Abstract
Most kidney diseases that ultimately lead to end-stage renal failure originate within the glomerulus and are associated with proteinuria. Treatment options are unspecific and offer partial cures at best because available therapies do not primarily treat glomerular cells but rather act systemically and thus cause many side effects. Most glomerulopathies directly stem from injury to podocytes, cells that have a key role in the maintenance of the glomerular filter. Thus, these cells constitute an obvious and promising target for the development of novel kidney-protective drugs. During the last decade, enormous advances have been made in the understanding of podocyte structure and function. A number of pathways that are altered during glomerular diseases may be targeted by novel small- and large-molecule drugs as well as biologicals that have been identified in nephrology and other areas of drug development. Cultured podocytes provide a valuable model for high-throughput drug screening assays. Furthermore, podocytes have been shown to possess many features that make them particularly good target cells for renal protection. This mini-review discusses some of the most recent promising data related to potential drug therapy for proteinuria and kidney disease through direct podocyte targeting.
Keywords: glomerulopathy, pathophysiology of renal disease and progression, podocyte, proteinuria, renal protection
MOLECULAR PATHWAYS IN GLOMERULAR DISEASES
During the last decade, knowledge of podocyte biology and SD structure has advanced tremendously through genetic studies in humans and rodents3 as well as available podocyte cell culture systems.4 While genetic studies have contributed invaluably to our understanding of podocyte function, genetic defects account for only a minority of glomerular diseases. In the following section, we will therefore focus on pathways that have been shown to be involved in the more common acquired human glomerular diseases. The key molecules of these pathways may be divided into several categories: cell membrane receptors, ion channels, growth factors, and proteases (Table 1 and Figure 1).
Table 1.
Pathways involved in acquired glomerular diseases
| Molecular pathways | Human diseases |
|---|---|
| Transmembrane receptors | |
| Nephrin | DNP, MCD |
| B7-1 | LN |
| uPAR | FSGS, DNP |
| Notch | FSGS, DNP |
| PLA2R | MN |
| Ion channels | |
| TRPC6 | MCD, MN |
| Growth factors | |
| VEGF-A | Preeclampsia |
| TGF-β | DNP |
| Proteases | |
| Cathepsin L→dynamin, synaptopodin | MN, FSGS, DNP |
Abbreviations: DNP, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; LN, lupus nephritis; MCD, minimal change disease; MN, membranous nephropathy; TGF-β, transforming growth factor-β; TRPC6, transient receptor potential cation channel-6; uPAR, urokinase plasminogen-activator receptor; VEGF-A, vascular endothelial growth factor A.
Only those pathways that have been shown to be involved in acquired human glomerular diseases are listed.
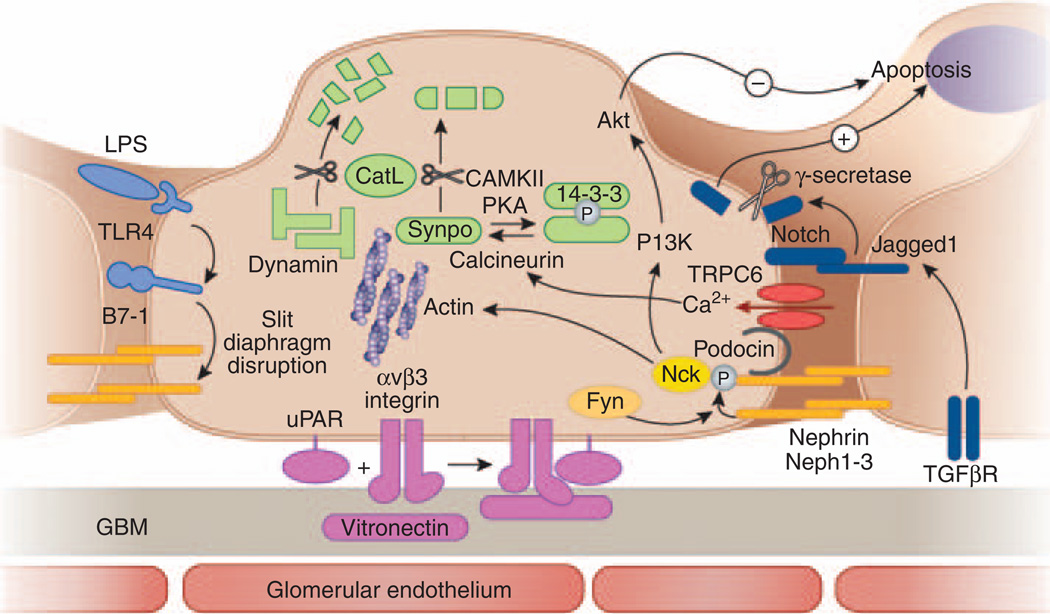
Figure 1. Pathways involved in acquired glomerular diseases representing targets for podocyte-specific drugs.
