Abstract
Lipoxygenase (LOX) activity has been implicated in neurodegenerative disorders such as Alzheimer's disease, but its effects in Parkinson's disease (PD) pathogenesis are less understood. Gene-environment interaction models have utility in unmasking the impact of specific cellular pathways in toxicity that may not be observed using a solely genetic or toxicant disease model alone. To evaluate if distinct LOX isozymes selectively contribute to PD-related neurodegeneration, transgenic (i.e. 5-LOX and 12/15-LOX deficient) mice can be challenged with a toxin that mimics cell injury and death in the disorder. Here we describe the use of a neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which produces a nigrostriatal lesion to elucidate the distinct contributions of LOX isozymes to neurodegeneration related to PD. The use of MPTP in mouse, and nonhuman primate, is well-established to recapitulate the nigrostriatal damage in PD. The extent of MPTP-induced lesioning is measured by HPLC analysis of dopamine and its metabolites and semi-quantitative Western blot analysis of striatum for tyrosine hydroxylase (TH), the rate-limiting enzyme for the synthesis of dopamine. To assess inflammatory markers, which may demonstrate LOX isozyme-selective sensitivity, glial fibrillary acidic protein (GFAP) and Iba-1 immunohistochemistry are performed on brain sections containing substantia nigra, and GFAP Western blot analysis is performed on striatal homogenates. This experimental approach can provide novel insights into gene-environment interactions underlying nigrostriatal degeneration and PD.
Keywords: Medicine, Issue 83, MPTP, dopamine, Iba1, TH, GFAP, lipoxygenase, transgenic, gene-environment interactions, mouse, Parkinson's disease, neurodegeneration, neuroinflammation
Introduction
Use of gene-environment interaction models provides an approach to mimic risk factors that likely influence idiopathic Parkinson’s disease (PD) and affords an opportunity to discern mechanistic insights that are unlikely to be elucidated by use of a genetic or toxicant system alone1,2. Here we illustrate this point and describe application of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of nigrostriatal degeneration3 to better understand the selectivity of lipoxygenase (LOX) isozyme activity on neuroinflammation and toxicity4. While a role for LOX isozymes has been widely evaluated in peripheral disorders5,6 as well as CNS disease including stroke7 and Alzheimer’s disease8,9, the role of the family of isozymes in nigrostriatal function and degeneration related to PD is not well understood and warrants study. The MPTP neurotoxin demonstrates preferential degeneration of the nigrostriatal pathway and recapitulates the striatal dopamine depletion and nigral dopaminergic cell loss that underlie motoric impairments in PD patients10. While this model does not reproduce the full cadre of nonmotor and motor PD behaviors and frank α-synuclein-positive Lewy body pathology, it has been useful to elucidate novel mechanistic targets that contribute to nigrostriatal damage and for early-stage translational testing as it is the best characterized noninvasive model available to reliably produce nigral cell death accompanied by striatal dopamine loss11-15. Wide use of the MPTP mouse, with paradigms ranging from acute, subacute to chronic16-18, has allowed for standardization of dosing to result in mild to severe nigrostriatal damage19,20 with activation of different mechanisms of toxicity depending on the treatment regimen18,21,22. Consequently, this permits a ‘window of lesioning’ to be targeted that may result in enhanced or reduced nigrostriatal injury depending on the therapeutic agent or transgenic model utilized23-25.
Also essential for translational and discovery biology studies are the techniques used to assess damage and the evidence such methods provide. For the MPTP mouse model, established metrics to evaluate lesioning are measurement of markers of striatal dopaminergic tone, including dopamine and its metabolites by HPLC, and Western blot analysis of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis, and indicators of degenerative events such as glial activation using Western blot analysis and immunohistochemistry4. Although these are classical neurochemical, biochemical, and histological procedures, the techniques provide critical and reproducible readouts on the extent of damage within the nigrostriatal dopaminergic pathway, indicate mechanisms of toxicity, and have proven to be valuable tools in understanding degenerative events in PD.
Protocol
Note: All animal procedures and animal care methods should be approved by the institution’s Institutional Animal Care and Usage Committee (IACUC). Study described here was performed in accordance to the guidelines established by SRI International’s IACUC.
1. Acquisition and maintenance of LOX-deficient mice
Purchase 5-LOX-deficient or 12/15-LOX-deficient mice and respective strain and sex-matched controls at age 7-8 weeks and allow several days for acclimation to facility after arrival.
Maintain mice in group housing on a 12-h light-dark cycle and give food and drinking water ad libitum.
2. MPTP precautions, storage, preparation, decontamination and disposal
Note: MPTP intoxication from intravenous exposure in humans has been shown to cause parkinsonism10; MPTP is highly lipophilic and can readily cross the blood-brain barrier26. Precautionary measures should be taken to ensure safe handling, detoxification and disposal. Its metabolism involves multiple steps including conversion to 1-methyl-4-phenyl-2,3-dihydropyridinium by the enzyme monoamine oxidase B (MAO-B)27. MAO-B inhibitors may be utilized in case of accidental human intoxication.
Ensure that personnel have appropriate safety and handling training before utilizing MPTP as dictated by the institution’s Health and Safety Committee. Each institution may establish and implement its own standard operating procedures for use of MPTP, based on recommendations comprehensively outlined in the literature17,22.
Prior to preparation, don appropriate personal protective equipment (PPE) including the following: disposable lab coat or full body suit, double nitrile gloves, surgical mask, hair cover, safety goggles, and disposable shoe covers. Proper PPE should be worn during preparation of MPTP, the injection paradigm and 72 h post-injection when handling animals and/or bedding.
- Perform calculations prior to preparation. Institutional guidelines for dosing volumes should be followed; delivery of 100-150 µl is typically used for 25-30 g mice.
