Summary
The nervous system adapts to experience by inducing a transcriptional program that controls important aspects of synaptic plasticity. Although the molecular mechanisms of experience-dependent plasticity are well characterized in excitatory neurons, the mechanisms that regulate this process in inhibitory neurons are only poorly understood. Here, we describe a transcriptional program that is induced by neuronal activity in inhibitory neurons. We find that while neuronal activity induces expression of early-response transcription factors such as Npas4 in both excitatory and inhibitory neurons, Npas4 activates distinct programs of late-response genes in inhibitory and excitatory neurons. These late-response genes differentially regulate synaptic input to these two types of neurons, promoting inhibition onto excitatory neurons while inducing excitation onto inhibitory neurons. These findings suggest that the functional outcomes of activity-induced transcriptional responses are adapted in a cell type-specific manner to achieve a circuit-wide homeostatic response.
Introduction
Experience-dependent synaptic plasticity underlies multiple aspects of learning and memory, and is important early in life during critical periods when sensory experience is necessary for the development of cortical circuits (Hensch, 2005; Wiesel and Hubel, 1963). Research over several decades has revealed that physical changes at synapses that form on excitatory and inhibitory neurons are important for the nervous system’s adaptive responses to sensory input. While specific molecular mechanisms by which neuronal activity modifies synapses on excitatory neurons have been identified, it remains to be determined how synapses on cortical inhibitory neurons adapt to changing levels of neuronal activity. Since inhibition is critically important for experience-dependent plasticity and normal cognitive function (Lewis et al., 2005), identifying these molecular mechanisms in inhibitory neurons is key to understanding how cortical circuits respond to sensory input.
Inhibitory neurons regulate cortical function by controlling action potential generation, preventing runaway excitation, sharpening excitatory neuron tuning, and entraining oscillatory firing of cohorts of excitatory neurons (Isaacson and Scanziani, 2011; Somogyi and Klausberger, 2005). Subtypes of inhibitory neurons differ from each other with respect to their developmental lineage, morphology, gene expression program, electrophysiological properties, and post-synaptic targets (Markram et al., 2004). Despite this diversity, inhibitory neurons can be broadly grouped into three non-overlapping functionally distinct subtypes based on whether they express somatostatin (SST), parvalbumin (PV) or the 5HT3a receptor (Rudy et al., 2010). A variety of cellular mechanisms have been identified that mediate the response of inhibitory neurons to sensory input: for instance, sensory experience promotes the maturation of inhibitory neurons by increasing their membrane excitability during brain development and by promoting the growth of dendritic and axonal arbors (Chattopadhyaya et al., 2004; Chen et al., 2011; Okaty et al., 2009). Excitatory synaptic inputs to inhibitory neurons also undergo changes in response to activity including short- and long-lasting plasticity (Kullmann et al., 2012). Despite this increased understanding of the cellular basis of inhibitory neuron plasticity, the molecular mechanisms by which neuronal activity affects the development and plasticity of excitatory synapses onto inhibitory neurons are poorly characterized.
Studies of excitatory neurons have revealed that neuronal activity regulates synapse development and function through several distinct mechanisms, including the transcriptional induction of regulators of synaptic function (Flavell and Greenberg, 2008). Upon membrane depolarization, calcium enters neurons through NMDA receptors and L-type calcium channels and initiates a signaling cascade that activates pre-existing transcription factors. These factors then induce the transcription of early-response genes, which are enriched for additional transcription factors (e.g. Fos, Npas4, Zif268) that subsequently promote the transcription of late-response genes. These late-induced genes include regulators of synaptic connectivity that act locally at synaptic sites (e.g. Bdnf, Cpg15/Nrn1, Homer1). In excitatory neurons, this gene network functions to promote neuronal survival and dendritic morphogenesis, as well as to restrict the number of excitatory synapses and increase the number of inhibitory synapses that form on excitatory neuron (Bloodgood et al., 2013; Hong et al., 2008; Lin et al., 2008).
Npas4 is an early-response transcription factor that is enriched in the brain, induced in excitatory neurons specifically upon calcium influx, and has been suggested to regulate excitatory-inhibitory balance within neural circuits (Bloodgood et al., 2013; Coutellier et al., 2012). Npas4 deletion leads to an impairment of several forms of neuronal plasticity, suggesting that expression of Npas4 is necessary for the nervous system to adapt to sensory input (Maya-Vetencourt et al., 2012; Ploski et al., 2011; Ramamoorthi et al., 2011).
We show here that neuronal activity induces a distinct Npas4-dependent gene program in inhibitory neurons that functions to promote the development of excitatory synapses on SST-positive inhibitory neurons. While neuronal activity induces the same early-response transcription factors such as Npas4 in both excitatory and inhibitory neurons, it induces distinct but overlapping sets of late-response genes in these two types of neurons. This allows the synapses that form on inhibitory and excitatory neurons to be modified by neuronal activity in a manner specific to their function within a circuit. In excitatory neurons, Npas4 activates transcription of Bdnf, thereby promoting an increased number of inhibitory synapses on excitatory neurons. In SST neurons, Npas4 regulates a distinct set of target genes that serve to increase excitatory input onto SST neurons, likely resulting in enhanced feedback inhibition within cortical circuits. Thus the same activity-regulated transcription factor, by controlling distinct networks of genes, differentially regulates synaptic input to excitatory and inhibitory neurons, thereby facilitating appropriate circuit responses to sensory experience.
Results
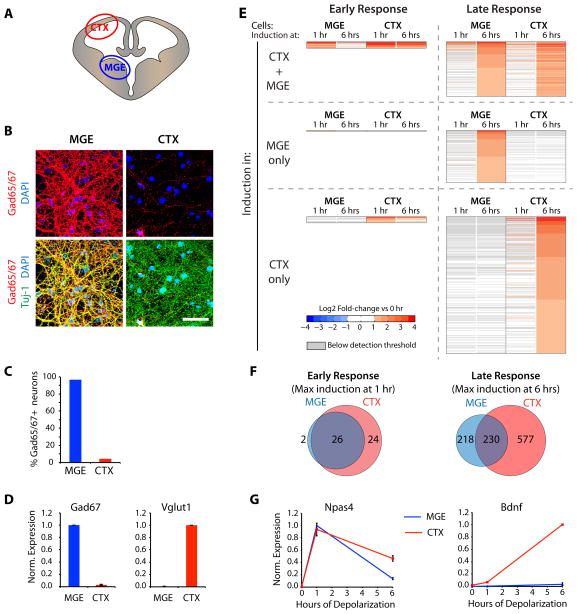
To study activity-dependent gene expression in inhibitory neurons, we prepared neuronal cultures highly enriched for these neurons by taking advantage of the fact that early in their development, inhibitory neurons are localized to specialized regions of the embryonic brain, the ganglionic eminences, that are devoid of excitatory neurons (Wonders and Anderson, 2006). We dissected the medial ganglionic eminence (MGE) at embryonic day 14 (E14), dissociated the tissue, and maintained the resulting cells in vitro for ten days (Fig 1A). We find by immunostaining, western blot, quantitative RT-PCR (qPCR), and microarray analysis (see below) that MGE cultures are highly enriched for the inhibitory markers Gad65/67 (Fig 1B–D, S1A, G). Conversely, MGE cultures are almost completely devoid of markers of excitatory neurons such as Vglut1 and Tbr1, and glia-specific GFAP (Fig 1D, S1A, C, G). Moreover, by immunostaining with antibodies directed against markers of various inhibitory neuron subtypes, we find that MGE cultures contain a variety of inhibitory neuron subtypes that match the subtypes found in MGE in vivo (Fig S1C) (Markram et al., 2004). Finally, using double immunolabeling for pre- and post-synaptic marker proteins (Fig S1D) and electrophysiological recordings of miniature synaptic currents (data not shown), we detect a large number of inhibitory synapses, but very few excitatory synapses in MGE cultures. For the purpose of comparison, we also prepared cultures that are devoid of inhibitory neurons by dissecting and dissociating the mouse cortex at E14, a time during brain development before most inhibitory neurons have migrated to the cortex (named from here on CTX-cultures, Fig 1A). DIV10 CTX cultures are almost completely devoid of GAD65/67-positive neurons and they contain substantially more Vglut1 mRNA than MGE cultures (Fig 1B–D, Fig S1G). Thus MGE- and CTX-cultures are highly enriched for inhibitory and excitatory neurons, respectively, and as such should be useful for examining how neuronal activity affects gene expression in these two subsets of neurons.
