200 |
| N | 9 | 7 | ||||||||
| Unfamiliar face minus common objects | ||||||||||
| Patient Z.D. | ||||||||||
| None* | None* | |||||||||
| Normal participants | ||||||||||
| S01 | 38 | −49 | −16 | 4.58 | 107 | −36 | −58 | −14 | 4.29 | 47 |
| S02 | ||||||||||
| S03 | 45 | −49 | −18 | 4.52 | 116 | −47 | −50 | −21 | 3.67 | 10 |
| S04 | 42 | −47 | −17 | 3.77 | 17 | −42 | −41 | −17 | 3.40 | 20* |
| S05 | ||||||||||
| S06 | ||||||||||
| S07 | 38 | −46 | −23 | 3.26 | 33* | |||||
| S08 | 38 | −45 | −16 | 5.35 | 125 | −34 | −61 | −20 | 4.76 | 145 |
| S09 | 34 | −35 | −20 | 5.10 | 102 | −38 | −43 | −18 | 3.68 | 16 |
| S10 | 47 | −49 | −14 | 3.34 | 15* | |||||
| Mean | 40 | −46 | −18 | 4.27 | 74 | −39 | −51 | −18 | 3.96 | 48 |
| SD | 5 | 5 | 3 | 0.83 | 49 | 5 | 9 | 3 | 0.55 | 56 |
| N | 7 | 5 | ||||||||
Note: RFFA, the right fusiform face area; LFFA, the left fusiform face area; N, number.
*P < 0.005 uncorrected; **P < 0.0001 uncorrected. The size of voxel: 2 × 2 × 2 mm3.
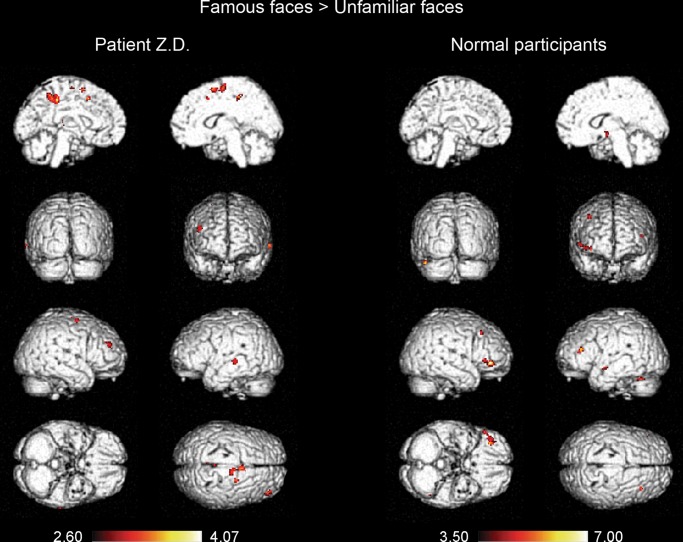
Additionally, to reveal the neural correlates of face identity processing, we compared the activation for famous faces to that for unfamiliar faces for Z.D. and normal participants, respectively. For patient Z.D., several brain region were identified using this comparative method, including the bilateral medial frontal gyrus (MeFG, BA 10) which extended to the left anterior cingulated cortex (ACC, BA32), right precuneus (BA 31), right superior temporal gyrus (STG, BA 22), right FG (BA 37), left middle cingulated gyrus (BA 32), and right posterior lobe in the cerebellum (Fig. 4 left and Table 3). Similarly, the brain regions activated in the normal participants by the famous faces relative to the unfamiliar faces included the bilateral lateral MFG (BA 46/9), left inferior frontal gyrus (IFG, BA 45), right parietal lobule (BA 7), bilateral STG (BA 13/22), right middle temporal gyrus (MTG, BA 39), right FG (BA 20/37), right posterior cingulated cortex (PCC, BA 31), left caudate, and left anterior lobe in the cerebellum (Fig. 4 right and Table 3).
Figure 4.