- To prepare a 3 mg/ml MPTP solution in saline, apply a correction factor (1.211 MPTP-HCl: 1.0 MPTP free base); the concentration of MPTP-HCl in saline vehicle is 3.633 mg/ml.
- Prepare all equipment, supplies and reagents needed for MPTP preparation and potential spill decontamination prior to handling the neurotoxin.
- Remove MPTP-HCl source vial from proper storage location. Note: MPTP should be maintained at RT in a closed vial within a secondary container, and stored within a locked cabinet, labeled “MPTP.”
- Cover area surrounding scales with pads or paper towels dampened with 10% bleach solution to reduce the risk from spilled powder. Keep tissues and 10% bleach solution nearby as a precaution.
- Using an analytical balance located in a fume hood, weigh 50 mg of MPTP-HCl into glass vial with secondary containment containing 10% bleach-soaked tissue. Label the glass vial “MPTP” with concentration and date.
- Close source vial and wipe the outside with 10% bleach.
- Slowly add 13.763-ml sterile saline to vial and close completely for mixing. (Solution is 3.633mg/ml MPTP-HCl.)
- In the hood, carefully sterilize MPTP solution by filtration using a 0.22 µm filter into labeled injection vial within a secondary container with 10% bleach-soaked tissue. Clean any spill with bleach-soaked tissue.
- Carefully dispose of sharps and vial in appropriately labeled biohazard container. Maintain MPTP solution in vial in the secondary container with bleach-soaked tissue for transport to animal procedure room. Note: do not autoclave MPTP solution for sterilization as its vaporization is an inhalation hazard.
Return MPTP source vial to its secondary container and place in storage location. Decontaminate spatula, analytical scale and hood surface for 10 min using 10% bleach.
3. MPTP administration
- Prepare disposable cages with standard microisolator perforated lids containing polyester filtered liners marked “MPTP” (minus wire food grill), disposable cage liners, pre-wet food pellets and hydrogel as water source. Weigh all animals and record volume of 3 mg/ml MPTP in saline required for 15 mg/kg injection. Note: MPTP metabolites are detectable in excreta for 3 days following injection28,29; however, mice excrete MPTP n-oxide, a non-toxic derivative that does not cross membranes because of its hydrophilicity30.
- Wearing all PPE listed above and holding mice over a disposable absorption pad, inject saline vehicle or MPTP solution for 15 mg/kg dose into the intraperitoneal cavity (i.p.), daily for 4 days with a disposable 26-gauge tuberculin syringe.
- Do not recap syringe after use; dispose into a dedicated and labeled MPTP biohazardous sharps container. Maintain 10% bleach and tissues nearby to clean any accidental drips.
- Place animals injected with MPTP in disposable cages, up to 5 mice per cage, and house on open racks. Do not use ventilated racks.
- Place MPTP use placards on rack containing mice and housing room door until 72 h after last injection. Note: ensure temperature of housing room is between 22.2 - 24.4oC, as mice experience transient hypothermia up to 12h following MPTP intoxication.31
- Decontaminate work surface with bleach after each use (see step 2.8), dispose of leftover MPTP solution by addition of a volume equivalent of 10% bleach, and discard contents as biohazardous liquid waste.
- Discard all used PPE in dedicated disposal bin. If needed, spray with 10% bleach before disposal.
- Check animals regularly and refresh food and water source daily during injection paradigm and 72 h after the last injection. Note: although no contamination is anticipated in the area immediately surrounding the outside of the cage, this region is treated with the bleach solution as a precaution (as described in Step 2.8).Care should be taken when handling all mice and equipment during this period.
- Three days after the last injection, dispose of all cages and liners in appropriately labeled biohazard bag. Resume use of regular PPE and normal housing for animals; remove door and shelf placards.
4. Tissue harvesting
Euthanize animals by cervical dislocation 7 days following the last MPTP injection. Immediately dissect brain on ice using pre-chilled brain mold with 1-mm slots in the coronal plane.
- Dissect the striatum from a forebrain slice 2-mm thick at the level of the anterior commissure.
- Remove surrounding cortical and subcortical regions using a scalpel. Snap-freeze striatal tissue from each hemisphere in a separate 1.5-ml microcentrifuge tube for neurochemical or biochemical analysis.
Block midbrain/hindbrain in the coronal plane at the level of anterior hypothalamus and immersion-fix tissue in 4% formaldehyde aqueous solution.
5. Tissue processing
- Process for biochemistry
- Homogenize striatal tissue from one hemisphere using an ultrasonic cell disruptor in 200-µL Tris-EDTA lysis buffer (25mM Tris base, 1mM EDTA, 1:100 protease and phosphatase inhibitor cocktails) for 10 pulses at 10-12 Hz and centrifuged for 10 min at 4oC at 1000xg.
- Carefully aspirate the supernatant, and reconstitute the pellet in 150-µl lysis buffer. Fractions are used for biochemical analysis by SDS-PAGE and Western blotting. Note: use protease and phosphatase inhibitor-containing buffer within 1 h of preparation due to instability in aqueous solutions.
- Process for neurochemistry
- Utilize striatum dissected from other hemisphere from each sample stored at -80°C for HPLC. Thaw striatal tissues in microfuge tubes on ice, add 500-μl 0.3N perchloric acid (PCA) and homogenize using sonicator. Note: To make 100 ml of 0.3N PCA, measure 90-ml ddH2O, add to a 100-ml graduated cylinder, add 2.58 ml of 70% PCA, and bring to 100-ml mark. This sample buffer can be stored at 4°C for up to one month.
- Sonicate striatal tissue in 500-µl ice-cold 0.3N PCA, for 10 pulses at 10-12 Hz and place on ice. To ensure homogeneity, sonicate samples a second time for 10 sec, then centrifuge for 12 min at 4oC at 16,100xg.
- Immediately decant supernatant to 1.5-ml microcentrifuge tubes and store at -80°C. Air-dry the pellet fraction in hood.
