Abstract
Maize is a major cereal crop worldwide. However, susceptibility to biotrophic pathogens is the primary constraint to increasing productivity. U. maydis is a biotrophic fungal pathogen and the causal agent of corn smut on maize. This disease is responsible for significant yield losses of approximately $1.0 billion annually in the U.S.1 Several methods including crop rotation, fungicide application and seed treatments are currently used to control corn smut2. However, host resistance is the only practical method for managing corn smut. Identification of crop plants including maize, wheat, and rice that are resistant to various biotrophic pathogens has significantly decreased yield losses annually3-5. Therefore, the use of a pathogen inoculation method that efficiently and reproducibly delivers the pathogen in between the plant leaves, would facilitate the rapid identification of maize lines that are resistant to U. maydis. As, a first step toward indentifying maize lines that are resistant to U. maydis, a needle injection inoculation method and a resistance reaction screening method was utilized to inoculate maize, teosinte, and maize x teosinte introgression lines with a U. maydis strain and to select resistant plants.
Maize, teosinte and maize x teosinte introgression lines, consisting of about 700 plants, were planted, inoculated with a strain of U. maydis, and screened for resistance. The inoculation and screening methods successfully identified three teosinte lines resistant to U. maydis. Here a detailed needle injection inoculation and resistance reaction screening protocol for maize, teosinte, and maize x teosinte introgression lines is presented. This study demonstrates that needle injection inoculation is an invaluable tool in agriculture that can efficiently deliver U. maydis in between the plant leaves and has provided plant lines that are resistant to U. maydis that can now be combined and tested in breeding programs for improved disease resistance.
Keywords: Environmental Sciences, Issue 83, Bacterial Infections, Signs and Symptoms, Eukaryota, Plant Physiological Phenomena, Ustilago maydis, needle injection inoculation, disease rating scale, plant-pathogen interactions
Introduction
Fungal diseases of plants represent one of the most eminent threats to agriculture. The need to develop crops with improved disease resistance is increasing due to the food needs of a growing world population. Plant pathogens naturally infect crop plants in the field causing diseases that negatively impact crop yield6. It has been shown that identifying and utilizing resistant plants can improve resistance and decrease yield loss. Resistant cultivars have been identified in many plant species including maize, wheat, rice, and sorghum by inoculating the plants with a plant pathogen and selecting for resistant lines7. Therefore, development and use of an efficient inoculation method would allow many plants to be inoculated and screened for resistance. Various inoculation methods have been used including dip inoculation, pipetting the pathogen cell suspension culture into the whirl of the plant, and needle injection inoculation8-11. With each method, the pathogen must reliably be introduced in between the plant leaves where the pathogen enters the plant through the formation of appresoria to ensure pathogen development and plant infection12,13.
The dip inoculation method involves submerging a plant seedling into a pathogen cell suspension culture, while the pipetting method requires placing the pathogen cell suspension culture into the whirl of the plant seedling. However, there are issues with both methods. First, both methods depend on the natural movement of the pathogen from the leaf surface into the plant tissue which is highly variable. Most pathogens naturally enter the plant through stomatal openings or wounds on the plant leaf surface. However, there is significant variability in the pathogens ability to penetrate the plant leaf surface through the stomata and/or wounds on the leaf surface. Therefore, pathogen penetration cannot be controlled with either inoculation method potentially resulting in inconsistent data. Second, when screening a large number of plants, submerging the seedlings into a pathogen cell suspension culture can be time consuming and may limit the number of plants that can be screened. Conversely, the needle injection inoculation protocol described herein delivers the pathogen cell suspension culture in between the plant leaves facilitating the formation of appressoria14. The pathogen then utilizes the newly developed appressoria to enter the plant eliminating the pathogen penetration issue. Additionally, the needle injection inoculation protocol provides a range of phenotypes for maize and teosinte plants that have been inoculated with U. maydis and demonstrate good infection. The phenotypes can be used as a marker to determine the best concentration for the pathogen cell suspension culture resulting in consistent plant phenotypes within and between different experiments.
