Abstract
This report summarizes recent findings of environmental arsenic (As) contamination and the consequent health effects in a community located near historic gold mining activities in the Mangalur greenstone belt of Karnataka, India. Arsenic contents in water, hair, nail, soil and food were measured by FI-HG-AAS. Elemental analyses of soils were determined by ICP-MS (inductively coupled plasma-mass spectrometry). Of 59 tube-well water samples, 79% had As above 10 μg L−1 (maximum 303 μg L−1). Of 12 topsoil samples, six were found to contain As greater than 2000 mg kg−1 possibly indicating the impact of mine tailings on the area. All hair and nail samples collected from 171 residents contained elevated As. Arsenical skin lesions were observed among 58.6% of a total 181 screened individuals. Histopathological analysis of puncture biopsies of suspected arsenical dermatological symptoms confirmed the diagnosis in 3 out of 4 patients. Based on the time-course of arsenic-like symptoms reported by the community as well as the presence of overt arsenicosis, it is hypothesized that the primary route of exposure in the study area was via contaminated groundwater; however, the identified high As content in residential soil could also be a significant source of As exposure via ingestion. Additional studies are required to determine the extent as well as the relative contribution of geologic and anthropogenic factors in environmental As contamination in the region. This study report is to our knowledge one of the first to describe overt arsenicosis in this region of Karnataka, India as well as more broadly an area with underlying greenstone geology and historic mining activity.
Keywords: Arsenic, Groundwater, Greenstone, Gold mine, Karnataka, Arsenical skin lesions
1. Introduction
Chronic arsenic (As) exposure through ingestion can cause severe adverse effects on human health, especially via the consumption of contaminated groundwater [1–5]. Epidemiological and experimental evidence is furthermore mounting that in utero or early life exposure to As may affect fetal development or increase rates of several malignant and non-malignant diseases [6,7]. Arsenic with a naturally low average crustal abundance (1.7 mg kg−1) is substantially concentrated in certain sediments adsorbed to hydrous iron oxides or in the form of sulfide-bearing minerals [8]. The natural dispersion of As in the environment is governed by a combination of region and site-specific biogeochemical and hydrological factors [9,10]. The mining of gold and base metals, frequently associated with sulfide mineralization, has in several settings induced or exacerbated localized As contamination [11–13]. Historically active mines abandoned with little rehabilitation are particularly notorious for the pollution of the surrounding soil and groundwater with toxic elements [14–16]. Elevated As in biomarkers, such as urine and toenails, has been reported in former or current gold mining areas in Australia [17,18], Ghana [19], Canada [20], France [21], Slovakia [22] and Brazil [23]. In areas of historic mining activity however, the relative contribution and interaction of anthropogenic and natural processes in environmental arsenic contamination are known to vary greatly depending on the locality and a host of related factors [24,25].
In the present study area in a northeastern region of Karnataka, India, factors impacting the natural secondary geochemical dispersion of As in groundwater and soil have been found to include: topography, water table depth, drainage, soil type, and underlying geology [26]. This area also has several historic gold mines with some presently active and many abandoned [27]. An understanding of the nature of the source, mobilization, and distribution of environmental arsenic in the region as well as the extent of human exposure to this contamination is essential to effective mitigation efforts and future planning. The purpose of the present study was the assessment of environmental (groundwater, soil, and food) and human biomarker arsenic levels as well as the health effects of arsenic in a community with previously reported arsenicosis cases.
The earliest cases of groundwater As contamination in Karnataka were identified in villages of the greenstone belts of Yadgir District, a district recently created in late 2009 from a southern region of Gulbarga District. Studies conducted by UNICEF and Government of Karnataka (GOK) [28,29] discovered several villages with well water supplies containing As exceeding the World Health Organization (WHO) guideline value (10 μg L−1). One of these studies hypothesized that local gold mining might be a contributing factor to As contamination [30].
The occurrence of arsenicosis and As-related cancers in Kiradalli Tanda village was first identified in early July 2009. Several patients from the community were admitted to the District Hospital, Gulbarga with dermatological symptoms. Attending physicians suspected As in drinking water could be the cause and carried out further investigations. Water samples were collected by GOK from the community and As levels were found up to 160 μg L−1; these reports along with cases of the adverse health impacts were published in a regional daily newspaper [31,32].
2. Materials and methods
2.1. Study area and timeline
Kiradalli Tanda (16°37′7.9″N, 76°36′47.3″E) is a small village (total population 535) situated in a remote region of Yadgir district in Karnataka, India. Traces of arsenopyrite are noticed in and around villages such as Godryal and Heggandoddi and specifically in the proximity of abandoned gold mines. Kiradalli Tanda is situated in the Mangalur greenstone belt (16°30′ to 16°44′N, 76°32′30″ to 76°40′E), one of the four Archean greenstone belts (Kolar, Ramagiri, Hutti-Maski and Mangalur) of the gold-bearing eastern province (Fig. 1) of the Dharwar Craton [33].