- Maintain the supernatant at -80°C until use for measurement of dopamine and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) by HPLC with electrochemical detection.
- Once dry, reconstitute the pellet fraction in 0.5N NaOH (500 µL), and briefly sonicate. Store reconstituted pellet fraction at 4°C until use for determination of total protein using the Lowry method.
- Process for immunohistochemistry
- Immersion-fix mid- and hind-brain blocks overnight in 4% formaldehyde aqueous solution and cryoprotect in graded sucrose solutions (10% in phosphate-buffered saline (0.01 M PBS; 0.138 M NaCl, 0.0027 M KCl, and ddH2O, pH 7.4) for 24 h, then 30% in PBS until until the tissue sinks) at 4°C. Briefly blot cryoprotected blocks to remove excess sucrose solution, freeze on dry ice and store at -80°C until use.
- Microtome sectioning of brain blocks containing mid- and hind-brain in a cryostat.
- Remove tissues from -80°C, equilibrate at -16°C for 1 h in cryostat, and mount in tissue embedding media.
- Collect coronal sections at 40-µm thickness into cryoprotectant solution (0.01M PBS with 30% sucrose, 30% ethylene glycol, pH 7.4) at -16°C. Collect tissue serially into 6 1.5-ml microcentrifuge tubes at 240-um intervals and store at -20°C.
6. Immunoblotting
Determine protein concentration in striatal supernatant fraction (prepared as described in Section 5.1) by BCA assay.
Calculate dilution required for each sample to yield 10 µg per well delivered in 20 µl of volume.
- Dilute supernatant protein with 4X Laemmli buffer and homogenization buffer to yield 10 µg protein in 1X Laemmli buffer solution. Example calculation:
- Determine final concentration of protein and volume required. In this case, 10 µg in 20 µl (0.5 mg/ml) is required.
- Determine the final volume of the protein solution needed. Although 20 µl is required for loading, extra volume should be prepared (30 µl).
- Since the final volume and concentration are known, and the concentration of the protein supernatant fraction is known (determined by the BCA assay), calculate the volume of the protein supernatant required using the equation: Vsupernatant = (Vfinal x Cfinal)/C supernatant Thus, for a supernatant protein concentration of 1.5 mg/ml, Vsupernatant = (30 µl x 0.5 mg/ml)/1.5 mg/ml Vsupernatant = 10 µl
- Calculate amount of 4X Laemmli buffer required to yield a 1x concentration in 30 µl of volume (30 µl / 4 = 7.5 µl). Note: 4X Laemmli buffer must be diluted to 1X strength in the sample preparation.
- Calculate amount of homogenization buffer required to bring volume to 30 µl (and obtain desired final protein concentration of 0.5 mg/ml):
- Prepare sample by vortexing then pipetting 10 µl of protein supernatant into 12.5 µl of homogenization buffer. Add 7.5 µl of 4X Laemmli buffer for a 30-µl sample working solution and 0.5 mg/ml concentration.
Prepare all samples using procedure described, vortex and boil for 5 min. Note: Use screw-top microcentrifuge tubes to prevent leakage and opening due to pressure during boiling.
- After 5 min, immediately place on ice. Load 20 µl of sample working solution per gel well (10 µg of protein). Separate protein samples by SDS-PAGE on a 12% Tris-glycine gel.
- Use a pre-stained protein ladder for confirmation of molecular weights. Running buffer is 25 mM Tris-base, 192 mM glycine, 0.1% SDS, and ddH2O, pH 8.3.
- Separate proteins by electrophoresis: run at 80 V to clear the wells (10 min) and 125 V (approximately 1 h; until protein ladder has fully separated) to resolve the proteins.
Perform protein-to-nitrocellulose transfer using 0.2 µm nitrocellulose membrane in Tris-glycine transfer buffer (25 mM Tris, 192 mM glycine, 0.04% SDS, 20% methanol and ddH2O, pH 8.3) overnight for 40 V at 4°C.
Wash nitrocellulose blots in 1x Tris saline (TS; 25mM Tris, 0.9% NaCl in ddH2O, pH 7.4) for 10 min at RT. lncubate in Ponceau S for 5 min then rinse in ddH2O to provide a rough verification of equivalent protein loading. Scan image to retain record for notebook and destain using PBS. Note: Alternatively, wash blot in Milli-Q or ddH2O containing HCl (0.2%) for approximately 5 min. Scan for record. Remove Ponceau S stain from membrane by subsequent incubation step (i.e. place in the blocking buffer).
Block blots in 5% non-fat milk powder in TS for 1 h at RT and incubate 24h at 4ºC with rabbit anti-tyrosine hydroxylase (1:1000), anti-β-actin (1:200); anti-GFAP (1:1000) in 5% milk-TS.
Wash blots 3 x 5 min in TS with 0.05% Tween-20 and 1 x 5 min in TS, then incubate in HRP-conjugated secondary antibody (1:5000 in TS containing 5% milk), matched to the species of the primary, for 2 h at RT.
Prepare chemiluminescent substrate using equal volumes of luminol and peroxide solutions and apply for 1-3 min. In a darkroom, place blot in a plastic sleeve for film exposure and expose to film; film is then developed using an automatic processor.
Strip blots with commercially available stripping buffer at 37°C for 30 min, wash 3x 10 min in TS with 0.05% Tween-20, and 1x 10 min in TS and then reprobe for loading control or other protein of interest.
Quantify the signals by Image J software for optical density measurements and normalize to the internal loading control β–actin.
7. Neurochemistry
- Tissue for analysis is prepared as described above in Section 5.2. Please refer to Table 1 for mobile phase recipe. Note: All reagents must be ≥99.0% pure and of HPLC grade. Ensure buffer integrity with clean, dedicated glassware and stir bars. Mobile phase buffer should be utilized within seven days of preparation.
