Abstract
Drosophila melanogaster is a powerful model system that has been widely used to elucidate a variety of biological processes. For example, studies of both the female and male germ lines of Drosophila have contributed greatly to the current understanding of meiosis as well as stem cell biology. Excellent protocols are available in the literature for the isolation and imaging of Drosophila ovaries and testes3-12. Herein, methods for the dissection and preparation of Drosophila testes for microscopic analysis are described with an accompanying video demonstration. A protocol for isolating testes from the abdomen of adult males and preparing slides of live tissue for analysis by phase-contrast microscopy as well as a protocol for fixing and immunostaining testes for analysis by fluorescence microscopy are presented. These techniques can be applied in the characterization of Drosophila mutants that exhibit defects in spermatogenesis as well as in the visualization of subcellular localizations of proteins.
Keywords: Basic Protocol, Issue 83, Drosophila melanogaster, dissection, testes, spermatogenesis, meiosis, germ cells, phase-contrast microscopy, immunofluorescence
Introduction
Drosophila testes are an ideal model system for the study of many biological processes including the regulation of stem cells, meiosis, and sperm development13-18. The spermatocytes and their meiotic spindles are large and hence convenient for cytological analysis, and relaxed cell cycle checkpoints during spermatogenesis facilitate the study of mutations in cell cycle genes. Different cell types can be observed in ordered progression along the length of the testes, and any disruption in spermatogenesis can lead to changes in this overall arrangement. These features combined with Drosophila genetic tools have facilitated the mutational analysis of spermatogenesis21-23.
The stages of Drosophila spermatogenesis have been well defined. Germline cells that develop synchronously within cysts progress sequentially through the stages of spermatogenesis along the length of the testis. During both the mitotic and meiotic divisions of the male germ cells, cytokinesis occurs incompletely such that the daughter cells remain connected by cytoplasmic bridges known as ring canals (Figure 1). The apical tip of the testis contains a population of germline stem cells that gives rise to spermatogonial cells, which undergo four mitotic divisions with incomplete cytokinesis to generate 16-cell cysts of primary spermatocytes. After premeiotic S phase, primary spermatocytes enter G2, a prolonged growth period of ~90 hr during which cellular volume increases ~25-fold. Progression through meiosis I and meiosis II results in the formation of 32-cell cysts of secondary spermatocytes and 64-cell cysts of haploid spermatids, respectively. The immature, round spermatids undergo extensive cellular remodeling to form mature sperm. Post-meiotic cells, in particular the bundles of elongating and mature spermatids, occupy much of the volume of the testis.
The successful transport of functional sperm to female flies requires coordination between the different parts of the male reproductive system, which is composed of several paired structures (the testes, seminal vesicles, and accessory glands) and a single ejaculatory duct (Figure 2). Sperm are produced within the testes and stored within the seminal vesicles until copulation24. The accessory glands contain secretory cells that produce seminal fluid. The sperm migrating from the seminal vesicles are mixed with seminal fluid within the ejaculatory duct, which is connected to both the seminal vesicles and the accessory glands. This mixture of sperm and seminal fluid is ultimately pumped out of the male into the vagina of the female fly through the ejaculatory bulb located at the posterior end of the male abdomen25. Proteins within the seminal fluid are essential for prolonged storage of sperm within specialized organs known as spermathecae in the reproductive tract of Drosophila females26.
Excellent methods for the isolation of Drosophila testes and visualization of cells at various stages of spermatogenesis are available in the scientific literature3-12. We herein add to this body of knowledge by presenting examples of these protocols with an accompanying video demonstration. The protocol for preparation of live testes samples for phase-contrast microscopy is based on a previously described method27. The protocol for formaldehyde fixation and immunostaining of testes is also based on a previously described method28. The approaches described herein have been used in many studies of Drosophila spermatogenesis (for example, to assess the roles of dynein, a minus-end-directed microtubule motor, during Drosophila spermatogenesis).
In addition to the basic protocols, suggestions are provided for varying the dissection so as to enrich for spermatogonia, spermatocytes, or mature sperm. Different methods for processing the testes such that cysts either remain intact or are disrupted as needed are described. An advantage in using Drosophila testes as a model system is that, compared to Drosophila oocytes and embryos, antibodies and dyes can easily penetrate cells following their dispersal from the testes, and fewer washing steps are required; thus, protocols can be performed in a relatively short time.
Protocol
1. Testes Dissection
Anesthetize flies in a bottle or vial using a stream of CO2 and transfer to a fly pad.
Sort flies under a dissecting microscope using a small paintbrush, and collect an appropriate number (depending on the experiment) of Drosophila males of the desired genotypes. Young males (0-2 days old) are ideal for examining cells throughout the earlier stages of spermatogenesis (e.g. spermatogonia, spermatocytes, and early post-meiotic spermatids), whereas slightly older males (2-5 days old) are ideal for examining cells in the final stages of spermatogenesis (in particular, mature sperm).
