Abstract
The circadian clock regulates many aspects of life, including sleep, locomotor activity, and body temperature (BTR) rhythms1,2. We recently identified a novel Drosophila circadian output, called the temperature preference rhythm (TPR), in which the preferred temperature in flies rises during the day and falls during the night 3. Surprisingly, the TPR and locomotor activity are controlled through distinct circadian neurons3. Drosophila locomotor activity is a well known circadian behavioral output and has provided strong contributions to the discovery of many conserved mammalian circadian clock genes and mechanisms4. Therefore, understanding TPR will lead to the identification of hitherto unknown molecular and cellular circadian mechanisms. Here, we describe how to perform and analyze the TPR assay. This technique not only allows for dissecting the molecular and neural mechanisms of TPR, but also provides new insights into the fundamental mechanisms of the brain functions that integrate different environmental signals and regulate animal behaviors. Furthermore, our recently published data suggest that the fly TPR shares features with the mammalian BTR3. Drosophila are ectotherms, in which the body temperature is typically behaviorally regulated. Therefore, TPR is a strategy used to generate a rhythmic body temperature in these flies5-8. We believe that further exploration of Drosophila TPR will facilitate the characterization of the mechanisms underlying body temperature control in animals.
Keywords: Basic Protocol, Issue 83, Drosophila, circadian clock, temperature, temperature preference rhythm, locomotor activity, body temperature rhythms
Introduction
Temperature is a ubiquitous environmental cue. Animals exhibit a variety of behaviors in order to avoid harmful temperatures and seek comfortable ones. Drosophila exhibit a robust temperature preference behavior6,7. When flies are released into a temperature gradient from 18-32 °C, the flies avoid both warm and cold temperatures and finally choose a preferred temperature of 25 °C in the morning3. The warm temperature sensors are a set of thermosensory neurons, AC neurons, which express Drosophila transient receptor potential (TPR) channel, TRPA16,9. The cold temperature sensors are located in the 3rd antennal segments, since ablating the 3rd antennal segments causes the lack of cold temperature avoidance6. Recently, the TRPP protein Brivido (Brv) was identified10. Since Brv is expressed in the 3rd antennal segments and mediates cold detection, Brv is a possible cold sensing molecule, which is critical for the temperature preference behavior. In sum, the flies use these two temperature sensors to avoid the warm and cold temperatures and find a preferred temperature.
While mammals generate heat to regulate their body temperature, ectotherms generally adapt their body temperatures to the ambient temperature11. Some ectotherms are known to exhibit a daily TPR behavior which is believed to be a strategy for the ectotherms to regulate their BTR12. To determine whether the flies exhibited TPR, we repeated the temperature preference behavioral analysis at various points during a span of 24 hr. We found that Drosophila exhibit a daily TPR, which is low in the morning and high in the evening and follows a pattern similar to that of BTR in humans13.
In Drosophila, there are ~150 clock neurons in the brain. The clock neurons that regulate locomotor activity are called M and E oscillators. However, interestingly, M and E oscillators do not regulate TPR, instead, we showed that DN2 clock neurons in the brain regulate TPR but not locomotor activity. These data indicate that TPR is regulated independently from locomotor activity. Notably, mammalian BTR is also independently regulated from locomotor activity. Ablation studies in rats show that BTR is controlled through specific SCN neurons that target a different subset of subparaventricular zone neurons than those that control locomotor activity14. Therefore, our data considers the possibility that the mammalian BTR and the fly TPR are evolutionally conserved3, since both fly TPR and mammalian BTR exhibit circadian clock-dependent temperature rhythms, which are independently regulated from locomotor activity.
Here, we describe the details of how to analyze the TPR behavioral assay in Drosophila. This method allows for the investigation of not only the molecular mechanism and neural circuits of TPR, but also how the brain integrates different environmental cues and inner biological clocks.
Protocol
1. Preparation of Flies
- Light Dark (LD) Experiments
- Raise flies in incubators (25 °C/40-60%relative humidity (RH)) under light 12 hr/dark 12 hr (LD) cycles. The light intensity of the incubators is ~500-1,000 lux.