Regions that show greater activation in response to famous faces compared with unfamiliar faces for patient Z.D. (left) and 10 normal participants at the group level (right) (P < 0.001 uncorrected, k ≥ 12).
Table 3.
The peak activations of famous faces relative to unfamiliar faces for patient Z.D. and 10 normal participants at the group level (P < 0.001, uncorrected, k > 12 voxels)
| Brain region | Hem | BA | Cluster voxels | Talairach |
T | ||
|---|---|---|---|---|---|---|---|
| x | Y | Z | |||||
| Famous faces minus unfamiliar faces for Z.D. | |||||||
| Frontal lobe | |||||||
| Medial frontal gyrus | R | 10 | 21 | 5 | 57 | 21 | 4.88 |
| Medial frontal gyrus | L | 10/32 | 150 | −6 | 48 | −4 | 6.12 |
| −3 | 37 | −5 | 5.57 | ||||
| −3 | 53 | 4 | 4.72 | ||||
| Parietal lobe | |||||||
| Precuneus | R | 31 | 20 | 19 | −73 | 23 | 3.83 |
| Temporal lobe | |||||||
| Superior temporal gyrus | R | 22/42 | 85 | 58 | 4 | 6 | 4.70 |
| 62 | −9 | 14 | 3.57 | ||||
| Superior temporal gyrus | R | 22 | 34 | 58 | −25 | 7 | 4.18 |
| Superior temporal gyrus | R | 22 | 16 | 51 | −15 | 0 | 3.74 |
| Fusiform gyrus | R | 37 | 14 | 42 | −40 | −10 | 3.56 |
| Limbic lobe | |||||||
| Cingulate gyrus | L | 32 | 46 | −7 | 26 | 28 | 4.25 |
| Posterior lobe | |||||||
| Declive | R | 105 | 4 | −79 | −14 | 5.12 | |
| 12 | −73 | −20 | 3.52 | ||||
| Famous faces minus unfamiliar faces for normal participants | |||||||
| Frontal lobe | |||||||
| Middle frontal gyrus | R | 46/9 | 156 | 45 | 34 | 24 | 7.81 |
| 47 | 24 | 29 | 7.03 | ||||
| 41 | 17 | 23 | 6.43 | ||||
| Middle frontal gyrus | L | 46 | 177 | −46 | 19 | 22 | 9.91 |
| Inferior frontal gyrus | L | 45 | 107 | −45 | 30 | 3 | 7.88 |
| Parietal lobe | |||||||
| Superior parietal lobule | R | 7 | 131 | 33 | −59 | 51 | 10.29 |
| 32 | −60 | 42 | 6.87 | ||||
| Temporal lobe | |||||||
| Superior temporal gyrus | R | 13 | 24 | 45 | −43 | 15 | 5.28 |
| Superior temporal gyrus | L | 22 | 20 | −58 | −26 | 3 | 6.74 |
| Middle temporal gyrus | R | 39 | 16 | 45 | −63 | 17 | 5.32 |
| Fusiform gyrus | R | 20 | 25 | 32 | −38 | −15 | 5.67 |
| Fusiform gyrus | R | 37 | 28 | 43 | −45 | −12 | 5.46 |
| 43 | −57 | −9 | 4.82 | ||||
| Limbic lobe | |||||||
| Posterior cingulate | R | 31 | 16 | 10 | −52 | 21 | 4.96 |
| 8 | −61 | 18 | 4.59 | ||||
| Sublobar | |||||||
| Caudate | L | 14 | −16 | −3 | 22 | 5.95 | |
| Anterior lobe | |||||||
| Culmen | L | 18 | −32 | −37 | −20 | 5.45 | |
Note: The size of voxel: 2 × 2 × 2 mm3.
As indicated in Table 3, a particular region of the cortex in the right middle FG showed increased activation in response to famous faces (as opposed to unfamiliar faces) in both patient Z.D. (Talairach coordinate: 42, −40, −10) and in normal participants (Talairach coordinate: 32, −38, −15). It should be noted that the loci of this region sensitive to famous face (henceforth referred to as the famous face region) was located anterior to the FFA that was identified by famous faces relative to common objects for both patient Z.D. and normal participants.