Use forceps to remove the wings from each fly (to prevent the flies from floating in liquid during dissection).
Add ~500 ml of phosphate-buffered saline (130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4; PBS) in a drop to a silicone-coated dissection dish on a black background. Other aqueous solutions have been successfully used for testes dissection3.
Point forceps towards the anterior of the fly, grasp it by the thorax, and immerse it in the PBS drop. While viewing through the dissecting microscope, use another pair of forceps to grasp and pull the external genitalia (dark brown structure located at the posterior end of the ventral abdomen) posteriorly until it detaches from the abdomen. In most cases, the testes, seminal vesicles, and accessory gland will be removed from the abdomen along with the external genitalia; if not, insert a single pair of forceps into the abdomen and tease out the testes.
Separate testes from accessory gland and external genitalia using two pairs of forceps in the PBS drop (Figure 2). Wild-type testes are easily distinguished from neighboring white tissues by their yellow color. Proceed immediately to step 2.
To isolate testes from pharate males (i.e. enclosed within the pupal case), an additional step must first be performed that involves removing the fly from the pupal case; this step has been previously described elsewhere31. Proceed with dissection as for the adult testes beginning at step 1.2.
To isolate testes from larval males, perform a modification of a protocol for isolating Drosophila larval ovaries32. Briefly, male larvae can be distinguished from female larvae by the presence of a pair of large, clear, oval structures (larval testes) embedded in the posterior third of the fat body. To isolate larval testes, partially flay open the male larva to isolate the testes and the surrounding fat body from the abdomen as described for the isolation of larval ovaries. Proceed immediately to step 2 of the protocol described herein; the testes can later be removed from the fat body just prior to mounting (steps 2.3 or 3.14) as described for the ovaries32.
2. Sample Preparation and Live Imaging
Use a pair of forceps to gently place 2-3 pairs of testes in a drop of 4-5 ml of PBS on a square glass cover slip. Note that the ratio of testes number to PBS volume may need to be adjusted: too much liquid will prevent cells from spreading properly when squashed, whereas too little liquid will cause cells to burst when squashed. Optional: Use siliconized cover slips to minimize adherence of tissue to cover slip in step 3.2.
Use a pair of forceps to tear open each testis at an appropriate position so as to maximize the presence of the desired germline cell types in the preparation (note that the contents of the testis will mostly egress from the torn region onto the slide during the squashing step.) To enrich for spermatogonia and spermatocytes, tear open the testis adjacent to its apical tip (level 1, Figure 2B). To enrich for spermatocytes and spermatids, tear open the testis at a position slightly basal to level 1 (level 2, Figure 2B). To enrich for more mature germline cells, tear open the testis closer to where the curvature begins (level 3, Figure 2B).
Gently place a glass microscope slide over the cover slip to squash the testes; do not apply pressure manually as the weight of the cover slip alone is sufficient to obtain a properly squashed sample. Try to avoid trapping air bubbles. Optional: Use poly-L-lysine coated microscope slides to promote adherence of tissue to slide in step 3.2.
Use preparation immediately (ideally within 15 min of preparation) to observe live cells by phase-contrast microscopy; for transgenic flies with expression of fluorescently tagged proteins in the testes, live cells can be examined by fluorescence microscopy at this step. Alternatively, proceed with fixation and antibody staining (Protocol 3).
Gently wick any excess liquid from under the coverslip using a cleaning wipe to allow flattening of the preparation until the germ cells are clearly in focus.
3. Formaldehyde Fixation and Antibody Staining
Snap freeze each slide containing squashed testes (from Protocol 2) using a pair of metal tongs to immerse it briefly in liquid nitrogen (until liquid nitrogen stops bubbling).
Remove the cover slip immediately using a razor blade.
Use metal tongs to transfer slides to a prechilled glass slide rack filled with ice-cold 95% ethanol (spectrophotometric grade, methanol-free). Store at -20 °C for 10 min.
Use metal tongs to transfer slides to a glass slide rack filled with 4% formaldehyde in PBS plus 0.1% Triton X-100 (PBS-T). Store at room temperature for 7 min.
Use metal tongs to transfer slides to a glass slide rack filled with PBS. Wash slides in PBS for 5 min at room temperature. Repeat 1x. Perform all washes by discarding solution in the glass slide rack (i.e. by pouring it out) and replacing with fresh solution.
Discard the PBS and immerse slides in PBS-T for 30 min at room temperature to permeabilize cell membranes.
Wash slides in PBS for 5 min at room temperature. Repeat 2x.
Blocking step (optional): Immerse slides in PBS plus 1% BSA for 45 min at room temperature.
Use a hydrophobic barrier pen to draw a circle on the slide around squashed tissue (easily visible by eye) in order to confine the antibody solutions (added in steps 3.9 and 3.11). The tissue should be kept moist at all times while performing immunostaining.