Following plant inoculation with a pathogen cell suspension culture, plants are typically screened to detect a resistant or susceptible phenotype8-11,15. While disease rating scales have being used extensively to screen and classify plant phenotypes, rating scales differ depending on the pathogen being analyzed. Therefore, a disease rating scale protocol establishment for U. maydis and maize interactions can be utilized for similar fungal pathogens16.
The present series of protocols details needle injection inoculation with a U. maydis cell suspension culture and disease resistance reaction screening of maize, teosinte, and maize x teosinte introgression lines. The present protocols are not limited to needle injection inoculation of U. maydis into maize plants but can be utilized for relatively any fungal pathogen and plant species. Therefore, including the details of both methods in the same protocol will enable researchers to directly utilize the protocols for inoculation and screening or to manipulate the original protocols to better fit the pathogen and plant species of interest.
Protocol
1. Growth of Plant Material
Select plant lines for inoculation and screening. Two maize lines, five teosinte lines, and forty maize x teosinte lines with uncharacterized resistance to U. maydis were used for this work (Table 1).
Plant seeds for experimental (U. maydis injection) and control (water injection) needle injection inoculation experiments. Do this for each plant line.
Plant four seeds (replicates) for each plant line in small flats by pushing the seeds about ½ inch into the soil with finger and covering with soil lightly (Figures 1A and 1B). Do not pack the soil over the seed. Planting the seed deeper or packing the soil over the seed may cause problems with seedling emergence.
Water the seeds into the soil. Ensure that the soil is soaked and the seeds remain under the soil after watering.
After watering, place plants in a growth chamber with day and night environments of 28/20 °C temperature and 14/10 hr of photoperiod, respectively and approximately 500 μmol/m2 sec photosynthetically active radiations at the top of the canopy. Maintain the relative humidity during the day and night at approximately 70% and 90%, respectively.
Keep all plants in the same growth chamber to maintain a growth environment that is congruent across the experiment.
After 10 days, remove the plants from the growth chamber and inoculate the plants with the U. maydis cell suspension culture using a needle injection inoculation method. Note: Maize plants can be inoculated 7 days after planting8-10. However, the teosinte plants are too small after 7 days. Therefore, inoculate both maize and teosinte plants 10 days after planting for consistency within the experiment (see step 2.12).
2. Needle Injection Inoculation
Do all work in a laminar flow hood. Remove U. maydis glycerol stocks from freezer storage. Use a sterile loop and streak glycerol stocks of U. maydis wild-type strains ½ (mating type a1b1) and 2/9 (mating type a2b2, near isogenic to ½) on to potato dextrose agar (PDA) plates. Maintain strains separately.
Place PDA plates streaked with U. maydis in a 30 °C incubator for two days. If using a different biotrophic pathogen use the appropriate strain, media and growth conditions. Monitor the growth of the pathogen over the two day period to ensure that the U. maydis strain is growing well.
Remove the PDA plates from the incubator after two days. The plates should have good pathogen growth and contain single colonies (Figure 2A). It is important to obtain single colonies. If single colonies are not present restreak the plates at a lower concentration.
Do all of the work in a laminar flow hood. Use a sterile toothpick to select a single colony for each strain from the PDA plates. Place the toothpick containing a single colony into a 3 ml potato dextrose broth (PDB). It is advised to have 2-3 cultures.
Place the 3 ml PDB cultures into a 30 °C incubator/shaker for two days at 200 rpm. Monitor the growth of the culture over the two day period to ensure growth of the culture. The culture should appear very cloudy.
Remove the liquid cultures from the incubator/shaker and measure the concentration at OD600 to ensure that the cells were grown to an OD of 1.0 (~1 x 107 cells/ml)17.
Bring the U. maydis cell suspension cultures to a final concentration of 1 x 106 cells/ml, using water in a final 30 ml culture volume. This concentration consistently results in good infection of the plants with the pathogen cell suspension culture.17
Note: Various cell suspension concentrations should be tested when using different pathogen strains to determine the appropriate cell titer needed for inoculation18,19. The given final concentration for the cell suspension culture can be used as a starting point for tittering. The appropriate concentration of the pathogen cell suspension culture should be verified by visualizing the plant phenotypes with good infection (Figures 3A-E).