- Weigh the dihydrogen phosphate and citric acid, add 1 L of ddH2O and mix in a 2-L graduated cylinder. Filter the solution through a 0.22-µm GSTF membrane. Note: This maximizes the extraction of contaminants in the phosphate and citric acid, which helps to minimize background currents.
- Add the OSA followed by the acetonitrile and EDTA solution. Add HPLC grade ddH2O to approximately 1900 ml prior to adjusting the pH to 3.0 with phosphoric acid.
- Add HPLC grade ddH2O to the 2-L mark, and then pour into a 2-L flask. Add a dedicated stir bar, and degas the mobile phase by stirring it under vacuum for > 10 min.
- Prepare the standards for dopamine, and DOPAC and HVA for standard curves. Stock solutions of standards are prepared at 1 mg/ml in 0.3 N PCA.
- Weigh 12.4 mg of dopamine-HCl, and add 10.0 ml of 0.3 N PCA to make 1 mg/ml dopamine.
- Weigh 10.0 mg of DOPAC, and add 10.0 ml of 0.3 N PCA to make 1 mg/ml DOPAC.
- Weigh 10.0 mg HVA, and add 10.0 ml of 0.3 N PCA to make 1 mg/ml HVA.
- Prepare working standard solutions from the stock solutions. Five-point standards are used to generate a concentration curve.
- For measurement of striatal dopamine, prepare standard concentrations of 12.5 ng/ml, 25 ng/ml, 50 ng/ml, 100 ng/ml, and 200 ng/ml.
- For striatal DOPAC, prepare standard concentrations of 2.5 ng/ml, 5 ng/ml, 10 ng/ml, 20 ng/ml, and 40 ng/ml.
- For striatal HVA, prepare standard concentrations of 5 ng/ml, 10 ng/ml, 20 ng/ml, 40 ng/ml, and 80 ng/ml.
- Generate five-point standards: dilute 1 mg/ml dopamine stock solution to 5 µg/ml using 0.3N PCA. Dilute 1 mg/ml DOPAC stock solution to 1 µg/ml using 0.3N PCA, and dilute 1 mg/ml HVA stock solution to 2 µg/ml using 0.3N PCA.
- Transfer 1 ml of 5 µg/ml dopamine, 1 ml of 1 µg/ml DOPAC, and 1 ml of 2 µg/ml HVA into a 25-ml volumetric flask and bring volume to 25 ml mark with 0.3 N PCA. Note: The mixed standards in the volumetric flask contain the highest concentration of the five-point standards: 200 ng/ml of dopamine, 40 ng/ml of DOPAC, and 80 ng/ml of HVA.
- Transfer 1 ml of the mixed standards into clean 1.5-ml microcentrifuge tubes. Perform 1:1 serial dilution four times to generate the subsequent four-point standards.
- For example, to 250 μl of the mixed standards, add 250 μl of sample buffer and vortex thoroughly to produce mixed standards at 50% of original concentrations; repeat process until desired dilutions are achieved.
- Prepare the HPLC system (pump, autosampler, reverse-phase column (C18, 150×3.2 mm, 3 µm)). Purge the pump with fresh mobile phase to replace all previous solution in the HPLC system and trapped air bubbles with fresh mobile phase. Chill the autosampler to 4°C. Warm the column to 35°C, which reduces the total pressure.
- Set the voltage at -150 mV and 220 mV for the first and second electrodes of the electrochemical detector, respectively. Equilibrate the system for at least 2 h, and ideally overnight, with the mobile phase at a flow rate of 0.2 ml/min. Note: During equilibration the cells should be on, and the baseline reading monitored. Stable reading indicates that the system is ready to use. For the two hours of the equilibration phase, allow the mobile phase to go into a waste container instead of recirculating it. After this period, the mobile phase can be recycled overnight for equilibration.
- Once equilibration is complete, adjust the mobile phase flow rate to 0.6 ml/min. Inject 20 µl of each of the five-point standards.
Thaw striatal samples on ice, and inject 2 x 20 µl of each sample into the HPLC system. Utilize standards at the beginning and randomly throughout each set of striatal samples.
Use the software to analyze the area under dopamine, DOPAC, and HVA peaks produced by the standards and samples. Identify analytes by retention time and measure by comparing the area under peak to that of 5-point curve for its corresponding standard.
Prepare pellet fraction as described in Section 5.2.3. Perform a Lowry protein assay on the striatal pellet. Note: This assay will measure the amount of protein present in the striatal samples in mg/ml; this information is used to determine the ng/mg concentration of analyte.
8. Immunohistochemistry
- GFAP/TH dual immunofluorescence
- Remove tubes containing sectioned midbrain from -20°C freezer and bring to RT. Remove tissue sections containing substantia nigra from cryopreservative solution in a tray containing PBS.
- Place tissue sections in polystyrene inserts with polyester mesh bottoms for free-floating immunochemistry. Wash 3 x 5 min in PBS.
- Transfer sections to 96-well plate with 180-µl block solution (5% normal donkey serum, 1% PVP, 1% BSA and 0.3% Triton X-100 in PBS) in each well. Incubate 40 min at RT. Note: All wash and incubation steps are performed with light agitation on shaker.
- Transfer sections to wells containing the primary antibody solution (180 µl per well). Incubate in primary antibody cocktail, rabbit anti-GFAP (1:1000) and sheep anti-TH (1:400) diluted in PBS with 1% BSA and 0.3% TX-100, for 24 h at 4°C. IgG from the primary species serves as the negative immunostaining control.
- Incubate in secondary antibody cocktail (1:200 donkey anti-rabbit 568 and 1:200 FITC donkey anti-sheep in PBS with 0.1% TX-100). Use foil to protect solution from light during preparation and incubation; incubate at RT for 2 h.