A schematic cross-section of a podocyte foot process with the corresponding cell body, the glomerular basement membrane, and the glomerular endothelium is shown. Pathways involved in podocyte injury that may be drug-targeted are depicted in different colors. Sustaining nephrin expression and phosphorylation (yellow) might contribute to both antiapoptotic signaling and actin polymerization. The B7-1 pathway (light blue) may be targeted by (1) toll-like receptor-4 antagonists or (2) blocking the binding of B7-1 to slit diaphragm structure proteins. Urokinase plasminogen-activator receptor (uPAR)-induced podocyte motility (violet) could be inhibited by (1) interfering with binding of uPAR to αvβ3 integrin, (2) inhibiting β3 integrin activation, or (3) inhibiting binding of αvβ3 integrin to vitronectin. The notch pathway (dark blue) can be targeted by (1) interfering with its upstream activation by blocking the TGF-β1 effect, (2) inhibiting γ-secretase, which is required for proteolytic receptor activation, or (3) interfering with target gene transcription. TRPC6 channels (red) may be targeted by (1) channel blockers or (2) inhibiting their expression. The CatL pathway (green) could be targeted by (1) specifically inhibiting CatL expression or activity, (2) shifting the equilibrium of synaptopodin toward the phosphorylated form by inhibiting calcineurin-mediated dephosphorylation or enhancing PKA or CAMKII-mediated phosphorylation, (3) protecting synaptopodin and dynamin by compounds that bind to the CatL cleavage site, or (4) delivering cleavage-resistant synaptopodin or dynamin mutants.
Cell membrane receptors
The transmembrane protein nephrin was the first SD-associated protein to be identified through a genome-wide mutation screen in families affected by Finnish-type congenital nephrotic syndrome. Nephrin molecules from adjacent FPs are thought to interact with each other in the middle of the slit through their extracellular immunoglobulin G-like domains.3 There is growing evidence that nephrin acts in addition to its structural function as an outside-in signaling molecule through phosphorylation by the Src kinase Fyn, exerting both antiapoptotic stimuli via the phosphoinositide-3 kinase (PI3K) pathway and modulating the actin cytoskeleton via the adaptor protein Nck.5 Reduced levels of nephrin have been found in human and experimental diabetic nephropathy, which may be mediated by protein kinase C α signaling6 Nephrin phosphorylation was decreased in the rat model of puromycin aminonucleoside nephrosis preceding proteinuria and coinciding with actin depolymerization, and in glomeruli from patients with minimal change disease.7
B7-1 is a transmembrane protein usually expressed on the surface of B cells and other antigen-presenting cells, where it acts as a costimulatory molecule. An upregulation of B7-1 was found by differential-display PCR in podocytes of α3-integrin-deficient mice that serve as a model for FP effacement.8 Podocyte expression of B7-1 correlates with the severity of human lupus nephritis. B7-1 induction in podocytes is mediated by lipopolisacharide signaling through toll-like receptor-4 and leads to reorganization of the podocyte actin cytoskeleton and essential SD proteins. Mice lacking B7-1 were protected from lipopolisacharide-induced proteinuria. This T- and B-cell-independent pathway may have a physiological function in allowing the excretion of pathogens and cytokines during inflammation, but leads to glomerular disease if persistently activated.
The development of FP effacement requires a highly dynamic reorganization of cell–matrix adhesion, which is reflected by an increased motility of cultured podocytes.9 The urokinase plasminogen-activator receptor has an important function in cancer cell motility, another situation in which cells can be hyperdynamic, allowing tissue invasion and metastasis. Urokinase plasminogen-activator receptor was therefore studied in the context of proteinuric diseases and found to be significantly upregulated in glomeruli of patients with diabetic nephropathy and FSGS, as well as in rodent models of proteinuria.10 Urokinase plasminogen-activator receptor is dispensible for normal renal function but required for podocyte motility in cell culture and for the development of FP effacement and proteinuria in vivo. These effects of urokinase plasminogen-activator receptor are independent of its major ligand urokinase, but require the activation of αvβ3-integrin within lipid rafts and αvβ3-integrin-mediated binding to high-affinity ligands such as vitronectin. This mechanism can be antagonized in vivo with small molecule integrin inhibitors or antibodies, leading to potent reduction of proteinuria in a mouse model.10
The notch transmembrane receptor is part of a highly conserved signaling pathway. Ligand binding renders the notch receptor susceptible to metalloprotease- and γ-secretase-mediated proteolytic cleavage and release of the intracellular domain, which subsequently translocates to the nucleus and induces target gene transcription. The notch pathway is crucial in podocyte development but usually not active in the mature kidney.11 However, in experimental and human glomerular diseases, the intracellular notch domain (ICN) was strongly upregulated in podocytes, most likely as a consequence of transforming growth factor-β1 (TGF-β1)-induced transcription of the notch ligand Jagged1.12 Conditional expression of ICN in mature podocytes in vivo and in vitro induced proteinuria, FP effacement, and podocyte apoptosis. In contrast, podocyte-specific inactivation of Rbpj, encoding a notch transcriptional-binding partner, or treatment with a γ-secretase inhibitor that interferes with notch activation, protected mice and rats from streptozotocin- and puromycin aminonucleoside-induced glomerular damage, respectively.