Fig. 1.
Location of the surveyed villages and local historic gold mining activities [27] in Yadgir District, Karnataka, India. (A) Karnataka State (gold), India; (B) Gulbarga District, after the creation of Yadgir District in 2009 (dark red), Yadgir District (medium red), and Raichur District (pink), Karnataka; (C) Mangalur and Hutti-Maski greenstone belts [33,41] located within Yadgir and Raichur Districts, respectively; the border between these two districts is the Krishna River; (D) surveyed villages and historic gold mine workings within the Mangalur greenstone belt.
The Mukangavi (Mangalur) gold mine, is located 4 km south of Kiradalli Tanda and was in operation between 1887 and 1913 and was reclaimed in 1980 to resume mining operations. The mine was closed in 1994 primarily due to heavy water inflow into the mining galleries [27] and remains closed to this day. Following the newspaper publication of possible arsenicosis in Kiradalli Tanda, an initial survey of the community, consisting of patient interviews as well as the collection of 12 groundwater and a small number of hair and nail samples, was conducted in July 2009. With the identification of elevated arsenic levels in groundwater and biological samples, a more detailed survey with a complete medical team was organized in September 2009.
2.2. Sampling and analysis
Water samples from tube-wells and dug-wells as well as samples of food grains and soil were collected from Kiradalli Tanda and the surrounding communities. Water samples were collected in acid pre-washed 10 ml polyethylene bottles. Immediately after sample collection, a drop of nitric acid (1:1) was added as preservative. Soil samples (n=12) were collected from residential areas in and around Heggandoddi, Kiradalli, Kiradalli Tanda, Jainapur and Gowdigeri (Fig. 2). Additionally, spot sampling of groundwater sources was conducted in Raichur District (Fig. S1 in Supplementary Data).
Fig. 2.
Locations and levels of As in water and soil samples collected from Kiradalli Tanda and the surrounding communities during the second more detailed survey of the study area in September 2009. Two groundwater samples, which are not plotted, were collected without spatial information: Kiradalli Tanda (144 μg/L) and Gowdigeri (13 μg/L).
A medical camp was organized in Kiradalli Tanda in September 2009 to assess recent As exposure and consequent health effects. Each individual visiting the camp was thoroughly screened for arsenicosis symptoms by dermatologists and other medical personnel. Hair and nail samples as well as other general information were also collected from these individuals during personal interviews. Puncture biopsies of suspected As-related cancers were collected from willing patients and histopathological analysis was performed at Al Ameen Medical College. We were unable to analyze urine samples, which indicate recent As exposure, as individuals were reluctant to submit urine.
All samples except punch biopsies were analyzed at SOES for As using FI-HG-AAS. The total concentration of As in the water (arsenite and arsenate) was measured after potassium bromate oxidation [34]. For hair, nail, soil and food samples, total As was determined after teflon-bomb digestion. Sample collection, processing, digestion of samples, analytical procedures and details of the instrumentation including the flow-injection system have been reported earlier [34–38]. Additionally, six soil samples were also analyzed at CERAR – University of South Australia by ICP-MS (inductively coupled plasma-mass spectrometry) (Agilent 7500ce) following microwave digestion (CEM Microwave Accelerated Reaction System 5, MARS5) in order to assess the levels of As and other elements in the soil samples.
2.3. Quality assurance and quality control
For quality control, inter-laboratory tests were performed on water, hair, food grain and soil samples as reported in earlier publications [34,37–39]. USEPA standardized water reference materials were also analyzed as reported previously [38]. National Institute of Standard and Technology (NIST) standard reference material (SRM) 2711 (Montana soil) was used to validate the results of As and other metals in soil. NIST 1643e (trace elements in water), NIST 2709 (San Joaquin Soil), SRM GBW 07601 (human hair, china) and NIST 1568a (rice) were routinely analyzed to validate the results during analysis of water, soil, hair and food grain samples [34]. Spikes and duplicates were also analyzed.
2.4. Statistical analysis
Descriptive statistics were calculated for the baseline characteristics of the affected population. All statistical analyses were performed using SPSS. Spearman two-tailed p value of <0.05 was considered to be statistically significant.
