Abstract
The stress hormone cortisol (CORT) is slowly incorporated into the growing hair shaft of humans, nonhuman primates, and other mammals. We developed and validated a method for CORT extraction and analysis from rhesus monkey hair and subsequently adapted this method for use with human scalp hair. In contrast to CORT "point samples" obtained from plasma or saliva, hair CORT provides an integrated measure of hypothalamic-pituitary-adrenocortical (HPA) system activity, and thus physiological stress, during the period of hormone incorporation. Because human scalp hair grows at an average rate of 1 cm/month, CORT levels obtained from hair segments several cm in length can potentially serve as a biomarker of stress experienced over a number of months.
In our method, each hair sample is first washed twice in isopropanol to remove any CORT from the outside of the hair shaft that has been deposited from sweat or sebum. After drying, the sample is ground to a fine powder to break up the hair's protein matrix and increase the surface area for extraction. CORT from the interior of the hair shaft is extracted into methanol, the methanol is evaporated, and the extract is reconstituted in assay buffer. Extracted CORT, along with standards and quality controls, is then analyzed by means of a sensitive and specific commercially available enzyme immunoassay (EIA) kit. Readout from the EIA is converted to pg CORT per mg powdered hair weight. This method has been used in our laboratory to analyze hair CORT in humans, several species of macaque monkeys, marmosets, dogs, and polar bears. Many studies both from our lab and from other research groups have demonstrated the broad applicability of hair CORT for assessing chronic stress exposure in natural as well as laboratory settings.
Keywords: Basic Protocol, Issue 83, cortisol, hypothalamic-pituitary-adrenocortical axis, hair, stress, humans, monkeys
Introduction
Measurement of CORT in plasma, saliva, or occasionally in urine or feces has been used as an index of physiological stress since Selye's discovery of the role of the HPA axis in stress1. Although numerous papers have been published relating HPA activity to acutely stressful situations, the field has been hampered by the lack of a simple and reliable index of chronic physiological stress. This problem arises because plasma and saliva both yield "point" estimates of HPA activity that are subject to circadian variation and can be confounded by environmental disturbances. Urinary and fecal samples yield measurements of CORT and/or metabolite excretion that span a number of hours up to a full day in some cases. Collection of multiple samples using any of these matrices may provide a rough composite index of CORT levels over time; however, none of these approaches provides a truly long-term index of HPA activity and the responsiveness of this system to chronic stressors.
Measuring CORT in hair has begun to fill this important need in the stress literature. Initial studies by several laboratories demonstrated the presence of CORT in human hair but did not investigate whether hair CORT levels changed as a function of stress2. As our laboratory has been interested for many years in the regulation of the rhesus monkey HPA axis by various social and behavioral factors3, we set out to establish and validate methods for extraction and analysis of rhesus monkey hair4. Based on the premise that blood-borne CORT is slowly and continuously incorporated into growing hair, the purpose of this new method was to use levels of hair-derived CORT as an integrated index of HPA activity over periods of weeks to months.
Several methodological challenges were encountered in developing the present protocol. First, previous studies had shown that small amounts of circulating CORT are excreted in sweat and sebum and therefore could coat the outside of the hair shaft2. In order to eliminate this potential confound, we developed a mild wash procedure that appears to remove external CORT while having a minimal effect on CORT present within the growing hair shaft. Thus, monkey hair subjected to this procedure (i.e. two 3-min washes with isopropanol) lost approximately 7-8% of the total hair CORT content, and a third wash removed less than 1% more steroid from the sample4. There appears to be more external CORT in human hair, since the same procedure removed an average of 27% total CORT content from the samples (K. Rosenberg and J. Meyer, unpublished). Like monkey hair, however, an additional wash contained much less CORT (about 7%) than the first two washes. Therefore, results from both monkey and human hair support the contention that most (if not all) external CORT can be removed while maintaining a major fraction of CORT within the internal hair matrix. Second, our pilot studies also showed that grinding the hair prior to extraction significantly increased CORT recovery from the sample, presumably by breaking open the complex proteinaceous matrix of the hair shaft as well as increasing the surface area available for solvent penetration. Two different grinding methods were developed, each with advantages and disadvantages. Method 1, which uses a ball mill, has the advantage of producing the finest powder. However, a ball mill is a relatively expensive equipment item and, if used with standard grinding jars and balls, it is capable of grinding only two samples at a time. Small samples are also difficult to process using a ball mill with standard grinding jars. Method 2, which uses a beadbeater, is less effective in its grinding ability. As a result, average CORT recovery is approximately 10% lower using this method compared to the ball mill (unpublished data). On the other hand, a beadbeater is considerably less expensive than a ball mill, 16-24 samples can be ground at once depending on the model, and the method is well suited for small samples. Because of the above mentioned differential recovery, it is advisable to use the same grinding method for all samples within a particular study.