Yet another transmembrane receptor, M-type phospholipase A2 receptor, has very recently been identified as target antigen in membranous nephropathy.13 However, the primary pathogenic role of M-type phospholipase A2 receptor in membranous nephropathy seems to be the participation in an antigen–antibody complex, which then leads to nocuous complement activation. The physiological function of M-type phospholipase A2 receptor and whether this function is affected in membranous nephropathy remains under investigation.
Ion channels
The transient receptor potential cation channel-6 (TRPC6) is a Ca2+ permeable ion channel expressed at the SD. Mutations in the TRPC6 gene which increase Ca2+ permeability were identified in families with autosomal dominant FSGS.14,15 The interaction of TRPC6 with nephrin and podocin,14 whose homolog mec-2 is a contributor to mechanosensation in the worm Caenorhabditis elegans, suggests a role of TRPC6 in a putative mechanosensor complex at the SD that could participate in monitoring glomerular pressure and filtration rate.16 TRPC6 expression levels and channel function also contribute to the pathogenesis of acquired forms of nephrotic syndrome. Induced expression of wild-type TRPC6 is a common feature of human proteinuric kidney diseases, and cultured podocytes that are exposed to complement upregulate TRPC6.17
Growth factors
Two growth factors have primarily been implicated in the pathogenesis of glomerular diseases: TGF-β and vascular endothelial growth factor (VEGF). TGF-β has long been recognized as a central player in renal fibrogenesis. This cytokine induces podocyte apoptosis leading to progressive glomerulosclerosis in transgenic mice overexpressing TGF-β,18 and glomerular TGF-β expression is increased in human and experimental rodent diabetic nephropathy.19 Currently, a neutralizing antibody against TGF-β is being tested for the treatment of primary FSGS in a phase I study (NCT00464321).
Podocytes, similar to pericytes of other fenestrated vascular beds, express VEGF-A in mature glomeruli. Homozygous podocyte-specific deletion of VEGF-A leads to severe endotheliosis followed by podocyte FP effacement and proteinuria, and heterozygous mice show a similar but milder phenotype, suggesting a ‘dose’ dependent effect of VEGF-A.20 Similar glomerular lesions associated with thrombotic microangiopathy have been described in patients treated with bevacizumab, a VEGF inhibitor used for cancer therapy.21 The glomerular endothelial lesions of VEGF-A-deficient mice further resemble the pathological lesions seen in preeclampsia, and interestingly, elevated levels of placental soluble fms-like tyrosine kinase 1, an endogenous inhibitor of VEGF, have been found in preeclamptic patients.22 In contrast, podocyte-specific overexpression of VEGF causes collapsing glomerulopathy, the lesion seen in HIV-associated nephropathy.20 Thus, tight regulation of VEGF-A signaling seems critical for establishment and maintenance of the glomerular filtration barrier.
Proteases
Cathepsin L (CatL) has long been known as a potent endoprotease primarily responsible for end-stage protein breakdown within lysosomal compartments.23 Recently, a cytosoplasmic variant of CatL generated by alternative translation and devoid of the lysosomal targeting sequence was found to be upregulated during experimental podocyte injury and in human glomerular diseases.24 Although lysosomal CatL unspecifically and efficiently cleaves a variety of proteins at a low pH, cytosolic short CatL shows remarkable substrate and sequence specificity at a higher pH.25 So far, two substrates of cytosolic CatL have been identified: dynamin24 and synaptopodin,26 which both stabilize the podocyte actin cytoskeleton and are required for normal glomerular function. In vivo delivery of cleavage-resistant mutant dynamin or synaptopodin or podocyte-specific transgenic expression of a synaptopodin mutant that lacks CatL cleavage sites protected mice from experimental proteinuria. Of interest, synaptopodin is physiologically protected from cleavage by CatL through phosphorylation-dependent binding to the chaperone-like protein 14-3-3. The calcineurin inhibitor cyclosporine blocks calcineurin-mediated dephosphorylation of synaptopodin, and thereby protects it from CatL-mediated cleavage.26 Thus, the beneficial effects of calcineurin inhibitors in proteinuric diseases may arise from a direct effect on podocytes rather than through their immunosuppressive properties.