- Two incubators are necessary to complete the behavior assays over a 24 hr period. Both incubators should have a programmable light with ON OFF functions. They should also have solid doors that are not permeable to light (i.e. no glass or plexiglass). Note: One incubator should be designated a “day” incubator and set to a LD cycle of 12 hr light and 12 hr dark. The second incubator should be designated a “night” incubator and set to the inverse of the first with 12 hr dark followed by 12 hr light. The night incubator must be placed in the room that is accessible in the dark, in such a way flies experiencing night conditions can be easily accessed for the experiments.
- Place the fly vials in either day or night incubators. Collect newly hatched flies in a fresh vial, 20-30 per assay and keep in the same incubator for 2-3 days.
- After 2-3 days, use flies for the temperature preference behavioral assay.
- For day (Zeitgeber time (ZT) 0-12) experiments, collect flies from the day incubator.
- Right before the behavioral experiments, take the collected fly vials out of the day incubator.
- For night (ZT 13-24) experiments, collect flies from the night incubator.
- Right before the behavioral experiments, take the collected fly vials out of the night incubator, wrap with aluminum foil and place in a box in the dark room under a red lamp. Note: Since the temperature preference behavioral assay is performed under darkness for the night experiments, light exposure to the flies must be prevented until the end of the behavioral experiments. Note: The flies should not be exposed to carbon dioxide on the day the experiments are to take place.
- Constant Darkness (DD) Experiments
- DD Day
- An extra incubator is necessary for DD day experiments, which we refer to as a "transition" incubator for the rest of the manuscript. The transition incubator must be placed in the room that is accessible in the dark, in such a way flies experiencing DD conditions can be easily accessed for the experiments. An example light schedule for a transition incubator would have the light ON from 1 pm-7 pm and the light OFF at 7 pm-1 pm (Figure 1).
- Collect the flies which have been raised in the day incubator. Place the fly vials in the transition incubator between 1 pm-7 pm, when the light is ON. In this way, the flies are properly exposed to light until 7 pm, at which time the light shuts OFF.
- The next day, before 1 pm, under dark conditions, take the fly vials out of the transition incubator, wrap them with aluminum foil and place them in a box. Keep the box in any incubator for one more days.
- DD Night
- Collect the flies which have been raised in the night incubator in the dark under a red lamp. Or additionally collect the flies when the night incubator's light is ON.
- Wrap collected vials with aluminum foil in the dark anytime the lights are OFF and place the vials in a box. Keep the box in any incubator for two more days (Figure 1B). Note: Since the temperature preference behavioral assay is performed under darkness for the night experiments, light exposure to the flies must be prevented until the end of the behavioral experiments.
- Constant Light (LL) Experiments
- LL Day
- An extra incubator is necessary for LL day experiments. This incubator maintains the LL condition (25 °C, 800 lux), with the light ON continuously.
- Collect flies which have been raised in the day incubator. Place the fly vials in the LL incubator anytime during their "day".
- LL Night
- Collect flies which have been raised in the night incubator. Transfer the fly vials from the night incubator to the LL incubator during the time the light in the night incubator is ON. Note: For example, the light turns OFF at 7 am in the night incubator. Transfer the fly vials from the night incubator to the LL incubator before 7 am and keep the vials in the LL incubator for 4 more days. Note: At day 4 in LL conditions, the oscillation of the locomotor activity is abolished15,16, while the TPR is still sustained 3.
2. The Apparatus for the Temperature Preference Behavioral Assay
Place a plexiglass cover (29 cm x 19.2 cm) (Figure 4) on an aluminum plate.
Monitor the air temperature between the plate and the cover. Six temperature probes are attached at various positions on the inside of the cover within one of the lanes (Figure 2). Note: Make sure the probes do not touch either the aluminum plate or the plexiglass cover. The air temperature should be set to a gradient from 18-32 °C.
Place the apparatus in an environmental room maintained at 25 °C/65-75% RH. This room needs to be sealed off from any outside light. An environmental room is normally equipped with fans to maintain a certain temperature and humidity. Note: The air from the fan likely disturbs a stable temperature gradient on the apparatus. To prevent this, we use a transparent sheet that covers the area surrounding the apparatus.
Prepare a thermometer and hygrometer to check the temperature and humidity in the environmental room. Note: Light influences the temperature preference of Drosophila3. The same intensity of light should be supplied on the apparatus uniformly. The intensity of our environmental room lights is ~800 lux. Note: When the behavioral experiments are done, place a tube connected from the CO2 tank or supply near the hole of the top of the apparatus in order to anesthetize and get rid of the flies.