Once the hair samples have been processed, they are extracted with methanol and CORT in the extracts is analyzed by means of a sensitive and specific commercial EIA kit originally designed to measure salivary CORT. The extraction and assay procedures were validated in part by demonstrating that serial dilutions of extracts from monkey hair samples yielded EIA readings that closely paralleled the readings obtained from authentic CORT standards. We then showed that hair CORT (in addition to plasma and salivary CORT) was sensitive to the major life stressor of an administratively mandated relocation of the monkeys to new housing quarters4,5. The present paper provides a detailed account of the methods used routinely in our laboratory to process human and monkey hair samples and to extract and analyze CORT from such samples.
Protocol
1. Sample Collection and Storage
- Human hair
- Secure the entire length of the hair to be sampled (up to a pencil-width in diameter) with a rubber band or clip. Cut hair as close to the scalp as possible (taking care not to nick the skin) with a clean scissors. Note: The standard sampling area is the posterior vertex of the skull.
- Some investigators have reported a decline in human hair CORT levels with distance from the scalp6, which may be due to washout from repeated exposure to water and shampoo7 (though see Manenschijn et al.8 and Thomson et al.9 for contrary results in which no segmental decline was observed) To minimize the impact of this potential "washout effect", we recommend collecting hair segments proximal to the scalp with a length no greater than 3 cm (assuming an average growth rate of 1 cm/month10, a 3 cm segment contains CORT that has been deposited over approximately the last 3 months). To accomplish this, use a ruler to measure 3 cm from the first cut and cut again to yield the 3 cm sample. Note: Once the hair has been cut to length, it is no longer necessary to keep the strands in alignment unless the study involves cutting the sample into separate 1 cm long segments to establish a retrospective calendar of CORT deposition over the time period prior to sampling6.
- Place the hair sample in a pouch made of aluminum foil, a clean paper envelope, or a 15 ml screw-cap polypropylene centrifuge tube of the type used for sample washing. As CORT is extremely stable in hair2, samples can be stored indefinitely at -20 °C and, if necessary, shipped overnight at ambient temperature.
- The hair collection procedure should be practiced on volunteers and the practice samples should be weighed before embarking on a full study. Note: Hair samples as small as 5-10 mg can be analyzed using the methods described here, although it is desirable to collect samples >10 mg to minimize the likelihood of obtaining EIA readings below the lowest CORT standard.
- Monkey hair
- Shave hair (100-250 mg) from the nape of the neck using a standard animal clipper. Shave as close to the skin as possible, being careful not to nick the skin and cause bleeding because blood CORT levels are extremely high compared to those found in hair. Note: The neck area was chosen for routine sampling because it is generally a good source of hair and can be readily observed for hair regrowth prior to resampling.
- Place the hair sample in a pouch made of aluminum foil or a 15 ml screw-cap polypropylene centrifuge tube. Monkey hair samples should be stored and shipped in the same manner as described above for human hair samples.
- Because monkey hair, unlike human scalp hair, grows to a certain length and then stops growing11, it is generally not feasible in this case to use distance from the skin as a calendar for CORT deposition. However, if an animal can be sampled more than once (for example, during periodic routine health exams), it is desirable to perform an initial shaving to set a baseline time point and then reshave the same area after the desired time period has elapsed. CORT in the second sample was deposited during the shave-reshave interval, which permits a precise attribution of the sample's CORT content to HPA activity over that interval5.
2. Sample Washing and Drying
- Washing
- Place each hair sample into a 15 ml screw-cap polypropylene centrifuge tube.
- Add 5 ml of high performance liquid chromatography (HPLC)-grade isopropanol to each tube followed by repeated inversion for 3 min using a rotator.