- For a negative control, incubate sections in IgG from the primary species diluted to the same concentration as used for the primary antibodies.
- Wash 2 x PBS and 1 x TS for 5 min each at RT. Mount tissue sections on plus slides in mounting medium with Hoechst stain and coverslip.
- Incubate in secondary antibody cocktail (1:200 donkey anti-rabbit 568 and 1:200 FITC donkey anti-sheep in PBS with 0.1% TX-100). Use foil to protect solution from light during preparation and incubation; incubate at RT for 2 h.
- Wash 2 x PBS and 1 x TS for 5 min each at RT. Mount tissue sections on plus slides in mounting medium with Hoechst stain and coverslip.
- Protect slides from light and oxidation until imaging by storing in a slide box at 4°C.
- Analyze the negative control first to determine the background level of fluorescence, then evaluate for positive immunoreactivity using fluorescent microscopy.
- Iba1 immunohistochemistry with chromagen precipitation
- Remove tubes containing sectioned midbrain from -20°C freezer and bring to RT. Remove tissue sections containing substantia nigra from cryopreservative solution in a tray containing PBS.
- Place tissue sections in polystyrene inserts for free-floating immunochemistry. Wash sections 3 x 5 min in PBS, and place sections directly into 12-well plate containing pre-heated 10 mM citric acid monohydrate, pH 9.0, 80°C for 30 min, for epitope retrieval.
- Cool plate 20 min at RT. Return sections to inserts and wash 3 x 5 min in PBS.
- Transfer sections to 96-well plate containing 180-µl blocking solution (10% normal goat serum, 1% BSA in PBS) per well, and incubate 40 min at RT.
- Transfer sections to wells containing 180-µl diluted primary antibody (1:1000 in 1% BSA-PBS). Incubate overnight at 4°C.
- Transfer sections to inserts. Wash 3 x 5 min PBS and apply biotinylated secondary antibody (1:200 in 1.5% normal serum-PBS) for 1 h at RT.
- Prepare ABC solution 30 min before use. Wash 3 x 5 min PBS and transfer to ABC solution for 1 h at RT.
- Prepare DAB solution in hood. Wash 2x 5 min in PBS and 1x 5 min in TS, and incubate sections in DAB.
- Develop for 3-4 min. Negative control should remain light in color. Note: perform this step in fume hood as DAB is a known carcinogen. A solution of 0.2M potassium permanganate in ddH2O for decontamination should be maintained in proximity in case of accidental drips.
- Rinse sections briefly in ddH2O, then in TS 3 x 5 min. Mount sections on plus slides and air-dry overnight in fume hood.
- Decontaminate DAB waste with an equal volume of 0.2M potassium permanganate in ddH2O, vortex, and incubate in a fume hood overnight before storing in appropriately labeled DAB waste bin in fume hood. Note: Bleach solution does not eliminate the mutagenic properties of DAB.
- Decontaminate items to be reused (i.e. polystyrene inserts) and wash with detergent.
- Dehydrate/counterstain lightly with Cresyl violet (CV). All dehydration/wash steps are 3 min: ethanol 70%, 95%, 100%, 95%, ddH2O, CV (30 seconds), ddH20 x 2, 95% ethanol + glacial acetic acid (0.1%) x 2, 100% ethanol, and xylene.
- Coverslip in distyrene-toluene-xylene mounting medium and dry in fume hood.
- Observe positive immunoreactivity by a light microscope. IgG from the primary species serves as the negative immunostaining control.
9. Statistics
Assess differences among means by one-way analysis of variance (ANOVA) to compare differences between genotype and by two-way ANOVA to compare differences between genotype and toxicant treatment. Utilize Tukey’s HSD post hoc analysis when differences are observed by ANOVA testing (p≤0.05). Note: experiments (with n = 6-9 mice/group) are performed a minimum of two times to ensure reproducibility.
Representative Results
This toxin exposure paradigm can produce a significant and detectable 20% striatal dopamine depletion in MPTP- vs. saline-injected animals. It is important to note that different lots of MPTP may yield slightly more or less lesioning; thus, for better precision, a preliminary experiment in wildtype mice is recommended prior to use in transgenics when a new lot of neurotoxin is utilized. The use of mild-to-moderate lesioning allows for impact of the transgene to be observed; a severe lesion may produce a 'floor effect' with injury too robust to attenuate or so damaging that it eclipses the effect of a deleterious genetic alteration. The effects of MPTP on striatal dopamine were significantly different in 5-LOX isozyme-deficient mice, but not the 12/15-LOX isozyme-deficient mice (Figure 1). Furthermore, with these methods, we were able to discern a significant difference in dopamine levels due to the 5-LOX deficiency in saline-treated mice (Figure 1).
Immunoblotting for TH and GFAP allowed for confirmation of damage and neuroinflammation, respectively, at the level of the striatum in wildtype mice, an effect that was diminished in the 5-LOX isozyme-deficient striatum (Figures 3A and 3B). The lesion is also discernible at the level of the substantia nigra (Figure 2A and 2B). In the same mice, the depletion of TH-positive neurons and increased GFAP immunoreactivity is observable using dual-label immunofluorescent labeling (Figure 2A). Furthermore, markedly elevated microglial activation (i.e. Iba1 immunoreactivity) in wildtype, but not 5-LOX isozyme-deficient, mice is apparent in the substantia nigra following MPTP exposure (Figure 2B). Thus, the MPTP model can provide a useful tool to assess the impact of genetic predisposition to nigral degeneration and inflammation.