3. Results and Discussion
3.1. Water analysis
During community interviews, elderly residents recalled that dug-wells were the primary source for all water needs over 20 years ago. Due to endemic guinea worm disease, hand-pumps and deep tube-wells were installed in the districts of Gulbarga, Raichur, and Bijapur through the Guinea Worm Eradication Programme starting in 1984 [40]. Community members also reported that skin lesions similar to those diagnosed by the medical team as arsenical first appeared roughly 15 years ago.
The first column of Table 1 shows the distribution of As in water sources in the present survey. Arsenic concentration in the hand tube-well thought to be the main source of contamination in Kiradalli Tanda was 303 μg L−1 in July 2009, and many villagers reported using this source for an extended time period. The source was abandoned after our initial survey, and an alternative groundwater source supplied by pipeline was installed that contained 63 μg L−1 of As. The primary large dug-well used by the community before the installation of other sources is, still present but not in use; water from this well was found to have an arsenic content of 245 μg L−1. While the villagers of Kiradalli Tanda appeared to have used this dug-well for many years, we are unaware of when it became As-contaminated. During our surveys in Kiradalli Tanda, the local Public Health Engineering Department was informed about the presence of arsenicosis patients, and they quickly arranged As-safe water for the village initially by tanker and later through a newly dug bore-well. Furthermore, the local Panchayat Raj and Health Departments arranged for regular medical follow-up visits to the community by physicians from a nearby hospital.
Table 1.
Summary of recent groundwater quality surveys investigating As contamination in Gulbarga, Yadgir, and Raichur districts, Karnataka.
| Present Study | GOK (2008) | GOK (2008) | |
|---|---|---|---|
| Districts sampled | Yadgir and Raichur | Gulbarga | Raichur |
| n | 59 | 464 | 316 |
| Arsenic concentration (μg L−1) | |||
| <3 | 3 (5.2%) | 264 (56.9%) | 237 (75.0%) |
| 3–10 | 10 (17.2%) | 175 (37.7%) | 3 (0.9%) |
| >10–50 | 28 (48.3%) | 16 (3.4%) | 52 (16.5%) |
| >50–100 | 11 (19.0%) | 5 (1.1%) | 18 (5.7%) |
| >100–200 | 4 (6.9%) | 3 (0.6%) | 3 (0.9%) |
| >200–300 | 2 (3.4%) | 0 (0%) | 2 (0.6%) |
| >300–400 | 1 (1.7%) | 0 (0%) | 0 (0%) |
| >400–500 | 0 (0%) | 0 (0%) | 0 (0%) |
| >500 | 0 (0%) | 1 (0.2%) | 1 (0.3%) |
In separate surveys in 2008, Zilla Panchayat Groundwater Division, Government of Karnataka and Department of Mines & Geology, Government of Karnataka in collaboration with UNICEF [28,29] also analyzed hand tube-well water samples in Gulbarga and Raichur Districts, respectively (Table 1). It is important to note that the sampling methodologies significantly differed between the present study and the GOK-UNICEF surveys. In the present study, the majority of collected groundwater samples were concentrated around an area in Yadgir District with identified arsenic-related health effects. The larger GOK-UNICEF studies systematically surveyed a large number of communities within Gulbarga (Shorapur Taluka only) and Raichur Districts. The GOK-UNICEF surveys identified not only areas with considerably higher As outside of Kiradalli Tanda but also an additional five villages in Gulbarga and 10 villages in Raichur with drinking water As concentration >50 μg L−1. Furthermore, these surveys identified a total of 14 additional villages in Gulbarga and 39 in Raichur with drinking water As > 10 μg L−1. It is not known whether these contaminated water supplies continue to be used and importantly whether individuals from these areas have been screened for As-related health effects.
3.2. Soil samples
During recent inspection (July 2012) of the Mukangavi mine site, one shaft was observed measuring about 150 m with two drives of about 450 m in length and 1.5 m in width on the north and south sides of the shaft. The shaft and drives are filled with loose excavated material. Only a small heap of material, not a large amount of tailings, was observed near the shaft. The host rock has been described previously as amphibolites crossed by quartz stringers with sulphides, including pyrite, pyrrhotite and arsenopyrite. Additionally, the ore zone was estimated at 1.5–2 mega ton, and as of 1996, three lodes had been identified with over 600 meters in length accessible to mining [41].
The study area is located within the command area of the Upper Krishna Project (UKP), an irrigation development project initiated in 1965, consisting of dams on the Krishna River as well as canals and associated irrigation schemes that aimed to develop irrigation potential in the Upper Krishna River Basin, including the districts of Raichur and Gulbarga [42]. Water table fluctuation following the hydrologic changes from the UKP likely resulted in the filtration of water through soil and fractures of amphibolite and associated minerals in the parental rock or the remnants of excavated mining material. It is hypothesized that this contributed to the release of these minerals into the groundwater due to disintegration, leachification, and chemical decomposition of arsenic from arsenopyrite associated with amphibolites.