3. Preparation of Apparatus for Use
Turn on the apparatus for at least 30 min in order to establish the temperature gradient properly on the surface of the plate (Figure 3).
Coat the cover of the behavior apparatus with water repellent to prevent flies from climbing the walls or ceiling of the cover. Wipe the excess water repellent off and leave the cover for 25-30 min to dry.
Remove any condensation on the aluminum plate. Place the plexiglass cover on the aluminum plate and secure with six C-clamps (Figure 2). Note: It is very important that the cover is sealed well, if necessary double stick tape may be used.
Leave the cover for at least 15 min. The air temperature gradient between the aluminum plate and the cover is created from 18-32 °C.
4. Temperature Preference Behavior Assay
Load the flies into the space between the aluminum plate and the plexiglass cover of the apparatus through small holes in the center of each lane of the cover (Figures 2 and 4). Cover the holes with cover slips to prevent the flies from escaping.
For the dark conditions, turn OFF all the lights in the environmental room. A red lamp can be used when the flies are placed into the apparatus. Make sure the flies are not exposed to any lights except a red lamp until the behavioral experiments are done.
For each trial, use 20-25 adult flies, which are not to be reused in subsequent trials. The behavioral assay is performed for 30 min. Take a few pictures with or without a flash. Be careful not to make much noise or any sudden movements during the experiments.
Record the temperature of all six probes on the apparatus. Take note of the room temperature as well as the humidity.
Anesthetize the flies in the apparatus with carbon dioxide gas, loosen the clamps, remove the plexiglass cover, and remove the flies from the plate. After each experiment, the flies are discarded. Wipe any condensation or moisture off the plate. Replace the cover on the plate and tighten with the clamps in preparation for the next experiment.
In order to have a representation of the temperature preference throughout the entire day, the 24 hr period is split into eight time zones, four during the day and four at night. For example, we use these ZT or CT 1-3, 4-6, 7-9, 10-12, 13-15, 16-18, 19-21 and 22-24. Note: Since phenotype variations caused by masking effects are expected right after the light is turned ON (ZT0) or OFF (ZT12), we do not examine the temperature preference behavior during these times (ZT or CT 0-1, 11.5-13 and 23.5-24). At least five trials must be done in each time zone in order for the results to be statistically sound.
5. Data Analysis
Calculate the temperature gradient as follows: Determine where the temperature probes are placed based on the two rulers that are placed on the top and bottom side of the plexiglass cover along the edges (Figure 2A).
The temperature gradient between the temperature probes is estimated to be linear. Based on the locations of the temperature probes, as well as their corresponding recorded temperatures, draw lines representing each degree of temperature in the appropriate position on the pictures. Count the number of flies located in each degree interval. Exclude any flies on the walls or the ceiling of the cover.
Calculate the percentage of the flies in each temperature range of each lane. A mean preferred temperature is calculated by summing the products of each interval's percentage of flies and temperature, as shown below: % of flies x 18.5 °C + % of flies x 19.5 °C + % of flies x 20.5 °C ….+ % of flies x 31.5 °C + % of flies x 32.5 °C.
Calculate the average preferred temperature in each time zone: Temperature preference behavior is performed >5 times during each time zone (ZT 1-3, 4-6, 7-9, 10-12, 13-15, 16-18, 19-21, and 22-24). To calculate the average preferred temperature in each time zone, the mean preferred temperature of each trial are averaged together. The s.e.m. error bars are equal to the error between the trials.
Representative Results
An example of the temperature preference rhythm is shown in Figure 5. If the behavior procedure is successfully done, the flies should exhibit a TPR in which they prefer a low temperature in the morning and higher temperature in the evening. The ~1-1.5 °C increase during the daytime in temperature preference should be observed during the course of the day, regardless of the genetic background, since we showed that w1118, yw and Canton S flies exhibit a similar temperature preference during the daytime3.
