Abstract
A lengthening in meal duration can be used to measure an increase in orofacial mechanical hyperalgesia having similarities to the guarding behavior of humans with orofacial pain. To measure meal duration unrestrained rats are continuously kept in sound attenuated, computerized feeding modules for days to weeks to record feeding behavior. These sound-attenuated chambers are equipped with chow pellet dispensers. The dispenser has a pellet trough with a photobeam placed at the bottom of the trough and when a rodent removes a pellet from the feeder trough this beam is no longer blocked, signaling the computer to drop another pellet. The computer records the date and time when the pellets were taken from the trough and from this data the experimenter can calculate the meal parameters. When calculating meal parameters a meal was defined based on previous work and was set at 10 min (in other words when the animal does not eat for 10 min that would be the end of the animal's meal) also the minimum meal size was set at 3 pellets. The meal duration, meal number, food intake, meal size and inter-meal interval can then be calculated by the software for any time period that the operator desires. Of the feeding parameters that can be calculated meal duration has been shown to be a continuous noninvasive biological marker of orofacial nociception in male rats and mice and female rats. Meal duration measurements are quantitative, require no training or animal manipulation, require cortical participation, and do not compete with other experimentally induced behaviors. These factors distinguish this assay from other operant or reflex methods for recording orofacial nociception.
Keywords: Behavior, Issue 83, Pain, rat, nociception, myofacial, orofacial, tooth, temporomandibular joint (TMJ)
Introduction
Animal models have been used to study pain and nociception associated with orofacial damage and or inflammation1,2, but a lack of appropriate animal models results in an incomplete understanding of the mechanisms. Although current models help us to understand various mechanisms involved in acute and chronic orofacial pain, there are strengths and weaknesses to these animal models.
Many models measure behavioral nociceptive responses for short durations. Face grooming is a known behavioral response following constriction of facial nerves3. Others studies measured facial rubbing with the ipsilateral fore or hindpaw, as well as, flinching of the head after administering formalin injections into the temporomandibular joint (TMJ) or lip4-7. Head withdrawal latencies is another model for measuring nociceptive behavior where a modified tail flick analgesia meter is used to quantitate the nociceptive response (i.e. head withdrawal) after applying heat to the shaved vibrissae pad of a rat8. Digastric and masseter muscle activity has also been recorded as a correlate of pain after glutamate injections into the TMJ9. Another study has measured changes in sleep parameters to assess nociceptive responses in male and female rats with an inflamed TMJ, these parameters included sleep latency, rapid-eye-movement (REM), percentage of non-REM sleep, and percentage of REM sleep10. Most animal models that measure behavioral nociceptive responses utilize a short time frame, i.e. minutes to hours per day11-14. In addition, most animal models testing occurs during the light phase and in a nocturnal animal, like a rat, this can cause stress which can confound the nociceptive results15-18. The above assays measure nociceptive response in varying orofacial conditions but for short duration and hence can only be used to study acute disorders. An alternative assay has used facial expression as a measure of nociception of moderate duration, but this methodology can be subjective19.
To evaluate persistent or chronic orofacial nociception some have used the application of a von Frey filament on the surface of the skin to assess mechanical sensitivity of animals subjected to nerve constriction or TMJ inflammation3,20. Liverman et al. 2009 measured withdrawal responses using graded monofilaments following CFA injections into the masseter muscle of rats 21,22. Yamazaki et al. 2008 injected the TMJ with CFA and then over 14 days quantified nociceptive behaviors to mechanical or heat or cold stimulation applied over the TMJ region. Unfortunately, these nociceptive behavioral assays involve animal restraint, which produce stress hormones, learning or alternative behaviors that may interfere with the measured outcomes.
Models to measure nociception in teeth utilize the jaw opening reflex but this method can be unreliable23 or imprecise24. Electromyographic activity has been used to measure tooth nociception25, but this method typically requires that the animal be unconscious, although in one study tooth nociception was investigated in freely moving rats26. In 2008, Khan studied the relationship between dental nociception and masticatory function using a sensitive strain gauge27 but this bite duration model requires restraining the animal from normal activity 28. Bite force is a reliable measure of tooth pain in humans but because rats require training and/or restraint to measure bite force a source of stress is introduced which can produce findings with questionable physiological significance29-31
Some limitations of restraint and stress can be overcome by using an operant design to assess nociceptive behaviors. One operant model uses avoidance of an uncomfortable temperature to evaluate and characterize orofacial nociception32-35. This reward-conflict model is based on a reward of sweetened milk to induce the rodent to position its face voluntarily against a heated or cooled thermal probe34,36. However, the test requires animal training, but a strength of the assay is the data is collected in an automated fashion.