Table 2 reports the concentrations of As and other elements of 6 topsoil samples (analyzed by ICP-MS) collected from the more densely populated areas of Heggandoddi, Jainapur, Kiradalli Tanda (samples 1–3) and the outskirts of Jainapur village (samples 4–6). These residential samples (samples 1–3 of Table 2) were all found to exceed 2000 mg kg−1 with a maximum concentration of 9136 mg kg−1 possibly indicating the presence of mine tailings. The analysis for As of 4 additional soil samples, also from the residential areas of these three villages revealed three samples with elevated levels of As (range: 2203–4264 mg/kg) much greater than the typical background level of 5–10 mg kg−1 in soil [10]. Three samples collected from Gowdigeri village had As levels well within the background levels in soil. This community is farther away from known historic gold mining activity than the other surveyed villages and was also found to contain significantly lower groundwater As concentrations (Fig. 2). Based on this limited spatial information, variable natural geochemical dispersion as well as historic mining activities appear to have resulted in heterogeneous local environmental arsenic contamination; however, additional and more detailed groundwater and soil studies are needed to determine the nature and extent of the source and mobilization of As.
Table 2.
Concentration of As and other elements (mg kg−1) in soils measured by ICP-MS. Variation of As concentration of the same samples measured at SOES laboratory by FI-HG-AAS method is within the 10% error margin on the reported values by the two methods.
| Soil ID | GPS location | Na | Mg | Al | K | Ca | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Heggandoddi village soil | N16° 35.858′, E76° 38.049′ | 286 | 12086 | 17149 | 5007 | 34241 | 88 | 6039 | 35016 | 26 | 53 | 73 | 157 | 5884 | 30 |
| 2. Jainapur village soil | N16° 36.960′, E76° 37.026′ | 230 | 12188 | 17534 | 5031 | 29143 | 89 | 689 | 38165 | 33 | 58 | 82 | 92 | 9136 | 34 |
| 3. Kiradalli Tanda village soil | N16° 37.132′, E76° 36.788′ | 272 | 12232 | 17615 | 5002 | 36576 | 87 | 656 | 35390 | 27 | 52 | 72 | 90 | 6711 | 30 |
| 4. Jainapur village soil 4 | N16° 36.813′, E76° 37.408′ | 81 | 4134 | 23494 | 2235 | 4886 | 88 | 962 | 45250 | 30 | 77 | 80 | 63 | 334 | 6 |
| 5. Jainapur village soil 5 | N16° 36.878′, E76° 37.313′ | 81 | 3652 | 33647 | 1814 | 4984 | 112 | 1185 | 50383 | 27 | 91 | 75 | 61 | 313 | 10 |
| 6. Jainapur village soil 6 | N16° 36.746′, E76° 37.493′ | 117 | 6442 | 24051 | 2532 | 7648 | 107 | 762 | 44397 | 30 | 76 | 93 | 92 | 99 | 9 |
3.3. Food grains
Fourteen samples of local staple foods (pearl millet or bajra and pigeon pea or red gram/arhar) were collected from in and around Kiradalli Tanda village and analyzed for As. The agricultural cultivations in this area are on the sides of hillocks where the average As concentration of soil is 4.5 mg kg−1. As the hillside agricultural plots are above the groundwater table and the topology of the land results in the drainage of rainwater down from these slopes to the valleys, As and other heavy metals were expected to be flushed from the soil and only minimally enter into the food supply. Arsenic concentrations of the food grains ranged between 12 to 112 μg kg−1, considerably lower than the recommended upper limit of As in rice (1000 μg kg−1) [43].
3.4. Hair and nail samples
Elevated As levels in hair and nail samples are an indicator of exposure over the last 3–18 months, depending on the growth rate and what section of hair or nail is analysed. For individuals with no known As exposure, concentrations of As in hair generally range from 20 to 200 μg kg−1 and in nails from 20 to 500 μg kg−1 [4]. Of 170 samples, 100% of both hair and nails were found to exceed the upper limit of unexposed individuals (Table 3). Of the 170 individuals that provided samples, 43 were below 18 years of age. Despite having an elevated level of biological As, many are not showing arsenical skin lesions and are possibly sub-clinically affected. Arsenic concentration between genders was highly similar for both hair (p = 0.937) and nail samples (p = 0.927). The spearman correlation (necessitated due to skewed distributions) was positive and strong between age and As concentrations in the collected samples of hair (r = 0.682, p <0.0001) and nail (r = 0.685, p <0.0001). Arsenic levels of these biomarkers strongly correlated with each other (r = 0.937, p <0.0001). The high levels of As in the hair and nails of the children from the study area after the drinking water source has been changed indicate that besides drinking water there exists some other source of As. It is possible that the extremely high concentrations of As in the residential soil may be a significant source of As for small children as well as other community members via ingestion.