Psychophysiological Interaction Analysis
As suggested by Haxby et al. (2000) and Ishai et al (2005), the recognition of familiar faces relies on the functional connectivity from a “core system” (e.g., the middle FG) to an “extended system” (e.g., prefrontal cortex [PFC]). We therefore used the PPI (Friston et al. 1997) method to estimate the influence of the famous face region on the other brain regions when normal participants or Z.D. viewed famous faces as opposed to when they viewed unfamiliar faces. The PPI analysis is a method used to calculate the effective connectivity between brain regions. It can quantitatively measure the influence of a given region on other brain regions, which can be induced by experimental manipulation (e.g., famous faces vs. unfamiliar faces). In terms of PPI analysis, the influence that one region (the seed region) exerts on another regions is defined by the degree to which the response of that region is predicted on the basis of the activities of the seed region (Friston et al. 1997). Thus, the PPI analysis can identify the regions (voxel by voxel) that are influenced to a greater degree by the seed region for one experimental condition relative to another.
In the present study, we selected the regions within the right middle fusiform which was identified, at the group level, by famous faces relative to unfamiliar faces as the reference regions for the seed volume of interest (VOI) (i.e., the famous face region: 42, −40, −10 for patient Z.D. and 32, −38, −15 for normal participants). To allow for individual differences in the peak activation locations, the VOI was defined as a 4-mm radius sphere centered on the peak activation of each individual contrast image, which was the nearest local maximum within 15 mm of the local maximum of the group analysis. The physiological activity was obtained by extracting the first eigenvariate across all voxel time courses within each VOI. The psychophysiological interaction term (PPI regressor) was defined as the cross-product of the physiological activity and a vector (psychological variable) that coded the effects of experimental manipulation (e.g., A > B; 1 for A and −1 for B). Thus, a GLM was constructed using the psychophysiological interaction term, the physiological activity, and the psychological variable as the regressors.
A whole-brain analysis was performed using SPM8 to identify the brain regions to which the seed region presented enhanced connectivity dependent on an experimental manipulation (famous faces minus unfamiliar faces). For each seed region, PPI analysis was first performed for individual participants, and then the group results of the PPI analysis were obtained by averaging all individual contrast images using a random effects analysis with a statistical threshold of P < 0.005 (uncorrected) and cluster threshold of k ≥ 20. Figure 5 and Table 4 show the results of the PPI analysis. For Z.D., when comparing famous faces to unfamiliar faces, the right middle FG showed enhanced coupling to the right MeFG (BA 6), left superior frontal gyrus (SFG, BA6), right SFG (BA9), and right MFG (BA6), left precuneus (BA7) which extended to the left cingulated gyrus (BA 31), left MTG (BA 21), and some sublobar regions such as the left thalamus and left lentiform nucleus (Fig. 5 left). For normal participants, when comparing famous faces to unfamiliar faces, their right middle FG showed more influence on the bilateral lateral MFG (BA 46/8), right IFG (BA 45/47), and some sublobar regions such as the left insula and right lentiform nucleus, and left anterior lobe in the cerebellum (Fig. 5 right).
Figure 5.
Regions influenced by the right middle fusiform gyrus identified by psychophysiological interaction (PPI) when comparing famous faces to unfamiliar faces for patient Z.D. (left) and 10 normal participants at the group level (right) (P < 0.005 uncorrected, k ≥ 20).
Table 4.