Still another animal model used nociception-induced gnawing dysfunction as an index of orofacial nociception37. However, the rodent is confined to a tube and its only escape is to gnaw through a dowel to exit. An advantage of this model is that it measures jaw function after acute or chronic jaw injury in mice. However, the rodent is confined, which adds a confounding alternate competing behavior, i.e. escape, which would be stressful and thus could influence the nociception assay results.
Meal duration has been used to measure nociception in animals with TMJ arthritis38-41, tooth pulp exposure42, and muscle damage43. A rodent who experienced orofacial nociception ate more slowly after the animal initiated a meal. Patients experiencing TMJ pain also take longer to chew their food and the cycle length shortens when TMJ pain is diminished44-46. The lengthening of meal duration when TMJ pain is present is expected to be a "guarding behavior", operationally defined as nociceptive behavior47.
Meal duration measures TMJ nociception using a noninvasive method for up to 19 days in male and female rats and 6 days (longest period tested) in male mice and could be described as a biological marker of nociception38-41. In support that meal duration measures nociceptive responses, nociception can be reduced by pharmacological intervention causing the animal's meal duration to return to normal38,40,41. This was also confirmed when nociceptive neurons were destroyed using capsaicin; after nerve destruction the animals meal duration was not increased following injection of CFA into the TMJ 40.
Below is the protocol on how to obtain and statistically analyze meal duration data.
Protocol
In this model the rats or mice were given food and water ad libitum. The Texas A&M University Baylor College of Dentistry Institutional Animal Care and Use Committee approved all the experimental protocols. Below specific settings are shown in italics and are utilized specifically for the rat TMJ arthritis model. Mice can also be utilized in this model and alternative tooth pain and myogenic orofacial pain animal models can be used as well42,43.
1. Software Settings
Load the Animal Monitor software for the feeder units onto the computer.
The Animals Monitor software is now opened by clicking on the icon and under the file menu selection choose the "configuration" pull down option.
In the "Animal Monitor Configuration" window uncheck the box entitled "Pellet Delivered Input" (Figure 1A). Note: This box is usually checked at the factory by default. Deselect this option. Once the investigator deselects this option the results are being recorded by removal of a pellet from the trough rather than when a pellet is being dispensed. Note: When the "Automatic File Naming" box is checked the software will automatically name the files (Figure 1A). This box is usually checked at the factory by default.
Set the timer lights to turn on at 06:00 (6:00 am) and to turn off at 20:00 (8:00 pm). Note: the hardware for the feeders units was modified so that the lights within the boxes are not controlled by the software but are wired instead to an isolated 24 hr timer. Thus, the "House Light" indicated on the configuration software was not functional in these examples.
Select the edit pull down menu and choose Experiment. A window with the title "Box 01 -" appears (Figure 1B).
Input the file name under which the data will be saved within this window. Note: if no file name is entered the data files will be automatically named by the software. Details of the experiment can be added in this window and saved with the data files. The information on the window can also be saved and used for subsequent experiments.
Enter a number greater than the total time of the experiment in the entry box entitled "Experiment Length (Days):". Note: This will ensure that the software saves the data until the experiment is complete, a mistake can be in setting this value too short, which will stop the software from recording the data even though animals are still in the feeding modules.
Enter 24 in the entry box entitled "Number of Hours in a Day". Note: the length can be modified to the experimenter's specifications.
Enter 10 in the box entitled "Meal Period End Criteria (min):" Note: for rats a meal was defined using a 10 min end of meal criterion based on previous studies48 (i.e. a meal was bracketed before and after by a 10 min period of no pellets being taken) and the minimum meal size was set at three pellets per meal in this software package.
Enter 45 in the box entitled "Pellet Size (mg)". If an individual wanted to use mice input 20 within this window.
For rats add 45 mg rodent chow pellets to the feeder dispenser hopper. Note: for mice add 20 mg rodent chow pellets to the chow hopper.
In the "Phase" section of the Experiment window enter the term Day in the "Name" field and in the "# of Hours" field type 24 (Figure 1B). Note: below the "Phase" section there are two large open fields. The first large open field will be populated by the text entered in the previous "Phase" and "Name" fields.
The next large open field will have the "Day Phase" header Enter in the field entitled "Name" the word Light and enter in the field entitled "Percent" the number 60. Note: this entered text will populate the large field below.