Table 3.
Arsenic concentrations in hair and nail samples of individuals from Kiradalli Tanda.
| Hair (μg kg−1) | Nail (μg kg−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| n | P10 | P50 | P90 | Max | Min | N | P10 | P50 | P90 | Max | Min | ||
| Male | 87 | 903 | 2168 | 4158 | 6265 | 456 | 87 | 1236 | 3390 | 5922 | 8213 | 696 | |
| Gender | Female | 83 | 900 | 2062 | 5262 | 6976 | 464 | 83 | 1237 | 3196 | 6748 | 9815 | 920 |
| 1st (up to 18) | 43 | 582 | 1001 | 1764 | 2965 | 456 | 43 | 941 | 2032 | 2981 | 3265 | 696 | |
| Age Quartiles (Yrs) | 2nd (19–28) | 43 | 974 | 1920 | 4413 | 6321 | 795 | 43 | 1857 | 3005 | 6077 | 7726 | 1029 |
| 3rd (29–42) | 42 | 1305 | 2877 | 4246 | 6162 | 1007 | 42 | 3021 | 4073 | 7155 | 8213 | 2402 | |
| 4th (43–75) | 42 | 1689 | 3007 | 6027 | 6976 | 1025 | 42 | 2980 | 4341 | 7026 | 9815 | 2062 | |
P10, P50, and P90 are the 10th, 50th, and 90th percentiles, respectively.
Based on the time-course of As-like symptoms reported by the community as well as the presence of overt arsenical symptoms in recent surveys, we are hypothesizing that the primary route of exposure in the study area was via contaminated groundwater used for drinking. Arsenical symptoms were reported by the elderly residents of Kiradalli Tanda as emerging first around 15 years ago, corresponding with the drilling of tubewells and a transition in the drinking water supply source. As mining operations at Mukangavi gold mine were resumed from 1980–1994, it is possible that these activities or naturally present soil As in high concentrations were however the major factors in the emergence of arsenicosis. Unlike contaminated groundwater, we are unaware of residential human exposure to arsenic-contaminated soil leading to overt arsenicosis, notably dermatological symptoms as in the present study. Reports have been published though on increased As biomarker levels with soil exposure [17] as well as contaminated soil being associated with decreased weeks of gestation for newborns [44] or significant increased risks of multiple types of cancer [45,46].
3.5. Clinical investigations
A total of 181 individuals were screened for symptoms of chronic As toxicity and complete demographic information was collected for 171. High rates of arsenicosis were identified with 58.6% of screened individuals presenting with at least one related symptoms (Table 4). Dermatological symptoms were not found to be significantly different between genders with the exception of mucous membrane melanosis, which was only observed in 8 females; we are uncertain whether As alone is responsible for this. The majority of dermatological symptoms were found to sharply increase with increasing age quartiles, reaching a similar prevalence in the oldest two quartiles where individuals were 29 years and older (Fig. 3). Table 5 presents the clinical details of 12 patients having pre-cancer (Bowen’s disease), gangrene (amputated) and non-healing ulcer. Punch biopsies were performed for the suspected cancers of four willing adult arsenicosis patients. Some clinically suspected carcinoma patients refused biopsy. Among the four completed histopathological reports, two samples showed nuclear atypia, mitosis and disarray in cellular arrangement and hyperchromatic nuclei.
Table 4.
Age distribution of individuals screened and identified with arsenical skin lesions
| Age wise distribution of population
|
No. people who came to the medical camp and were screened | No. of people having arsenic skin lesions | |
|---|---|---|---|
| Age range (Yrs) | No. of people | ||
| > 25 | 216 | 94 (43.5% of 216) | 85 (90.4%) |
| 20 – 25 | 60 | 42 (70% of 60) | 20 (47.6%) |
| <20 | 259 | 45 (17.4% of 259) | 1 (2.2%) |
| Total | 535 | 181 (33.8% of 535) | 106 (58.6%) of 181 population screened |
Fig. 3.