Regions to which the regions showing preferential response to the famous faces in the right middle fusiform gyrus showed enhanced connectivity when comparing famous faces to unfamiliar faces for patient Z.D. and 10 normal participants at the group level (P < 0.005, uncorrected, k > 20 voxels)
| Brain region | Hem | BA | Cluster voxels | Talairach |
T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Famous faces minus unfamiliar faces for Z.D. | |||||||
| Frontal lobe | |||||||
| Medial frontal gyrus | R | 6 | 48 | 4 | −4 | 62 | 4.07 |
| Medial frontal gyrus | R | 6 | 63 | 6 | −17 | 59 | 3.30 |
| Superior frontal gyrus | L | 6 | 24 | −2 | 7 | 48 | 3.55 |
| Superior frontal gyrus | R | 9 | 36 | 40 | 40 | 32 | 3.18 |
| Middle frontal gyrus | R | 6 | 29 | 20 | −9 | 61 | 3.32 |
| Parietal lobe | |||||||
| Precuneus | L | 7/31 | 208 | −15 | −45 | 43 | 3.54 |
| 0 | −38 | 46 | 3.18 | ||||
| −11 | −37 | 40 | 2.99 | ||||
| Temporal lobe | |||||||
| Middle temporal gyrus | L | 21 | 40 | −60 | −28 | 2 | 3.26 |
| Sublobar | |||||||
| Thalamus | L | 48 | −27 | −25 | 7 | 3.01 | |
| Lentiform nucleus | L | −21 | −13 | 8 | 2.92 | ||
| Famous faces minus unfamiliar faces for normal participant | |||||||
| Frontal lobe | |||||||
| Middle frontal gyrus | L | 46 | 61 | −31 | 28 | 24 | 7.00 |
| −42 | 31 | 19 | 5.81 | ||||
| Middle frontal gyrus | R | 8 | 20 | 34 | 18 | 46 | 6.20 |
| Inferior frontal gyrus | R | 45/47 | 112 | 31 | 34 | 4 | 6.79 |
| 49 | 26 | 9 | 5.25 | ||||
| 38 | 30 | 2 | 4.44 | ||||
| Sublobar | |||||||
| Insula | L | 13 | 21 | −43 | −4 | −6 | 5.00 |
| Lentiform nucleus | R | 24 | 18 | −8 | −5 | 5.71 | |
| Anterior lobe | |||||||
| Culmen | L | 37 | −43 | −53 | −27 | 5.21 | |
Note: The size of voxel: 2 × 2 × 2 mm3.
Discussion
Patients with prosopagnosia offer a unique opportunity for the investigation of the neural correlates of covert face processing due to their inability to consciously recognize the identity of familiar faces. In the present study, neither famous nor unfamiliar faces were recognized by the patient, Z.D., even though the famous faces were highly familiar to him before the onset of his disease. Nevertheless, his bilateral FFA showed greater activation for familiar faces than for common objects. Our findings are largely consistent with the findings of the recent fMRI studies of another prosopagnosic patient P.S., who also failed to identify faces but showed greater normal FFA activation for faces (Rossion et al. 2003). However, different from Z.D., P.S. showed enhanced activities in FFA for both famous faces and unfamiliar faces relative to common objects (Simon et al. 2011), suggesting that Z.D. might have a more severe impairment than P.S. Nevertheless, the findings from these 2 studies taken together suggest that the FFA plays an important role in covert processing of familiar faces.
Our patient data, along with data from P.S. significantly extend understanding of the role of the FFA in covert face processing. Existing studies with normal participants have implicated the FFA in covert processing of unfamiliar faces (e.g., Lehmann et al. 2004; Jiang and He 2006, Morris et al. 2007). However, these studies failed to include familiar faces in their design to assess whether face familiarity plays any role in the activation of the FFA during covert face processing. Extensive fMRI studies have examined the effect of face familiarity on overt face processing in normal individuals. Although results are not always consistent, familiar faces tend to elicit a greater degree of activation in the middle FG than unfamiliar faces (e.g., Grill-spector et al. 2004; Rotshtein et al. 2005; Gobbini and Haxby 2007). In line with this tendency, the normal participants in the present study also showed similar patterns of activation. The existing evidence regarding the role of familiarity in normal individuals' overt face processing and prosopagnosic patients' covert face processing taken together suggests a central role of the middle fusiform regions including the FFA in familiar face processing.