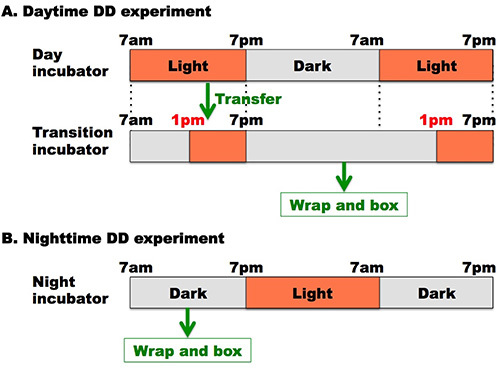 Figure 1. A schematic of the fly preparation in DD day. (A) An example of a DD daytime experiment. The light is ON from 1 pm-7 pm and light is OFF from 7 pm-1 pm in the transition incubator. Collect the flies which have been raised in the day incubator. Place the fly vials in the transition incubator sometime between 1 pm-7 pm. The next day before 1 pm, take the fly vials out of the transition incubator in the dark, wrap them with aluminum foil and place them in a box. (B) An example of a DD nighttime experiment. Collect the flies which have been raised in the night incubator either in the dark during 7am to 7pm or in the light during 7pm to 7am. Take the fly vials out of the night incubator in the dark between 7am and 7pm, and wrap them with aluminum foil and place them in a box
Figure 1. A schematic of the fly preparation in DD day. (A) An example of a DD daytime experiment. The light is ON from 1 pm-7 pm and light is OFF from 7 pm-1 pm in the transition incubator. Collect the flies which have been raised in the day incubator. Place the fly vials in the transition incubator sometime between 1 pm-7 pm. The next day before 1 pm, take the fly vials out of the transition incubator in the dark, wrap them with aluminum foil and place them in a box. (B) An example of a DD nighttime experiment. Collect the flies which have been raised in the night incubator either in the dark during 7am to 7pm or in the light during 7pm to 7am. Take the fly vials out of the night incubator in the dark between 7am and 7pm, and wrap them with aluminum foil and place them in a box
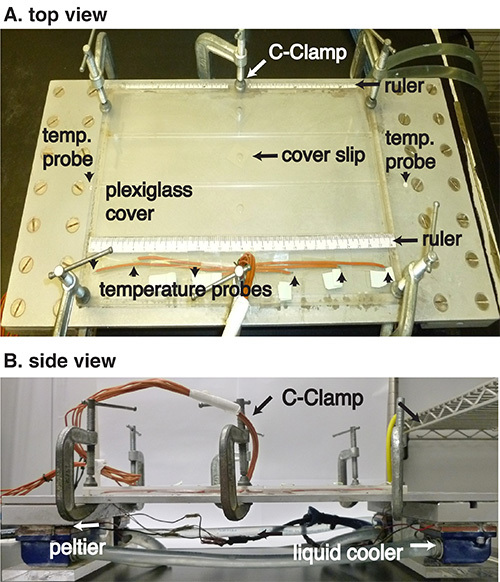 Figure 2. Temperature preference behavioral apparatus. (A) Top view. The plexiglass cover is placed on the aluminum plate with six C-clamps. Six temperature probes are attached at various positions on the inside of the cover within one of the lanes. Two rulers are placed on the top and bottom of the plexiglass cover along the edges to determine the temperature gradient. (B)Side view. Four Peltier devices are placed underneath an aluminum plate (44 cm x 22 cm). Each Peltier device is connected to the temperature controllers that generate cold or hot temperatures. To prevent the Peltiers from overheating, the computer cooling system is connected to water tubes, air-cooling fans, and power supplies. Temperature probes are embedded into the edge of the aluminum plate and are connected to the temperature controllers to directly control temperatures on the aluminum plate. For our current apparatus, the cold and hot sides are set at 12 °C and 36 °C, respectively.
Figure 2. Temperature preference behavioral apparatus. (A) Top view. The plexiglass cover is placed on the aluminum plate with six C-clamps. Six temperature probes are attached at various positions on the inside of the cover within one of the lanes. Two rulers are placed on the top and bottom of the plexiglass cover along the edges to determine the temperature gradient. (B)Side view. Four Peltier devices are placed underneath an aluminum plate (44 cm x 22 cm). Each Peltier device is connected to the temperature controllers that generate cold or hot temperatures. To prevent the Peltiers from overheating, the computer cooling system is connected to water tubes, air-cooling fans, and power supplies. Temperature probes are embedded into the edge of the aluminum plate and are connected to the temperature controllers to directly control temperatures on the aluminum plate. For our current apparatus, the cold and hot sides are set at 12 °C and 36 °C, respectively.