Next enter in the field entitled "Name" the word Dark and enter in the field entitled "Percent" the number 40. Note: with these entries 60% of the day will be attributed to the light phase and 40% will be attributed to the dark phase. When the software calculates the meal patterns this information will be used. These settings typical for cycling female animals that are kept on a 14:10 light/dark cycle.
Select the "Set All Boxes Like This" button. Save this information and then hit OK.
The "Start Boxes" screen appears, select the feeders to activate and hit OK (Figure 1C).
Next the Animal Monitor Run-Time windows will appear with the meal pattern data (Figure 1D). Note: monitor and record the "# of Pellets Dispensed" from this window to determine the current health of the rat. A healthy male rat weighing around 300 g will typically eat between 300-800 45 mg pellets a day.
Files are generated daily and automatically saved that have a .CSV extension. Open these files to retrieve meal pattern data such as food intake, meal number, meal duration, meal size, or intermeal interval. The intervals for these meal patterns can be calculated for the entire day or for a phase of the day such as the Dark and Light phase. As noted above, the settings are for a 14:10 light/dark cycle. The raw data of when each pellet was removed from the trough is also recorded as a raw .CSV file. Note: In older versions of the software a minimum meal size of 3 pellets is not used in the calculations for generating the .CSV file. Moreover, to get the average meal duration using the older software you must subtract 10 min from the values in the Average Meal Duration column of the .CSV file. When the software is operating the operator can manually select the file pull down menu option and daily select "save raw data". This will save the raw data for a 24 hr period rather than for the entire experiment. This raw data can be processed by alternative software at the user's discretion. Note: in the results shown we used alternative software to include a minimum meal size of 3 pellets.
2. Meal Duration Assay
Place individual rats in the sound-attenuated chambers equipped with photobeam computer-activated pellet feeders. Note: in these feeding units there are graduated water bottles and waste pans in which a sheet of thick absorbent paper is placed. In the feeder dispenser hopper 45 mg rodent chow pellets can be added for rats or 20 mg rodent chow pellets can be added for mice. Chow pellets are dispensed into a V shaped feeding trough and at the bottom of this trough is a photobeam. A pellet dispensed into the trough will be detected by breaking this photobeam. Once a rat removes this pellet from the feeder trough the photobeam is restored and this signals the computer to drop another pellet. Restoration of the photobeam also triggers the computer to record the date and time and keeps a running tally of the pellets dispensed. This tally of pellets is then analyzed to determine food intake, meal number, meal duration, meal size, or intermeal interval during any part of the day using Med Assoc. Inc. software. Again the raw .CSV data file can be analyzed by outside software39,40,49-51.
Record the total number of pellets eaten, the amount of water consumed and the weight of the animals to discern the general health of the rats during the experimentation.
Rinse water bottles and fill with fresh water daily and add chow to the feeder hopper when needed.
Dump waste pan and the thick absorbent paper beneath the cage daily and blow the dust from moving part of the feeder daily using high pressure air. Note: personal protection equipment (e.g. gowns, gloves, masks, and masks) is required.
Remove floors, waste pans and water bottles after the experiment is completed and wash these components. Also, remove the feeding electronics from the caging wash by hand or in a dishwasher.
3. Induction of TMJ Arthritis
Place animals in the feeders at least 4 days before experimentation. Note: this data will be reported as predays to obtain a baseline feeding behavior. Then the animals are removed from the feeder for treatment. One type of treatment was to induce an arthritic TMJ. For this model rats are injected with complete Freund's adjuvant (CFA) at 08:00 (i.e. beginning of the light phase) after the rats are anesthetized with isoflurane (5% flow).
Inject 250 mg of CFA in 50 L bilaterally into the periarticular space of each TMJ. Note: in the example (Figure 2) 250 mg of CFA in 50 L was injected into each TMJ, but doses as low as 10 mg in volumes as small as 15 μl are effective over shorter periods of time52.
Inject control rats TMJ with 50 L of 0.9% saline. Note: all animals were mobile within 5 min or less after induction of anesthesia. In the event that a smaller dose of CFA is given in a lesser volume the control rats would receive this same volume of saline.