CULTURED PODOCYTES AS TOOLS FOR HTS
HTS has emerged as a vital technique for early-stage discovery of disease targets and therapeutics.27 Many biochemical assays have been miniaturized for use in the HTS format, allowing purified protein-based, cell-based, or even whole-organism-based (such as Drosophila melanogaster, zebrafish, and C. elegans) drug discovery assays.
As described in the previous section, research in the last decade has identified a number of podocyte proteins as particularly suitable for therapeutic targeting. However, most of these proteins are rather large, contain many domains, and have a poorly defined molecular structure. In addition, some of them do not have a known enzymatic function, but rather serve in a structural role or as scaffolds/adaptor proteins for dynamically assembling protein complexes and can only be evaluated in the context of the whole cell. Furthermore, many podocyte proteins are difficult to purify in large enough quantities for undertaking an HTS campaign. Thus, purified protein-based assays are not ideal for discovery of novel podocyte-specific therapeutics. Conversely, the availability of both murine28 and human29 podocyte cell lines make cellbased HTS assays the most practical alternative. In addition, the remarkable similarity of the zebrafish pronephros to vertebrate nephrons,30 and of D. melanogaster nephrocytes31 and even C. elegans touch receptor neurons16 to vertebrate podocytes, renders these classical model organisms potentially suitable for whole-organism-based HTS.32 However, important questions remain about how closely these organisms can represent human disease processes and how reliably such results translate to humans.
Culturing podocytes has long been hampered by the lack of cell-type-specific markers, the rapid dedifferentiation of podocytes in vitro and the limited ability of differentiated podocytes to propagate in cell culture. The growing number of identified podocyte proteins can now be used as markers for these cells. By avoiding repeated subcultivation, differentiation of primary podocytes in culture can be achieved, but is associated with an irreversible growth arrest.33 However, using a temperature-sensitive transgene, conditionally immortalized mouse28 and human29 podocyte cell lines could be established that are able to proliferate under a permissive temperature (33°C), whereas under the nonpermissive temperature (37°C), the cells show growth arrest and key features of podocyte differentiation. Although they cannot fully replicate the in vivo kidney filtration barrier, cultured podocytes provide a validated model system4 that reliably recapitulates critical aspects of rodent and human glomerular disease.
How can podocyte damage be visualized in vitro? As mentioned in the introduction section, most glomerular diseases are, regardless of their etiology, characterized by reversible podocyte FP effacement leading to proteinuria at early stages, and a progressive loss of podocytes leading to glomerulosclerosis at late stages. Podocyte depletion in vivo can be reflected by a reduced viability of cultured cells. FP effacement involves a dynamic reorganization of attachment to the glomerular basement membrane and of a specialized actin-based cytoskeleton supporting the elaborate structure of these cell processes.34 These two characteristics translate into increased motility of cultured podocytes9 and a loss of actin stress fibers (parallel actin bundles) in vitro, respectively. Particularly, the latter is a very suitable read-out for drug testing. Several substances have been used to target some of the above discussed pathways of podocyte injury in cultured podocytes and rodent disease models,10,24,26 and the rescue of actin stress fibers in vitro correlated well with protection from FP effacement and proteinuria in vivo.
In addition to such phenotypic (actin structure) and functional (cell viability, motility) assays, reporter-based assays can serve to identify podocyte-targeting drugs. Yamauchi et al.35 recently used immortalized murine podocytes that were stably transfected with a reporter gene encoding secreted alkaline phosphatase under the control of the nephrin promoter. The established reporter cells were exposed to various substances, and culture media were subjected to a secreted alkaline phosphatase assay to identify regulators of nephrin gene expression. An inherent problem of quantitative reporter-based assays, however, is the long duration required for cultured podocytes to fully differentiate,4 which could potentially lead to high well-to-well and plate-to-plate variability. In contrast, imaging-based phenotypic assays are relatively independent of cell number per well. To achieve higher accuracy for drug screening, multiple measurements obtained from a single well can be combined by high-content screening27 (Figure 2).