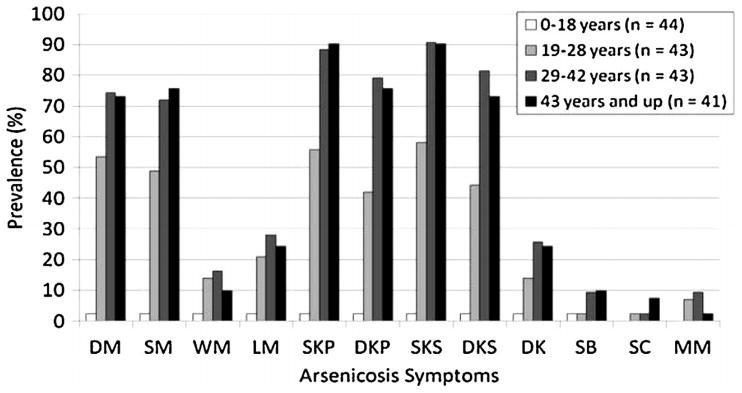
Comparative prevalence of dermatologic symptoms of identified individuals of Kiradalli Tanda with arsenicosis (age quartiles). Abbreviations: DM, diffuse melanosis; SM, spotted melanosis; WM, whole body melanosis; LM, leucomelanosis (white-spotted pigmentation alongside black-spotted); SKP, spotted keratosis palm; DKP, diffuse keratosis palm; SKS, spotted keratosis sole; DKS, diffuse keratosis sole; DK, dorsal keratosis; SB, suspected Bowen’s Disease; SC, suspected skin cancer; MM, mucous melanosis.
Table 5.
Clinical investigation (mainly dermatological symptoms) of 12 patients having suspected cancer, pre-cancer (Bowen’s), gangrene (amputated) and non-healing ulcer (-- not present, + mild, ++ moderate, and +++ severe)
| Patient’s No. |
Age (Yr) /Sex |
Melanosis | Leuco- melanosis |
Keratosis (palm) | Keratosis (sole) | Dorsal Keratosis |
Suspected Bowen’s/ Pre-cancer/ Gangrene |
Suspected Cancer (type) |
Other including bronchitis |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Diffuse | Spotted | Whole body |
Spotted | Diffuse | Spotted | Diffuse | |||||||
| 4 | 35 /F | + | ++ | -- | + | +++ | +++ | +++ | +++ | +++ | Whole body Bowen’s | Suspected Cancer | |
| 12 | 14 /F | + | ++ | + | ++ | ++ | + | ++ | ++ | + | Multiple suspected Bowen’s | Bronchitis -2 years | |
| 13 | 22 /F | ++ | ++ | ++ | + | +++ | ++ | +++ | ++ | + | Suspected cancer | ||
| 14 | 45 /F | + | + | -- | ++ | ++ | + | +++ | ++ | + | Operated for gangrene | ||
| 18 | 46 /F | + | ++ | ++ | -- | ++ | ++ | ++ | ++ | Non healing ulcer | Bronchitis -2 years | ||
| 30 | 35 /M | ++ | ++ | - | ++ | ++ | ++ | ++ | ++ | Suspected Bowen’s | Leg amputated | ||
| 34 | 45 /F | ++ | ++ | + | + | ++ | +++ | +++ | +++ | +++ | Suspected multiple Bowen’s | Suspected cancer | Bronchitis –2 years; Leg amputated |
| 35 | 75 /F | + | + | + | + | ++ | + | +++ | +++ | + | Suspected Bowen’s | Bronchitis | |
| 58 | 65 /M | ++ | ++ | -- | -- | + | + | ++ | + | Suspected Bowen’s | |||
| 64 | 40 /F | + | + | -- | -- | ++ | ++ | ++ | ++ | + | Suspected Bowen’s | ||
| 66 | 38 /M | + | ++ | -- | ++ | ++ | ++ | ++ | ++ | Suspected Bowen’s | |||
| 67 | 45 /M | + | ++ | -- | -- | +++ | ++ | ++ | ++ | ++ | Suspected cancer on palm | ||
From histopathological observations, three patients were found to have confirmed arsenical keratosis and one patient was confirmed with features highly consistent with Bowen’s disease (in situ carcinoma). The pathological analysis of the suspected Bowen’s disease biopsy revealed an epidermis composed of keratinized stratified squamous epithelium, which is thickened. The basement membrane is intact and there is no evidence of infiltration (Fig. 4A). Furthermore, there was moderate hyperkeratosis, moderate acanthosis with focal dyskeratosis and keratin plugging. The cells exhibited cellular as well as nuclear atypia throughout the full thickness of the epidermis and were arranged in a ‘windblown appearance’. The cells appeared large and round having hyperchromatic nuclei containing prominent nucleoli with moderate eosinophilic cytoplasm. The cells also exhibited mild-moderate pleomorphism with disoriented maturation order (Fig. 4B). Since the health effects of chronic As exposure take years to manifest possibly as carcinomas, further detailed histopathological evaluation is needed in this population to rule out other non-melanoma skin cancers.
