Abstract
The peripheral taste response of insects can be powerfully investigated with electrophysiological techniques. The method described here allows the researcher to measure gustatory responses directly and quantitatively, reflecting the sensory input that the insect nervous system receives from taste stimuli in its environment. This protocol outlines all key steps in performing this technique. The critical steps in assembling an electrophysiology rig, such as selection of necessary equipment and a suitable environment for recording, are delineated. We also describe how to prepare for recording by making appropriate reference and recording electrodes, and tastant solutions. We describe in detail the method used for preparing the insect by insertion of a glass reference electrode into the fly in order to immobilize the proboscis. We show traces of the electrical impulses fired by taste neurons in response to a sugar and a bitter compound. Aspects of the protocol are technically challenging and we include an extensive description of some common technical challenges that may be encountered, such as lack of signal or excessive noise in the system, and potential solutions. The technique has limitations, such as the inability to deliver temporally complex stimuli, observe background firing immediately prior to stimulus delivery, or use water-insoluble taste compounds conveniently. Despite these limitations, this technique (including minor variations referenced in the protocol) is a standard, broadly accepted procedure for recording Drosophila neuronal responses to taste compounds.
Keywords: Neuroscience, Issue 84, Drosophila, insect, taste, neuron, electrophysiology, labellum, extracellular recording, labellar taste sensilla
Introduction
The sense of taste allows an insect to detect a vast range of soluble chemicals and plays an important role in the acceptance of a nutritious substance, or the rejection of a noxious or toxic one. Taste is also thought to play a role in mate selection, through the detection of pheromones1-5. These important and diverse functions have made the insect taste system a compelling target of investigation into how sensory systems translate environmental cues into relevant behavioral outputs.
The primary unit of the Drosophila melanogaster taste system is the taste hair, or sensillum. Molecules enter the sensillum via a pore at its tip2,6. Sensilla are found on the labellum, the legs, the wing margin, and the pharynx6. On the labellum, the number and location of sensilla is stereotyped. There are three morphological classes of sensilla based on length: the long (L), intermediate (I), and short (S) sensilla7,8. Each sensillum contains either two (I-type) or four (L- and S- type) gustatory receptor neurons (GRNs)9. Different GRNs respond to different categories of taste stimuli: bitter, sugar, salt and osmolarity7,10 and express different subsets of gustatory receptors8,11-13. Only I and S-type sensilla contain bitter-responsive GRNs8,10. The GRNs project to the subesophageal ganglion (SOG) and their activation by taste molecules is relayed to the higher central nervous system for decoding, resulting in a behavioral response6. The relatively small number of neurons and the amenability to molecular and behavioral analysis make the Drosophila taste system an excellent model for the investigation of gustatory systems in general. The relative ease with which the system can be manipulated via genetic mutation or the GAL4-UAS expression system also serves as a valuable tool14,15.
Because these sensilla protrude from the surface of the labellum, they make excellent targets for electrophysiology. The firing of the GRNs can be monitored using extracellular recording. Historically, the side-wall recording method, which uses a glass electrode inserted into the sensillum to record neuronal activity,26 has been used. However, this method is technically challenging to perform, and it is difficult to record for long from each preparation. The tip-recording method, which measures the response of the neurons with an electrode that simultaneously delivers a tastant, has since become the method of choice9,16. It has been utilized to investigate the taste system of Drosophila melanogaster8,10,17,18 as well as a number of other insect species19-23. It has been greatly facilitated by the development of the tastePROBE amplifier, which overcame one of the major drawbacks of the tip-recording method by compensating for the large potential difference between the reference electrode and the insect sensillum, allowing the GRN action potentials to be recorded without excessive amplification or filtering24. Another important development was the use of tricholine citrate as the recording electrolyte25. TCC suppresses responses from the osmolarity-sensitive GRN and does not stimulate the salt-sensitive GRN, making responses generated by bitter and sugar tastants much easier to analyze25.