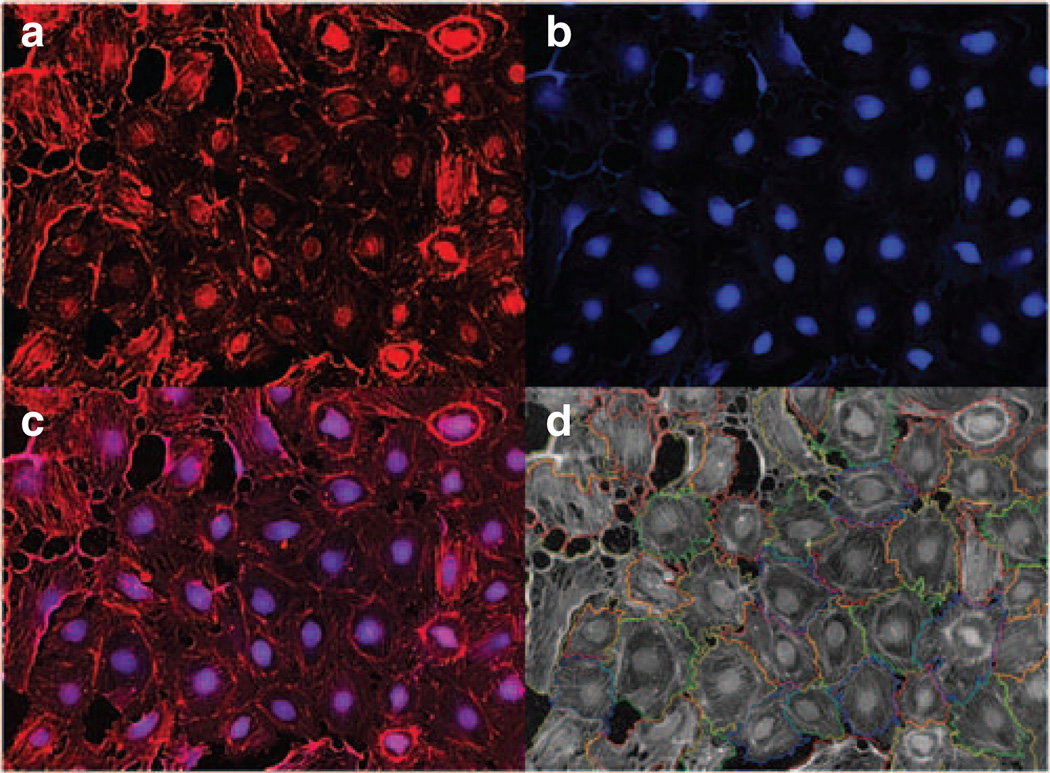
Figure 2. Phenotypic podocyte assays used for high-content screening (HCS).
Cultured podocytes can be used in HCS assays for automated detection of cellular morphology and stress fiber formation in a high-throughput environment. (a) An HCS captured image from a well of a 96-well plate with podocytes stained with phalloidin to detect actin-stress fibers. (b) Same image as in panel a stained with Cell Mask Blue (to stain cell nuclei and cytoplasm). (c) An overlay of images in panels a and b. (d) An overlay of automatically calculated cellular boundaries that can be used in measuring various cellular characteristics (such as cell number, cell size, and intensity of stress fibers per cell) on the raw image (a) from the 96-well plate.
A typical workflow for an HTS campaign with podocytes is depicted in Figure 3. Podocyte cell lines are grown on a large scale under permissive conditions and then transferred to HTS assay plates where the culture environment is switched to nonpermissive conditions that render cells growth arrested and fully differentiated. The differentiated podocytes in 96-well plates can be incubated with compound libraries for the desired time period, and the effects of compounds on cells can be measured using one of the readout formats discussed above.
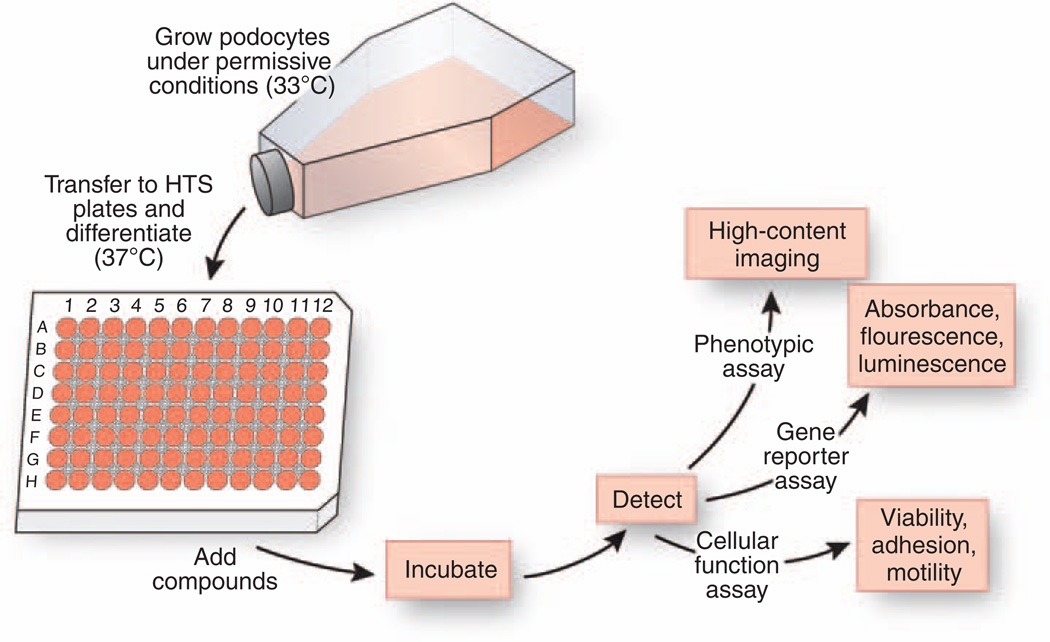
Figure 3. Schematic of a workflow for a primary drug screen with cultured podocytes.