Representative Results
Meal duration is a behavioral correlate of orofacial pain and meal duration measurements have been applied to animals with TMJ arthritis (Figure 2) and tooth decay (Figure 3). In one experiment, rats had TMJ arthritis after administering a high 250 mg dose of CFA and this treatment induced a significant increase in meal duration for 19 days (Figure 2). A lower dose of CFA (10 mg) injected into each TMJ joint produced a smaller increase in meal duration for only 2-3 days52 indicating a dose response for CFA administration using this meal duration assay. Meal duration measured nociceptive responses in the orofacial region but did not detect a response from arthritis within the knee (Figure 2).
In a second experiment, meal duration has also detected nociceptive responses in rats with pulp exposure (Figure 3). Pulp exposure resulted in tooth decay and was a model for tooth ache in humans42. Other feeding patterns, such as food intake, meal number, and meal size, do not change to as great an extent nor for as long a period as meal duration suggesting these other meal patterns are not as sensitive a measure for the nociceptive response as meal duration39. As meal duration significantly increases there are typically nonsignificant trends in meal number and meal size that result in food intake being near normal such that the bodyweight of the treated animal is equal to that of the sham or control animals.
From the data in Figure 1 and previous mean and standard deviation data38-42 to calculate a significant difference between treatment groups of at least 2 min with an 80% power (using ANOVA) would require about 9 animals/treatment group.
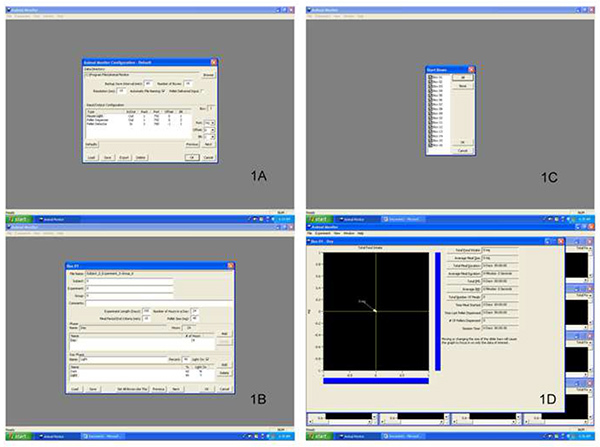 Figure 1. Screen shots of the Animal Monitor Software. Panel 1A is the Animal Monitor Configuration window. Panel 1B is the window that appears when the edit pull down menu is selected and Experiment is chosen from this menu. Panel 1C is the window that allows for the selective activation of particular feeder units. Panel 1D is next window that appears and is entitled Animal Monitor. This window shows real time calculation for meal parameters for the active feeder units. Click here to view larger image.
Figure 1. Screen shots of the Animal Monitor Software. Panel 1A is the Animal Monitor Configuration window. Panel 1B is the window that appears when the edit pull down menu is selected and Experiment is chosen from this menu. Panel 1C is the window that allows for the selective activation of particular feeder units. Panel 1D is next window that appears and is entitled Animal Monitor. This window shows real time calculation for meal parameters for the active feeder units. Click here to view larger image.
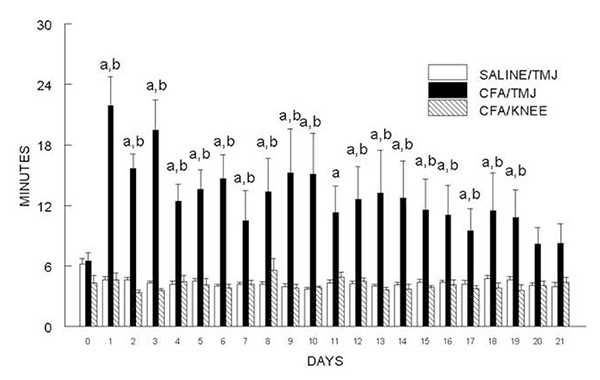 Figure 2. Meal duration was significantly lengthened for 19 days in male rats with arthritic temporomandibular joints (TMJ). For the control group Sprague Dawley rats were given an injection of 50 L saline into each TMJ (SALINE/TMJ, n = 13). In the experimental group 250 g of complete Freund's adjuvant (CFA) was injected into the TMJ (CFA/TMJ, n = 14) or knee (KNEE/CFA, n = 7). Meal duration data was calculated for one day before injection (0) and for days 1-21 (1, 2, 3, etc.) after TMJ or knee injection. Each joint was examined by dissection after the behavioral test to verify the site of injection. Values are given as the means ± SEM. Two-way ANOVA with repeated measures having independent variables treatment (saline and CFA) and time and the dependent variable meal duration was used in these studies. A significant main effect was observed for CFA treatment, F(2, 31)=4.7, p<0.05. Data was further analyzed using Duncan's post hoc test. For a = p<0.05 a comparison was made between the SALINE/TMJ group and the CFA/TMJ group. For b = p<0.05 a comparison was made between the CFA/TMJ group and the CFA/KNEE group.