Findings from recent fMRI studies with normal participants and prosopagnosic patients suggest that activation of the FFA alone is not sufficient for overt face identification (Steeves et al. 2006). Rather, the successful recognition of a familiar face relies on a neural network that is distributed throughout the brain (Haxby et al. 2000; Ishai et al. 2005). The present study provided new evidence supporting such hypothesis. In the present study, though the bilateral FFAs of patient Z.D. showed more response to famous faces than to common objects, he did not recognize the famous faces with which he had been familiar before the onset of his disease, suggesting that the enhanced activation of the FFA did not automatically lead to the successful face identification.
The whole-brain analysis revealed familiar face recognition to involve additional regions for both Z.D. and normal participants. However, the exact cortical regions that showed differential responses to famous faces relative to unfamiliar faces differed greatly between the normal participants and patient Z.D. The major differences were in the PFC and the parietal lobule. Among the normal participants, the bilateral MFG, left IFG, and right superior parietal lobule (SPL) showed more activation for famous faces than for unfamiliar faces. A large body of evidence has suggested that the lateral prefrontal cortex (LPFC) is involved in memory processing (Curtis and D'Esposito 2003). Specifically, recent fMRI studies have shown that certain regions in the PFC were related to the processing of faces that required the recollection of face identity or autobiographical information. Further, the loci of these regions are highly similar to the regions identified in the normal participants in the present study, for famous faces relative to unfamiliar faces (for convenience of comparison, the loci of the latter are displayed in italic font in the square bracket in the following text.). For example, Ishai et al. (2005) found that in the left IFG (Talairach coordinate: −47, 19, 22 [−46, 19, 22]), the famous faces elicited greater activation than unfamiliar faces. Denkova et al. (2006) also reported that the left IFG (Talairach coordinate: −46, 28, 2 [−45, 30, 3]) showed greater response when participants successfully recalled an autobiographical episode of a famous face than when they did not. Elfgren et al. (2006) also found that the bilateral IFG/MFG showed more activation for familiar faces than for unfamiliar ones (Talairach coordinates: left −46, 31, 4 [−45, 30, 3]; right 55, 27, 26 [47, 24, 29]). Additionally, using an old/new paradigm, increased activation was observed in the bilateral MFG (Talairach coordinates: left −50, 24, 12 [−46, 19, 22]; right 52, 22, 16; [47, 24, 29]) and the left IFG (Talairach coordinate: −40, 24, 8 [−45, 30, 3]) for remembered faces but not for forgotten ones (Sergerie et al. 2005). A recent neuropsychological study also found that damage to the frontal lobe could lead to impaired face encoding and retrieval (Rapcsak et al. 2001).
The right SPL was seldom reported to be related to face processing. However, converging evidence suggests that the parietal lobule (including the SPL and inferior parietal lobule [IPL]) is involved in the retrieval of episodic memory (Wagner et al. 2005). Especially, Vilberg and Rugg (2007) found that the right SPL showed enhanced activation when studied pictures were recollected, whose locus (33, −60, 57) was highly consistent with that in the present study ([33, −59, 51]) when participants identified famous faces compared with unfamiliar faces. Thus, our findings from the normal participants suggest that the bilateral PFC and the right SPL are perhaps involved in the storage and retrieval of identity information for famous faces during overt face processing. In addition to the PFC and SPL, for normal participants, the activation elicited by famous faces relative to unfamiliar faces was also observed in the right middle FG, bilateral posterior STG, right posterior MTG and right PCC. These regions have also been suggested to be involved in the processing of familiar faces (Gobbini and Haxby 2007).
In contrast, for patient Z.D., activation for famous faces minus activation for unfamiliar faces was observed mainly in the bilateral medial frontal cortex, an area that did not show a differential response in the normal participants. Additionally, the activation for famous faces minus activation for unfamiliar faces was also found to occur in the right STG, right middle FG, and right precuneus. Given the crucial roles of the lateral PFC and SPL in overt face recognition, it is reasonable to speculate that Z.D.'s face identification impairment may be, at least partially, due to the absence of the involvement of the lateral PFC and the SPL.