Here we describe how tip recording of Drosophila labellar sensilla is currently performed in the Carlson laboratory. This protocol will explain how to establish a suitable electrophysiology rig, how to prepare the fly, and how to perform taste recordings. We also present some representative data obtained by recording from subsets of Drosophila sensilla, as well as some common issues and potential solutions that may be encountered when using this technique.
Protocol
The following protocol complies with all the animal care guidelines of Yale University.
1. Reagents and Equipment Preparation
- Recording equipment setup (Figure 1A).
- Choose a room for rig setup that is free of large variations in temperature or humidity and also isolated from sources of electrical and mechanical noise, such as refrigerators and centrifuges.
 Figure 1. (A) Overview of recording rig setup. Stereomicroscope (a) is mounted on anti-vibration platform (b). Reference electrode holder (c) is mounted on platform opposite the headstage (d), via micromanipulators. An outlet plastic tube (e) delivering humidified air stream directed at the fly preparation is also mounted on the platform. The headstage is connected to the amplifier (f), which is connected to the digital acquisition system (DAS) (g), which is connected to a PC (h). (B) Configuration of electrodes and outlet tube: reference electrode on the left, recording electrode on the right, and air stream outlet tube directed at fly preparation. Click here to view larger image.
Figure 1. (A) Overview of recording rig setup. Stereomicroscope (a) is mounted on anti-vibration platform (b). Reference electrode holder (c) is mounted on platform opposite the headstage (d), via micromanipulators. An outlet plastic tube (e) delivering humidified air stream directed at the fly preparation is also mounted on the platform. The headstage is connected to the amplifier (f), which is connected to the digital acquisition system (DAS) (g), which is connected to a PC (h). (B) Configuration of electrodes and outlet tube: reference electrode on the left, recording electrode on the right, and air stream outlet tube directed at fly preparation. Click here to view larger image.
- Mount stereomicroscope to center of anti-vibration table or platform.
- Attach micromanipulators for the reference electrode/insect preparation and headstage/recording electrode to the left and right of the microscope, respectively, using magnetic stands.
- Mount outlet plastic tube in a third micromanipulator to the rear of the microscope, oriented such that the tube opening is pointed toward location of fly prep (see Figure 1B).
- Using flexible plastic tubing, attach outlet plastic tube to a vacuum flask partially filled with water. Connect a small aquarium pump to bubble air through the water in the flask, generating a humidified air stream through the outlet plastic tube towards the fly.
- Mount fiber optic light source off the vibration table, orienting the outputs to illuminate the preparation by reflecting light via a piece of white card paper directly below the preparation. Ensure that the light source does not rest on the table. Note: the benefit of reflecting the light source on a paper disc is two-fold: it improves the contrast, making sensilla easier to visualize, and it prevents heating of the preparation that would result from direct light.
- Plug tastePROBE amplifier into the digital acquisition system (DAS), and the DAS into a personal computer, according to supplier manual. Plug foot pedal trigger in and arrange under workspace. Note: Electrically isolated wall sockets for the amplifier and DAS are highly desirable.
- Electrically ground microscope, micromanipulators, and light source by connecting metal components to table using alligator clips and lengths of insulated electrical wire and electrical tape. Electrically ground metal platform by connecting to building ground or DAS, which is grounded through power supply plug.
- Install appropriate acquisition software for the DAS of choice on the personal computer. Note: Ensure that the digital acquisition drivers are compatible with the operating system on the PC.
- Configure software amplification (10-100x), signal filtering (typically a Bessel bandpass filter set from 100 Hz-3,000 Hz), and sampling rate (at least 10 KHz). Note: Signal amplitudes from gustatory neurons are typically in the 0.5-2 mV range, so the display scale is set to facilitate their visualization. Note: The 100 Hz filter helps to exclude extraneous electrical noise; however, it changes the shape of spikes and can make advanced spike sorting more challenging. Alternatively, a 1 Hz filter can be used.