Figure 2. Meal duration was significantly lengthened for 19 days in male rats with arthritic temporomandibular joints (TMJ). For the control group Sprague Dawley rats were given an injection of 50 L saline into each TMJ (SALINE/TMJ, n = 13). In the experimental group 250 g of complete Freund's adjuvant (CFA) was injected into the TMJ (CFA/TMJ, n = 14) or knee (KNEE/CFA, n = 7). Meal duration data was calculated for one day before injection (0) and for days 1-21 (1, 2, 3, etc.) after TMJ or knee injection. Each joint was examined by dissection after the behavioral test to verify the site of injection. Values are given as the means ± SEM. Two-way ANOVA with repeated measures having independent variables treatment (saline and CFA) and time and the dependent variable meal duration was used in these studies. A significant main effect was observed for CFA treatment, F(2, 31)=4.7, p<0.05. Data was further analyzed using Duncan's post hoc test. For a = p<0.05 a comparison was made between the SALINE/TMJ group and the CFA/TMJ group. For b = p<0.05 a comparison was made between the CFA/TMJ group and the CFA/KNEE group.
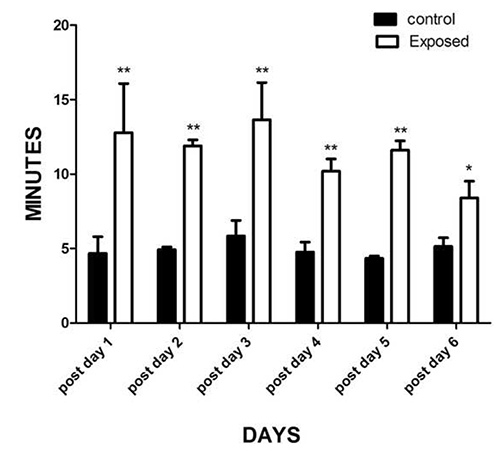 Figure 3. Six maxillary molars of male Spraque Dawley rats were exposed and meal duration was measured for 6 days after surgery. Control rats did not have the pulp exposure surgery but did have anesthesia administered. Two-way ANOVA with repeated measures using the independent variables of treatment (control, exposure) and time and the dependent variable meal duration was used to analyze the meal duration data. A significant main effect was observed for pulp exposure, F(1, 12)=66, P<0.001. Data was further analyzed using Duncan's post hoc test. When comparing the control versus the rat who had their molars exposed * = p<0.05, ** = p<0.01. Means ± SEM. Five rats were in each treatment group.
Figure 3. Six maxillary molars of male Spraque Dawley rats were exposed and meal duration was measured for 6 days after surgery. Control rats did not have the pulp exposure surgery but did have anesthesia administered. Two-way ANOVA with repeated measures using the independent variables of treatment (control, exposure) and time and the dependent variable meal duration was used to analyze the meal duration data. A significant main effect was observed for pulp exposure, F(1, 12)=66, P<0.001. Data was further analyzed using Duncan's post hoc test. When comparing the control versus the rat who had their molars exposed * = p<0.05, ** = p<0.01. Means ± SEM. Five rats were in each treatment group.
Discussion
TMJ patients with orofacial pain report increased pain with increased chewing time, such that, the chewing cycle lengthens the longer the individual has been chewing45,53-56. Our behavioral assay allows for similar testing in rats and mice when measuring meal duration39. A recent unpublished study suggested that von Frey filament testing had greater sensitivity than meal duration measurements, showing a significant change for a longer period but von Frey filament testing can have a reflex response component whereas the meal duration measurements require processing by regions of the central nervous system. Thus, sensitivity might be greater with von Frey filament tests but the response could reflect, in part, a reflex. Although, treatment with drugs that have central effects modify the filament test results suggesting the assay reflects some aspects of central pain processing57.
In the meal duration assay the total number of pellets dispensed should be monitored daily. A male rat typically will ingest 400-800 of the 45 mg pellets and a female rat will ingest 300-600 pellets. In the event that the daily pellet value is less than these typical values the experimenter should check the feeder units, if the pellet dispenser drops 5 pellets upon removal of a pellet from the trough; the pellet sensor near the pellet hopper (not in the trough) may be dusty and require cleaning. Even though the dispenser is dropping 5 pellets the computer will indicate that only one pellet was dropped (giving the low count). Thus, whether five or one pellet is in the trough the computer only records one event. After cleaning the sensor a single pellet should be dispensed upon removal of a pellet from the feeder trough. Alternatively, one can replace the sensor. If the daily pellet value is higher than these typical values the sensors on the feeding trough may be dusty and require cleaning. Clean the sensors and check the next day to determine if the number of pellets dropped fell within the typical range.