Figure 1. Activity-induced transcription in inhibitory neurons.
A–D) Separate dissection of the E14 Medial Ganglionic Eminence and cortex yield cultures highly enriched for either inhibitory (MGE) or excitatory (CTX) neurons. A) Schematic coronal cross-section of E14 brain and the regions dissected to prepare MGE- and CTX-cultures. B) DIV10 MGE and CTX cultures immunostained for Gad65/67 (red, inhibitory neuron marker) and Tuj-1 (green, pan-neuronal marker) (DAPI = blue, scale bar = 50 μm). C) Quantification of Tuj-1 positive neurons that stain positive for GAD65/67 in MGE or CTX cultures. D) qPCR-analysis for Gad67 and Vglut1 in DIV10 MGE (blue) and CTX (red) cultures (Data represented as mean ± SEM of 3 bioreps). EG) Genome-wide analysis of the gene program induced by neuronal activity in inhibitory and excitatory neurons: shared early-induced genes and cell type-specific late-induced genes. E) Heat maps showing results of the microarray analysis of membrane depolarized MGE or CTX cultures. Displayed are all probesets changing by 2-fold or more in at least one condition, each horizontal line represents the fold-changes of one probe-set. F) Venn diagrams displaying the number of probesets induced after one or six hours of membrane depolarization specifically in MGE cultures (blue), specifically in CTX cultures (red) or commonly in both types of cultures (purple). G) qPCR-analysis for Npas4 and Bdnf in DIV10 MGE (blue) and CTX cultures (red) after 0, 1, or 6 hours of membrane depolarization (Data represented as mean ± SEM of 3 bioreps). (See also Figure S1)
Activity-induced gene expression in inhibitory neurons
To identify the activity-dependent gene program in inhibitory neurons, MGE cultures were incubated with TTX and AP-5 to block sodium channels and NMDA receptors, respectively, and then exposed to elevated levels of potassium chloride (55 mM KCl) to induce membrane depolarization and calcium influx. RNA was purified from samples after 0, 1 and 6 hours of stimulation and microarray analysis was performed. We also performed a similar depolarization experiment using CTX-cultures so that we could compare the activity-dependent gene program induced in those two neuronal subpopulations.
In MGE cultures, almost all of the probesets maximally induced after one hour of membrane depolarization (26 out of 28) were also induced in CTX-cultures one hour after depolarization, indicating that the early transcriptional response to membrane depolarization is very similar in MGE and CTX cultures (Fig 1E–F). As in excitatory neurons, the set of early-response genes in MGE cultures is significantly enriched for transcriptional regulators (Gene Ontology term ‘Transcription Regulator Activity’, p = 2.7×10−4). Strikingly, 11 of the 12 transcriptional regulators acutely induced by neuronal activity in MGE-derived cultures are also induced in excitatory neurons, and include immediate-early genes such as Fos, FosB, Egr1-3, Nr4a1 and Npas4 that are known to robustly respond to neuronal activity and to mediate important neuronal functions (Supplemental Table 3; Flavell and Greenberg, 2008). Thus, despite the opposing functions of excitatory and inhibitory neurons within a neural circuit, neuronal activity induces a common early-response gene program in excitatory and inhibitory neurons that is enriched for transcriptional activators (Fig 1E–F, S1B).
We next asked whether the shared early-response transcription factors induced in both excitatory and inhibitory neurons regulate the same set of late-response genes, or if they regulate cell type-specific sets of late-response genes that might mediate the response of specific neuronal subtypes to sensory input. To this end, we identified probesets maximally induced six hours after membrane depolarization in MGE and CTX cultures, and found a large number of late-induced probesets in both cultures (MGE: 438 probesets; CTX: 808 probesets); however, only ~25% of these probesets (230 out of 1025) are induced in both MGE and CTX cultures, indicating that neuronal activity induces distinct sets of late-response genes in excitatory and inhibitory neurons (Fig 1E–F). These findings were corroborated by high-throughput sequencing of MGE cultures (RNA-Seq) and qPCR experiments (Fig 1G, S1F). Stimulation of MGE and CTX cultures with glutamate confirmed that common sets of early-response genes and distinct sets of late-response genes are activated by multiple activity paradigms in vitro (Fig S1E). Taken together, these analyses indicate that inhibitory and excitatory neurons share a common set of rapidly induced transcription factors that likely regulate distinct sets of late-response genes.
To determine if neuronal activity driven by sensory experience in vivo also induces shared early-response genes and distinct sets of late-response genes in inhibitory and excitatory neurons, we generated mice heterozygous for alleles of either Emx1-Cre (expressed specifically in excitatory neurons) or Gad2-Cre (expressed specifically in inhibitory neurons) harboring the Rpl22-HA (RiboTag) allele, which expresses an HA-tagged ribosomal subunit specifically in Cre-expressing neurons (Sanz et al., 2009). The resulting animals permit the immunopurification of ribosomally associated RNAs selectively from either excitatory or inhibitory neurons using anti-HA antibodies. qPCR analysis of cell type-specific marker genes showed that samples immunopurified from Emx1-Cre mice were highly enriched for Tbr1 and Vglut1 mRNA, whereas samples from Gad2-Cre mice were highly enriched for Gad65 and Gad67 mRNA (Fig 2A).
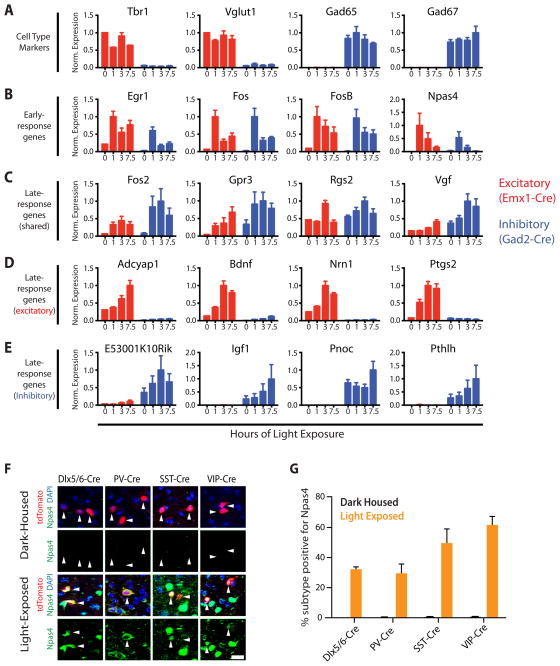
Figure 2. Experience-dependent gene induction in inhibitory neurons in vivo.
A–E) Sensory experience induces shared sets of early-response transcription factors and distinct sets of late-response effector genes in inhibitory and excitatory neurons in vivo. Normalized expression of ribosome-associated RNA purified from the visual cortex of Emx1-Cre (red) or Gad2-Cre (blue) expressing neurons in dark-housed mice (0 hrs) and after light exposure (1, 3, 7.5 hrs) (Data represented as mean + SEM of 3 bioreps). A) Cell type-specific marker genes. B) Early-response genes predicted by in vitro experiments to be induced in both excitatory and inhibitory neurons. C–E) Late-response genes predicted by in vitro experiments to be induced in C) both excitatory and inhibitory neurons, D) only in excitatory neurons, E) only in inhibitory neurons. F–G) Npas4 is induced by sensory experience in multiple inhibitory neuron subtypes. F) Immunostaining of visual cortices of mice dark-housed and light-exposed for 2.5 hours. Inhibitory neuron subtypes are labeled by Cre-dependent expression of tdTomato (red, driven by Dlx5/6-Cre, PV-Cre, SST-Cre, or VIP-Cre), and with antibodies against Npas4 (green) (Coronal sections, scale bar = 5 μm). G) Quantification of (F), data represented as mean + SEM of 3 bioreps. (See also Figure S2)
With this approach we examined the cell type-specific regulation of selected activity-responsive genes in vivo. RiboTag animals maintained in the dark were exposed to light for 0, 1, 3, or 7.5 hours, their visual cortices dissected, and ribosomal-associated RNA immunopurified from either excitatory or inhibitory neurons. qPCR analysis of the resulting immunoprecipitates confirm the induction of a common set of early-response factors, including Egr1, Fos, FosB and Npas4, in both inhibitory and excitatory neurons (Fig 2B). While a subset of late-response genes shared between excitatory and inhibitory cell types is apparent (e.g. Fosl2, Gpr3, Rgs2, Vgf), we also observe cell type-specific regulation of late-response genes that recapitulated the expression patterns observed in vitro, including late-response genes specific to either inhibitory (e.g. E530001K10RIK, Igf1, Pnoc, Pthlh) or excitatory (e.g. Adcyap1, Bdnf, Nrn1, Ptgs2) neurons (Fig 2C–E). Taken together, these experiments demonstrate that sensory experience induces shared sets of early-response genes and distinct but overlapping sets of late-response genes in excitatory and inhibitory neurons in vivo in an intact neural circuit. Furthermore, these in vivo experiments confirm that MGE and CTX cultures are a useful paradigm for studying the cell type-specific molecular events that occur in response to neuronal activity in inhibitory and excitatory neurons.