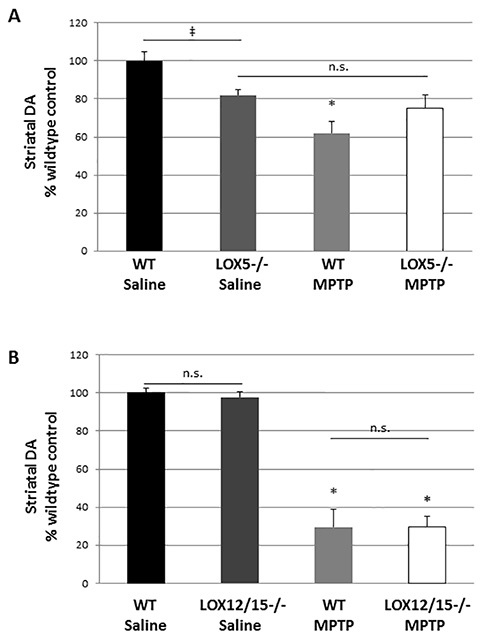 Figure 1.LOX isozyme-selective effects on striatal dopamine after MPTP challenge. (A) Striatal homogenates were used to measure dopamine (DA) by HPLC from WT and 5-LOX-/- littermates given saline or MPTP (n=6-8/group). ‡, marks a significant effect due to genotype; p<0.05. *, marks a significant effect due to genotype and treatment; p<0.05. No significant difference due to treatment was noted in 5-LOX-/- mice (n.s.) indicating that MPTP did not produce striatal DA depletion in this transgenic line. (B) Striatal homogenates were used to measure DA in WT and 12/15-LOX-/- littermates given saline or MPTP (n=6-9/group). No significant difference in DA levels due to genotype was observed. A significant, and similar, reduction due MPTP treatment was noted in both genotypes. *, p<0.01. Data are shown as mean ± SEM.
Figure 1.LOX isozyme-selective effects on striatal dopamine after MPTP challenge. (A) Striatal homogenates were used to measure dopamine (DA) by HPLC from WT and 5-LOX-/- littermates given saline or MPTP (n=6-8/group). ‡, marks a significant effect due to genotype; p<0.05. *, marks a significant effect due to genotype and treatment; p<0.05. No significant difference due to treatment was noted in 5-LOX-/- mice (n.s.) indicating that MPTP did not produce striatal DA depletion in this transgenic line. (B) Striatal homogenates were used to measure DA in WT and 12/15-LOX-/- littermates given saline or MPTP (n=6-9/group). No significant difference in DA levels due to genotype was observed. A significant, and similar, reduction due MPTP treatment was noted in both genotypes. *, p<0.01. Data are shown as mean ± SEM.
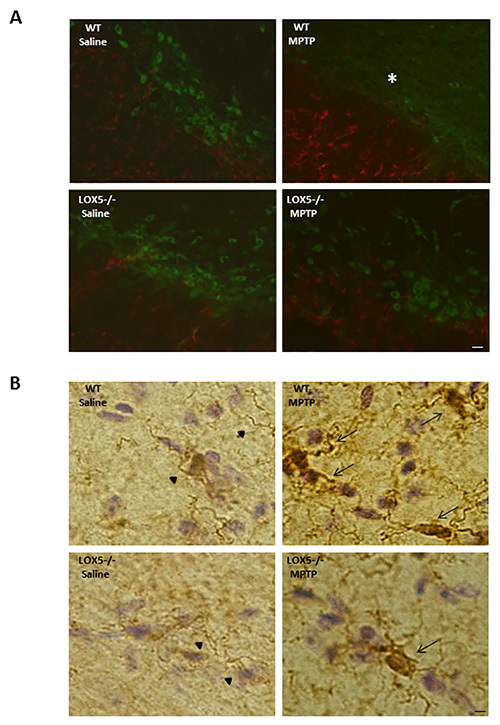 Figure 2.5-LOX isozyme effects on nigral TH and astroglia following MPTP challenge. (A) Immunofluorescence staining for TH (FITC; green) and GFAP (568; red) immunoreactivities was performed in nigral brain sections from WT and 5-LOX-/- littermates given saline or MPTP. Fewer TH-positive cell bodies (*) and enhanced GFAP immunoreactivity are apparent in the WT-MPTP group. Bar = 25 µm. (B) Immunohistochemical histology for the Iba-1 on microglia was detected using DAB (brown chromatgen); sections were counterstained by Cresyl violet. Nigral sections from WT and 5-LOX-/- littermates treated with saline or MPTP were assessed. Microglia with ramified cell bodies and long, branching processes are observed in substantia nigra from saline-treated mice (arrow heads). Activated microglia with rounded cell bodies and short, thickened processes, was observed in substantia nigra from MPTP-treated mice (arrows). Bar = 10 µm.
Figure 2.5-LOX isozyme effects on nigral TH and astroglia following MPTP challenge. (A) Immunofluorescence staining for TH (FITC; green) and GFAP (568; red) immunoreactivities was performed in nigral brain sections from WT and 5-LOX-/- littermates given saline or MPTP. Fewer TH-positive cell bodies (*) and enhanced GFAP immunoreactivity are apparent in the WT-MPTP group. Bar = 25 µm. (B) Immunohistochemical histology for the Iba-1 on microglia was detected using DAB (brown chromatgen); sections were counterstained by Cresyl violet. Nigral sections from WT and 5-LOX-/- littermates treated with saline or MPTP were assessed. Microglia with ramified cell bodies and long, branching processes are observed in substantia nigra from saline-treated mice (arrow heads). Activated microglia with rounded cell bodies and short, thickened processes, was observed in substantia nigra from MPTP-treated mice (arrows). Bar = 10 µm.