Fig. 4.

Histopathological section showing, A) thickened epidermis (10x) and B) Bowen’s disease (carcinoma in situ) (45x).
Most residents of Kiradalli Tanda village are primarily daily wage laborers. There was no history of loss of appetite among the clinically examined group. There is no apparent nutritional deficiency in the community; however, additional study is required to assess the prevalence of malnutrition in the area, which may play a significant role in the occurrence of arsenicosis [47]. When asked about deceased family members and skin lesions similar to arsenicosis, 12 individuals were named that had died with comparable symptoms in the last 10 years. Furthermore, four individuals who had skin lesions and died of cancer in the last 5 years were also reported. Death certificates for these individuals, however, could not be obtained. Fig. 5 shows the dermatological symptoms of three severe arsenicosis patients with associated photographs.
Fig. 5.
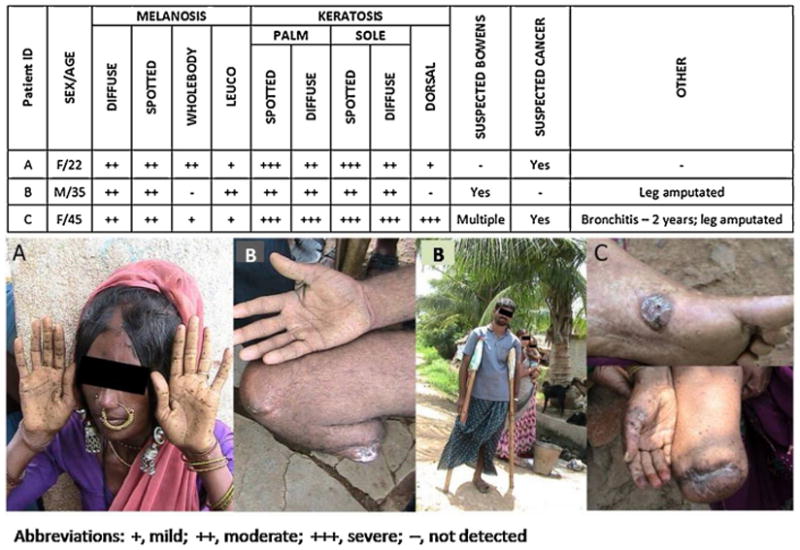
Characteristics of three individuals from Kiradalli Tanda suffering from chronic As toxicity.
4. Conclusions
Elevated biomarker arsenic levels in communities with underlying greenstone geology as well as historic gold mining activity have been reported previously [17–23]. This study report, however, is to our knowledge one of the first to describe overt arsenicosis in such an area as well as in this region of Karnataka, India. In the present study, 106 of the 181 (58.6%) individuals of Kiradalli Tanda village screened for arsenical symptoms presented with at least one skin lesion specifically related to chronic As toxicity. Of these individuals including children, 94.5% volunteered for hair and nail analysis with 100% of these found to have elevated As levels in these samples. This evidence indicates that those without identified symptoms are potentially sub-clinically affected.
While groundwater contamination is hypothesized to be the main route of As exposure, the presence of highly elevated As levels in soil samples from the area warrants further investigation of other possible significant routes of exposure as well as their relative contributions to toxicity. The historic gold mine workings of the Mangalur belt may be a significant contributor to localized pollution in this and other similar regions in Karnataka. Consequently, additional studies to determine, temporal variation, the nature of As mobilization, particularly whether previous and ongoing mining activities are playing a role, are crucial in guiding efforts to identify at-risk communities and remedy the effects of this contamination.
If historic gold mining has played a role in contamination, increasing interest in the reopening of previously closed mines [48] and the prospecting of new regions in the Mangalur belt and around Karnataka [49] should be conducted along with sound monitoring and assessment of the environment and the health effects resulting from its degradation. As the GOK-UNICEF surveys identified many additional communities with elevated groundwater arsenic, environmental contamination appears to be more widespread than the present study area. Both medical screening and regular water quality analysis programs should be continued or organized in the region immediately.
Supplementary Material
Acknowledgments
Thanks to the Hamdard National Foundation, India for the award to Dipankar Chakraborti of INR 5 million to continue As research work in India. The authors would also like to thank Prof. Ravi Naidu, Director, CERAR, Australia for the opportunity to use the microwave digestion and ICP-MS for soil analysis.
Footnotes
Ethical committee permission
Ethical approval for this study was obtained from the Institutional Ethical Committee of Al-Ameen Medical College, Karnataka, India as per the ethical guidelines for Biomedical Research on human participants by the Indian Council for Medical Research [50]. Informed consent was obtained from the participants before the study started.