Npas4 is induced by neuronal activity across inhibitory neuron subtypes
Rather than focus on a single late-response gene induced selectively in MGE cultures, to assess the general function of the activity-dependent gene program in inhibitory neurons, we investigated the function of a shared early-response transcription factor, Npas4. Since Npas4 promotes inhibitory synapse development on excitatory neurons by activating Bdnf, but Bdnf is not expressed in inhibitory neurons in vitro or in vivo, we hypothesized that Npas4 induces distinct sets of late-response genes in excitatory or inhibitory neurons, thereby mediating cell type-specific responses that are tuned to the function of a neuron within a circuit. By identifying the specific function of Npas4 and its transcriptional targets in inhibitory neurons, we might characterize the molecular response of inhibitory neurons to activity.
We first asked which inhibitory neuron subtypes express Npas4 in vitro in response to membrane depolarization and in vivo in response to sensory stimulation. We membrane depolarized E16.5 mixed cortical cultures and immunostained for Npas4 and marker proteins that are selectively expressed in distinct inhibitory neuron subtypes. Upon membrane depolarization each of the inhibitory neuron subtypes examined expresses Npas4 (Fig S2A–B). To assess Npas4 expression in vivo, we generated mice in which genetically defined subtypes of inhibitory neurons were fluorescently labeled. These mice were dark-housed for several days and perfused either without having been exposed to light (dark-housed), or after 2.5 hours of light exposure (light-exposed). We immunolabeled brain sections with anti-Npas4 antibodies and quantified the percentage of each inhibitory neuron subtype that stained positive for Npas4. No Npas4-positive inhibitory neurons were observed in the visual cortex of dark-housed mice; however, upon light exposure we find induction of Npas4 expression in all inhibitory neuron types analyzed (e.g. Dlx5/6, PV, SST, VIP) (Fig 2F). Consistent with our in vitro results, Npas4 is expressed in SST- and VIP-positive neurons and in a smaller fraction of PV-positive neurons (Fig 2G). This pattern of Npas4 induction is also observed when subtypes of inhibitory neurons were identified using antibodies that recognize proteins that are selectively expressed in specific inhibitory neuron subtypes (Fig S2C). Taken together, these data demonstrate that Npas4 is induced in multiple inhibitory neuron subtypes in vitro upon membrane depolarization and in vivo after sensory stimulation.
Npas4 regulates the development of excitatory synapses onto SST neurons
To investigate the function of Npas4 in inhibitory neurons, we focused on SST-expressing neurons because Npas4 is highly induced in these neurons, and because SST-neurons mediate feedback inhibition to pyramidal neuron dendrites (Silberberg and Markram, 2007). Since SST neurons receive local excitatory input and form dense networks of inhibitory synapses onto nearby pyramidal neurons (Fino and Yuste, 2011), we hypothesized that they are optimally situated within a cortical circuit to utilize activity-induced transcriptional pathways to read out local levels of activity and mount a homeostatic response. To test this idea, we selectively removed Npas4 from SST neurons and prepared dissociated E16.5 mixed cortical cultures from embryos heterozygous for an allele expressing Cre recombinase under the control of the endogenous Somatostatin locus (SST-Cre), and an allele harboring a Cre-dependent tdTomato fluorescent reporter. These embryos were homozygous for either a wild-type allele of Npas4 (WT) or an Npas4 allele flanked by LoxP-sites allowing for Cre-dependent Npas4 deletion (cKO) (Lin et al., 2008). SST-Cre functions effectively in SST-expressing neurons, as most tdTomato-positive neurons were also positive for staining with anti-somatostatin antibodies (Fig S3A). Furthermore, SST-Cre efficiently excised the floxed Npas4 allele, as KCl-induced Npas4 expression in labeled SST neurons is abolished in cKO cultures (Fig S3B).
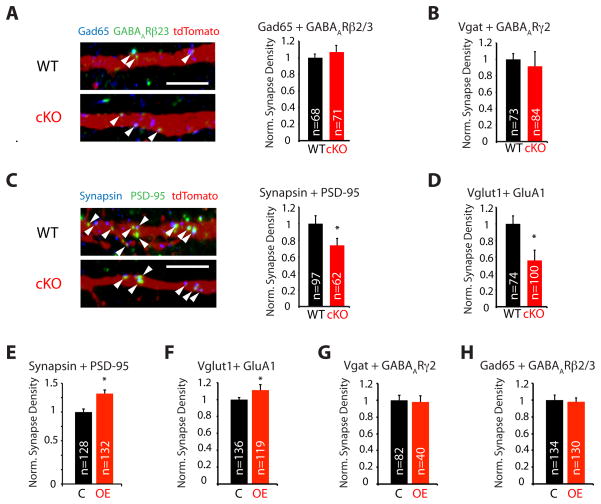
To assess if Npas4 regulates the development of inhibitory synapses formed onto SST-positive neurons, as it does in excitatory neurons, we quantified the density of inhibitory synapses formed onto SST neurons by immunolabeling WT and cKO cultures with antibodies directed against two sets of markers for inhibitory synapses, Gad65/GABAARβ2/3 and Vgat/GABAARγ2. We then counted the number of pre- and post-synaptic marker co-clusters that overlapped with fluorescently labeled SST neuron dendrites and divided by the total dendritic area. This analysis revealed that, in contrast to the effect of Npas4 deletion in excitatory neurons, deletion of Npas4 has no effect on the density of inhibitory synapses formed onto SST-expressing neurons (Fig 3A–B).
Figure 3. Npas4 regulates excitatory, but not inhibitory, synapse number in SST neurons.
Synapse assays in E16.5 mixed cortical cultures immunostained for pairs of pre- and post-synaptic markers. Synapse density was determined by quantifying colocalized puncta of pre- and post-synaptic markers that overlap with either tdTomato- (A–D) or GFP- (E–H) positive SST neuron dendrites (A, C: Arrowheads indicate synapses, scale bars = 5 μm). Data were normalized to the mean synapse density in each control condition, data represent the mean + SEM of 3 bioreps (* = significant change, p < 0.05). A–D) cKO of Npas4 in SST neurons reduces the number of excitatory but not inhibitory synapses formed onto Npas4 cKO SST neurons. Cultures were prepared from littermate Npas4 WT or cKO embryos heterozygous for alleles of SST-Cre and Cre-inducible tdTomato. (A) Inhibitory synapse markers GABAAR β2/3 (green) + Gad65 (blue) and (B) Vgat and GABAARγ2, (C) Excitatory synapse markers PSD-95 (green) + Synapsin (blue) and (D) Vglut1 + GluA1. E–H) Overexpression of Npas4 in SST neurons increases the number of excitatory but not inhibitory synapses formed onto SST neurons. Cultures were prepared from SST-Cre embryos, infected at DIV 4 with lentiviral constructs driving in a Cre-dependent manner the expression Npas4 and GFP (OE) or only GFP (C) and maintained until DIV 8. (E) Excitatory synapse markers PSD-95 + Synapsin and (F) Vglut1 + GluA1, (G) Inhibitory synapse markers Vgat + GABAAR γ 2 and (H) GABAARβ2/3 + Gad65. (See also Figure S3)
To determine the effect of Npas4 loss on the development of excitatory inputs onto SST neurons, we quantified the density of excitatory synapses formed onto SST neurons. We find that selective deletion of Npas4 in SST neurons results in a significantly lower density of excitatory synapses on SST neurons as measured by the overlap of two independent sets of excitatory synapse markers, Synapsin-1/PSD-95 and Vglut1/GluA1 (Fig 3C–D). Importantly, the effect of Npas4 deletion on excitatory synaptic connections onto SST neurons is not due to an indirect effect of Npas4 deletion on neuronal survival, cell size, or the complexity of proximal dendrites (Fig S3C–D and data not shown).