Mix equal volumes of the two U. maydis strains prior to inoculation. If using one pathogen strain proceed to step 2.9. Prepare fresh U. maydis cell suspension cultures for each inoculation experiment and discard cell suspension cultures after two days.
For the experimental needle injection inoculation, fill a 3 ml syringe with the U. maydis cell suspension culture by drawing the cell suspension culture into the syringe.
For the control needle injection inoculation, fill a 3 ml syringe with water17. Use the same procedure for the experimental needle injection inoculation.
Attach a 0.457 mm x 1.3 cm hypodermic needle to the end of each 3 ml syringe. The selected needle size will deliver the cell suspension culture in between the plant leaves with minimal damage to the plant tissue.
Remove the experimental and control plants from the growth chamber 10 days after planting in preparation for needle injection inoculations (Figure 2B) (see step 1.7).
Carefully insert the hypodermic needle containing the U. maydis cell suspension culture into the stem of an experimental plant at a 90° angle just above the soil line. Insert the needle until it is in the middle of the stem. Do not push the needle through the stem (Figure 2C).
Inject the experimental plant with about 100 μl of the U. maydis cell suspension culture18,19. This will vary slightly depending on the height of the seedling. The cell suspension culture will push through the stem and move into the whirl of the plant. The cell suspension culture will be visible in the whirl of the plant. Continue injecting 100 μl of the cell suspension culture into each individual plant until the 3 ml syringe is empty.
After the injection, carefully remove the needle from the plant stem. Remove the needle from the now empty 3 ml syringe and fill with water. Attach the needle back to the syringe and push the water through the needle to remove any plant tissue that may be caught in the needle tip.
Repeat steps 2.9-2.15 for each experimental plant. Follow the same protocol for the control plants by injecting water.
Place the inoculated experimental and control plants back into the growth chamber. Water the plants daily by wetting the soil not the plant tissue.
Check the plants daily to detect pathogen development and plant resistance reactions.
3. Resistance Reaction Screening
Score and record the resistance reactions for each plant 7, 10, 14, and 21 days post inoculation (dpi) using a 1 to 5 resistance reaction rating scale. Disease severity increases as the numerical values on the rating scale increases (Table 2). A 1C (Leaf chlorosis), 1A (Leaf anthocyanin production), or 2 (small leaf galls) resistance reaction indicates resistance. A 3 (stem galls), 4 (basal gall), or 5 (plant death) resistance reaction indicates susceptibility (Figures 3A-E and Table 2)18,19.
Score both experimental and control plants and record resistance reaction ratings.
Compare the resistance reactions of the experimental and control plants. Select experimental plants with a 1C, 1A, or 2 resistance reaction rating. These plants are considered to be resistant to U. maydis18,19.
Repeat the entire experiment to verify the plant phenotypes.
Representative Results
A successful needle injection inoculation can be determined by visualizing the phenotype of the plants inoculated with U. maydis (experimental). The majority of the experimental plants were susceptible to U. maydis infection. The susceptible plants showed very severe disease development demonstrated by stem and basal gall formation with black teliospores (Figures 3D and 3E, Table 2). Several plants were dead after inoculation due to the severity of the disease. Three maize x teosinte introgression lines that were resistant to U. maydis were identified. For plants resistant to U. maydis, a successful inoculation was demonstrated by minor chlorosis, anthocyanin production or, minor leaf gall formation. (Figures 3A-C and Table 2).
To verify that the phenotype observed for the experimental plants was the result of the inoculation, the phenotypes of the experimental and control plants (water inoculated) were compared. The experimental plants showed pathogen development on the leaf and/or stem area as described above for the resistant and susceptible plants. Conversely, the control plants did not demonstrate a phenotype. The controls plants were very clean and did not show pathogen development on any part of the plant, indicating that pathogen development on the experimental plants was due to the needle injection inoculation with U. maydis.