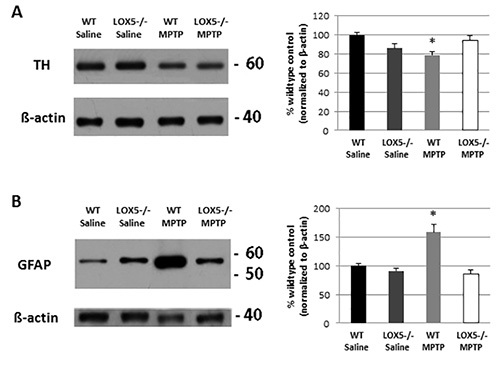 Figure 3.5-LOX isozyme effects on striatal TH and inflammatory following toxic insult. (A) Striatal TH protein levels were semi-quantitatively measured by Western blot analyses of homogenate from WT and 5-LOX-/- littermates given saline or MPTP (n=6-8/group). Immunoreactivity, measured by optical density, was normalized to β-actin. *, p<0.05. (B) Similarly, GFAP was semi-quantitatively measured by Western blot analyses of striatal homogenate from WT and 5-LOX-/- littermates given with saline or MPTP (n=6-8/group) and normalized to β-actin. *, p<0.05. Data are noted as mean ± SEM.
Figure 3.5-LOX isozyme effects on striatal TH and inflammatory following toxic insult. (A) Striatal TH protein levels were semi-quantitatively measured by Western blot analyses of homogenate from WT and 5-LOX-/- littermates given saline or MPTP (n=6-8/group). Immunoreactivity, measured by optical density, was normalized to β-actin. *, p<0.05. (B) Similarly, GFAP was semi-quantitatively measured by Western blot analyses of striatal homogenate from WT and 5-LOX-/- littermates given with saline or MPTP (n=6-8/group) and normalized to β-actin. *, p<0.05. Data are noted as mean ± SEM.
Discussion
The design of this gene-environment interaction study allowed us to gain new information regarding the dual nature of the 5-LOX isozyme in the nigrostriatal pathway. By performing HPLC to measure striatal monoamines after saline or MPTP treatment in transgenics lacking the 5-LOX isozyme and their wildtype littermates, we were able to note that its deficiency appears to be protective under toxic conditions (Figure 1), but under normal conditions, lack of the enzyme reduces striatal dopamine levels and may be deleterious. Thus, we are able to demonstrate that the 5-LOX isozyme contributes to striatal dopaminergic tone under normal conditions, but can contribute to damage following toxicant challenge4.
While further evaluation should lend novel mechanistic insights into the role of LOX isozymes in nigrostriatal toxicity, Western blot analyses (Figure 3) as well as immunohistochemical studies (Figure 2) revealed that neuroinflammation markers were, at least in part, attenuated in the 5-LOX isozyme-deficient cohort exposed to MPTP. These findings, using classical biochemical and histological techniques, indicate a critical role of 5-LOX products in potentiation of micro- and astro-glial activation4.
Depending on gene-environment interaction investigated, pathological readouts in addition to glial activation may be analyzed. Of particular importance in PD is loss of dopaminergic neurons in the substantia nigra and potentially pathological accumulation and aggregation of α-synuclein. Along this line, the impact of toxicant exposure in transgenic mice with α-synuclein overexpression has been monitored by evaluation of nigral cell death (i.e. using unbiased stereological cell counting) and insoluble α-synuclein deposition32-35.
The MPTP dose used in the paradigm described here produces a mild lesion with modest, but significant, striatal injury (Figure 1) and glial activation in both striatum and substantia nigra (Figures 2 and 3). Typically, higher doses of the toxicant are used to produce robust nigral dopaminergic cell loss and striatal dopamine depletion23,24,32,36-38,39. It is important to note that toxicity of MPTP can vary between vendors and lots; consequently doses may need to be adjusted to produce the desired lesion. Furthermore, other factors which must be considered are the strain and sex of animals utilized for the studies. Selective sensitivity to the neurotoxin has been demonstrated in distinct background strains of mice, a phenomenon due, at least in part, to differences in activation of subcellular pathways that mediate degeneration, including JNK and c-Jun36-39. Sex-dependent differences in MPTP toxicity have also been reported40,41, and may contribute to variability in studies using transgenic mice in which both sexes are used for poor-breeding lines. In such an instance, use of sex-matched wildtype controls is critical4. Such sex-related effects may account for the difference in lesioning observed in the WT mice between experiments testing the impact of 5- and 12/15-LOX-deficient mice in which one sex was used for one line and both sexes for the other (Figure 1). For gene-environment interaction studies, an MPTP challenge that produces severe injury (e.g. >80% reduction in striatal dopamine) is not recommended as this may mask a possible genetic effect.
While MPTP exposure produces nigral cell death3,42, striatal dopamine depletion3, complex I inhibition43-45, and glial activation46,47 that have been reported in human PD, in mouse, a slowly progressive degeneration that produces stable parkinsonism (i.e. motor deficits) and frank α-synuclein pathology (i.e. Lewy bodies and neurites) fully recapitulating hallmark features of the disease does not occur in the model. However, it is important to note that the MPTP mouse has played a critical role in understanding subcellular pathways that contribute to PD-related neurodegeneration. Variation in exposure paradigms, for example, has revealed activation of distinct mechanisms of toxicity: low-dose, subacute exposure promotes apoptotic cell death48 with a limited immune response49 whereas acute treatment with higher doses produces marked microglial activation50. Indeed, such factors should be considered when utilizing the model for efficacy studies and, relevant to the current study, to unmask the impact of a potential genetic risk factor.
Disclosures
There is nothing to disclose.
Acknowledgments
This work was funded by the National Institutes of Health NIGMS 056062.