We next determined whether overexpression of Npas4 in SST neurons is sufficient to drive an increase in excitatory synapse density. We infected E16.5 mixed cortical cultures from SST-Cre embryos with lentiviral constructs that express in a Cre-dependent manner either Npas4 together with GFP or GFP alone. After confirming that expression of Npas4 and/or GFP in these cultures is restricted to SST neurons (Fig S3E), we quantified the density of synapses as before. Overexpression of Npas4 in SST neurons causes an increase in the density of excitatory synapses, but has no effect on the number of inhibitory synapses that form on SST neurons (Fig 3E–H). These findings indicate that Npas4 controls the number of excitatory, but not inhibitory, synaptic inputs onto SST neurons. This function is different from Npas4’s previously described function in excitatory neurons (Bloodgood et al., 2013; Lin et al., 2008) and suggests that Npas4 functions in a cell type-specific manner to control excitatory-inhibitory balance.
To determine whether these morphological changes reflect alterations in functional excitation, we used the genetic strategy described above to conditionally delete Npas4 from SST neurons and obtained whole-cell patch-clamp recordings from these cells in acute slices from P10-12 WT or cKO animals (Fig 4A–F), an age range that closely matches the developmental stage of the neurons analyzed in culture. At P10-12, calcium waves sweep through the cortex before eye opening, providing a source of neuronal activity to activate Npas4 (Garaschuk et al., 2000), and widespread Npas4 expression is observed in SST neurons at P11 following kainate seizure induction (Fig S2D). Importantly, at this age SST-Cre efficiently excises the Npas4 floxed allele in vivo and promotes expression of tdTomato in SST, but not VIP- or PV-positive neurons (Fig S4B–C).
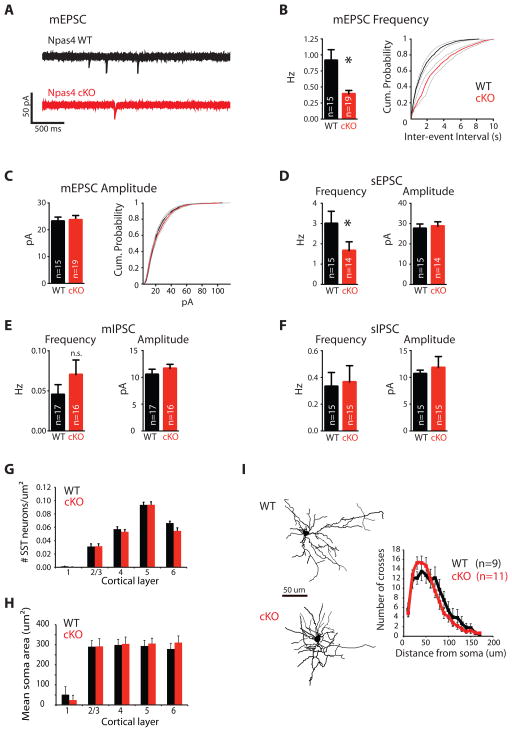
Figure 4. Npas4 regulates the development of functional excitatory inputs onto SST neurons.
A–F) Deletion of Npas4 in SST neurons reduces functional excitation but not inhibition onto SST neurons in the visual cortex in vivo. Whole-cell patch clamp recordings were obtained from tdTomato-labeled SST-neurons in acute slices of Npas4 WT (black) or cKO (red) P10-12 mice (data presented as bar graph of mean + SEM or as cumulative distribution function CDF of mean ± SEM; * = significant change, p < 0.05). A) Example traces of mini EPSCs (mEPSCs) (recorded at −70 mV in the presence of 0.5 μM TTX, 50 μM PTX and 25 μM CTZ; scale bar = 50 pA, 500 ms). B) Left, mEPSC frequency (p < 0.005, Mann-Whitney U-Test). Right, CDF of the mEPSC inter-event intervals of all neurons sampled in each genotype. C) Left, mEPSC amplitude (p = 0.847, Mann-Whitney U-Test). Right, CDF of the mEPSC event amplitudes of all neurons sampled in each genotype. D) Left, spontaneous EPSC (sEPSC) frequency (recorded at −70 mV in the presence of 50 μ M PTX; p = 0.027, Mann-Whitney U-Test). Right, sEPSC amplitude (p = 0.499, Mann-Whitney U-Test). E) Left, mIPSC frequency (recorded at 0 mV in the presence of 0.5 μM TTX, 25 μM NBQX, and 50 μM CPP; p = 0.261, Mann-Whitney U-Test). Right, mIPSC amplitude (p = 0.386, Mann-Whitney U-Test). F) Left, sIPSC frequency (recorded at 0 mV in the presence of 25 μM NBQX and 50 μM CPP; p = 0.734, Mann-Whitney U-Test). Right, sIPSC amplitude (p = 0.409, Mann-Whitney U-Test). G–I) Deletion of Npas4 in SST neurons does not affect the layer distribution, soma size or dendritic complexity of SST neurons in the visual cortex. Density (G) and soma size (H) of tdTomato-labeled Npas4 WT (black) or cKO (red) SST neurons by cortical layer in P10-12 visual cortices (data represented as mean + SEM of 3 bioreps). I) Sholl analysis of dye-filled and reconstructed Npas4 WT (black) or cKO (red) SST neuron dendrites in the P11 visual cortex. Left: example images (scale bar = 50 μm), Right: quantification (p = 0.244, repeated measures ANOVA). (See also Figure S4)
Conditional deletion of Npas4 results in a significant decrease in the frequency of pharmacologically isolated miniature excitatory postsynaptic currents (mEPSCs) (WT: 0.91 ± 0.168 Hz, n = 15; cKO 0.39 ± 0.054 Hz, n = 19; p = 0.003, Fig 4A–B), which are blocked by NBQX and CPP treatment (Fig S4D, n = 3). We observe no change in mEPSC amplitude or kinetics (WT: 23.19 ± 1.54 pA, n = 15; cKO 23.64 ± 1.68 pA, n = 19; p = 0.847) (Fig 4C, S4E), suggesting that deletion of Npas4 from SST neurons selectively alters mEPSC frequency. This decrease in mEPSC frequency could be due to decreased presynaptic probability of release or result from a decrease in the number of functional excitatory synapses onto SST neurons. Given that this effect is observed following the selective deletion of Npas4 from SST neurons, together with the observed reduction in the density of excitatory synaptic connections formed onto SST neurons in vitro when Npas4 is deleted, our data are consistent with a change in the number of functional excitatory synapses onto SST neurons in vivo when Npas4 is deleted from SST neurons.
To investigate the effect of selective Npas4 deletion on the functional excitatory drive impinging upon SST neurons, we recorded EPSCs in the absence of bath-applied TTX [e.g. spontaneous EPSCS (sEPSCs)] from SST neurons in acute visual cortex slices of P10-12 WT or cKO mice. Loss of Npas4 results in a significant decrease in the frequency of spontaneous EPSCs (WT: 2.99 ± 0.608 Hz, n = 15; cKO 1.66 ± 0.437 Hz, n = 14; p = 0.027) but does not affect the amplitude of these events (WT: 27.57 ± 2.24 pA, n = 15; cKO 28 ± 2.169 pA, n = 14; p = 0.499) (Fig 4D), indicating that SST neurons lacking Npas4 receive reduced excitatory drive, which likely results in reduced firing.