Add 30-40 ml of primary antibody (diluted in PBS-T, 1:400 to 1:50, depending on antibody) to tissue within the circle. If blocking was performed, dilute primary antibody in PBS-T plus 1% BSA. Anti-gamma-tubulin antibody (for staining of centrosomes in Figure 4) was diluted 1:100. Incubate in a moist, dark chamber (e.g. closed plastic box with damp paper towels) for 2 hr at room temperature or overnight at 4 °C.
Wash slides in PBS for 5 min at room temperature 3x. If blocking was performed, wash twice in PBS-T and once in PBS (5 min at room temperature each).
Add 30-40 ml of fluorophore-conjugated secondary antibody (diluted 1:400 in PBS) to the tissue and incubate in the dark for 1-2 hr at room temperature.
Wash slides in PBS for 5 min at room temperature. Repeat 2x.
Add 30-40 ml of DAPI solution (0.2 mg/ml in PBS) to the tissue within the circle.
Gently place a glass cover slip over the tissue, taking care to avoid trapping air bubbles. If air bubbles should appear, carefully move around the cover slip without destroying the squash until the bubbles escape from the sides of the cover slip.
Use a cleaning wipe to blot excess DAPI from the edges of the slide.
Seal the cover slip to the slide using clear nail polish.
Use this preparation within the next 3-4 hr to view immunostained cells by fluorescent microscopy. For longer-term storage of slides (up to at least several weeks), use a glycerol-based hard mount media with DAPI, and store slides at -20 ˚C.
Alternatively, fix samples in methanol instead of formaldehyde (depending on the antigen). After completing the entire protocol through step 3.2, immerse slides of squashed testes in methanol for 10 min at -20 °C and proceed with step 3.5 onward.
Representative Results
An example of a properly dissected pair of Drosophila male reproductive organs is shown in Figure 2A. Testes removed from the abdomen of the adult male fly are typically attached to the ejaculatory duct (brown, Figure 2A') and a pair of accessory glands (green, Figure 2A') via a pair of seminal vesicles (blue, Figure 2A'). To separate the testes from most of the accompanying somatic tissue, the ejaculatory duct and the accessory glands should be detached and discarded such that only the testes pair and the seminal vesicles remain (Figures 2B and 2B'). The seminal vesicles can also be removed, leaving only the testes pair to be processed further.
The age of the adult males selected for testes dissection is an important consideration. In young males (0-2 days after eclosion), the seminal vesicles are small and nearly empty, and the testes are at their maximal diameter (as in Figure 2). Using younger males ensures a relatively large number of dividing germline cells (undergoing either mitosis or meiosis) and early post-meiotic spermatids. When older males (3-5 days after eclosion) are used for dissection (image not shown), the seminal vesicles are more prominent because they are bulging with sperm, and the testes are narrower than in younger males. Older males are preferable if the goal is to visualize mature sperm.
Owing to the ordered progression of developmental events along the length of the Drosophila testes, germline cells at specific stages of spermatogenesis can be enriched based on the position at which dissected testes are torn prior to squashing. For example, tearing open the testis near its apical tip (level 1, Figure 2B') yields an abundance of cells in early stages of spermatogenesis. Figure 3A shows a phase-contrast image of a representative population of spermatogonia (white arrow), early primary spermatocytes (yellow arrow), and late primary spermatocytes (red arrow) obtained in this way. Alternatively, tearing open the testis at a slightly more basal position (level 2, Figure 2B') yields primarily spermatocytes and spermatids. Figure 3B shows a phase-contrast image of a representative cell population of primary spermatocytes (red arrow), round spermatids (green arrow), elongating spermatids (orange arrow), and mature sperm bundles (blue arrow) obtained in this way.
Phase-contrast imaging of testes can be used to characterize defects in Drosophila spermatogenesis resulting from mutations in genes that are critical for this process. The round spermatids have a very stereotypical appearance when viewed through a phase-contrast microscope. Each of these immature spermatids has a single, phase-light, round nucleus and a single, phase-dark, round mitochondrial aggregate of roughly the same size (marked by purple arrowhead and arrow, respectively, in Figure 3C). Variation in the relative numbers or sizes of these organelles can result from aberrant meiotic divisions (see discussion). An example of such a defect is shown in Figure 3D. Round spermatids from adult males with mutation of the asunder (asun) gene contain a single large mitochondrial aggregate and multiple small nuclei as a result of defects in cytokinesis and chromosome segregation 29.
Fluorescence microscopy is another powerful approach for studying Drosophila spermatogenesis. An example of a fluorescent image of fixed cells from a squashed testes sample is shown in Figure 4. Testes were obtained from transgenic flies expressing GFP-beta1-tubulin (product of bTub56D gene fused at its C-terminal end to GFP and under control of the Ubi-p63E ubiquitin gene promoter; gift from H. Oda and Y. Akiyama-Oda, JT Biohistory Research Hall, Osaka, Japan) in order to label the microtubules (green in Figure 4D, grayscale in Figure 4A). The transgenic testes were immunostained using a mouse antibody against gamma-tubulin (secondary antibody: anti-mouse Cy3) to mark centrosomes (red in Figure 4D, grayscale in Figure 4C) and DAPI-stained to mark DNA (blue in Figure 4D, grayscale in Figure 4B). Cells at different stages of spermatogenesis can be readily identified by examining the arrangement of the microtubules, centrosomes, and chromosomes (see legend).