References
- Manning-Bog AB, Langston JW. Model fusion, the next phase in developing animal models for Parkinson's disease. Neurotox. Res. 2007;11:219–240. doi: 10.1007/BF03033569. [DOI] [PubMed] [Google Scholar]
- Vance JM, Ali S, Bradley WG, Singer C, Di Monte DA. Gene-environment interactions in Parkinson's disease and other forms of parkinsonism. Neurotoxicology. 2010;31:598–602. doi: 10.1016/j.neuro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Hess A, Duvoisin RC. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science. 1984;224:1451–1453. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- Chou VP, Holman TR, Manning-Bog AB. Differential contribution of lipoxygenase isozymes to nigrostriatal vulnerability. Neuroscience. 2013;228:73–82. doi: 10.1016/j.neuroscience.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg. Med.Chem. 2006;14:4295–4301. doi: 10.1016/j.bmc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Weaver JR, et al. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Mol. Cell Endocrinol. 2012;358:88–95. doi: 10.1016/j.mce.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Yigitkanli K, et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann. Neurol. 2013;73:129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leyen K, et al. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci. Res. 2008;86:904–909. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Pratico D. 5-lipoxygenase as an endogenous modulator of amyloid beta formation in vivo. Ann. Neurol. 2011;69:34–46. doi: 10.1002/ana.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Bove J, Perier C. Neurotoxin-based models of Parkinson's disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Beal MF. Neuroprotective effects of creatine. Amino Acids. 2011;40:1305–1313. doi: 10.1007/s00726-011-0851-0. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Blesa J, Przedborski S. Animal models of Parkinson's disease. Parkinsonism Relat. Disord. 2012;18:183–185. doi: 10.1016/S1353-8020(11)70057-8. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Shimoji M, Yu SW, Dawson TM, Dawson VL. Apoptosis inducing factor and PARP-mediated injury in the MPTP mouse model of Parkinson's disease. Ann. N.Y. Acad. Sci. 2003;991:132–139. doi: 10.1111/j.1749-6632.2003.tb07471.x. [DOI] [PubMed] [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Przedborski S, et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J. Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Thomas B, et al. Mitochondrial permeability transition pore component cyclophilin D distinguishes nigrostriatal dopaminergic death paradigms in the MPTP mouse model of Parkinson's disease. Antioxid. Redox. Signal. 2012;16:855–868. doi: 10.1089/ars.2010.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Heikkila RE. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur. J. Pharmacol. 1986;129:339–345. doi: 10.1016/0014-2999(86)90444-9. [DOI] [PubMed] [Google Scholar]
- Di Monte DA, et al. Relationship among nigrostriatal denervation, parkinsonism, and dyskinesias in the MPTP primate model. Mov. Disord. 2000;15:459–466. doi: 10.1002/1531-8257(200005)15:3<459::AID-MDS1006>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lee KW, et al. Apoptosis signal-regulating kinase 1 mediates MPTP toxicity and regulates glial activation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat. Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Bolin LM, Strycharska-Orczyk I, Murray R, Langston JW, Di Monte D. Increased vulnerability of dopaminergic neurons in MPTP-lesioned interleukin-6 deficient mice. J. Neurochem. 2002;83:167–175. doi: 10.1046/j.1471-4159.2002.01131.x. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, et al. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiol. Dis. 2007;27:141–150. doi: 10.1016/j.nbd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Quik M, Di Monte DA. Nicotine administration reduces striatal MPP+ levels in mice. Brain Res. 2001;917:219–224. doi: 10.1016/s0006-8993(01)02937-7. [DOI] [PubMed] [Google Scholar]
- Markey SP, Johannessen JN, Chiueh CC, Burns RS, Herkenham MA. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984;311:464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984;311:467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- Crampton JM, Runice CE, Doyle TJ, Lau YS, Wilson JA. MPTP in mice: treatment, distribution and possible source of contamination. Life Sci. 1988;42:73–78. doi: 10.1016/0024-3205(88)90625-x. [DOI] [PubMed] [Google Scholar]
- Yang SC, Markey SP, Bankiewicz KS, London WT, Lunn G. Recommended safe practices for using the neurotoxin MPTP in animal experiments. Lab. Anim. Sci. 1988;38:563–567. [PubMed] [Google Scholar]
- Lau YS, Novikova L, Roels C. MPTP treatment in mice does not transmit and cause Parkinsonian neurotoxicity in non-treated cagemates through close contact. Neuroscience research. 2005;52:371–378. doi: 10.1016/j.neures.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Satoh N, et al. Central hypothermic effects of some analogues of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 1-methyl-4-phenylpyridinium ion (MPP) Neurosci. Lett. 1987;80:100–105. doi: 10.1016/0304-3940(87)90503-9. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, et al. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J. Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, et al. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp. Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Thomas B, et al. Resistance to MPTP-neurotoxicity in alpha-synuclein knockout mice is complemented by human alpha-synuclein and associated with increased beta-synuclein and Akt activation. PloS one. 2011;6 doi: 10.1371/journal.pone.0016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M, Goloubeva O, Smeyne RJ. Strain-dependent susceptibility to MPTP and MPP(+)-induced parkinsonism is determined by glia. Glia. 2001;34:73–80. [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828:91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Sedelis M, et al. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav. Genet. 2000;30:171–182. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- Boyd JD, et al. Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction prior to loss of neurons in the substantia nigra pars compacta. Brain Res. 2007;1175:107–116. doi: 10.1016/j.brainres.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookubo M, Yokoyama H, Kato H, Araki T. Gender differences on MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in C57BL/6 mice. Molecular and cellular endocrinology. 2009;311:62–68. doi: 10.1016/j.mce.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Diwakar L, Annepu J, Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J. 2004;18:1102–1104. doi: 10.1096/fj.03-1075fje. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J. Neurochem. 1987;48:1787–1793. doi: 10.1111/j.1471-4159.1987.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Youngster SK, Kindt MV, Heikkila REMPTP. MPP+ and mitochondrial function. Life Sci. 1987;40:721–729. doi: 10.1016/0024-3205(87)90299-2. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Wu DC, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp. Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Furuya T, et al. Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J Neurosci. 2004;24:1865–1872. doi: 10.1523/JNEUROSCI.3309-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur. J. Neurosci. 2006;24:3174–3182. doi: 10.1111/j.1460-9568.2006.05192.x. [DOI] [PubMed] [Google Scholar]