- Decant the isopropanol into a waste container, taking care not to lose any of the sample.
- Repeat steps 2.1.2 and 2.1.3 once more.
- Drying
- Dry the hair for at least 2-3 days to ensure complete isopropanol evaporation.
3. Sample Grinding and CORT Extraction - Method 1 for Large Samples
- Sample grinding
- Place up to 250 mg of dried hair into a 10 ml stainless steel grinding jar along with a single 12 mm stainless steel grinding ball.
- Grind the sample for 6 min at a speed of 25 Hz using a ball mill.
- Weigh up to 50 mg of powdered hair on an analytical balance and then transfer it to a clean 2.0 ml polypropylene microcentrifuge tube for subsequent CORT extraction.
- CORT extraction
- Add 1.0 ml of HPLC-grade methanol to the microcentrifuge tube containing the powdered sample.
- Cap the tube and incubate the sample for 18-24 hr at room temperature with constant inversion using a rotator.
- Centrifuge the tubes at 14,000 rpm for 1 min at room temperature to pellet the powdered hair.
- Transfer 0.6 ml of the supernatant to a clean 1.5 ml microcentrifuge tube, taking care not to disturb the pellet of powdered hair.
4. Sample Grinding and CORT Extraction - Method 2 for Small Samples
- Sample grinding
- Place up to 60 mg of hair into a preweighed 2 ml microcentrifuge tube reinforced for bead beating.
- Reweigh the vial to obtain the sample weight.
- Add three 3.2 mm chrome steel beads to each vial and then grind the sample for at least 2 min in a bead beater. If visual inspection reveals that the sample is insufficiently pulverized, then perform additional grinding for 0.5 or 1 min. Note: No transfer of the ground hair is needed in this case because CORT extraction is performed in the same vial as grinding.
- CORT extraction
- Add 1.5 ml of HPLC-grade methanol to the microcentrifuge tube containing the powdered sample.
- Cap the tube and incubate the sample for 18-24 hr at room temperature with constant inversion using a rotator.
- Centrifuge the tubes at 10,000 rpm for 5 min at room temperature to pellet the powdered hair. Note: The chrome steel beads remain in the tube with the hair during the extraction and centrifugation steps, which accounts for the lower centrifugation speed compared to method 1.
- Transfer 1.0 ml of the supernatant to a clean 1.5 ml microcentrifuge tube, taking care not to disturb the pellet of powdered hair.
5. Solvent Evaporation and Sample Reconstitution
Dry down the methanol using either a vacuum evaporator or a stream of nitrogen gas if a vacuum evaporator is not available. If a vacuum evaporator is used, the methanol vapor can be trapped by means of a cold trap or a chemical trap equipped with an activated charcoal cartridge.
Following removal of the methanol, reconstitute the CORT extract in an appropriate volume of EIA assay buffer (assay diluent). If relatively high CORT values are anticipated, then use a buffer volume of 0.4 ml or greater. If relatively low values are anticipated, then the volume can be reduced to 0.2 ml or less to increase sensitivity. Note: 0.2 ml is sufficient volume to run duplicate aliquots of the sample at least twice in case a rerun of the sample is required.
Either assay the reconstituted sample immediately or freeze it at -20 °C for later analysis. Note: If the reconstituted sample is frozen, it is important to avoid sublimation during the freezing period since an alteration in sample volume will lead to a false CORT value when the final calculations are performed.
6. CORT Assay and Data Conversion
Assay the hair extracts for CORT using a high-sensitivity enzyme immunoassay (EIA) kit. Note: If using a commercial salivary CORT EIA kit, follow the manufacturer's recommendations as specified in the kit insert except that the test samples will be duplicate aliquots of each reconstituted hair extract instead of saliva samples.
- Prepare a quality-control (QC) hair extract for use in every assay.
- Collect a number of extra hair samples and either process them individually as in steps 4.1 and 4.2 or pool them for large sample processing as in steps 3.1 and 3.2.
- Evaporate the solvent and reconstitute the extracts as in steps 5.1 and 5.2, then pool the reconstituted extracts from several individual or pooled samples.
- Aliquot an appropriate volume of the pooled reconstituted extract (e.g. 0.10 ml) into individual microcentrifuge tubes and freeze for later use.