Meal duration is a behavioral assay that can be affected by species differences. In a previous study using mice with TMJ arthritis39 there were strains of mice that would hoard the pellets. The mice would take the pellets from the feeder trough and drop the pellets in the corner of the cage and into the waste pan rather than eat the pellet. Because of the hoarding behavior the meal pattern measurements would not reflect the speed at which the mouse was eating and not reflect the level of mechanical hyperalgesia of the mice with TMJ arthritis. One method of troubleshooting this hoarding behavior was to screen the mice in the pretreatment phase. From previous studies about 40-70% of the mice would hoard more than 5% of the total pellets taken. This resulted in significant changes in the meal pattern data. To eliminate the hoarding problem mice were preselected so that they hoarded less than 5% of their pellets. Experiments were performed with the preselected animals and hoarding behavior was monitored throughout the experiment. Animals that hoarded greater than 5% of their total food intake during any point of the experiment were eliminated from the results. Two problems with this preselection process were one, it takes time to prescreen enough mice to get the number of animals to complete the experiment and second, the process requires screening a lot of animals, a majority of which will not be used for experimentation resulting in excess cost.
In conclusion meal duration is a quantitative measure that is not subjective to the experimenter. Like operant methods eating is a behavior that requires cortical participation but eating is unlike many reflex measurements, such as scratching, von Frey hairs, or heat. When measuring meal duration the animal does not have to be trained prior to testing nor immobilized or handled which can compound stresses and alternate behaviors. Meal duration measurements are continuous so testing occurs in the dark as well as light phase, in contrast to testing in the light phase when the rodent normally sleeps. Meal duration measurements can occur for days as opposed to other methods where the test is performed briefly at specific time intervals. These advantages make the meal duration measurement a powerful tool for studying the mechanisms of nociception in the head region.
Disclosures
There is nothing to disclose.
References
- Khan A, Hargreaves KM. Animal models of orofacial pain. Methods Mol. Biol. 2010;617:93–104. doi: 10.1007/978-1-60327-323-7_8. [DOI] [PubMed] [Google Scholar]
- Fried K, Sessle BJ, Devor M. The paradox of pain from tooth pulp: low-threshold #34;algoneurons#34; Pain. 2011;152:2685–2689. doi: 10.1016/j.pain.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J. Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roveroni RC, Parada CA, Cecilia M, Veiga FA, Tambeli CH. Development of a behavioral model of TMJ pain in rats: the TMJ formalin test. Pain. 2001;94:185–191. doi: 10.1016/S0304-3959(01)00357-8. [DOI] [PubMed] [Google Scholar]
- Botelho AP, Gameiro GH, Tuma CE, Marcondes FK, deArruda Veiga MC. The effects of acute restraint stress on nociceptive responses evoked by the injection of formalin into the temporomandibular joint of female rats. Stress. 2010;13:269–275. doi: 10.3109/10253890903362645. [DOI] [PubMed] [Google Scholar]
- Fischer L, Arthuri MT, Torres-Chavez KE, Tambeli CH. Contribution of endogenous opioids to gonadal hormones-induced temporomandibular joint antinociception. Behav. Neurosci. 2009;123:1129–1140. doi: 10.1037/a0017063. [DOI] [PubMed] [Google Scholar]
- Multon S, et al. Lack of estrogen increases pain in the trigeminal formalin model: a behavioural and immunocytochemical study of transgenic ArKO mice. Pain. 2005;114:257–265. doi: 10.1016/j.pain.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Nag S, Mokha SS. Testosterone is essential for alpha(2)-adrenoceptor-induced antinociception in the trigeminal region of the male rat. Neurosci. Lett. 2009;467:48–52. doi: 10.1016/j.neulet.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Sim Y, Bereiter DA, Sessle BJ, Hu JW. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Res. 2002;957:338–344. doi: 10.1016/s0006-8993(02)03671-5. [DOI] [PubMed] [Google Scholar]
- Schutz TC, Andersen ML, Silva A, Tufik S. Distinct gender-related sleep pattern in an acute model of TMJ pain. J. Dent. Res. 2009;88:471–476. doi: 10.1177/0022034509334618. [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, Sigurdsson A, Light AR, Narhi M, Maixner W. Trigeminal c-Fos expression and behavioral responses to pulpal inflammation in ferrets. Pain. 2002;99:61–69. doi: 10.1016/s0304-3959(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Roveroni RC, Parada CA, Cecilia M, Veiga FA, Tambeli CH. Development of a behavioral model of TMJ pain in rats: the TMJ formalin test. Pain. 2001;94:185–191. doi: 10.1016/S0304-3959(01)00357-8. [DOI] [PubMed] [Google Scholar]