However, it should be noted that, for Z.D., in addition to the right middle FG, the remaining activated regions have also been reported to play a role in face processing among normal participants. For example, the locus of the STG was consistent with that of the STS, which was one of the regions in the “core system” of face recognition (Haxby et al. 2000). It is known that the precuneus was involved in the retrieval of episode memory (Cavanna and Trimble 2006). In addition, the precuneus and the medial frontal cortex were suggested to be involved in the retrieval of person knowledge (Gobbini and Haxby 2007). In other words, in Z.D.'s face recognition network, despite the absence of the lateral PFC and SPL, other regions may still be involved in certain aspects of face processing such as face perception and face memory retrieval. Though the activation of these residual regions does not yield successful conscious face recognition, it may be sufficient for covert face processing.
It should be further noted that although patient Z.D. was unable to recognize familiar faces behaviorally, he was able to successfully recognize the identities of celebrities, family members, and acquaintances according to their voices or clothes. Thus, Z.D. was clearly capable of storing and retrieving identity information. Additionally, as indicated by the DWI, there was little cytotoxic edema in Z.D.'s PFC, which would have explained his impairment in overt face identity recognition. One possible explanation for Z.D.'s impairment in face recognition is that, while the visual information from the familiar faces may have been adequately processed in the right middle FG, it might have failed to “trigger” the correct retrieval of personnel identity information stored in the PFC (Fox et al. 2008). Recently, several studies have revealed a hierarchically organized system for face recognition, wherein the “core system” (e.g., the middle fusiform gyrus) receives the face information from the primary visual cortex, and in turn exerts an influence on the extended system (e.g., the PFC, some sublobar regions) which results in further processing, for example, the retrieval of person knowledge or the decoding of emotions. Consistent with such face recognition mode, Fairhall and Ishai (2007) found that famous faces relative to unfamiliar faces enhanced the feed-forward connectivity from the face-preferential regions in the middle FG to the lateral PFC. We therefore speculated that the patient Z.D.'s face recognition impairment might be due to a functional disconnection between the right middle FG and the lateral PFC, where facial identity information is stored and needs to be retrieved for correct familiar face recognition. Indeed, when comparing famous faces to unfamiliar faces, though the right middle FG of both Z.D. and normal participants showed enhanced activation, the activation for Z.D. (42, −40, −10; T = 3.56) was evidently weaker than that for normal participants (32, −38, −15; T = 5.67). Thus, Z.D.'s failure to recognize familiar faces might be due to the fact that the residual activation in Z.D.' core system was too weak to drive the activation of the lateral PFC regions.
To further validate our hypothesis, we used PPI analysis to explore which brain regions in the high-level cognitive cortex were more influenced by the activities of such regions in the middle FG when processing famous faces than when processing unfamiliar faces. To the best of our knowledge, our study was the first to use functional connectivity analysis to examine the influence of the right middle FG on other cortical regions during a prosopagnosic patient's face processing. As revealed by the PPI results, the normal participants and patient Z.D. showed different coupling patterns enhanced by famous faces. For the normal participants, when comparing famous faces to unfamiliar faces, the region sensitive to famous faces in the middle fusiform, exerted enhanced influence on the bilateral MFG, the right IFG, and the left insula. As previously mentioned, these prefrontal regions are involved in the processing of face identity. Enhanced activation of the anterior insula has also been suggested to be involved in the processing of familiar faces (Gobbini and Haxby 2007). Such findings are consistent with the feed-forward network reported by Fairhall and Ishai (2007).