Consistent with our observations in cultured neurons, no significant effect on mIPSC frequency (WT: 0.045±0.012 Hz, n = 17; cKO: 0.07±0.018 Hz, n = 16, p = 0.261) or amplitude (WT: 10.53 ± 0.98 pA, n=17; cKO: 11.65 ± 0.772 pA, n = 16; p = 0.386) is observed in SST neurons in slice upon deletion of Npas4 (Fig 4E). Furthermore, spontaneous IPSCs in SST neurons are not affected by loss of Npas4 (Frequency: WT: 0.33 ± 0.1 Hz, n = 15; cKO: 0.365 ± 0.122 Hz, n = 15; p = 0.7349; Amplitude: WT: 10.66 ± 0.674 pA, n=15; cKO: 11.81 ± 2.06 pA, n = 15; p = 0.4989) (Fig 4F). Together, these data show that Npas4 regulates the amount of functional excitation, but not inhibition, on SST positive inhibitory neurons in acute cortical slices.
To determine if the effect of Npas4 on excitation onto SST neurons in vivo arises from a direct effect at the synapse or a secondary effect on cell health, we compared visual cortex development of P10-12 WT and cKO mice. The cortices of cKO mice are grossly normal as compared to their WT littermates (Fig S4A), and we find no change in the number or laminar distribution of SST neurons across the different cortical layers, indicating that Npas4 is not required for SST neuron migration, terminal differentiation, or survival (Fig 4G). Conditional deletion of Npas4 in SST neurons does not affect soma size (Fig 4H), and SST neurons lacking Npas4 extend and elaborate axons to target distal dendrites normally, as indicated by the presence of bright bands of tdTomato in layer 1 of the cortex of both genotypes (Fig S4A). In addition, Sholl analysis reveals that Npas4 does not regulate the dendritic complexity of SST neurons (WT: n = 9; cKO, n = 11; p = 0.244 repeated measures ANOVA) (Fig 4I). Taken together, this analysis demonstrates that removal of Npas4 from SST neurons affects neither overall cortical structure nor the number or morphology of SST neurons in the cortex.
In summary, our data indicate that, in contrast to Npas4’s ability to stimulate an increase in the number of inhibitory synapses onto excitatory neurons, this activity-dependent transcription factor promotes the development of excitation on SST-positive inhibitory neurons. Given that inhibitory neurons do not express Bdnf, a major Npas4 target gene in excitatory neurons, it seemed likely that Npas4 regulates SST neuron connectivity by activating the transcription of a distinct set of late-response genes in inhibitory neurons.
Npas4 Regulates Cell Type-Specific Activity-Dependent Transcriptional Programs
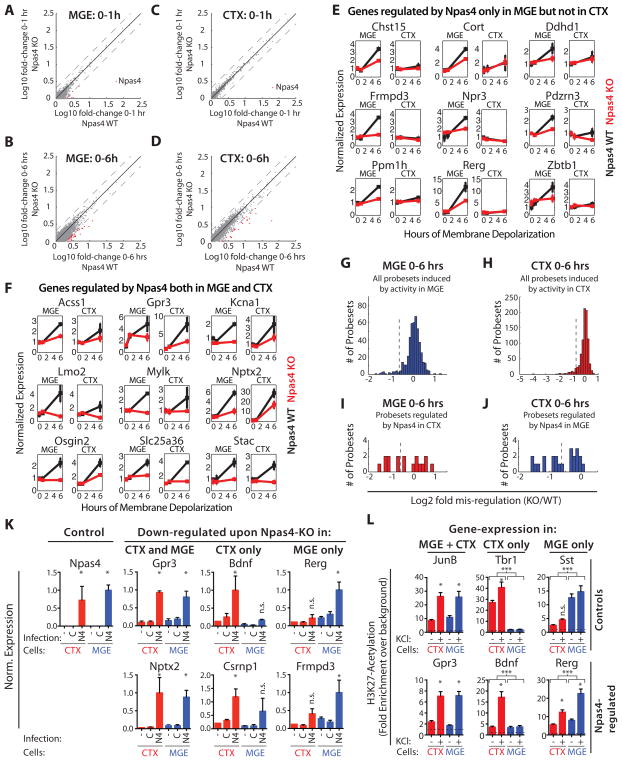
We next identified the genes that Npas4 regulates in inhibitory neurons and compared them to those regulated by Npas4 in excitatory neurons. MGE cultures from littermate Npas4 WT or KO embryos were membrane depolarized for 0, 1 or 6 hours (Fig S5A), and mRNA from these cultures was analyzed by microarray and subsequent qPCR validation (Fig S5B). Deletion of Npas4 in MGE neurons has no effect on the early transcriptional response to membrane depolarization, but the induction of a set of late-response genes is compromised in the absence of Npas4 (Fig 5A–B, G). To determine whether Npas4 specifically regulates these genes in inhibitory neurons as compared to excitatory neurons, we depolarized CTX cultures prepared from littermate Npas4 WT or KO embryos and performed microarray analysis. As in MGE cultures, loss of Npas4 has no effect on the induction of early-response genes in CTX cultures, but does result in decreased induction of a specific subset of late-response genes (Fig 5C–D, H). Comparative analysis revealed that Npas4-regulated genes in inhibitory neurons fall into two classes: some (e.g. Rerg, Frmpd3; Fig 5E) are induced by membrane depolarization only in MGE cultures, whereas others (e.g. Nptx2, Gpr3) are activity-induced in both MGE and CTX cultures (Fig 5F). In total, half of the genes induced by membrane depolarization in an Npas4-dependent manner in MGE cultures were regulated by Npas4 in a cell type-specific manner (9 genes Npas4-regulated only in MGE, 9 in MGE + CTX) (Fig 5E–J). Additionally, 34 genes were regulated by Npas4 specifically in excitatory neurons (e.g. Bdnf, Csrnp1; Fig S5C). We conclude that Npas4 controls the expression of distinct but overlapping sets of activity-induced genes in inhibitory and excitatory neurons.
Figure 5. Npas4 regulates a cell type-specific activity-induced transcriptional program in inhibitory neurons.
A–D) Microarray analysis of the depolarization-induced gene expression response in Npas4 WT or KO MGE and CTX cultures. Scatter plots show the fold-change of every expressed probeset in WT cultures against its fold-change in KO cultures. Black line = unity, dotted lines = two-fold changes in either direction. Probesets misregulated by more than two-fold in the KO are labeled in red (the single strongly misregulated probeset in A and C represents Npas4) A) Early response (0–1h) in MGE cultures. B) Late response (0–6h) in MGE cultures. C) Early response (0–1h) in CTX cultures. D) Late response (0–6h) in CTX cultures. E–J) Analyis of microarray-experiments: Npas4 controls the activity-dependent induction of distinct sets of late-response genes in inhibitory and excitatory neurons. E, F) Microarray-based line plots of genes regulated by Npas4 specifically in MGE-neurons (E) or in both MGE- and CTX-neurons (F). Normalized expression is plotted versus duration of stimulus, data normalized to WT MGE 0h and presented as mean ± SEM of two bioreps (WT: black, KO: red). G, H) Histograms of all probesets induced in MGE (G) and CTX (H) cultures showing the misregulation in Npas4 KO cultures. Probesets to the left of the dotted lines are considered as Npas4-regulated I) Histogram showing the misregulation in Npas4 KO MGE cultures of all genes regulated by Npas4 in CTX cultures. Probesets to the right of the dotted line are considered as Npas4-regulated specifically in CTX-neurons. J) Histogram showing the misregulation in Npas4 KO CTX cultures of all genes regulated by Npas4 in MGE cultures. Probesets to the right of the dotted line are considered as Npas4-regulated specifically in MGE-neurons. K) Npas4-overexpression (OE) induces cell type-specific Npas4 target gene expression in the absence of neuronal activity. qPCR analysis of expression levels of cell type-specific and shared Npas4-targets after Npas4-OE in CTX (red) or MGE (blue). The cultures were either not infected (-) or infected at DIV4 with constructs expressing GFP (C) or Npas4 (N4), quieted O.N. at DIV7 and harvested on DIV8. (Data represent the mean + SEM of 4 Bioreps; * = significantly changed, p < 0.05, one-way ANOVA with Sidak’s multiple comparison correction,). L) Cell type-specific activation status of gene regulatory elements associated with Npas4-target genes reflects the cell type-specific regulation by Npas4. H3K27Ac ChIP on DNA isolated from CTX (red) and MGE cultures (blue) − KCl-depolarization. qPCR was done either on regulatory regions associated with control genes or on Npas4-binding regulatory regions associated with genes regulated by Npas4. Data are presented as fold enrichment above background (dashed black line; n=4 for CTX, n=5 for MGE; * = significant change of H3K27Ac-levels within a cell type ; *** = significant difference of H3K27Ac across CTX and MGE. * and *** p < 0.05, one-way ANOVA with Sidak’s multiple comparison correction). (See also Figure S5)
We next asked whether Npas4 overexpression is sufficient to induce the genes identifed in the loss-of function-analysis in the absence of membrane depolarization, and if so, whether Npas4 functions in a cell type-specific manner. We overexpressed Npas4 or GFP by lentiviral infections in MGE and CTX neurons, silenced the cells with TTX and AP-5 and then assessed the expression of genes found to be misregulated in the absence of Npas4. qPCR analysis showed that infection with the Npas4-expressing construct leads to a large increase in Npas4 expression and to significantly increased levels of the shared Npas4-targets Gpr3 and Nptx2 in both MGE and CTX cultures (Fig 5K). Strikingly, Npas4 overexpression results in significantly increased levels of the excitatory-specific Npas4 targets Bdnf and Csrnp1 only in CTX cultures, whereas the levels of the MGE-specific Npas4 targets Frmpd3 and Rerg increase significantly upon Npas4-overexpression only in MGE cultures (Fig 5K). These findings indicate that overexpressed Npas4 is sufficient to induce target gene expression in a cell type-specific manner in the absence of other activity-regulated factors.