- Optionally, a Faraday cage can be set up around the whole vibration table. However, small sheets of aluminum foil are usually sufficient to reduce any noise generated by the external environment or investigator.
- Glass Electrode Preparation
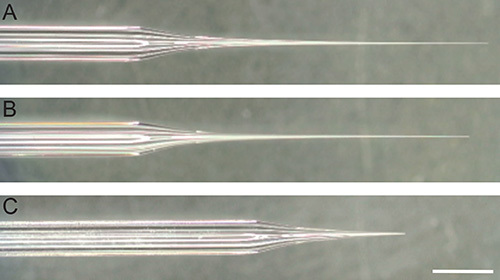 Figure 2. Reference and recording electrodes. Photograph under magnification of glass capillaries pulled into reference electrode, with (A) and without (B) tip broken, and recording electrode (C). White bar represents 2 mm. Click here to view larger image.
Figure 2. Reference and recording electrodes. Photograph under magnification of glass capillaries pulled into reference electrode, with (A) and without (B) tip broken, and recording electrode (C). White bar represents 2 mm. Click here to view larger image.
- Pull the reference electrode from a glass capillary using a pipette puller instrument. Note: The exact settings of the pipette puller program will vary from instrument to instrument. Try to achieve a very long gradual taper. The pore size at the tip is not crucial because the tip will be broken before fly preparation (Figures 2A and 2B); however, make sure that the diameter of the tapered length of the electrode is neither too thin, which will not allow for sufficient immobilization of the labellum, nor too large, which could damage the gustatory neurons or rupture the salivary glands.
- Pull recording electrode from a borosilicate glass capillary with filament using a pipette puller instrument. Try to achieve a taper that is shallower than that of the reference electrode, and a pore diameter of approximately 10-15 μm (Figure 2C)28.
- Tastant solutions preparation
- Use Beadle-Ephrussi Ringer solution (B&E) as the reference electrode electrolyte. To make one liter of B&E, dissolve 7.5 g NaCl, 0.35 g KCl, and 0.279 g CaCl2∙2H2O in one liter of ultrapure water. Store smaller aliquots at -20 °C.
- Use 30 mM tricholine citrate solution (TCC) as the recording electrode electrolyte and solvent for tastant solutions25, if bitter or sugar GRN responses are to be measured. Alternatively, 1-3 mM potassium chloride solution can be used if responses of the water cell are to be measured.
- To make tastant solutions, weigh appropriate amount of tastant in powder form and add to TCC to make an initial stock concentration. Use this to make serial dilutions from this initial stock to yield the desired concentration for testing. Note: If tastants do not readily dissolve in water, another solvent, such as ethanol, can be used to make initial stock concentration. An appropriate control solution of TCC and solvent without tastant should be used in this case.
- Store aliquots long term at -20 °C. Store one working aliquot of a tastant solution at 4 °C for recording use for up to a week, depending on chemical properties of tastant.
2. Drosophila Preparation
 Figure 3. Preparation of fly for recording. (A) Insertion position of reference electrode into dorsal thorax of fly. The white arrow indicates the reference electrode. (B) Intermediate position of reference electrode: advanced through neck and head, proboscis not yet extended. (C,D) Fly with reference electrode in final position with tip of electrode inside labellum, and proboscis fully extended. Click here to view larger image.
Figure 3. Preparation of fly for recording. (A) Insertion position of reference electrode into dorsal thorax of fly. The white arrow indicates the reference electrode. (B) Intermediate position of reference electrode: advanced through neck and head, proboscis not yet extended. (C,D) Fly with reference electrode in final position with tip of electrode inside labellum, and proboscis fully extended. Click here to view larger image.
Collect newly eclosed flies for recording from well-maintained fly cultures, grown under temperature- and humidity-controlled conditions, and age them 5-10 days in fresh culture vials before recording.
Chill microscope plate on ice for 15-30 min before preparing fly.