To verify the reproducibility and efficiency of the needle injection inoculation method, the experiment was performed twice consisting of 700 plants and compared the resistance reaction scores (phenotypes) for the experimental plants within and between experiments for each plant line. The four replicate plants from the same plant line within one experiment showed the same resistance reaction score for 99.8% of the plants. Additionally, the four replicate plants were compared between experiments and indicated that 99.4% of the plants showed the same resistance reaction score. This suggests that the needle injection inoculation method can efficiently deliver the U. maydis cell suspension culture in between the plant leaves and that the inoculations and phenotypes were consistent within and between experiments.
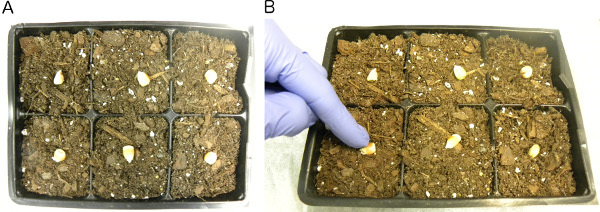 Figure 1. Maize seeds planted for inoculation. A) Six maize seeds placed on top of soil for planting. B) Push seeds ½ inch into the soil with finger.
Figure 1. Maize seeds planted for inoculation. A) Six maize seeds placed on top of soil for planting. B) Push seeds ½ inch into the soil with finger.
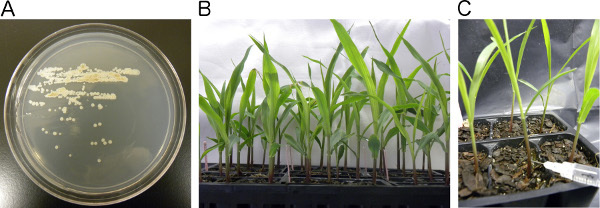 Figure 2. Flow chart of the needle injection inoculation process. A) Growth of U. maydis streaked on PDA plates after two day incubation at 30 °C. B) Flat of uninoculated 10 day old seedlings removed from the growth chamber. C) Needle injection inoculation in the stem of ten day old seedling with 100 μl of the U. maydis cell suspension culture.
Figure 2. Flow chart of the needle injection inoculation process. A) Growth of U. maydis streaked on PDA plates after two day incubation at 30 °C. B) Flat of uninoculated 10 day old seedlings removed from the growth chamber. C) Needle injection inoculation in the stem of ten day old seedling with 100 μl of the U. maydis cell suspension culture.
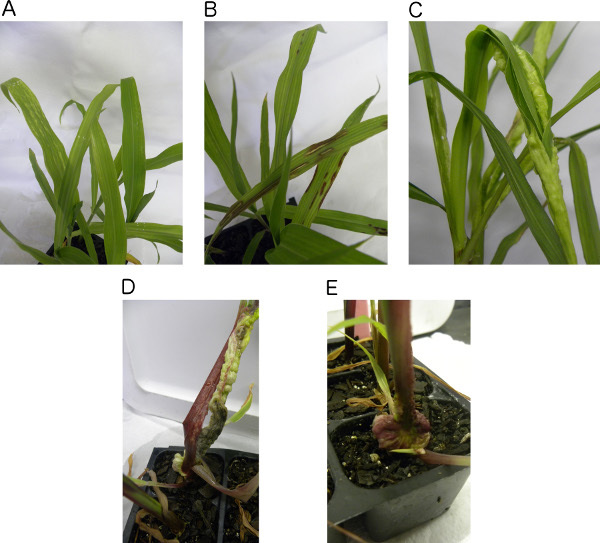 Figure 3. Plant phenotypic responses to U. maydis needle injection inoculation. A) Resistant teosinte plants with minor leaf chlorosis exhibited by white streaks on the leaves. The phenotype corresponds to a 1C resistance reaction rating score. B) Resistant teosinte plants with Anthocyanin production exhibited by the purple leaf color. The phenotype corresponds to a 1A resistance reaction rating score. C) Resistant teosinte plants with minor leaf gall development. The phenotype corresponds to a 2 resistance reaction rating score. D) Susceptible maize plants with severe stem gall development and black teliospores. The phenotype corresponds to a 3 resistance reaction rating score. E) Susceptible maize plants with severe basal gall development. The phenotype corresponds to a 4 resistance reaction rating score. Phenotypes correspond to the resistance reaction rating scale and disease symptoms in Table 2. Click here to view larger figure.