- Thaw and run one QC aliquot in duplicate on each immunoassay microplate to provide a reference CORT value for checking the quality of each run.
- An intra-assay coefficient of variation (CV; defined as the standard deviation divided by the mean of a set of sample values) can be calculated by analyzing 10-12 QC wells in the same run, whereas an inter-assay CV can be calculated using the QC values across a group of runs. If one's microplate reader software calculates a CV for the duplicate wells representing each hair extract, then an alternate method for determining intra-assay CV is to calculate the mean of those individual CV values across the entire plate.
- If any sample extract yields a reading greater than the highest CORT standard in the EIA, then another aliquot of the extract is diluted with an appropriate amount of assay buffer and the subsequent EIA reading is corrected for the dilution factor. Samples are also routinely reanalyzed if the CV for the duplicate wells is >10%.
- Because CORT EIA kits are designed to measure CORT values in liquid samples such as saliva or plasma, the output of the microplate reader software must be converted to amount of CORT per unit weight of powdered hair. The following formula converts assay output in μg/dl to pg CORT per mg hair: (A/B) * (C/D) * E * 10,000 = F where A = μg/dl from assay output; B = weight (in mg) of hair subjected to extraction; C = vol. (in ml) of methanol added to the powdered hair; D = vol. (in ml) of methanol recovered from the extract and subsequently dried down; E = vol. (in ml) of assay buffer used to reconstitute the dried extract; and F = final value of hair CORT concentration in pg/mg.
Representative Results
Figure 1 shows the printout from a representative set of human hair samples (adult male and female human subjects) processed using method 2 grinding and extraction. Computer software was used to generate the data output and to fit a 4-parameter sigmoidal curve to the CORT standards (Figure 2). The between-well CVs from this plate ranged from 0.01-5.73% with an average intra-assay CV of 1.34%. The inter-assay CV determined using the QC values from nine recent human hair assays was 4.41%. The 37 samples analyzed on this plate yielded a range of hair CORT values from 3.1-650 pg/mg (median = 10.2 pg/mg; mean±SD = 36.0±110 pg/mg).
Method 1 is used in our laboratory to process hair samples from nonhuman primates and other large animals. A representative assay of adult rhesus monkey hair (mostly from females) yielded a range of CORT values from 50.1-102 pg/mg (median = 75.0; mean±SD = 75.8±14.0 pg/mg). The average intra-assay CV for this assay was 2.08%, and the inter-assay CV determined using the QC values from nine recent monkey hair assays was 4.63%.
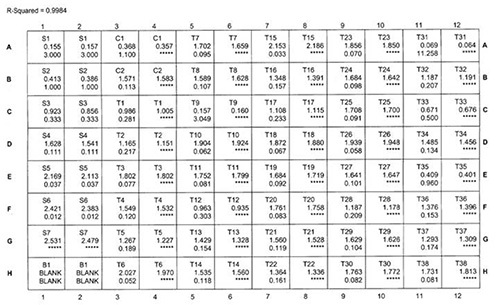 Figure 1. Software output for a representative human hair CORT assay. The duplicate wells are as follows: S1-S6 - CORT standards ranging from 0.012 μg/dl (S6) to 3.0 μg/dl (S1); S7 - 0 CORT wells; B1 - nonspecific binding (NSB) wells that lack anti-CORT antibody (the software automatically subtracts the average NSB optical density [OD] value from all other OD readings); C1 and C2 — high and low CORT calibrators provided by the manufacturer; T1 - QC; T2-T38 - test samples. From C1 to T38, the upper value in each cell of the array is the measured OD value from the corresponding well, and the lower value in the left-hand cell of the pair is the CORT value in μg/dl calculated from the mean OD value. Note that samples T9 and T31 yielded readings above the highest CORT standard. As a result, both samples were later diluted 4-fold in assay diluent and reanalyzed. The reanalyzed values were used after correction for the dilution factor. Click here to view larger image.