Cultured podocytes are grown under permissive conditions at 33°C to allow for proliferation. Next, cells are transferred to 96-well HTS microtiter plates and allowed to differentiate under nonpermissive conditions at 37°C. Fully differentiated podocytes are incubated with chemical compound libraries, and the effect of compounds on cells is measured using a variety of cell-based readout assays. These detection assays include high-content imaging to detect changes in cellular phenotypes, measurement of homogenous changes in absorbance, fluorescence- or luminescence-based gene reporter assays as well as determination of changes in cell viability and cellular function, such as adhesion and motility.
CLASSES OF PODOCYTE-SPECIFIC DRUGS
Podocytes can be targeted using a number of different classes of therapeutic molecules. First, cDNA coding for proteins of interest under the control of podocyte-specific promoters can be delivered to podocytes in vivo through plasmid transfer.10,24,26,36 Second, purified proteins may be directly delivered to and be taken up by podocytes to replace degraded or dysfunctional native proteins. Third, targeting podocytes with small hairpin RNA and RNA interference oligonucleotides for knockdown of protein expression can be used to reduce disease-causing overexpression of podocyte proteins. Finally, small molecules that specifically bind to selected target proteins are effective at modulating podocyte function, as has been shown in a number of recent studies. Cyclo-RGDfV (Figure 4), a specific inhibitor of integrin αvβ3, ameliorates proteinuria in mouse models of nephrotic syndrome by directly targeting the upregulated integrin αvβ3 on podocytes.10 Similarly, the calcineurin inhibitor cyclosporine reduces proteinuria by specifically stabilizing synaptopodin in podocytes.26 Furthermore angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers37 as well as glucocorticoids38 have recently been shown to directly act on podocytes in addition to their antihypertensive and immunosuppressive properties, respectively.
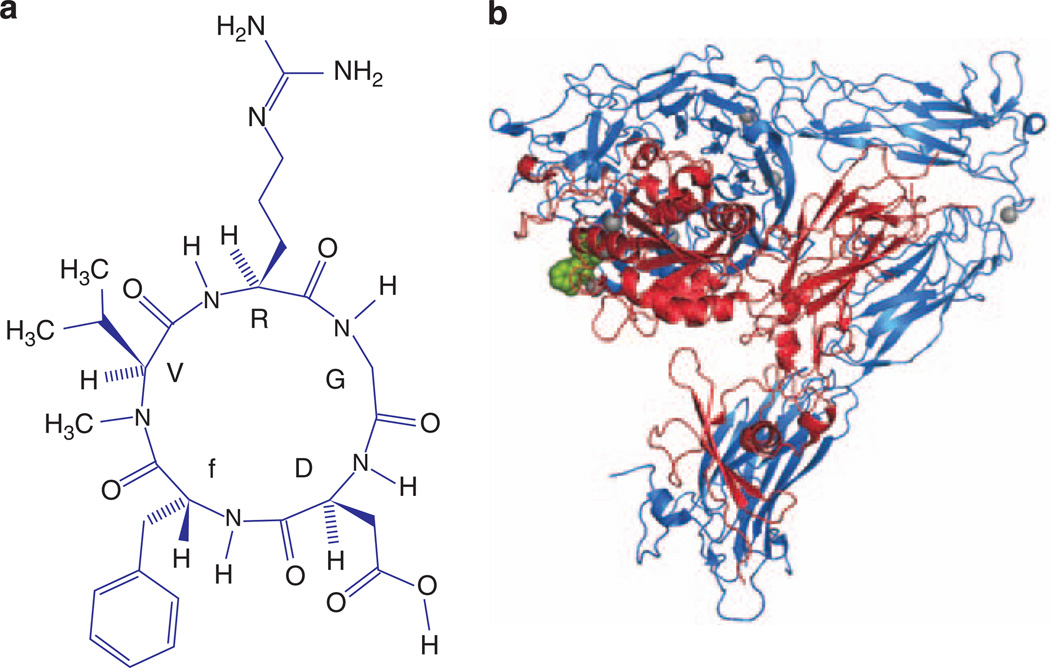
Figure 4. Cyclo-RGDfV as an example of a small molecule targeting podocytes.