Figure 3. Plant phenotypic responses to U. maydis needle injection inoculation. A) Resistant teosinte plants with minor leaf chlorosis exhibited by white streaks on the leaves. The phenotype corresponds to a 1C resistance reaction rating score. B) Resistant teosinte plants with Anthocyanin production exhibited by the purple leaf color. The phenotype corresponds to a 1A resistance reaction rating score. C) Resistant teosinte plants with minor leaf gall development. The phenotype corresponds to a 2 resistance reaction rating score. D) Susceptible maize plants with severe stem gall development and black teliospores. The phenotype corresponds to a 3 resistance reaction rating score. E) Susceptible maize plants with severe basal gall development. The phenotype corresponds to a 4 resistance reaction rating score. Phenotypes correspond to the resistance reaction rating scale and disease symptoms in Table 2. Click here to view larger figure.
| Plant Lines | Plant Species | Resistance Response |
| 1. Zea mays (NSL 30060) | Maize | Resistant |
| 2. Zea mays subsp. mays (PI511562) | Teosinte | Susceptible |
| 3. Zea mays subsp. Parviglumis | Teosinte | Susceptible |
| 4. Zea mays subsp. Diploperennis | Teosinte | Resistant |
| 5. Zea mays subsp. Luxurians | Teosinte | Resistant |
| 6. B73 (P1) | Maize | Susceptible |
| 7. Parviglumis (P2) | Teosinte | Susceptible |
| 8. Z031E0560 | Maize x Teosinte NIL | Resistant |
| 9. Z031E0560 | Maize x Teosinte NIL | Resistant |
| 10. Z031E0068 | Maize x Teosinte NIL | Resistant |
| 11. 37 maize x teosinte NILs | Maize x Teosinte NILs | Susceptible |
Table 1. Resistance responses of maize and teosinte lines inoculated with U. maydis. P1 indicates parent of the NILs. P2 indicates parent of the NILs. NIL indicates Near-isogenic-lines.
| Host Response | Disease Rating* | Disease Symptoms* |
| Resistant | 1C | Few chlorotic areas, no gall formation. |
| Resistant | 1A | Dark purple anthocyanin production, few galls formed. |
| Resistant | 2 | Minor leaf galls. |
| Susceptible | 3 | Severe stem galls with the formation of black teliospores. |
| Susceptible | 4 | Large basal galls with the formation of black teliospores |
| Susceptible | 5 | Death of plants with severe leaf, stem and basal galls. |
Table 2. Resistance reaction rating system used for U. maydis scoring. *Rating and disease symptoms correspond to the phenotypes in Figure 3.
Discussion
In this study the needle injection inoculation method used to deliver a strain of U. maydis into the stem of 700 maize and teosinte plants was successful. Additionally, a revised disease resistance rating scale was used to screen the plants and detect pathogen development. As a result of using both methods, plant lines that are resistant to U. maydis were identified among 700 maize and teosinte plants that can now be combined and tested in breeding programs for improved disease resistance.
As with most inoculation methods, the ability to reproduce the same resistance phenotype among plants from the same line is essential. Additionally, the same resistance phenotypes must be observed in at least two separate experiments20,21. Because the ability to obtain a plant phenotype, whether it is resistant or susceptible, is primarily determined by the ability of the pathogen to gain access into the plant tissue, it is very important to select an inoculation method that delivers the pathogen in between the plant leaves each inoculation. A few of the common issues researchers have faced with needle injection inoculation methods using biotrophic fungal pathogens such as U. maydis are: 1) Inappropriate concentration of fungal pathogen used for inoculation, 2) lack of reproducible phenotypes in multiple experiments, and 3) lack of an established resistance reaction scoring method. Here each of the issues is addressed separately.