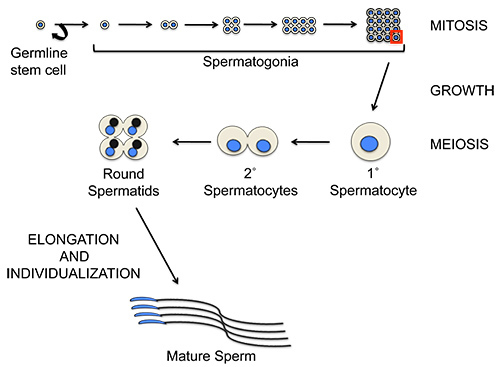 Figure 1.Schematic of germline cell divisions in Drosophila males. Each stem cell division produces a gonial cell that undergoes four rounds of mitotic divisions with incomplete cytokinesis to produce a 16-cell cyst of primary spermatocytes. Each primary spermatocyte undergoes a prolonged growth phase prior to undergoing the two meiotic divisions, again with incomplete cytokinesis, to form a 32-cell cyst of interconnected secondary spermatocytes followed by a 64-cell cyst of interconnected round spermatids. Round spermatids are characterized by the presence of a phase-light nucleus and a phase-dark mitochondrial aggregate (the Nebenkern) of similar sizes. The round spermatids undergo elongation and individualization to form the mature sperm. Mature sperm heads can be identified by the their needle-shaped nuclei. Nuclei are shown in blue; Nebenkerne (mitochondrial aggregates) in black; cytoplasm in tan. Click here to view larger image.
Figure 1.Schematic of germline cell divisions in Drosophila males. Each stem cell division produces a gonial cell that undergoes four rounds of mitotic divisions with incomplete cytokinesis to produce a 16-cell cyst of primary spermatocytes. Each primary spermatocyte undergoes a prolonged growth phase prior to undergoing the two meiotic divisions, again with incomplete cytokinesis, to form a 32-cell cyst of interconnected secondary spermatocytes followed by a 64-cell cyst of interconnected round spermatids. Round spermatids are characterized by the presence of a phase-light nucleus and a phase-dark mitochondrial aggregate (the Nebenkern) of similar sizes. The round spermatids undergo elongation and individualization to form the mature sperm. Mature sperm heads can be identified by the their needle-shaped nuclei. Nuclei are shown in blue; Nebenkerne (mitochondrial aggregates) in black; cytoplasm in tan. Click here to view larger image.
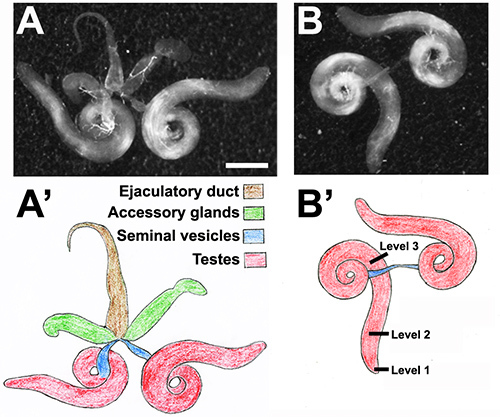 Figure 2.Reproductive organs of Drosophila males. Young adult males (0-2 days after eclosion) were selected for dissection. Light micrographs of isolated reproductive organs are shown in A and B with corresponding cartoons in A' and B'. (A, A') Drosophila testes (red) removed from the abdomen of an adult male fly are connected to the ejaculatory duct (brown) via the seminal vesicles (blue). A pair of accessory glands (green) is also attached to the ejaculatory duct. (B, B') The testes should be separated from the accessory glands before slide preparation. Prior to squashing, tear open the testes at level 1 to enrich for cells at early stages of spermatogenesis; at level 2 to obtain a mixed population of meiotic and post-meiotic germline cells; and at level 3 to enrich for mature sperm. Scale bar, 250 mm. Click here to view larger image.
Figure 2.Reproductive organs of Drosophila males. Young adult males (0-2 days after eclosion) were selected for dissection. Light micrographs of isolated reproductive organs are shown in A and B with corresponding cartoons in A' and B'. (A, A') Drosophila testes (red) removed from the abdomen of an adult male fly are connected to the ejaculatory duct (brown) via the seminal vesicles (blue). A pair of accessory glands (green) is also attached to the ejaculatory duct. (B, B') The testes should be separated from the accessory glands before slide preparation. Prior to squashing, tear open the testes at level 1 to enrich for cells at early stages of spermatogenesis; at level 2 to obtain a mixed population of meiotic and post-meiotic germline cells; and at level 3 to enrich for mature sperm. Scale bar, 250 mm. Click here to view larger image.