Npas4 regulates gene transcription by binding to the activity-dependent enhancers and promoters of its target genes (Kim et al., 2010). We hypothesized that Npas4 might achieve cell type-specific activation of its target genes by binding to gene regulatory regions that function in a cell type-specific manner in inhibitory and/or excitatory neurons. To test this idea, we identified cis-regulatory elements within 50 kb of Npas4 target genes that are inducibly bound by Npas4 upon membrane depolarization and exhibit histone modifications indicative of enhancer or promoter regions. ChIP-seq datasets (Kim et al., 2010) generated from mixed cortical cultures containing both excitatory and inhibitory neurons using anti-Npas4, -CBP, -H3K4me1 and -H3K4me3 antibodies revealed that most Npas4-regulated genes are located near candidate regulatory elements (6 out 9 genes regulated by Npas4 in MGE only, 9/9 regulated in MGE + CTX, 31/34 regulated in CTX only; Fig S5D).
To test whether these elements are activated in a cell type-specific manner upon membrane depolarization we conducted chromatin immunoprecipitation (ChIP) experiments on nuclear extracts from MGE and CTX cultures with antibodies to acetylated lysine 27 of histone H3 (H3K27Ac), a histone mark that demarcates transcriptionally active regulatory elements (Creyghton et al., 2010). H3K27Ac enrichment at the regulatory regions of Tbr1 (expressed only in CTX) and Sst (MGE-specific) accurately tracks with the respective expression of these loci in MGE and CTX cultures (Fig 5L). H3K27Ac levels also increase significantly at the Npas4-bound gene regulatory regions of JunB, an Npas4-independent early-response gene induced in both cell types, and of Gpr3, an Npas4-target in both excitatory and inhibitory neurons, in both CTX and MGE cultures in response to membrane depolarization. In contrast, at a regulatory element associated with Bdnf, a gene regulated by Npas4 specifically in excitatory neurons, H3K27Ac levels increased only in CTX cultures while H3K27Ac levels at the regulatory region of Rerg, regulated by Npas4 only in inhibitory neurons, shows selective enrichment of H3K27Ac in MGE cultures following membrane depolarization. These data suggest that the transcriptional outcome of Npas4-induction in excitatory and inhibitory neurons is defined at least in part by the cell type-specific status of the regulatory elements of Npas4 target genes.
Npas4 regulates an experience-induced gene program in SST neurons
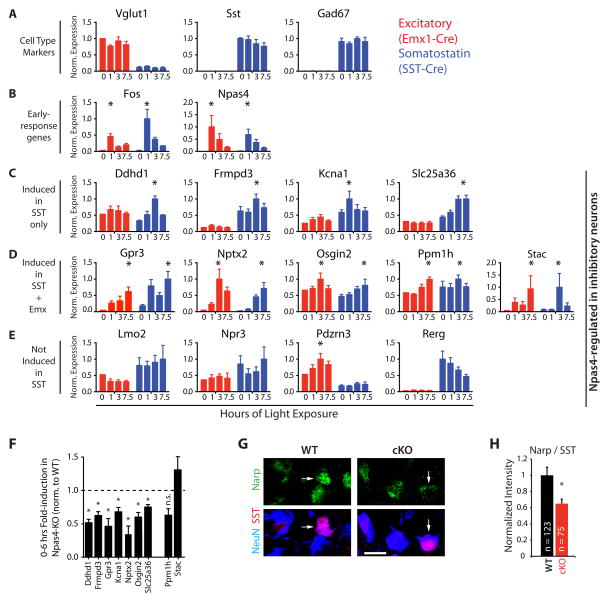
Finally, we sought to identify Npas4-regulated genes in SST neurons that might mediate the Npas4-dependent effects on neuronal connectivity in these cells. We employed the RiboTag-approach to immunopurify RNA from the visual cortex of dark-housed and light-exposed mice that express the RiboTag-allele in either SST-neurons (labeled by SST-Cre) or excitatory neurons (labeled by Emx1-Cre). qPCR analysis for a set of cell type-specific control genes indicated that the RNA immunopurified from SST-Cre expressing neurons is highly enriched for Sst and Gad67 mRNA, whereas RNA immunopurified from Emx1-Cre expressing neurons is enriched for Vglut1 mRNA (Fig 6A). As expected, light exposure results in a robust increase in the levels of the ribosome-associated mRNAs of the early-response genes Npas4 and Fos in both Emx1- and SST-Cre-expressing neurons (Fig 6B). We then examined 14 of the Npas4-regulated genes identified in MGE cultures. mRNA corresponding to four of these loci (Frmpd3, Slc25a36, Kcna1, Ddhd1) is significantly induced following light exposure in SST neurons but not in excitatory neurons, and the levels of six more Npas4-regulated genes (Stac, Osgin2, Nptx2, Ppm1h, Bach2, Gpr3) is increased upon light stimulation in both excitatory and SST neurons (Fig 6C–D, Induction: 1, 3, 7.5 hour >30% change from max 0 h value). Levels of the four remaining genes do not increase in response to light exposure in SST neurons, suggesting that elements of the Npas4 regulated gene program in MGE cultures may be specific to other inhibitory neuron subtypes (Fig 6E).
Figure 6. Npas4 regulates a cell type-specific set of target genes induced by sensory stimulation in SST neurons in vivo.
A–E) Npas4 target genes identified in inhibitory neurons in vitro are induced by sensory experience in a cell type-specific manner in vivo. Normalized expression of ribosome-associated mRNA from the visual cortex of Emx1-Cre (red) or SST-Cre (blue) expressing neurons in dark-housed mice (0 hrs) and after light exposure (1, 3, 7.5 hrs) (Data represent mean + SEM of 3 bioreps, * = induction defined as a mean >30% increase from the 0h time point). A) Cell type-specific marker genes. B) Early-response genes. C) Npas4-target genes induced selectively in SST neurons D) Npas4-target genes induced in both excitatory and SST neurons. E) Npas4-target genes that are not induced in SST neurons. F-H) Experience-induced Npas4 targets in SST neurons are misregulated in the absence of Npas4 in the visual cortex in vivo. F) Reduced induction of Npas4-targets in the visual cortex of Npas4-KO mice in response to sensory stimulation. KO and WT mice were dark-housed and light-exposed and qPCR analysis was performed on RNA isolated from the visual cortex. (Data represented as mean + SEM of the stimulus-induced fold-change in KO mice relative to the normalized fold-change observed in WT mice. n = 3 animals per genotype and time-point, * = significantly changed induction, p < 0.05, two-tailed t-test). G–H) The levels of Narp, the protein product of the Npas4 target gene Nptx2, are reduced in SST neurons lacking Npas4. G) Coronal sections from the visual cortex of P11 Npas4 WT and cKO mice lacking Npas4 specifically in SST neurons stained with antibodies directed against Narp (green) and the pan-neuronal marker NeuN (blue) (Scale bar = 10 μm). (H) Quantification of the intensity of Narp immunostaining in SST neuron somata (arrows) in (G). (Data represented as mean + SEM of normalized intensity value across all SST neurons imaged. * = significant difference, p < 0.005, Mann-Whitney U-Test). (See also Figure S6)
To identify which of these activity-responsive genes are dependent on Npas4 in vivo, we compared light-dependent induction of these genes in the visual cortex of dark-housed wild-type and Npas4 KO mice (Fig 6F). qPCR analysis demonstrates that all four of the Npas4 targets induced by light exposure specifically in SST neurons show reduced upregulation in Npas4 KO mice. In addition, three of the six Npas4-regulated genes that are induced upon light exposure in both SST and excitatory neurons are misregulated in Npas4 KO mice (Gpr3, Nptx2, Osgin2). To begin to test whether Npas4 regulates the expression of these genes specifically in SST neurons in vivo, we immunolabeled brain sections of mice that lack Npas4 in SST neurons with antibodies that recognize Narp, the protein product of the Npas4 target Nptx2. Quantitative immunofluorescence analysis confirmed that the level of Narp is reduced in SST neurons when Npas4 is deleted in these neurons (Fig 6G–H). In summary, these studies demonstrate that a distinct Npas4-driven gene program is induced in SST neurons in response to sensory experience. Intriguingly, several of these Npas4 target genes in SST neurons have been reported to act at postsynaptic sites of excitatory synapses (Nptx2, Kcna1) (Hoffman et al., 1997; O’Brien et al., 1999) or are homologous to related molecules (Frmpd3) (Lee et al., 2008), suggesting that the function of the Npas4-regulated gene program in SST neurons is to promote excitatory synapse development onto SST neurons.