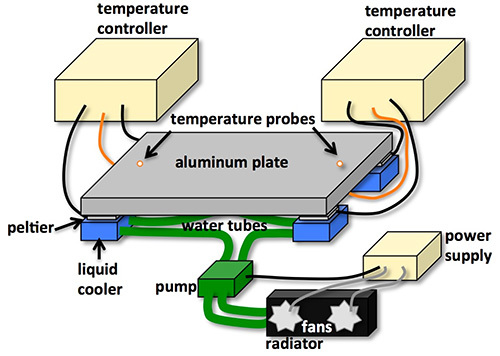 Figure 3. A diagram of the apparatus. The temperature probes are employed as a feedback control reading the temperature on the aluminum plate. The Peltier devices are connected to the temperature controllers. To prevent overheating of the Peltiers, the liquid coolers are directly placed underneath the Peltiers. The four liquid coolers are connected by water tubes which connect to the pump and the radiator. The radiator has two fans which cool down the temperature of water. The pump and radiator are connected to the power supply.
Figure 3. A diagram of the apparatus. The temperature probes are employed as a feedback control reading the temperature on the aluminum plate. The Peltier devices are connected to the temperature controllers. To prevent overheating of the Peltiers, the liquid coolers are directly placed underneath the Peltiers. The four liquid coolers are connected by water tubes which connect to the pump and the radiator. The radiator has two fans which cool down the temperature of water. The pump and radiator are connected to the power supply.
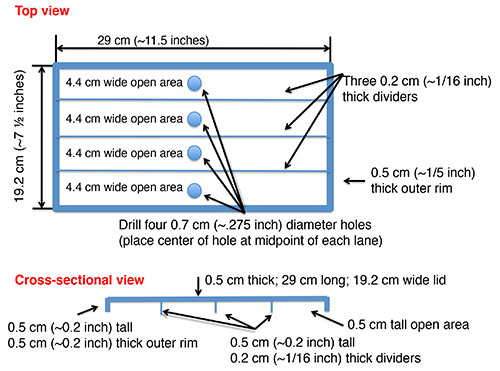 Figure 4. The plan of the plexiglass cover. This is the plan for the cover that is made of plexiglass. The cover has four lanes divided by three 0.2 cm thick dividers, and a 0.7 cm diameter hole is located in the center of the top panel on each lane (Figure 2A).
Figure 4. The plan of the plexiglass cover. This is the plan for the cover that is made of plexiglass. The cover has four lanes divided by three 0.2 cm thick dividers, and a 0.7 cm diameter hole is located in the center of the top panel on each lane (Figure 2A).
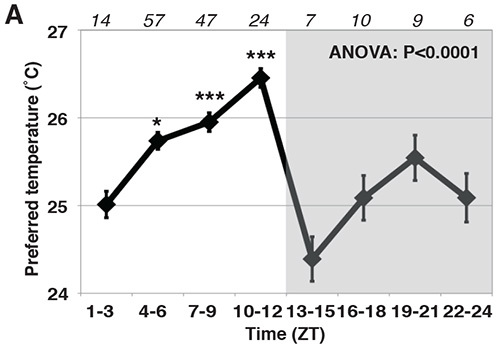 Figure 5. An example of the TPR behavioral data. TPR of w1118 flies over 24 hr. Preferred temperatures were calculated using the distribution of flies in the temperature preference behavior experiments. Data are shown as the mean preferred temperature in each time zone. Numbers represent the number of assays. ANOVA, P < 0.0001. Tukey-Kramer test compared to ZT1-3, ***P < 0.001, **P < 0.01 or *P < 0.05. This figure of the TPR phenotype is adapted from Kaneko et al.3 with permission.
Figure 5. An example of the TPR behavioral data. TPR of w1118 flies over 24 hr. Preferred temperatures were calculated using the distribution of flies in the temperature preference behavior experiments. Data are shown as the mean preferred temperature in each time zone. Numbers represent the number of assays. ANOVA, P < 0.0001. Tukey-Kramer test compared to ZT1-3, ***P < 0.001, **P < 0.01 or *P < 0.05. This figure of the TPR phenotype is adapted from Kaneko et al.3 with permission.
Discussion
Here, we illustrate the details of the temperature preference behavioral apparatus and analysis of the TPR behavior. Drosophila exhibit the salient, robust, and reproducible features of clock-controlled TPR. However, our data suggests that at least two factors, ambient light and age, significantly disturb the TPR behavioral phenotypes.