Backfill glass reference electrode with B&E solution using a long, thin plastic needle of 0.5 mm diameter, such as a spinal needle and 1 ml syringe and gently tap out any bubbles. Break small amount of tip off using forceps and use capillary action to draw out all remaining bubbles with a tissue, observing under dissecting microscope.
Slide B&E-filled reference electrode onto wire of reference electrode holder, taking care not to introduce air bubbles.
Aspirate fly into a P200 pipette tip, using fly aspirator built from tubing, mesh, and pipette tip;29 place in ice bucket and chill for 30-60 sec.
Remove microscope plate from ice, wipe off any moisture, and position underneath microscope. Gently tap fly out of pipette tip onto microscope plate. Note: the fly should be sufficiently immobilized to manipulate easily.
Under low magnification, gently remove the forelegs with one pair of forceps, while holding the thorax stable with the other pair of forceps. Position the fly on its ventral side, dorsal side facing up. Note: Always be careful to avoid touching the labellum with the forceps at all times during the preparation process to minimize mechanical damage.
While holding the fly in place with one pair of forceps, insert the reference electrode at the midline of the posterior dorsal thorax. A suggested angle of entry is approximately forty-five degrees, in the direction of the head (Figure 3A).
Secure the reference electrode holder with modeling clay such that the fly is visible underneath the microscope at high magnification. Maneuver and angle the glass electrode through the neck and head, by sliding the fly towards the reference electrode holder using two pairs of forceps. Note: Work quickly but smoothly; it is easier to complete this step while the fly is still immobilized from the cold (Figure 3B).
Gently extend the proboscis with one pair of forceps, while sliding the fly further down the glass reference electrode, until the tip of the electrode is inside the labellum and the proboscis is fully extended (Figures 3C and 3D). Note: Take care not to puncture any part of the proboscis tissue or distend the edge of the labellum with the reference electrode, as this may damage the fly and/or taste neurons and affect the recording quality.
3. Recording from Labellar Sensilla
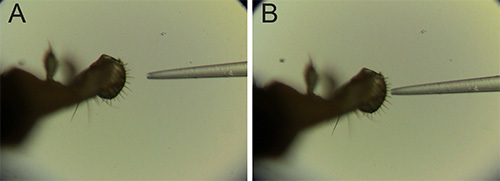 Figure 4. Recording from fly. (A) labellum of fly preparation on left with recording electrode aligned for contact on right, under high magnification. (B) recording electrode and single sensillum on labellum in contact, under high magnification. Click here to view larger image.
Figure 4. Recording from fly. (A) labellum of fly preparation on left with recording electrode aligned for contact on right, under high magnification. (B) recording electrode and single sensillum on labellum in contact, under high magnification. Click here to view larger image.
Always ground yourself by touching the metal surface of the anti-vibration table or platform prior to touching any equipment during recording process! Note: It is extremely important not to deliver a static charge to the headstage as that can damage the circuitry.
Secure reference electrode holder to micromanipulator mounted on air table of recording rig. Position one lobe of labellum in microscope field of view, under high magnification (typically at least 140X), and in line with humidified air stream.
Turn on humidified air stream, computer, DAS, and amplifier. Open acquisition software.
- Rinse and fill glass recording electrode with desired tastant.
- Rinse glass recording electrode with ultrapure water by using a syringe and plastic tubing28 to pull small amounts of water through the tube at least ten times.
- Rinse recording electrode with tastant at least five times. Fill recording electrode approximately one-third to halfway full with tastant and remove from tubing. If there are air bubbles, tap to release or simply refill the electrode.
- Slide electrode onto silver wire of the headstage quickly and smoothly so as not to introduce air bubbles.
- Stimulate single sensillum with tastant-filled recording electrode.
- Use the micromanipulator to bring the recording electrode aligned with sensillum of interest.
- Press foot pedal to trigger acquisition mode of the amplifier.