For patient Z.D., when comparing famous faces to unfamiliar faces, the region sensitive to famous faces failed to show an enhanced directional influence on the lateral PFC regions, as was seen among the normal participants, supporting our hypothesis. Interestingly, the signals from Z.D.'s right middle FG appeared robust enough to activate the left precuneus, the left MTG, and the medial frontal regions (i.e., the right MeFG and the bilateral superior frontal cortex). The precuneus and the MTG have been suggested to be related to the recognition of familiar faces (Gobbini and Haxby 2007), but the medial frontal cortex and the SFG have seldom been reported to be involved in face processing. This difference in PPI results between normal participants and patient Z.D. suggest that the functional connectivity from the right middle fuisform gyrus to the lateral MFG/IFG may play a crucial role in the overt recognition of famous faces. The absence of this coupling may lead to an impairment of overt face recognition despite enhanced activation of the right middle fusiform regions in response to familiar faces. However, some of Z.D.'s other residual regions, namely the precuenus and the MTG, can still receive face information from the middle FG. As previously discussed, these regions were related to the processing of face memory. Thus, Z.D.'s surviving connectivity from the right middle FG to these regions may account for the preservation of his ability to covertly or unconsciously recognize faces.
The exact cause of Z.D.'s abnormal functional connectivity is not clear. As indicated by Figure 1, Z.D. had cytotoxic edema in almost the whole right inferior temporal cortex and posterior parietal lobule. Cytotoxic edema sometimes leads to the dysfunction of neurons. Thus, damage to the connection between the ventral and dorsal pathway from the visual cortex to the extended system may be the cause of Z.D.'s impairment in overt face recognition. The findings of the present study make a strong case for further research on acquired prosopagnosia in patients who are suffering from mitochondrial encephalomyopathy. Many existing patient studies of acquired prosopagnosia tend to involve patients whose prosopagnosia tends to be permanent and nonreversible. In contrast, some abilities impaired by mitochondrial encephalomyopathy (e.g., prosopagnosia) can experience partially neural recovery. This makes it possible to track changes in the face-processing abilities of such patients longitudinally, in order to examine how patients' FFAs and their functional connectivity to other cortical regions change with increases or decreases in disease severity. Furthermore, mitochondrial encephalomyopathy is a genetic disease that is passed along on the maternal line and typically has midlife onset. Thus, it is possible to longitudinally track patients even before the onset of the disease, and to compare their abilities to those of affected relatives whose mitochondrial encephalomyopathy may affect a different brain region, as well as to their normal relatives on the paternal side. Systematic investigation of the face-processing abilities of such patients, and their relatives, would provide new insights into the neural bases of normal and abnormal overt and covert face processing. In particular, by recruiting more prosopagnosic patients with similar mitochondrial encephalomyopathy, we would be able to use the well-established covert face-processing paradigms (e.g., Jiang and He 2006) and perform group level comparisons between participants with vs. without prosopagnosia. Such research would thus overcome one of the limitations of the present study, that is, the involvement of a single patient, and offer deeper insight about the neural mechanisms underlying covert face processing.
Funding
This paper is supported by the National Basic Research Program of China (973 Program) under Grant 2011CB707700; the National Natural Science Foundation of China under Grant No. 81227901, 61231004, 30970771, 60910006, 31028010, 30970769, 81000640, 81271534, 81271565; and the Fundamental Research Funds for the Central Universities (2011JBM226) and NIH (R01HD046526 and R01HD060595).
Notes
Conflict of Interest: None declared.