Figure 1. Software output for a representative human hair CORT assay. The duplicate wells are as follows: S1-S6 - CORT standards ranging from 0.012 μg/dl (S6) to 3.0 μg/dl (S1); S7 - 0 CORT wells; B1 - nonspecific binding (NSB) wells that lack anti-CORT antibody (the software automatically subtracts the average NSB optical density [OD] value from all other OD readings); C1 and C2 — high and low CORT calibrators provided by the manufacturer; T1 - QC; T2-T38 - test samples. From C1 to T38, the upper value in each cell of the array is the measured OD value from the corresponding well, and the lower value in the left-hand cell of the pair is the CORT value in μg/dl calculated from the mean OD value. Note that samples T9 and T31 yielded readings above the highest CORT standard. As a result, both samples were later diluted 4-fold in assay diluent and reanalyzed. The reanalyzed values were used after correction for the dilution factor. Click here to view larger image.
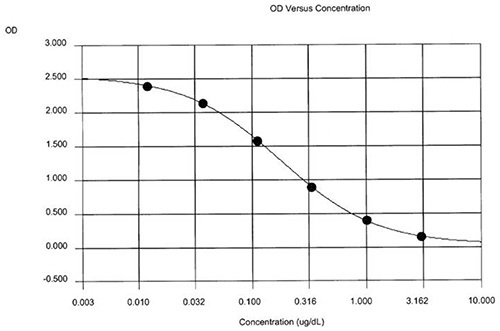 Figure 2. Standard curve of OD values versus log CORT concentration for the same human hair assay. The R-squared value shown in panel A is the calculated goodness of fit of the curve to the standards. Click here to view larger image.
Figure 2. Standard curve of OD values versus log CORT concentration for the same human hair assay. The R-squared value shown in panel A is the calculated goodness of fit of the curve to the standards. Click here to view larger image.
Discussion
The hair CORT procedure described above is simple to perform, is relatively inexpensive, makes use of readily available chemicals, reagents, and supplies, and requires equipment that, with one exception, is likely to be present in a typical analytical laboratory. The exception is a grinding apparatus such as a ball mill or mini-beadbeater. We note that some research groups mince hair samples into small fragments roughly 1 mm in length12, but based on our observations we recommend performing grinding instead of mincing if at all possible. Another area of methodological variation in the literature involves whether or not to wash the hair prior to CORT extraction13, and if so, what wash conditions to use. If washing is not performed, then one risks the possibility that the methanol extract will contain not only CORT incorporated slowly over the weeks or months of hair growth but also sweat- and/or sebum-derived CORT deposited recently on the surface of the hair. Studies on polar bear samples support the existence of two CORT fractions in hair: a loosely-bound fraction removable by brief isopropanol washing of intact hair that presumably represents mainly surface contamination, and a more tightly-bound fraction that is accessible by extensive methanol extraction of powdered hair samples14. The same conclusion can be drawn from the diminishing amounts of CORT extracted from monkey and human hair by repeated isopropanol washing (see Introduction). On the other hand, if washing is performed, then the choice of solvent and wash conditions can have a significant impact on the resulting CORT values. The Society of Hair Testing has published guidelines for selecting an appropriate solvent to minimize hair swelling and potential solute elution from the interior of the hair matrix during the washing process15. Although these recommendations were developed for application to drug testing in hair, they are relevant for steroid analysis as well.
Measuring CORT in hair rather than plasma or saliva offers a number of advantages2. Most importantly, this approach provides a biomarker of integrated CORT levels over periods of weeks to months that is uninfluenced by the time of day when samples are collected or by brief stress exposure prior to collection. Hair collection is noninvasive, although sampling of some animal species such as rhesus monkeys or bears may require anesthetization of the subject for safety reasons. Another advantage is that CORT is extremely stable in hair compared with other sample matrices, which permits analysis of historical or archival samples even if they have been stored at ambient temperature for a long period of time. Finally, CORT levels in human hair segments cut at successively greater distances from the scalp have sometimes been used to create a retrospective calendar of HPA activity over time. In such cases it is especially important to be aware of the previously mentioned “washout” effect produced by repeated hair washing. Although some studies have failed to replicate this effect8,9, it is worth noting that in those studies the samples were not washed prior to methanol extraction. This important methodological difference may help explain why hair CORT levels did not decline with distance from the scalp.