- Advance the recording electrode with the fine control knob of micromanipulator carefully until it makes contact with tip of sensillum and recording commences.
- Remove electrode after 1-2 sec.
- Repeat step 3.5 with other sensilla, if desired. Note: Wait at least 1 min in between presentations to the same sensillum. If recording with a single tastant for a prolonged period of time, the tastant solution may dry out and the solution in the tip may become more concentrated. This can be remedied by gently contacting the tip of the glass electrode with smooth paper to remove a small amount of liquid by capillary action.
To record responses to another tastant, rinse and load recording electrode with new tastant and repeat step 3.4. Note: Thoroughly rinsing the electrode between tastants is absolutely crucial to avoid cross-contamination.
Save data files periodically with identifying information, such as date, genotype, and tastants. Note: It is important to keep a written record of the tastant and sensillum identity of each presentation during recording session for data analysis.
Representative Results
Figure 5A shows the response of an L sensillum to a sugar, sucrose. The same sensillum does not respond to a bitter compound, berberine. Figure 5B shows that an I type sensillum, which contains a bitter responsive neuron, displays larger amplitude spikes in response to berberine, and smaller amplitude spikes in response to sucrose. L sensilla display a minimal background response to the solvent control, TCC, while I sensilla display virtually no response to TCC (Figure 5). For more information on salt and water responses of labellar GRNs, please refer to Hiroi10.
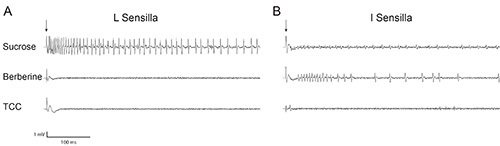 Figure 5. Representative traces of wild-type Drosophila labellar responses (A) L sensillum response to 100 mM sucrose (SUC), 1 mM berberine (BER), and 30 mM TCC. (B) I sensillar response to SUC, BER, and TCC. The arrowhead indicates the contact artifact that occurs at the beginning of each recording. Click here to view larger image.
Figure 5. Representative traces of wild-type Drosophila labellar responses (A) L sensillum response to 100 mM sucrose (SUC), 1 mM berberine (BER), and 30 mM TCC. (B) I sensillar response to SUC, BER, and TCC. The arrowhead indicates the contact artifact that occurs at the beginning of each recording. Click here to view larger image.
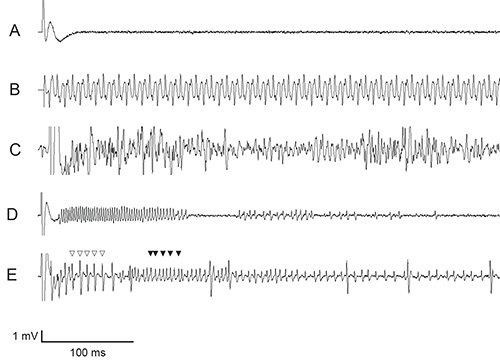 Figure 6. Representative suboptimal electrophysiological results. (A) complete lack of signal (B) 50/60 Hz “noise” (C) stochastic noise (D) mechanosensory neuron firing alone (E) bitter GRN (open triangles) and mechanosensory neuron (filled triangles) both firing. Click here to view larger image.
Figure 6. Representative suboptimal electrophysiological results. (A) complete lack of signal (B) 50/60 Hz “noise” (C) stochastic noise (D) mechanosensory neuron firing alone (E) bitter GRN (open triangles) and mechanosensory neuron (filled triangles) both firing. Click here to view larger image.
Discussion
Labellar sensilla vary in the ease of recording due to differences in morphology and anatomical organization. Sometimes a sensillum does not respond to any tastants, even one that is known to elicit a positive response. The frequency with which this occurs varies depending on sensillum type. L sensilla are most consistently responsive and are relatively easy to access due to their length. In general, S sensilla are consistently responsive, but their short length and position on the labellum make good contact challenging. I sensilla can be accessed more readily, depending on the angle of the preparation; however, they are more frequently unresponsive. On any given fly preparation, a greater proportion of I sensilla may be unresponsive than L or S sensilla. Genetic background can affect the consistency of taste responses as well. For example, some transgenic flies may display less consistent responses than wild-type, presumably because the transgenes affect the general health of the fly. We have observed that w- mutant flies are particularly challenging to record from.