References
- Barton JJS, Cherkasova M, O'Connor M. Covert recognition in acquired and developmental prosopagnosia. Neurology. 2001;57:1161–1168. doi: 10.1212/wnl.57.7.1161. [DOI] [PubMed] [Google Scholar]
- Bauer RM. Autonomic recognition of names and faces in prosopagnosia: a neuropsychological application of the guilty knowledge test. Neuropsychologia. 1984;22:457–469. doi: 10.1016/0028-3932(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Bobes MA, Lopera F, Díaz Comas L, Galan L, Carbonell F, Bringas ML, Valdés-Sosa M. Brain potentials reflect residual face processing in a case of prosopagnosia. Cogn Neuropsychol. 2004;21:691–718. doi: 10.1080/02643290342000258. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- De Haan EH, Bauer RM, Greve KW. Behavioural and physiological evidence for covert face recognition in a prosopagnosic patient. Cortex. 1992;28:77–95. doi: 10.1016/s0010-9452(13)80167-0. [DOI] [PubMed] [Google Scholar]
- Denkova E, Botzung A, Manning L. Neural correlates of remembering/knowing famous people: an event-related fMRI study. Neuropsychologia. 2006;44:2783–2791. doi: 10.1016/j.neuropsychologia.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Elfgren C, van Westen D, Passant U, Larsson EM, Mannfolk P, Fransson P. fMRI activity in the medial temporal lobe during famous face processing. NeuroImage. 2006;30:609–616. doi: 10.1016/j.neuroimage.2005.09.060. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Iaria G, Barton JJS. Disconnection in prosopagnosia and face processing. Cortex. 2008;44:996–1009. doi: 10.1016/j.cortex.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:89–210. [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Jiang Y, He S. Cortical responses to invisible faces: dissociating subsystems for facial-information processing. Curr Biol. 2006;16:2023–2029. doi: 10.1016/j.cub.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Phil Trans R Soc B. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouider S, Eger E, Dolan R, Henson RN. Activity in face-responsive brain regions is modulated by invisible, attended faces: evidence from masked priming. Cereb Cortex. 2009;19:13–23. doi: 10.1093/cercor/bhn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Mueller T, Federspiel A, Hubl D, Schroth G, Huber O, Strik W, Dierks T. Dissociation between overt and unconscious face processing in fusiform face area. NeuroImage. 2004;21:75–83. doi: 10.1016/j.neuroimage.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Face processing without awareness in the right fusiform gyrus. Neuropsychologia. 2007;45:3087–3091. doi: 10.1016/j.neuropsychologia.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, de Martin de Vivies X, Anzures G, Quinn PC, Slater AM, Tanaka JW, Lee K. Development of face processing. WIREs Cogn Sci. 2011;2:666–675. doi: 10.1002/wcs.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Nielsen L, Littrell LD, Glisky EL, Kaszniak AW, Laguna JF. Face memory impairments in patients with frontal lobe damage. Neurology. 2001;57:1168–1175. doi: 10.1212/wnl.57.7.1168. [DOI] [PubMed] [Google Scholar]
- Renault B, Signoret JL, DeBruille B, Breton F, Bolgert F. Brain potentials reveal covert facial recognition in prosopagnosia. Neuropsychologia. 1989;27:905–912. doi: 10.1016/0028-3932(89)90066-3. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Burton AM. Covert recognition and the neural system for face processing. Cortex. 2003;39:9–30. doi: 10.1016/s0010-9452(08)70071-6. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL. A face to remember: emotional expression modulates prefrontal activity during memory formation. NeuroImage. 2005;24:580–585. doi: 10.1016/j.neuroimage.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Simon SR, Khateb A, Darque A, Lazeyras F, Mayer E, Pegna AJ. When the brain remembers, but the patient doesn't: converging fMRI and EEG evidence for covert recognition in a case of prosopagnosia. Cortex. 2011;47:825–838. doi: 10.1016/j.cortex.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Steeves JKE, Culham JC, Duchaine BC, Pratesi CC, Valyear KF, Schindler I, Humphrey GK, Milner AD, Goodale MA. The fusiform face area is not sufficient for face recognition: evidence from a patient with dense prosopagnosia and no occipital face area. Neuropsychologia. 2006;44:594–609. doi: 10.1016/j.neuropsychologia.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Mangels J, Hirsch J. Mistaking a house for a face: neural correlates of misperception in healthy humans. Cereb Cortex. 2006;16:500–508. doi: 10.1093/cercor/bhi129. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Young AW, Hellawell D, De Haan EHF. Cross-domain semantic priming in normal subjects and a prosopagnosia patient. Q J Exp Psychol. 1988;40:561–580. doi: 10.1080/02724988843000087. [DOI] [PubMed] [Google Scholar]