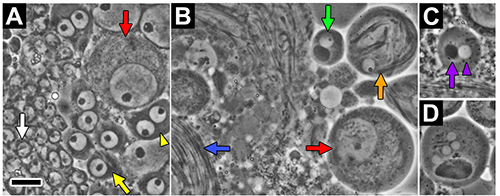 Figure 3.Phase-contrast images of Drosophila testes. (A) An abundance of cells at early stages of spermatogenesis, including spermatogonia (white arrow), early primary spermatocytes (yellow arrow), and late primary spermatocytes (red arrow) are typically released when the testis is torn at level 1 (see Figure 2B'). Two or more interconnected cells (a consequence of incomplete cytokinesis) often fuse completely as an artifact of the squashing procedure (yellow arrowhead marks a fusion of two early spermatocytes). (B) A combination of spermatocytes (red arrow marks late primary spermatocyte) and post-meiotic cells, including round spermatids (green arrow), elongating spermatids (orange arrow marks partial cyst), and mature sperm bundles (blue arrow), are typically released when the testis is torn at level 2 (see Figure 2B'). (C, D) Phase-contrast imaging of squashed testes can be used to readily identify defects in the abundant and stereotypical round spermatids. Wild-type spermatids have one nucleus (phase-light; marked by purple arrowhead) attached to a single mitochondrial aggregate (phase-dark; marked by purple arrow) of roughly equal size (C). Spermatids from asunf02815 testes contain multiple small nuclei and one large mitochondrial aggregate as a result of failed cytokinesis and errors in chromosome segregation (D). Scale bar, 10 mm. Click here to view larger image.
Figure 3.Phase-contrast images of Drosophila testes. (A) An abundance of cells at early stages of spermatogenesis, including spermatogonia (white arrow), early primary spermatocytes (yellow arrow), and late primary spermatocytes (red arrow) are typically released when the testis is torn at level 1 (see Figure 2B'). Two or more interconnected cells (a consequence of incomplete cytokinesis) often fuse completely as an artifact of the squashing procedure (yellow arrowhead marks a fusion of two early spermatocytes). (B) A combination of spermatocytes (red arrow marks late primary spermatocyte) and post-meiotic cells, including round spermatids (green arrow), elongating spermatids (orange arrow marks partial cyst), and mature sperm bundles (blue arrow), are typically released when the testis is torn at level 2 (see Figure 2B'). (C, D) Phase-contrast imaging of squashed testes can be used to readily identify defects in the abundant and stereotypical round spermatids. Wild-type spermatids have one nucleus (phase-light; marked by purple arrowhead) attached to a single mitochondrial aggregate (phase-dark; marked by purple arrow) of roughly equal size (C). Spermatids from asunf02815 testes contain multiple small nuclei and one large mitochondrial aggregate as a result of failed cytokinesis and errors in chromosome segregation (D). Scale bar, 10 mm. Click here to view larger image.
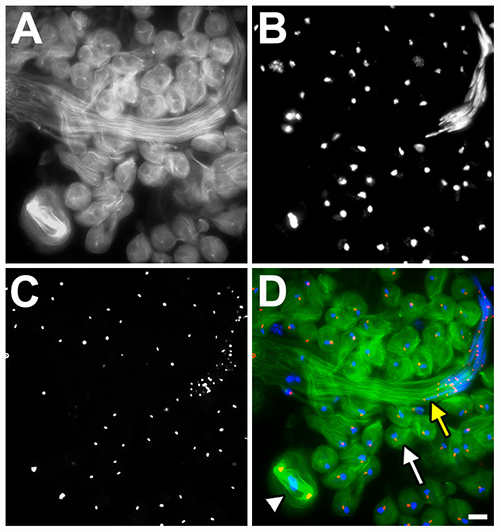 Figure 4.Fluorescent image of Drosophila testes. Preparations of squashed testes isolated from males expressing GFP-tagged beta1-tubulin (green in D, grayscale in A) were fixed and stained for both DNA (DAPI; blue in D, grayscale in B) and centrosomes (gamma-tubulin antibody; red in D, grayscale in C). A dividing spermatocyte (white arrowhead) can be seen next to a large field of round spermatids (white arrow marks a representative cells) and a bundle of mature sperm (yellow arrow). Scale bar, 10 mm. Click here to view larger image.
Figure 4.Fluorescent image of Drosophila testes. Preparations of squashed testes isolated from males expressing GFP-tagged beta1-tubulin (green in D, grayscale in A) were fixed and stained for both DNA (DAPI; blue in D, grayscale in B) and centrosomes (gamma-tubulin antibody; red in D, grayscale in C). A dividing spermatocyte (white arrowhead) can be seen next to a large field of round spermatids (white arrow marks a representative cells) and a bundle of mature sperm (yellow arrow). Scale bar, 10 mm. Click here to view larger image.