(a) Chemical structure of the anti-integrin αvβ3 drug cyclo-RGDfV. (b) Three-dimensional crystal structure of the extracellular domains of integrin αvβ3 (shown as a ribbon model with αv-chain in blue and β3-chain in red) bound to cyclo-RGDfV (green, shown as a CPK model). Bound metal ions are shown as gray spheres. Models a and b are based on the study by Dechantsreiter et al.44 and Xiong et al.,45 respectively. Cyclo-RGDfV has been successfully used to treat proteinuria in the lipopolysaccharide-mouse model.10
Cell-based HTS assays can be used to discover potential podocyte-targeting therapeutics from any of these classes of molecules. Screening chemical libraries against cultured podocytes remains the most promising approach for discovering novel podocyte-specific therapeutics because uptake of DNA, RNA, and protein by terminally differentiated podocytes in culture is relatively inefficient and variable,39 while small molecules can easily penetrate the podocyte cell membrane. However, ‘biologics’ (DNA, RNA, proteins, and antibodies) are valuable tools in deciphering the exact molecular signaling pathways and especially antibodies against cell-surface receptors hold considerable therapeutic promise.
PODOCYTES QUALIFY FOR EFFICIENT TARGETED DRUG DELIVERY
Any pharmacological compound designed to specifically target pathways of podocyte injury needs to be deliverable to podocytes in vivo with sufficient efficiency and specificity. Most drugs reach their site of action through the circulation after being injected or absorbed in the gut. Therefore, the perfusion of an organ is a critical pharmacokinetic determinant. The kidney is perfused by 25% of the total cardiac output while accounting for only 0.5% of total body weight. Hence, the perfusion rate of the kidney, amounting to 350 ml/min per 100 g tissue, is 70 times higher than that of the remaining body. Approximately 20% of the plasma volume perfusing the kidneys is filtered into Bowman’s space. Thus, podocytes are virtually flushed by 180 l of plasma ultrafiltrate per day. In contrast, drugs targeting other organs must usually leave the capillary bed by paracellular diffusion through endothelial cell junctions or by transcellular pinocytosis and reach their site of action by further diffusion through the interstitium. There remains controversy about the permeability of the glomerular layers before the podocyte SD. However, the fenestrated glomerular endothelium is certainly by far more permeable to macromolecules than most other vascular beds throughout the body. The successful use of gene24 and antibody10 delivery to podocytes in mice shows that the glomerular endothelium and the glomerular basement membrane are sufficiently permeable for the delivery of these macromolecules to podocytes in vivo.
Once having reached the podocytes, a podocyte-specific drug will need to be taken up by these cells efficiently (in the case of intracellularly acting compounds) and bind its molecular target with high affinity in order not to be lost into the urine. Affinity can be optimized using secondary drug screening assays with libraries of subclasses of compounds identified in a primary HTS, or through molecular modeling. Small molecular compounds with an intracellular target usually diffuse readily through the cell membrane, whereas specific drug delivery strategies are required for macromolecules, such as nucleic acids and proteins. During the last decade, substantial progress has been made in the design of new technologies to improve cellular uptake of therapeutic compounds, including the use of cell-penetrating peptides.40 Cell-penetrating peptides allow the cytosolic delivery of macromolecules including small hairpin RNA and proteins. While some of the carrier molecules transport their cargoes directly through the plasma membrane, others use the cell’s endocytosis machinery. In this respect, it is important to point out that the podocyte possesses an efficient endocytosis machinery41 capable to endocytose albumin42 and immunoglobulin G,43 which can likely be exploited therapeutically.
CONCLUSIONS
Significant advances have recently been made in the understanding of podocyte biology and the pathophysiology of glomerular diseases, providing promising target pathways for drug discovery. The ability to culture and manipulate podocytes in vitro can be implemented in modern HTS assays to identify compounds that specifically interact with podocyte proteins. Furthermore, podocytes are particularly amenable to efficient and specific drug delivery due to their anatomical location and physiological properties. Several molecular pathways involved in glomerular diseases have already been successfully targeted in rodent disease models. Taken together, there is justified hope that novel podocytetargeting pharmaceuticals will be developed and applied to patients in the near future.
ACKNOWLEDGMENTS
JR is supported by the US National Institutes of Health (NIH) Grant DK073495. VG is supported by US National Science Foundation (NSF) Grant CCF-0958490 and NIH Grants K01DK068253 and R03NS053659. ADK is supported by a scholarship from the Swiss National Science Foundation and an Amgen-FROMO fellowship in renal physiology.