Discussion
Activity-induced transcriptional networks in inhibitory neurons
Despite the importance of activity-dependent plasticity of inhibition for the development and function of cortical circuits, the study of stimulus-induced events in cortical inhibitory neurons has historically been difficult: widely used protocols for culturing cortical neurons yield cultures that are composed mainly of excitatory neurons, and more recent protocols that allow for the specific isolation of fluorescently labeled inhibitory neurons lack the temporal resolution necessary to accurately study transient transcriptional events (Batista-Brito et al., 2008; Okaty et al., 2009). By dissecting mouse embryos at an earlier developmental time point than that used for standard cortical cultures (E14 instead of E16–E18), and by culturing neurons derived from the MGE and from the nascent cortex separately over a prolonged period (~10 days), we were able to circumvent previous limitations and to establish separate cultures of inhibitory and excitatory neurons that proved useful for an initial analysis of acute stimulus-dependent processes in excitatory versus inhibitory neurons. Using this approach, we were able to identify a large number of activity-regulated genes that are specifically induced in inhibitory neurons. Additionally, by profiling of ribosome-associated RNAs, we were able to recapitulate these findings in vivo, showing that many of these genes are activated specifically in inhibitory neurons in response to sensory experience. It seems likely that these newly identified activity-regulated genes underlie the molecular mechanisms by which inhibitory neurons adapt to changes in neural activity. Strikingly, our gene expression analysis led to the finding that several of the best-studied late-response genes in excitatory neurons – including Bdnf, Homer1, and Cpg15/Nrn1 – are not induced by neuronal activity in inhibitory neurons. Accordingly, late-response genes that are induced specifically in inhibitory neurons – such as Igf1, Pthlh or Cacng5 – may serve previously unappreciated roles in specifying how inhibitory neurons adjust their synaptic inputs in response to sensory input.
Npas4 functions in a cell type-specific manner according to a circuit-wide homeostatic logic
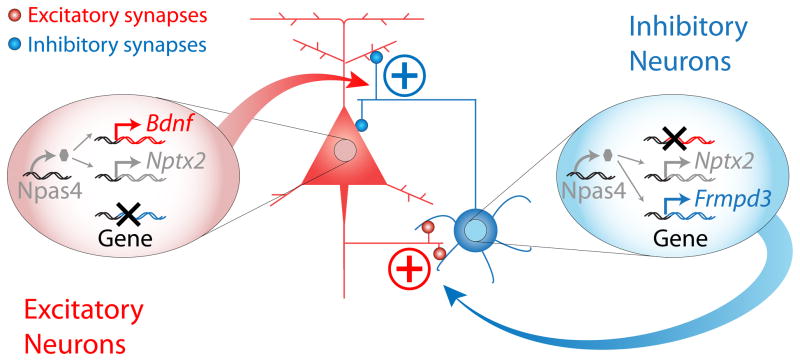
Our analysis of the activity-induced transcription factor Npas4 revealed that this factor is induced in both excitatory and inhibitory neurons and that it functions in SST neurons to positively regulate the function of excitatory, but not inhibitory, synapses on these neurons. The effect of Npas4 on the development of excitation onto SST neurons is the reciprocal of its role in excitatory neurons, where it positively regulates the number of inhibitory synapses on excitatory neurons (Lin et al., 2008). The directionality of Npas4’s cell type-specific functions can be understood in the context of neuronal homeostasis (Figure 7): in response to activity, Npas4 is induced in excitatory neurons, where it promotes increased numbers of inhibitory synapses, thereby reducing the activity level of the pyramidal neuron. In SST neurons, elevated activity also induces Npas4, which then acts to promote increased excitation onto the SST neuron. In isolation, this paradoxical form of homeostasis would result in continuously increasing levels of excitation of SST neurons; however, from a circuit-wide perspective, increased excitatory drive to inhibitory neurons should promote increased GABA release, and thereby result in decreased net excitation within the local circuit. SST neurons largely receive excitatory input from local cortical afferents, so levels of Npas4 in SST neurons may specifically reflect activity levels in local cortical circuits, and could potentially function to fine-tune the amount of feedback inhibition broadcasted by SST neurons throughout the local microcircuit (Silberberg and Markram, 2007). The reciprocal nature of Npas4 function in excitatory and inhibitory neurons demonstrates that key elements of activity-dependent transcriptional pathways are adapted to reflect the distinct function of a particular type of neuron in a neural circuit. Our findings on Npas4 function in SST neurons are consistent with previous reports of strengthening of excitatory synapses onto inhibitory neurons in response to elevated levels of circuit activity (Turrigiano, 2011), supporting the hypothesis that increasing excitation to inhibitory neurons may be a general principle governing the nervous system’s response to activity.
Figure 7. Cell type-specific functions of Npas4 are part of a circuit-wide homeostatic logic to restrict excitation.
Model demonstrating that the cell type-specific activity-induced transcriptional programs controlled by Npas4 in excitatory and inhibitory neurons function to regulate different types of synapses to achieve a common homeostatic goal of restricting network activity. In excitatory neurons, Npas4 activates a transcriptional program that consists of late-response genes selectively expressed in excitatory neurons (e.g. Bdnf, red) and commonly induced in excitatory and inhibitory neurons (e.g. Nptx2, grey). These Npas4 targets function together to promote increased numbers of inhibitory synapses onto excitatory neurons, thereby decreasing circuit activity. In inhibitory neurons, Npas4 activates a transcriptional program consisting of late response genes selectively expressed in inhibitory neurons (e.g. Frmpd3, blue), and genes that are commonly induced by activity in both excitatory and inhibitory neurons (e.g. Nptx2, grey). Together, Npas4-regulated late-response genes in inhibitory neurons function to promote increased excitation onto inhibitory neurons, thus increasing GABA release and lowering the overall levels of circuit activity.
Npas4 activates cell type-specific transcriptional programs of late-response genes
Our findings raise the question how can an activity-induced transcription factor that is ubiquitously expressed in neocortical neurons activate distinct gene programs in a cell type-specific manner. The ability of overexpressed Npas4 to induce target genes in a cell-type specific manner in the absence of neuronal activity indicates that cell-intrinsic mechanisms define the transcriptional outcome of Npas4 activation. Epigenetic chromatin marks on enhancers and promoters have previously been shown to contribute to cell type-specific gene expression (Whyte et al., 2013) and thus might also function to define the inducibility of Npas4 target genes in excitatory and inhibitory neurons. Accordingly, while Npas4 acts as a ubiquitous detector of circuit activity, the transcriptional program regulated by Npas4, and thus the specific synapses modulated by Npas4, may be determined by the cohort of accessible regulatory elements in a specific neuronal subtype.