It is important to determine the appropriate concentration of the fungal pathogen cell suspension culture used for inoculation8-11,22. Inoculation with high concentrations of the pathogen cell suspension culture will cause the death of both resistant and susceptible plants, while low concentrations will not show a phenotype on either plant type. However, the appropriate concentration of the fungal pathogen cell suspension culture used for inoculation will vary depending on several factors including the pathogen, pathogen strain, plant species, and plant accession. The present protocols provide phenotypes and a concentration for the U. maydis cell suspension culture to be used as a starting point to test the titer for needle injection inoculation. This results in consistent plant phenotypes within and between different experiments. The cell suspension culture concentration used for U. maydis inoculations can also be used as a starting concentration for inoculations with other biotrophic fungal pathogens. It is advisable to test different dilutions of the pathogen cell suspension culture when using other biotrophic fungal pathogens. This will facilitate the selection of the best concentration for the pathogen cell suspension culture being used for inoculation.
A large number of plants typically must be inoculated and screened from a plant population to potentially identify plants resistant to the pathogen of interest6,23. Therefore, it is important to utilize an inoculation method that reliably delivers the pathogen cell suspension culture in between the plant leaves and that this is done with relative ease and little manipulation of the plants. This will facilitate reproducible phenotypes in multiple experiments. The present protocols give a detailed outline of a needle injection inoculation in the stem of maize and teosinte plants with a U. maydis cell suspension culture. This method can also be used for inoculation of other plant species similar to maize and teosinte. In order to cause disease in the plant, U. maydis must move into the plant tissue7,21,24. During natural infection, U. maydis moves into the plant tissue through stomatal openings or wounds on the plant leaf surface. A dip inoculation and plant whirl pipetting method has also been used to mimic the U. maydis natural infection process but has had limited success due to the variability in the pathogens ability to penetrate the plant tissue8-10,25. However, the needle injection inoculation method delivers the U. maydis cell suspension culture in between the plant leaves eliminating the pathogen penetration issue.
Establishment of a resistance reaction rating scale for U. maydis in essential to identify plants resistant to the pathogen25. The present protocols give a detailed description of the 1 (resistant) to 5 (susceptible) disease rating scale established for U. maydis infection of maize and teosinte plants. It is impotent to first perform a test inoculation and screen a small number of plants prior to initiating a large scale experiment with hundreds of plants. The resistance reaction rating scale established in the present protocol demonstrated consist phenotypes for 700 plants in two different experiments. It is advised to repeat the inoculation and screening protocols at least twice to demonstrate consistency and reproducibility of the results.
The present needle injection inoculation method and the established resistant reaction rating scale will continue to be used to screen and select maize and/or teosinte plants that are resistant to U. maydis infection. As a result, the two methods have many important implications in agriculture that can be used in breeding programs for improved resistance to U. maydis infection decreasing yield losses in the U.S. and internationally.
Disclosures
Authors have nothing to disclose.
Acknowledgments
We thank Dr. Emir Islamovic for laboratory and greenhouse assistance. We also thank Dr. Sherry Flint-Garcia for providing the maize x teosinte introgression lines.
References
- Smith JT. Crop fungal resistance developed using genetic engineering and antifungal proteins from viruses. 2011. ISB News. report http://www.isb.vt.edu/news/2011/nov/cropfungalresistance.pdf.