Footnotes
DISCLOSURE
The authors declared no competing interests
REFERENCES
- 1.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 2.Reiser J, Kriz W, Kretzler M, et al. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 3.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 4.Shankland SJ, Pippin JW, Reiser J, et al. Podocytes in culture: past, present, and future. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- 5.Benzing T. The promise of well-being: stay in shape with N(i)ck. J Am Soc Nephrol. 2009;20:1425–1427. doi: 10.1681/ASN.2009040453. [DOI] [PubMed] [Google Scholar]
- 6.Menne J, Meier M, Park JK, et al. Nephrin loss in experimental diabetic nephropathy is prevented by deletion of protein kinase C alpha signaling in-vivo. Kidney Int. 2006;70:1456–1462. doi: 10.1038/sj.ki.5001830. [DOI] [PubMed] [Google Scholar]
- 7.Uchida K, Suzuki K, Iwamoto M, et al. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int. 2008;73:926–932. doi: 10.1038/ki.2008.19. [DOI] [PubMed] [Google Scholar]
- 8.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 10.Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 11.Cheng HT, Kim M, Valerius MT, et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 13.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragmassociated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 16.Huber TB, Schermer B, Muller RU, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moller CC, Wei C, Altintas MM, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 18.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, Nakamura T, Noble NA, et al. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett AJ, Kirschke H, Cathepsin B, Cathepsin H, cathepsin L. Methods Enzymol. 1981;80(Part C):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 24.Sever S, Altintas MM, Nankoe SR, et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest. 2007;117:2095–2104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmuller T, Wenzler D, Hagemann S, et al. Toward computer-based cleavage site prediction of cysteine endopeptidases. Biol Chem. 2003;384:899–909. doi: 10.1515/BC.2003.101. [DOI] [PubMed] [Google Scholar]
- 26.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An WF, Tolliday NJ. Introduction: cell-based assays for high-throughput screening. Methods Mol Biol. 2009;486:1–12. doi: 10.1007/978-1-60327-545-3_1. [DOI] [PubMed] [Google Scholar]
- 28.Mundel P, Reiser J, Zuniga Mejia Borja A, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 29.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 30.Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol. 2005;16:299–304. doi: 10.1681/ASN.2004090754. [DOI] [PubMed] [Google Scholar]
- 31.Weavers H, Prieto-Sánchez S, Grawe F, et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457:322–326. doi: 10.1038/nature07526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 33.Mundel P, Reiser J, Kriz W. Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol. 1997;8:697–705. doi: 10.1681/ASN.V85697. [DOI] [PubMed] [Google Scholar]
- 34.Faul C, Asanuma K, Yanagida-Asanuma E, et al. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi K, Takano Y, Kasai A, et al. Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int. 2006;70:892–900. doi: 10.1038/sj.ki.5001625. [DOI] [PubMed] [Google Scholar]
- 36.Mayer G, Boileau G, Bendayan M. Furin interacts with proMT1-MMP and integrin alphaV at specialized domains of renal cell plasma membrane. J Cell Sci. 2003;116:1763–1773. doi: 10.1242/jcs.00394. [DOI] [PubMed] [Google Scholar]
- 37.Reiser J, Mundel P. Dual effects of RAS blockade on blood pressure and podocyte function. Curr Hypertens Rep. 2007;9:403–408. doi: 10.1007/s11906-007-0074-7. [DOI] [PubMed] [Google Scholar]
- 38.Ransom RF, Lam NG, Hallett MA, et al. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 2005;68:2473–2483. doi: 10.1111/j.1523-1755.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 39.Kajiyama H, Titus S, Austin CP, et al. Tetracycline-inducible gene expression in conditionally immortalized mouse podocytes. Am J Nephrol. 2009;29:153–163. doi: 10.1159/000151770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris MC, Deshayes S, Heitz F, et al. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 41.Ina K, Kitamura H, Tatsukawa S, et al. Glomerular podocyte endocytosis of the diabetic rat. J Electron Microsc (Tokyo) 2002;51:275–279. doi: 10.1093/jmicro/51.4.275. [DOI] [PubMed] [Google Scholar]
- 42.Eyre J, Ioannou K, Grubb BD, et al. Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol. 2007;292:F674–F681. doi: 10.1152/ajprenal.00272.2006. [DOI] [PubMed] [Google Scholar]
- 43.Akilesh S, Huber TB, Wu H, et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA. 2008;105:967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dechantsreiter MA, Planker E, Matha B, et al. N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J Med Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 45.Xiong JP, Stehle T, Zhang R, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]