Discussion
Although the testes of wild-type flies can be readily identified due to their yellow color (in contrast the neighboring white tissues), the testes of white mutant flies are white and thus can occasionally be confused with the gut. Most transgenic strains, which are typically in a white background, also have white testes because the mini-white gene found in P-elements does not promote pigment accumulation in the testes. When Drosophila testes cannot be distinguished by color, other easily recognizable features include their spiral pattern and occurrence in pairs12. Note that some workers find it easier to use dissection needles instead of forceps to isolate the testes12.
A critical step of this protocol is the preparation of the squashed testes. One useful approach is to hover the slide a few millimeters over the cover slip containing the testes such that the surface tension of the PBS on the cover slip lifts up the cover slip containing the tissue towards the slide. This method tends to disrupt cysts and spread out cells on the slide, making it ideal for imaging individual cells. Alternatively, to maintain entire or partial cysts for imaging, the volume of PBS placed on the cover slip can be increased (from 4-5 ml to 7-8 ml).
If a particular Drosophila mutant of interest does not survive to adulthood, then larval or pupal testes can alternatively be used to study spermatogenesis. In the protocol section, references are provided for the isolation of pharate males and larval ovaries; a modification of the latter protocol can be used to isolate larval testes. The steps for tearing, squashing, and staining larval or pupal testes are essentially identical to that described herein for the adult testes. Even if the fly line to be studied can reach adulthood, isolation of larval or pupal testes may be preferable if the goal is to obtain cells at the earliest stages of spermatogenesis. As described in the representative results section, cells in early or late stages of spermatogenesis can be enriched as desired by varying the level at which the testis is torn prior to squashing.
Phase-contrast microscopy offers a relatively simple approach for studying Drosophila spermatogenesis. One advantage of phase-contrast microscopy over immunostaining and fluorescent microscopy is that less time is required to prepare the samples. All of the major stages of spermatogenesis can be readily identified using this technique24. Indeed, several groups have used phase-contrast microscopic analysis of testes as a primary screen for characterizing the phenotypes of Drosophila male-sterile mutant lines21-23. During meiotic divisions, mitochondria and chromosomes are equally partitioned to daughter cells. A unique feature of spermatogenesis in Drosophila and other insects involves formation of a mitochondrial aggregate (the Nebenkern) in round spermatids24. Round spermatids contain a phase-dark Nebenkern and a phase-light nucleus of roughly equal size in a 1:1 ratio (Figure 3). The diameter of the nucleus reflects the DNA content of the round spermatids, so errors in chromosome segregation during meiosis can lead to variability in nuclear size33. Disruption of meiotic cytokinesis following chromosome segregation results in multinucleated round spermatids in which the Nebenkerne fuse into a single aggregate34. Hence, any variation in the 1:1 ratio or in the size or shape of the Nebenkern and nucleus in round spermatids can be readily detected by phase-contrast microscopy of live testes and is often diagnostic of defects in meiosis. Fusion of two or more interconnected cells within a common cyst frequently occurs as an artifact of the squashing procedure (Figure 3A, yellow arrowhead); the 1:1 ratio of nuclei to Nebenkerne, however, is still maintained.
Immunostaining of fixed preparations of Drosophila testes can be performed to stage cells undergoing spermatogenesis as well as to assess expression patterns and subcellular localizations of proteins of interest. A refined scheme for classifying the stages of Drosophila spermatogenesis based on the cellular morphology, chromatin organization, and microtubule arrangements of testes cells immunostained for tubulin and DNA-stained has been described19. Drosophila primary spermatocytes are relatively large cells, and the meiotic spindles can be clearly observed using this approach. Coimmunostaining for centrosomes (for example, using antibodies against gamma-tubulin) can be performed to obtain a more precise view of the meiotic spindle structure. Formaldehyde is a suitable fixative when immunostaining testes with a wide range of antibodies (for example, anti-lamin and anti-dynein heavy chain). The morphology of microtubules and centrosomes, however, is better preserved with methanol; hence, methanol is typically used as the fixative when performing immunostaining with antibodies against alpha-tubulin and gamma-tubulin.
If antibodies against a protein of interest are unavailable, transgenic Drosophila lines expressing a fluorescently tagged (e.g. mCherry or GFP) version of the protein can be generated as an alternative approach. The subcellular localization of the protein can then be determined by using a microscope to view the GFP fluorescence in live or fixed preparations of tissue; alternatively, anti-GFP antibodies can be used to detect the fusion protein in fixed samples. In Figure 4, microtubules in a fixed testes preparation were visualized via the intrinsic fluorescence of GFP-tagged beta1-tubulin (expressed from a transgene under control of a globally expressed ubiquitin promoter). Alternatively, transgenic flies in which expression is under the control of a tissue-specific promoter; for example, the promoter of the beta2-tubulin gene have been used for testes-specific expression35. Alternatively, the expression of a protein of interest can be restricted to specific male germline cells by using the UAS-Gal4 system36. nanos-Gal4 can be used to induce the expression of a protein under the control of a UAS promoter within spermatogonial cells, while bam-Gal4 can be used to induce its expression within spermatocytes37. Knockdown of a protein of interest can also be induced in the testes by expressing an RNAi hairpin construct under the control of a UAS promoter in conjunction with these Gal4 drivers37.