Npas4-regulated genes induced by sensory experience in SST neurons can modify excitatory synapses
Despite the recent identification of molecules that shape the synaptic connectivity of inhibitory neurons (Fazzari et al., 2010; Sylwestrak and Ghosh, 2012), the molecular mechanisms underlying the plasticity of synaptic inputs to these neurons are still not well understood. Intriguingly, three of the experience-induced Npas4 targets in SST-neurons are well suited to regulate excitatory inputs to these cells. Kcna1 encodes the shaker-like potassium channel Kv1.1, which is expressed in the somatodendritic compartment of neurons and reinforces Hebbian plasticity of excitatory inputs by regulating the electrical properties of dendrites (Hoffman et al., 1997). Frmpd3 is a yet uncharacterized homologue of the scaffolding molecule Frmpd4 (e.g. Preso), which associates with PSD-95 and regulates dendritic spine morphogenesis (Lee et al., 2008). Finally, Nptx2 is an experience-induced Npas4 target gene in both SST neurons and excitatory neurons, the protein product of which (Narp) is present at excitatory synapses on SST neurons (Fig S6C). Considering Narp’s well-known role in promoting AMPA receptor accumulation and the stabilization of non-spiny excitatory synapses (Koch and Ullian, 2010; O’Brien et al., 1999), these data suggest that Npas4 executes its effect on excitatory synapses in SST neurons at least in part via transcriptional induction of Nptx2.
In contrast to our finding that Nptx2/Narp is induced in SST inhibitory neurons, a recent report indicates that Narp is not produced in Parvalbumin-expressing inhibitory neurons (Chang et al., 2010). This suggests that the activity-induced gene expression programs may differ not only between excitatory and inhibitory neurons, but also between distinct subtypes of inhibitory neurons. Supporting this idea, several genes that we identified as activity-induced in an Npas4-dependent manner in MGE-derived cultures were not induced by sensory stimulation in SST neurons in the visual cortex; a possible explanation for this observation is that these genes are instead induced by activity in inhibitory neuron subtypes that do not express somatostatin.
In conclusion, our findings indicate that in excitatory and inhibitory neurons neuronal activity induces a common set of transcriptional regulators and distinct sets of late-response genes. The early-induced transcriptional regulator Npas4 controls activation of distinct sets of effector genes in inhibitory and excitatory neurons to mediate plasticity responses that are tailored to the specific function of a particular neuronal cell type within a neural circuit. It is intriguing to speculate that this cell type-specific function of Npas4 may represent a general principle by which common activity-induced transcription factors can activate unique transcriptional programs in different neurons, thereby governing unique cell type-specific plasticity outcomes in response to activity.
Experimental Procedures
MGE- and CTX-cultures
Cultures were established by dissecting the MGE and CTX of E14 embryos, plating the dissociated cells onto glass bottom dishes (Mattek, Ashland, MA) and maintaining the cultures for 9–11 DIV.
Visual Stimulation
For ICC, P18-20 mice reared in standard conditions were dark-housed for four days and subsequently sacrificed either in the dark or after 2.5 hours of light-exposure. For Ribotag experiments, six-week-old mice reared in standard conditions were dark-housed for two weeks and subsequently sacrificed either in the dark or after 1, 3 or 7.5 hours of light-exposure.
RiboTag-analysis
Per time-point and genotype, the dissected visual cortices of three animals were pooled. Immunopurification of ribosome-associated RNA was performed as described (Sanz et al., 2009) with minor modifications. Purified RNA was amplified using the Ovation RNA Amplification System V2 (NuGEN, San Carlos, CA) and the resulting cDNA was used for qPCR-analysis.
Electrophysiology
Coronal sections were cut from P10-12 mouse visual cortex in choline dissection media and incubated in artificial cerebral spinal fluid(ACSF). Whole-cell voltage-clamp recordings were performed in ACSF at room temperature from tdTomato-labeled SST neurons identified under fluorescent and DIC optics. mEPSCs were isolated by holding neurons at −70 mV and exposing them to 0.5 μM tetrodotoxin, 50 μM picrotoxin and 25 μM cyclothiazide and were blocked by application of 25 μM NBQX and 50 μM CPP. mIPSCs were isolated by holding neurons at 0 mV and exposing them to 0.5 μM tetrodotoxin, 25 μM NBQX, and 50 μM CPP and were blocked by 50 μM picrotoxin. Spontaneous EPSCs (sEPSC) were recorded by holding neurons at −70 mV and exposing them to 50 μM picrotoxin, while sIPSCs were recorded by holding neurons at 0 mV and exposing them to 25 μM NBQX and 50 μM CPP. All cells were allowed to stabilize for at least three minutes and were recorded from for at least 25 minutes following stabilization.
Microarray Analysis and RNA-Seq
Total RNA was collected from MGE or E14 Cortical cultures using Trizol reagent following the RNEasy Micro Kit’s procedure (Qiagen, Valencia, CA) and RNA quality was assessed on a 2100 Bionalayzer (Agilent, Palo Alto, CA). Two independent biological replicates were performed for each microarray experiment in this study. Arrays from all experimental conditions and all replicates of a given condition were normalized to one another using the robust multichip averaging method (RMA normalization) using the MATLAB bioinformatics toolbox.
Supplementary Material
Highlights.
Inducible gene programs are adapted to reflect the function of a neuron in a circuit
Neuronal activity induces a distinct transcriptional response in inhibitory neurons
Npas4 promotes development of excitatory synapses on SST-positive inhibitory neurons
Npas4 activates a set of inducible genes in SST neurons that function at synapses
Acknowledgments
We thank F. Polleux and J. De Marchena for help with establishing the MGE cultures, C. Mandel-Brehm and C. Chen for help with electrophysiology experiments, J.M. Gray and A.M. Costa for help with preparing RNA-Seq libraries, B.L. Bloodgood for preliminary recordings from MGE cultures, N. Sharma for the lentiviral expression construct of Npas4, E. Griffith and T. Cherry for critical reading of the manuscript. and P. Zhang for managing the mouse colony. This work was funded by fellowships by the Human Frontiers Science Program and the Swiss National Science Foundation (I.S) and the National Institute of Health grant NS028829 (M.E.G.).
Footnotes
Author Contributions
Experiments were designed by I.S., A.R.M. and M.E.G. Experiments were conducted and analyzed by I.S., A.R.M., H.W.G., J.E.B., C.H.C., C.P.T. and D.A.H. The manuscript was written by I.S., A.R.M. and M.E.G.
Accession-numbers
Microarray and RNA-Seq data are available at NCBI GEO (Accession# GSE55591).
Additional experimental procedures and mouse strains are detailed in the Supplemental Information.
References
- Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–2317. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sharma N, Browne HA, Trepman AZ, Greenberg ME. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature. 2013;503:121–125. doi: 10.1038/nature12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13:1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14:587–594. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M. Npas4: A neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS ONE. 2012;7:e46604. doi: 10.1371/journal.pone.0046604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Nat Acad Sci. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Luján R, Lloyd K, Lerma J, Marín O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SM, Ullian EM. Neuronal Pentraxins mediate silent synapse conversion in the developing visual system. J Neurosci. 2010;30:5404–5414. doi: 10.1523/JNEUROSCI.4893-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012;75:951–962. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Lee HW, Choi J, Shin H, Kim K, Yang J, Na M, Choi SY, Kang GB, Eom SH, Kim H, et al. Preso, a novel PSD-95-interacting FERM and PDZ domain protein that regulates dendritic spine morphogenesis. J Neurosci. 2008;28:14546–14556. doi: 10.1523/JNEUROSCI.3112-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Tiraboschi E, Greco D, Restani L, Cerri C, Auvinen P, Maffei L, Castren E. Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. J Physiol. 2012;590:4777–4787. doi: 10.1113/jphysiol.2012.234237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploski JE, Monsey MS, Nguyen T, DiLeone RJ, Schafe GE. The Neuronal PAS Domain Protein 4 (Npas4) is required for new and reactivated fear memories. PLoS ONE. 2011;6:e23760. doi: 10.1371/journal.pone.0023760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, Otto T, Lin Y. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334:1669–1675. doi: 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Devel Neurobio. 2010;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwestrak EL, Ghosh A. Elfn1 regulates target-specific release probability at CA1-interneuron synapses. Science. 2012;338:536–540. doi: 10.1126/science.1222482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and Mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.