One common technical problem is a lack of signal, i.e. no spikes are observed (Figure 6A). First, sometimes one particular sensillum may be unresponsive, while others of that same class on the same fly may respond. Second, there may be an air bubble in the recording electrode or the reference electrode. If the recording electrode is suspected, this can be fixed by simply removing and refilling the glass electrode, tapping gently and inspecting under magnification to ensure no bubbles are present. If the reference electrode is suspected to contain an air bubble, remaking the prep with a new fly is the easiest way to resolve this issue. Third, sometimes the wires carrying the electrical signal may not be securely connected. Fourth, occasionally the voltage signal being received may be either higher or lower than the range the amplifier can measure. If using the tastePROBE amplifier, check to see if either the clip up or clip down indicator light is on. If the clip up indicator light is on, often removing and refilling the glass reference electrode, while taking care to fill not more than halfway and wiping down the outside to remove moisture will resolve the problem. Moisture on the outside of the glass electrode can make an electrical connection between the metal case of the electrode and the wire, sending the signal out of range of the amplifier. If that fails to solve the issue, or the clip down indicator light is on, consider suggestions in the following paragraph to combat electrical noise in the system. Fifth, sometimes a fly may die during preparation or is otherwise unresponsive despite the preparation’s healthy appearance. Growth conditions, such as humidity, temperature, age, food quality, and microbiota, as well as a less healthy genetic background could contribute to a higher proportion of “unresponsive” flies. Lastly, rarely, a piece of equipment may be nonfunctional. If signal is consistently not being achieved and all other possibilities have been exhausted, it may be necessary to investigate the functionality of each piece of equipment: headstage, amplifier, and digitizer. The easiest way to do this is to replace a piece of equipment with another from a rig that is known to be functional. If only one rig is present in a lab, a signal generator can be used to test functionality of the electronic components.
Another common technical issue is that of “noise,” which is an observed signal that does not appear to represent neuronal action potentials fired in response to a gustatory stimulus (Figures 6B-E). First, the signal may result from 50/60 Hz electrical noise from recording equipment or other equipment nearby (Figure 6B). With no fly on the reference electrode, directly connect the recording and reference electrodes through a drop of Ringer’s solution and enter the passthrough mode on the amplifier by pressing the up button. If noise is observable on the passthrough signal, this likely means that the noise is external to the fly preparation. Ensure that all rig equipment is properly grounded and that tin foil shields are in place. Try unplugging nearby equipment to see if the noise is eliminated, or shield additional components. Second, the noise may appear stochastic (Figure 6C). In this case, the steps detailed for 50/60 Hz noise should still be undertaken. Additionally, try unplugging or replacing different components of the recording equipment, particularly the headstage and/or amplifier. If no noise is observed when the electrodes are directly connected, the source is likely the fly preparation itself. It is usually simplest to prepare a new fly for recording, taking care to minimize damage to the fly. Third, activation of the mechanosensory neuron contained within the sensillum (Figures 6D and 6E) may be observed. The mechanosensory neuron can be activated if the sensillum is deflected or bent upon application of the recording electrode, or bumped during contact. The spikes are usually distinguishable from chemosensory spikes by their irregular pattern, which usually appears coordinated with the mechanical disruption, not the application of a gustatory stimulus. Mechanosensory firing can be minimized by aligning the recording electrode with the sensillum and advancing gently only as far as is necessary to make contact with the tip of the sensillum. Fourth, stochastic spike “bursting” may be observed; this appears similar to neuronal firing, but is of high frequency and amplitude, not coordinated in response to a stimulus. This usually results from the fly prep itself, not from the equipment, and may be due to a nerve disrupted by the reference electrode.