The development of methods for live-image analysis of Drosophila testes cells has expanded the range of questions that can be addressed using this system. The events of meiotic cytokinesis can be visualized by phase-contrast microscopy of spermatocytes cultured in fibrin clots inside a perfusion chamber in order to prolong the life of the cells35. Time-lapse confocal microscopy of Drosophila spermatocytes expressing fluorescently tagged proteins has also been a powerful approach (for example, to study meiotic spindle assembly)38. The use of confocal microscopy for live imaging of an earlier stage, the dividing germline stem cells of the Drosophila testes, has led to an increased understanding of stem cell regulation. In addition to the phase-contrast and immunofluorescence microscopy approaches presented herein, transmission electron microscopy has also been used extensively to study Drosophila spermatogenesis31.
In addition to imaging, Drosophila testes can be used as a source of material for biochemical analysis. For immunoblotting experiments, dissected fly testes can be homogenized in 6x sample buffer (5 ml/testes pair), boiled, and directly loaded on a SDS-PAGE gel (~4 testes pairs/lane). For example, this approach has been used to assess the levels of dynein-dynactin components in the testes of several Drosophila mutants. Testes extracts can alternatively be prepared by homogenizing the tissue in nondenaturing lysis buffer if it is important to maintain protein integrity. For example, Drosophila testes extracts have been used in coimmunoprecipitation experiments to demonstrate the presence of stable protein complexes in this tissue41-43.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The authors would like to thank Michael Anderson for establishing in the Lee lab these accepted methods for studying spermatogenesis with expert advice from Karen Hales. H. Oda and Y. Akiyama-Oda generously provided the γ-tubulin-GFP fly stock. This work was supported by an NIH R01 grant to L.A.L. (GM074044).
References
- McKim KS, Joyce EF, Jang JK. Cytological analysis of meiosis in fixed Drosophila ovaries. Methods Mol. Biol. 2009;558:197–216. doi: 10.1007/978-1-60761-103-5_12. [DOI] [PubMed] [Google Scholar]
- Weil TT, Parton RM, Davis I. Preparing individual Drosophila egg chambers for live imaging. J. Vis. Exp. 2012. [DOI] [PMC free article] [PubMed]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. In: Drosophila Protocols. Sullivan W, Ashburner M, Hawley RS, editors. Cold Spring Harbor Laboratory Press; 2000. pp. 87–109. [Google Scholar]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. Immunostaining of Drosophila testes. Cold Spring Harb. Protoc. 2011;2011:1273–1275. doi: 10.1101/pdb.prot065771. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. Methanol-acetone fixation of Drosophila testes. Cold Spring Harb. Protoc. 2011;2011:1270–1272. doi: 10.1101/pdb.prot065763. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. Preparation of live testis squashes in Drosophila. Cold Spring Harb. Protoc. 2011;2011 doi: 10.1101/pdb.prot5577. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. Formaldehyde fixation of Drosophila testes. Cold Spring Harb. Protoc. 2012;2012 doi: 10.1101/pdb.prot067355. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. Paraformaldehyde fixation of Drosophila testes. Cold Spring Harb. Protoc. 2012;2012:102–104. doi: 10.1101/pdb.prot067330. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Giansanti MG, Cenci G, Gatti M. F-actin staining of Drosophila testes. Cold Spring Harb. Protoc. 2012;2012:105–106. doi: 10.1101/pdb.prot067348. [DOI] [PubMed] [Google Scholar]
- Kibanov MV, Kotov AA, Olenina LV. Multicolor fluorescence imaging of whole-mount Drosophila testes for studying spermatogenesis. Anal. Biochem. 2013;436:55–64. doi: 10.1016/j.ab.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Singh SR, Hou SX. Immunohistological techniques for studying the Drosophila male germline stem cell. Methods Mol. Biol. 2008;450:45–59. doi: 10.1007/978-1-60327-214-8_3. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Ma S. Isolation of Drosophila melanogaster Testes. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]
- de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138:2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian L, Brill JA. Drosophila spermiogenesis: Big things come from little packages. Spermatogenesis. 2012;2:197–212. doi: 10.4161/spmg.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti MG, Sechi S, Frappaolo A, Belloni G, Piergentili R. Cytokinesis in Drosophila male meiosis. Spermatogenesis. 2012;2:185–196. doi: 10.4161/spmg.21711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis EL, Stine RR, de Cuevas M. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis. 2012;2:137–144. doi: 10.4161/spmg.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee BD, Yan R, Tsai JH. Meiosis in male Drosophila. Spermatogenesis. 2012;2:167–184. doi: 10.4161/spmg.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller R, Schulz C. The Drosophila cyst stem cell lineage: Partners behind the scenes. Spermatogenesis. 2012;2:145–157. doi: 10.4161/spmg.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci G, Bonaccorsi S, Pisano C, Verni F, Gatti M. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 1994;107:3521–3534. doi: 10.1242/jcs.107.12.3521. [DOI] [PubMed] [Google Scholar]