- Sher AF, MacNab AA. Vegetable diseases and their control. 2. New York, NY: John Wiley & Sons Inc; 1986. pp. 223–226. [Google Scholar]
- Crepet WL, Feldman GD. The earliest remains of grasses in the fossil record. Am. J. Bot. 1991;78:1010–1014. [Google Scholar]
- Iltis HH. Maize evolution and agricultural origins. In: Scoderstrom TR, Hilu KW, Campbell CS, Barkworth ME, editors. Grass systematic and evolution. Washington D.C.: Smithsonian Institution Press; 1997. pp. 195–213. [Google Scholar]
- Mangelsdorf PC, Reeves RG. The origin of corn. III. Modern races, the product of tesonite. Bot. Mus. Leafl. 1957;18:389–411. [Google Scholar]
- Agrios GN. Plant Pathology. New York: Academic Press; 1997. [Google Scholar]
- Dean R, et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada AE, Jonkers W, Kistler HC, May G. Interactions beteen Fusarium verticillioides, Ustilago maydis, and Zea mays: An endophyte, a pathogen, and their shared plant host. Fung. Genet. Biol. 2012;49:578–587. doi: 10.1016/j.fgb.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Freeman S, Rodriguez RJ. A rapid technique for assessing pathogenicity of Fusarium oxysporum f. sp niveum and F. o. melonis on cucrbits. Plant Dis. 1993;77:1198–1201. [Google Scholar]
- Gottwald TR, Graham JH. A device for precise and nondisruptive stomatal inoculation of leaf tissue with bacterial pathogens. Phytopathol. 1992;82:930–935. [Google Scholar]
- Posada F, Aime MC, Peterson SW, Rehner SA, Vega FE. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Asomycota: Hypocreales) Mycolog. Res. 2007;111:748–757. doi: 10.1016/j.mycres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bolker M, Bohnert HU, Braun KH, Gorl J, Kahmann R. Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated intergratior (REMI) Mol. Gen. Genet. 1991;6:274–283. doi: 10.1007/BF02423450. [DOI] [PubMed] [Google Scholar]
- Brachmann A, Weinzierl G, Kamper J, Kahmann R. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 2001;42:1047–1063. doi: 10.1046/j.1365-2958.2001.02699.x. [DOI] [PubMed] [Google Scholar]
- Christensen JJ. The American Phytopathological Society; 1963. Corn smut caused by Ustilago maydis. Monograph number 2. [Google Scholar]
- Skibbe DS, Doehlemann G, Fernandes J, Walbot V. Maize tumors caused by Ustilago maydis require organ-specific genes in host and pathogen. Sci. 2010;328:89–92. doi: 10.1126/science.1185775. [DOI] [PubMed] [Google Scholar]
- Kamper J, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444:97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- Allen A, Kaur J, Gold S, Shah D, Smith TJ. Transgenic maize plants expressing the Totivirus antifungal protein, KP4, are highly resistant to corn smut. Plant Biotechnol. J. 2011;8:857–864. doi: 10.1111/j.1467-7652.2011.00590.x. [DOI] [PubMed] [Google Scholar]
- Gold SE, Brogdon SM, Mayorga ME, Kronstad JW. The Ustilago maydis regulatory subunit of a cAMP-Dependent protein kinase is required for gall formation in maize. 1997. [DOI] [PMC free article] [PubMed]
- Gold SE, Kronstad JW. Disruption of two chitin syn- thase genes in the phytopathogenic fungus Ustilago maydis. Mol. Microbiol. 1994;11:897–902. doi: 10.1111/j.1365-2958.1994.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Brefort T, Doehlemann G, Mendoza-Mendoza A, Reissmann S, Djamei A, Kahmann R. Ustilago maydis as a Pathogen. Annu. Rev. Phytopathol. 2005;47:423–445. doi: 10.1146/annurev-phyto-080508-081923. [DOI] [PubMed] [Google Scholar]
- Doehlemann G, Wahl R, Vranes M, de Vries R, Kämper J, Kahmann R. Establishment of compatibility in the Ustilago maydis/maize pathosystems. J. Plant Physiol. 2008;165:29–40. doi: 10.1016/j.jplph.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Reineke G, Heinze B, Schirawski J, Buettner H, Kahmann R, Base CW. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumor formation. Mol. Plant Pathol. 2008;9:339–355. doi: 10.1111/j.1364-3703.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Espinoza A, García-Pedrajas MD, Gold SE. The Ustilaginales as Plant Pests and Model Systems. Fungal Genet. Biol. 2002;35:1–20. doi: 10.1006/fgbi.2001.1301. [DOI] [PubMed] [Google Scholar]
- Banuett F. Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annu. Rev. Genet. 1995;29:179–208. doi: 10.1146/annurev.ge.29.120195.001143. [DOI] [PubMed] [Google Scholar]
- Keen NT. A century of plant pathology: a retrospective view on understanding host-parasite interactions. Annu. Rev. Phytopathol. 2000;38:31–48. doi: 10.1146/annurev.phyto.38.1.31. [DOI] [PubMed] [Google Scholar]