A third common technical issue is that the preparation is mobile, causing the labellum to move, which makes connection with a sensillum difficult. First, the fly preparation may be unstable. Check that the reference electrode is correctly positioned, and readjust if necessary. Second, the reference electrode may be too thin at the tip to hold the proboscis and labellum immobile. Try breaking off a longer amount of the tip before preparing the fly. If that is not sufficient, readjust the pipette puller settings as needed to change the shape of reference electrode such that the taper is more gradual and the diameter is slightly increased. Third, the fly may be unusually active. Remake the preparation with a new fly.
For general electrophysiology information and more troubleshooting guidance, refer to Axon Guide30.
There are a few limitations to the tip-recording method outlined in this publication. One limitation is that the tastant must be water soluble, as it is delivered in the recording electrode along with the electrolyte. This increases the difficulty of recording with hydrocarbon compounds, though use of a solvent like DMSO has made some recording with pheromones possible4. Alternative approaches are to use a sharpened tungsten electrode to perform the recordings from the socket base of the sensillum, or use a glass electrode to perform recordings from the side wall of the sensillum, in both of which the tastant is delivered independently of the recording electrode26,27. However, these techniques are challenging and side-wall recordings are more injurious to the taste organ. Another limitation is the amount of time required to exchange the tastant solution (Protocol step 3.3), which reduces throughput, and limits the use of complicated stimulus paradigms often seen in olfactory recordings. Gustatory receptor neurons exhibit some variability in amplitude that is dependent on spike frequency. This feature can complicate assessment of neuronal identity and make advanced spike sorting more difficult25,31-33. In addition, because of the nature of the tip-recording method one cannot record the basal firing immediately prior to the delivery of a stimulus, as is commonly done in olfactory recordings. Despite these drawbacks, the tip-recording method has been successfully used to elucidate many of the principles of taste coding in Drosophila and other species8,10,17,19,21-23.
The fly preparation technique outlined here is just one possible approach. In this preparation method the proboscis is fixed in an extended position to facilitate contact of the recording electrode with the sensillum of interest, and the reference electrode is inserted into the animal. Other preparation methods include the mounting of the animal to a ball of modeling clay and the use of thin strips of tape to fix the proboscis34. Indeed, as long as the basic parameters of tissue stabilization and reference electrode placement are met, sensilla in other locations or from different species can be recorded from in much the same way. For example, leg sensilla can be recorded from by fixing the body of a fly to a sylgard-coated microscope slide with fine insect pins, splaying the legs off the edge of the glass slightly35. It is possible to deliver pharmacological agents to the sensilla via the recording electrode to investigate signal transduction in the gustatory receptor neurons. It is simply a task of experimentation to determine which approach works best for the desired outcome.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by an NRSA predoctoral grant 1F31DC012985 (to R.D.) and by NIH grants to J.C.
We would like to thank Dr. Linnea Weiss for helpful comments on the manuscript, Dr. Ryan Joseph for help compiling figures, and Dr. Frederic Marion-Poll for helpful technical advice. We would also like to acknowledge the helpful comments of four reviewers.