Some limitations of the hair CORT approach should also be mentioned. First, this approach cannot detect changes in the circadian rhythmicity of HPA activity (as seen in some depressed patients) or the awakening CORT response. Hair CORT levels also might not detect the impact of relatively brief stressors that occurred during the period of hormone incorporation. Hence, this approach should be thought of as complementary to measurements of salivary and/or plasma CORT, not as a replacement for such measurements. Second, whereas the use of hair CORT will likely be of particular value to researchers interested in psychosocial and environmental stressors, it is important to keep in mind that elevated HPA activity can occur under a variety of conditions, including physical exercise, metabolic abnormalities, and infectious disease. Third, whereas data exist from both humans and monkeys supporting the hypothesis that hair CORT is derived mainly from the bloodstream2, this hypothesis has not yet been proven. Indeed, Ito and coworkers16 have demonstrated the existence of a functional HPA-like system in microdissected human hair follicles maintained in organ culture. The degree to which hair follicles contribute to the CORT measured in the hair shaft remains unknown at this time.
In just a few years since its inception as a new biomarker of HPA axis activity, hair CORT has been used in a wide variety of applications across numerous species. Many of these applications fall within several major themes aimed at determining how long-term HPA activity is related to chronic stress, endocrine disorders such as Cushing’s disease, or neuropsychiatric disorders such as post-traumatic stress disorder2,17-19. Other studies have used hair CORT to examine HPA axis function in relation to behavioral temperament, normal development, the influence of developmental factors such as early childhood experiences in humans or different rearing conditions in monkeys, environmental conservation of wild-living animals, and retrospective investigation of historical or archival samples. Species studied to date with respect to hair CORT include humans, several species of nonhuman primates (macaques, vervet monkeys, and baboons), dogs, cats, cattle, horses, and several species of bears. It is likely that the use of hair CORT to assess long-term HPA activity, whether to investigate the physiological response to chronic stress or to address other experimental questions, will continue to expand and to be applied to an even greater range of species.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgments
We thank Kymberlee O'Brien, Celia Moore, and Edward Tronick (Department of Psychology, University of Massachusetts, Boston) for providing the human hair samples analyzed in this study, and Stephen Suomi and Amanda Dettmer (Laboratory of Comparative Ethology, NICHD) for providing the rhesus monkey hair samples. Initial development and continued use of this method has been supported by NIH RR11122 to M.A.N.
References
- Selye H. Stress and the general adaptation syndrome. Br. Med. J. 1950;1(4667):1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Novak MA. Minireview: Hair cortisol: A novel biomarker of hypothalamic- pituitary-adrenocortical activity. Endocrinology. 2012;153:4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. The physiology and neurochemistry of self-injurious behavior: A nonhuman primate model. Front. Biosci. 2005;10:1–11. doi: 10.2741/1500. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. A rhesus monkey model of self injury: Effects of relocation stress on behavior and neuroendocrine function. Biol. Psychiatry. 2008;63:990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production - Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA. Effects of shampoo and water washing on hair cortisol concentrations. Clin. Chim. Acta. 2011;412:382–385. doi: 10.1016/j.cca.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenschijn L, Koper JW, Lamberts SW, van Rossum EF. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76:1032–1036. doi: 10.1016/j.steroids.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SH. Hair analysis provides a historical record of cortisol levels in Cushing’s syndrome. Exp. Clin. Endocrinol. Diabetes. 2010;118:133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau MA, Montgomery MA, Brewer JD. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci. Int. 2011;210:110–116. doi: 10.1016/j.forsciint.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Dolnick EH. Variability of hair growth in Macaca mulatta. In: Montagna W, Dobson RL, editors. Adv. Biol. Skin. IX. Pergamon Press; 1969. pp. 121–128. [Google Scholar]
- Sauvé B, Koren G, Walsh G, Uum Tokmakejian SVan, H S. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Invest. Med. 2007;30:183–191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci. Int. 2010;196:32–37. doi: 10.1016/j.forsciint.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Bechshøft TØ, Sonne C, Dietz R, Born EW, Novak MA, Henchey E, Meyer JS. Cortisol levels in hair of East Greenland polar bears. Sci. Total Environ. 2011;409:831–834. doi: 10.1016/j.scitotenv.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GAA, Kronstrand R, Kintz P. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci. Int. 2012;218:20–28. doi: 10.1016/j.forsciint.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C. Analysis of cortisol in hair - State of the art and future directions. Brain Behav. Immun. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology. 2013;38:1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]