- Chidiac JJ, et al. Nociceptive behaviour induced by dental application of irritants to rat incisors: a new model for tooth inflammatory pain. Eur. J. Pain. 2002;6:55–67. doi: 10.1053/eujp.2001.0305. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Byers MR. Behavioural responses following tooth injury in rats. Arch. Oral Biol. 2005;50:333–340. doi: 10.1016/j.archoralbio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Suarez-Roca H, Quintero L, Arcaya JL, Maixner W, Rao SG. Stress-induced muscle and cutaneous hyperalgesia: differential effect of milnacipran. Physiol. Behav. 2006;88:82–87. doi: 10.1016/j.physbeh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quintero L, et al. Repeated swim stress increases pain-induced expression of c-Fos in the rat lumbar cord. Brain Res. 2003;965:259–268. doi: 10.1016/s0006-8993(02)04224-5. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Kordower JH, Wallace MM, Tamir H. Stress and morphine analgesia: alterations following p-chlorophenylalanine. Pharmacol. Biochem. Behav. 1981;14:645–651. doi: 10.1016/0091-3057(81)90126-x. [DOI] [PubMed] [Google Scholar]
- Von KM, Dworkin SF, Le RL, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32:173–183. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- Langford DJ, et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Ren K, Shimada M, Iwata K. Modulation of paratrigeminal nociceptive neurons following temporomandibular joint inflammation in rats. Exp. Neurol. 2008;214:209–218. doi: 10.1016/j.expneurol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009;29:729–741. doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman CS, et al. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009;29:520–531. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P, Strassman A, Maciewicz R. Is the jaw-opening reflex a valid model of pain. Brain Res. 1985;357:137–146. doi: 10.1016/0165-0173(85)90003-7. [DOI] [PubMed] [Google Scholar]
- Rajaona J, Dallel R, Woda A. Is electrical stimulation of the rat incisor an appropriate experimental nociceptive stimulus. Exp. Neurol. 1986;93:291–299. doi: 10.1016/0014-4886(86)90190-1. [DOI] [PubMed] [Google Scholar]
- Sunakawa M, Chiang CY, Sessle BJ, Hu JW. Jaw electromyographic activity induced by the application of algesic chemicals to the rat tooth pulp. Pain. 1999;80:493–501. doi: 10.1016/S0304-3959(98)00241-3. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Pollin B, Azerad J. Microinfusions of excitatory amino acid antagonists into the trigeminal sensory complex antagonize the jaw opening reflex in freely moving rats. Brain Res. 1993;614:155–163. doi: 10.1016/0006-8993(93)91029-r. [DOI] [PubMed] [Google Scholar]
- Khan J, et al. Bite force and pattern measurements for dental pain assessment in the rat. Neurosci. Lett. 2008;447:175–178. doi: 10.1016/j.neulet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong FW, Satoh M, Takagi H. A newly devised reliable method for evaluating analgesic potencies of drugs on trigeminal pain. J. Pharmacol. Methods. 1982;7:271–278. doi: 10.1016/0160-5402(82)90080-8. [DOI] [PubMed] [Google Scholar]
- Khan AA, et al. Measurement of mechanical allodynia and local anesthetic efficacy in patients with irreversible pulpitis and acute periradicular periodontitis. J. Endod. 2007;33:796–799. doi: 10.1016/j.joen.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Khan AA, et al. The development of a diagnostic instrument for the measurement of mechanical allodynia. J. Endod. 2007;33:663–666. doi: 10.1016/j.joen.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Khan J, et al. Bite force and pattern measurements for dental pain assessment in the rat. Neurosci. Lett. 2008;447:175–178. doi: 10.1016/j.neulet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, et al. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Neubert JK, et al. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behav. Brain Res. 2006;170:308–315. doi: 10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Neubert JK, et al. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Mol. Pain. 2008;4 doi: 10.1186/1744-8069-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Vierck CJ, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Mol. Pain. 2006;2(37) doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TA, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behav. Brain. Res. 2010. [DOI] [PMC free article] [PubMed]