We observe that light significantly affects temperature preference in Drosophila. It is consistent with the fact that w1118 flies kept in LD prefer higher temperatures during the daytime than those kept in DD, although the rhythmic changes of preferred temperature are still maintained under LD and DD3. Therefore, light affects the fly's temperature preference independent of the circadian clock. Since it is not clear how much light intensity is required and what mechanisms regulate this light dependent temperature preference, we use the same light intensity (~500-1,000 lux) during the experiments to obtain reproducible results.
The flies' ages also affect temperature preference. We avoid using day 1 flies because the TPR phenotypes of the day 1 flies (one day after hatching) are variable. Although day 4 and older flies show constant TPR behavior, they prefer lower temperatures than 2 or 3 day old flies. Therefore, it is very important not to mix wide-ranging aged flies. We use the day 2-3 flies or the day 4-5 flies group as necessary.
In our current TPR behavior method, we only examine temperature preference behaviors for 30 min. The reason for this is because the flies kept >1 hr in the temperature gradient tend to prefer a lower temperature. This maybe due to the lack of the food and water in the apparatus. Therefore, we discard the flies after each 30 min behavioral experiment. It would be a huge advantage if the TPR behavior could be measured continuously for at least 24 hr, ideally ~15 days. In this case, the TPR behavior assay would be easily done without transferring the fly vials to the different incubators. More importantly, TPR phenotypes would be more efficiently compared to other circadian behaviors such as locomotor activity.
Animals are very sensitive to small changes in the environment. We showed that the flies temperature preference behavior is not only regulated by the clock but is strongly influenced by light. TPR might be a behavioral output that is integrated by all of the environmental cues and internal states. Drosophila is a sophisticated model system to dissect fundamental mechanisms of brain functions by using the variety of genetic tools, relatively simple brain structure and versatile behavioral assays. Therefore, studying temperature preference behavior assays could shed light on the fundamental mechanisms of how the brain integrates different information to produce optimal behaviors.
Furthermore, our recently published data suggest that the fly TPR shares features with the mammalian BTR3. Because the mechanisms controlling sleep in flies are analogous to those controlling mammalian sleep17-20, we believe that further exploration of Drosophila TPR will contribute to a greater understanding of circadian rhythm and sleep behavior.
Disclosures
There is nothing to disclose.
Acknowledgments
We are grateful to Drs. Aravinthan Samuel and Marc Gershow who helped develop the initial version of the behavioral apparatus and Matthew Batie who modified the behavioral apparatus. This research was supported by Trustee Grant from Cincinnati Children’s Hospital, JST/PRESTO, March of Dimes and NIH R01 GM107582 to F.N.H.
References
- Krauchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med. Rev. 2007;11:439–451. doi: 10.1016/j.smrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Krauchi K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol. Behav. 2007;90:236–245. doi: 10.1016/j.physbeh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kaneko H, et al. Circadian Rhythm of Temperature Preference and Its Neural Control in Drosophila. Curr. Biol. 2012;22:1851–1857. doi: 10.1016/j.cub.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong ST, et al. cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature. 2008;454:771–775. doi: 10.1038/nature07090. [DOI] [PubMed] [Google Scholar]
- Dillon ME, Wang G, Garrity PA, Huey RB. Review: Thermal preference in Drosophila. J. Therm. Biol. 2009;34:109–119. doi: 10.1016/j.jtherbio.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, et al. Opposite thermosensor in fruitfly and. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144:614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RD. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 1985;126(3) [Google Scholar]
- Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol. Behav. 1992;51:613–637. doi: 10.1016/0031-9384(92)90188-8. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am. J. Physiol. 1998;275:1478–1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Pittendrigh C, Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J. Neurogenet. 1989;6:1–10. doi: 10.3109/01677068909107096. [DOI] [PubMed] [Google Scholar]
- Qiu J, Hardin PE. per mRNA cycling is locked to lights-off under photoperiodic conditions that support circadian feedback loop function. Mol. Cell Biol. 1996;16:4182–4188. doi: 10.1128/mcb.16.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Sehgal A. Genetic analysis of sleep. Genes Dev. 1220;24:1220–1235. doi: 10.1101/gad.1913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Parisky KM, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]