- Rebollo E, Gonzalez C. Visualizing the spindle checkpoint in Drosophila spermatocytes. EMBO Rep. 2000;1:65–70. doi: 10.1093/embo-reports/kvd011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, et al. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135:489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti MG, et al. Genetic dissection of meiotic cytokinesis in Drosophila males. Mol. Biol. Cell. 2004;15:2509–2522. doi: 10.1091/mbc.E03-08-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto BT, Lindsley DL, Herrera C. Toward a comprehensive genetic analysis of male fertility in Drosophila melanogaster. Genetics. 2004;167:207–216. doi: 10.1534/genetics.167.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT. In: The Development of Drosophila melanogaster. Bate M, Martinez-Arias A, editors. Cold Spring Harbor Laboratory Press; 1993. pp. 71–147. [Google Scholar]
- Wolfner MF. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem. Mol. Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- Tram U, Wolfner MF. Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics. 1999;153:837–844. doi: 10.1093/genetics/153.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues KJ, Raff EC, Raff RA, Kaufman TC. Mutation in a testis-specific beta-tubulin in Drosophila: analysis of its effects on meiosis and map location of the gene. Cell. 1980;21:445–451. doi: 10.1016/0092-8674(80)90481-x. [DOI] [PubMed] [Google Scholar]
- Gunsalus KC, et al. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J. Cell Biol. 1995;131:1243–1259. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, et al. Asunder is a critical regulator of dynein-dynactin localization during Drosophila spermatogenesis. Mol. Biol. Cell. 2009;20:2709–2721. doi: 10.1091/mbc.E08-12-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram P, Anderson MA, Jodoin JN, Lee E, Lee LA. Regulation of dynein localization and centrosome positioning by Lis-1 and asunder during Drosophila spermatogenesis. Development. 2012;139:2945–2954. doi: 10.1242/dev.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AR, Machado P, Callaini G, Bettencourt-Dias M. Microscopy methods for the study of centriole biogenesis and function in Drosophila. Methods in cell biology. 2010;97:223–242. doi: 10.1016/S0091-679X(10)97013-1. [DOI] [PubMed] [Google Scholar]
- Maimon I, Gilboa L. Dissection and staining of Drosophila larval ovaries. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]
- Gonzalez C, Casal J, Ripoll P. Relationship between chromosome content and nuclear diameter in early spermatids of Drosophila melanogaster. Genet. Res. 1989;54:205–212. doi: 10.1017/s0016672300028664. [DOI] [PubMed] [Google Scholar]
- Liebrich W. The effects of cytochalasin B and colchicine on the morphogenesis of mitochondria in Drosophila hydei during meiosis and early spermiogenesis. An in vitro study. Cell Tissue. Res. 1982;224:161–168. doi: 10.1007/BF00217275. [DOI] [PubMed] [Google Scholar]
- Wong R, et al. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr. Biol. 2005;15:1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- White-Cooper H. Tissue cell type and stage-specific ectopic gene expression and RNAi induction in the Drosophila testis. Spermatogenesis. 2012;2:11–22. doi: 10.4161/spmg.19088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo E, Llamazares S, Reina J, Gonzalez C. Contribution of noncentrosomal microtubules to spindle assembly in Drosophila spermatocytes. PLoS Biol. 2004;2 doi: 10.1371/journal.pbio.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Hunt AJ. Time-lapse live imaging of stem cells in Drosophila testis. Curr. Protoc. Stem Cell. Biol. 2009;2:10–1002. doi: 10.1002/9780470151808.sc02e02s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–3376. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni G, et al. Mutations in Cog7 affect Golgi structure, meiotic cytokinesis and sperm development during Drosophila spermatogenesis. J. Cell Sci. 2012;125:5441–5452. doi: 10.1242/jcs.108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Cho B, Min SH, Lee D, Chung YD. The THO complex is required for nucleolar integrity in Drosophila spermatocytes. Development. 2011;138:3835–3845. doi: 10.1242/dev.056945. [DOI] [PubMed] [Google Scholar]
- Wang Z, Mann RS. Requirement for two nearly identical TGIF-related homeobox genes in Drosophila spermatogenesis. Development. 2003;130:2853–2865. doi: 10.1242/dev.00510. [DOI] [PubMed] [Google Scholar]