- Dolan JC, Lam DK, Achdjian SH, Schmidt BL. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J. Neurosci. Methods. 2010;187:207–215. doi: 10.1016/j.jneumeth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerins C, Carlson D, McIntosh J, Bellinger L. A role for cyclooxygenase II inhibitors in modulating temporomandibular joint inflammation from a meal pattern analysis perspective. J. Oral Maxillofac. Surg. 2004;62:989–995. doi: 10.1016/j.joms.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Kerins CA, Schneiderman E, Bellinger LL. Measuring persistent temporomandibular joint nociception in rats and two mice strains. Physiol. Behav. 2010;99:669–678. doi: 10.1016/j.physbeh.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger LL, et al. Capsaicin sensitive neurons role in the inflamed TMJ acute nociceptive response of female and male rats. Physiol. Behav. 2007;90:782–789. doi: 10.1016/j.physbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kerins CA, Spears R, Bellinger LL, Hutchins B. The prospective use of COX-2 inhibitors for the treatment of temporomandibular joint inflammatory disorders. Int. J. Immunopathol. Pharmacol. 2003;16:1–9. [PubMed] [Google Scholar]
- Kramer PR, He J, Puri J, Bellinger LL. A Non-invasive Model for Measuring Nociception after Tooth Pulp Exposure. J. Dent. Res. 2012;91:883–887. doi: 10.1177/0022034512454297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL. Reduced GABA receptor alpha6 expression in the trigeminal ganglion enhanced myofascial nociceptive response. Neuroscience. 2013;245C:1–11. doi: 10.1016/j.neuroscience.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir R, Bakke M. Joint tenderness, jaw opening, chewing velocity, and bite force in patients with temporomandibular joint pain and matched healthy control subjects. J. Orofac. Pain. 2004;18:108–113. [PubMed] [Google Scholar]
- Bakke M, Hansdottir R. Mandibular function in patients with temporomandibular joint pain: a 3-year follow-up. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;106:227–234. doi: 10.1016/j.tripleo.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Pereira LJ, Steenks MH, de WA, Speksnijder CM, van Der BA. Masticatory function in subacute TMD patients before and after treatment. J. Oral Rehabil. 2009;36:391–402. doi: 10.1111/j.1365-2842.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Wachterman MW. Fillingim RB, editor. Ch. 7 Sex, Gender and Pain. Progress in pain research and management. 2000. pp. 71–88.
- Castonguay TW, Kaiser LL, Stern JS. Meal pattern analysis: artifacts, assumptions and implications. Brain Res. Bull. 1986;17:439–443. doi: 10.1016/0361-9230(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Kerins CA, et al. Specificity of meal pattern analysis as an animal model of dermining temporomandibular joint inflammation/pain. Int. J. Oral Maxiollofac. Surg. 2005;34:425–431. doi: 10.1016/j.ijom.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Guan G, Kerins CC, Bellinger LL, Kramer PR. Estrogenic effect on swelling and monocytic receptor expression in an arthritic temporomandibular joint model. J. Steroid Biochem. Mol. Biol. 2005;97:241–250. doi: 10.1016/j.jsbmb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL. The effects of cycling levels of 17β-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150:3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerins CA, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern changes associated with temporomandibular joint inflammation/pain in rats; analgesic effects. Pharmacol. Biochem. Behav. 2003;75:181–189. doi: 10.1016/s0091-3057(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Gavish A, et al. Experimental chewing in myofascial pain patients. J. Orofac. Pain. 2002;16:22–28. [PubMed] [Google Scholar]
- Karibe H, Goddard G, Gear RW. Sex differences in masticatory muscle pain after chewing. J. Dent. Res. 2003;82:112–116. doi: 10.1177/154405910308200207. [DOI] [PubMed] [Google Scholar]
- Stegenga B, de Bont LG, Boering G. Temporomandibular joint pain assessment. J. Orofac. Pain. 1993;7:23–37. [PubMed] [Google Scholar]
- Dao TT, Lund JP, Lavigne GJ. Pain responses to experimental chewing in myofascial pain patients. J. Dent. Res. 1994;73:1163–1167. doi: 10.1177/00220345940730060601. [DOI] [PubMed] [Google Scholar]
- Guo W, et al. Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: a model of myogenic orofacial. 2010;6 doi: 10.1186/1744-8069-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]