References
- Glendinning JI, Jerud A, Reinherz AT. The hungry caterpillar: an analysis of how carbohydrates stimulate feeding in Manduca sexta. The Journal of experimental biology. 2007;210:3054–3067. doi: 10.1242/jeb.004671. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Zhao X, Dickson BJ. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell reports. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell and tissue research. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoological Science. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The Molecular and Cellular Basis of Bitter Taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R, Bleiser-Avivi N, Atidia J. Labellar taste organs of Drosophila melanogaster. Journal of Morphology. 1976;150:327–341. doi: 10.1002/jmor.1051500206. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Meunier N, Marion-Poll F, Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. Journal of neurobiology. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science (New York, N.Y.) 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6:e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development (Cambridge, England) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Parks AL, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nature genetics. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Hodgson ES, Lettvin JY, Roeder KD. Physiology of a primary chemoreceptor unit. Science (New York, N.Y.) 1955;122:417–418. doi: 10.1126/science.122.3166.417-a. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoins C, Marion-Poll F. Electrophysiological responses of gustatory sensilla of Mamestra brassicae (Lepidoptera, Noctuidae) larvae to three ecdysteroids: ecdysone, 20-hydroxyecdysone and ponasterone. A. J Insect Physiol. 1999;45:871–876. doi: 10.1016/s0022-1910(99)00057-8. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Davis A, Ramaswamy S. Contribution of different taste cells and signaling pathways to the discrimination of "bitter" taste stimuli by an insect. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7281–7287. doi: 10.1523/JNEUROSCI.22-16-07281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JL, Shields VD, Dickens JC. Gustatory receptor neuron responds to DEET and other insect repellents in the yellow-fever mosquito, Aedes aegypti. Die Naturwissenschaften. 2013;100:269–273. doi: 10.1007/s00114-013-1021-x. [DOI] [PubMed] [Google Scholar]
- Merivee E, Must A, Milius M, Luik A. Electrophysiological identification of the sugar cell in antennal taste sensilla of the predatory ground beetle Pterostichus aethiops. J Insect Physiol. 2007;53:377–384. doi: 10.1016/j.jinsphys.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Popescu A, et al. Function and central projections of gustatory receptor neurons on the antenna of the noctuid moth Spodoptera littoralis. Journal of comparative physiology. A, Neuroethology. 2013;199:403–416. doi: 10.1007/s00359-013-0803-0. [DOI] [PubMed] [Google Scholar]
- Marion-Poll F, Der Pers JVan. Un-filtered recordings from insect taste sensilla. Entomologia Experimentalis et Applicata. 1996;80:113–115. [Google Scholar]
- Wieczorek H, Wolff G. The labellar sugar receptor of Drosophila. J. Comp. Physiol. A. Neuroethol Sens. Neural Behav. Physiol. 1989;164:825–834. [Google Scholar]
- Morita H. Initiation of spike potentials in contact chemosensory hairs of insects. III. D.C. stimulation and generator potential of labellar chemoreceptor of calliphora. Journal of cellular and comparative physiology. 1959;54:189–204. doi: 10.1002/jcp.1030540209. [DOI] [PubMed] [Google Scholar]
- Lacaille F, et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS One. 2007;2:e661. doi: 10.1371/journal.pone.0000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Dahanukar A. Electrophysiological recording from Drosophila taste sensilla. Cold Spring Harbor protocols. 2011;2011:839–850. doi: 10.1101/pdb.prot5631. [DOI] [PubMed] [Google Scholar]
- Pellegrino M, Nakagawa T, Vosshall LB. Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. J. Vis. Exp. 2010. p. e1725. [DOI] [PMC free article] [PubMed]
- Axon Instruments. The Axon Guide for Electrophysiology & Biophysics Laboratory Techniques. 1993.
- Fujishiro N, Kijima H, Morita H. Impulse frequency and action potential amplitude in labellar chemosensory neurones of Drosophila melanogaster. Journal of insect physiology. 1984;30:317–325. [Google Scholar]
- Marion-Poll F, Tobin TR. Software filter for detecting spikes superimposed on a fluctuating baseline. Journal of neuroscience. 1991;37:1–6. doi: 10.1016/0165-0270(91)90015-r. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Lansky P, Rospars JP. Estimation of the individual firing frequencies of two neurons recorded with a single electrode. Chem Senses. 2003;28:671–679. doi: 10.1093/chemse/bjg059. [DOI] [PubMed] [Google Scholar]
- Marion-Poll Lab Website. 2013. Available from: http://taste.versailles.inra.fr/fred.
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. Journal of neurobiology. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]