Competing interests: None declared.
References
- 1.Mukherjee A, Sengupta MK, Hossain MA, Ahamed S, Das B, Nayak B, Lodh D, Rahman MM, Chakraborti D. Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr. 2006;24:142–163. [PubMed] [Google Scholar]
- 2.IPCS. Arsenic and Arsenic Compounds. 2. World Health Organization; 2001. Environmental Health Criteria 224. [Google Scholar]
- 3.Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, Geen AV, Graziano J, Ahsan H. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376:252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NRC. Arsenic in Drinking Water. National Academy of Sciences; Washington, DC: 1999. pp. 182–184. [Google Scholar]
- 5.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Drinking-water Disinfectants and Contaminants, including Arsenic. Vol. 84. World Health Organization - International Agency for Research on Cancer; Lyon: 2004. Arsenic in drinking-water; pp. 39–270. [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, Ehrenstein OV, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vahter M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- 8.Nordstrom DK. Worldwide occurrences of arsenic in ground water. Sci. 2002;296:2143–2145. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- 9.Ravenscroft P, Brammer H, Richards K. Arsenic Pollution: a Global Synthesis. 1. Wiley-Blackwell; West Sussex: 2009. [Google Scholar]
- 10.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. App Geochem. 2002;17:517–568. [Google Scholar]
- 11.Williams M. Arsenic in mine waters: an international study. Environ Geol. 2001;40:267–278. [Google Scholar]
- 12.Eisler R. Arsenic hazards to humans, plants, and animals from gold mining. In: Ware GW, editor. Rev Environ Contam Toxicol. Vol. 180. Springer-Verlag; New York: 2004. pp. 133–166. [DOI] [PubMed] [Google Scholar]
- 13.Garelick H, Jones H, Dybowska D, Valsami-Jones E. Arsenic pollution sources. In: Whitacre DM, Garelick H, Jones H, editors. Rev Environ Contam Toxicol, Arsenic Pollution and Remediation: An International Perspective. Vol. 197. Springer; New York: 2008. pp. 41–44. [Google Scholar]
- 14.Haffert L, Craw D. Mineralogical controls on environmental mobility of arsenic from historic mine processing residues, New Zealand. App Geochem. 2008;23:1467–1483. [Google Scholar]
- 15.Pauwels H, Pettenati M, Greffié C. The combined effect of abandoned mines and agriculture on groundwater chemistry. J Contam Hydrol. 2010;115:64–78. doi: 10.1016/j.jconhyd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu CP, Luo CL, Gao Y, Li FB, Lin LW, Wu CA, Li XD. Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China. Environ Poll. 2010;158:820–826. doi: 10.1016/j.envpol.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Hinwood AL, Sim MR, Jolley D, de Klerk N, Bastone EB, Gerostamoulos J, Drummer OH. Hair and toenail arsenic concentrations of resident living in areas with high environmental arsenic concentrations. Environ Health Perspect. 2003;111:187–193. doi: 10.1289/ehp.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce DC, Dowling K, Gerson AR, Sim MR, Sutton SR, Newville M, Russell R, McOrist G. Arsenic microdistribution and speciation in toenail clippings of children living in a historic gold mining area. Sci Total Environ. 2010;408:2590–2599. doi: 10.1016/j.scitotenv.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Basu N, Nam D, Kwansaa-Ansah E, Renne EP, Nriagu JO. Multiple metals exposure in a small-scale artisanal gold mining community. Environ Res. 2011;111:463–467. doi: 10.1016/j.envres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Kusiak RA, Springer J, Ritche AC, Muller J. Carcinoma of the lung in Ontario gold miners: possible aetiological factors. Br J Industrial Med. 1991;48:808–817. doi: 10.1136/oem.48.12.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonato L, Moulin JJ, Javelaud B, Ferro G, Wild P, Winkelmann R, Saracci R. A retrospective mortality study of workers exposed to arsenic in a gold mine and refinery in France. Am J Industrial Med. 1994;25:625–633. doi: 10.1002/ajim.4700250503. [DOI] [PubMed] [Google Scholar]
- 22.Rapant S, Dietzová Z, Cicmanová S. Environmental and health risk assessment in abandoned mining area, Zlata Idka, Slovakia. Environ Geol. 2006;51:387–397. [Google Scholar]
- 23.Matschullat J, Borba RP, Deschamps E, Figueiredo BR, Gabrio T, Schwenk M. Human and environmental contamination in the Iron Quadrangle. Brazil App Geochem. 2000;15:181–190. [Google Scholar]
- 24.Oyarzun R, Lillo J, Higueras P, Oyarzun J, Maturana H. Strong arsenic enrichment in sediments from the Elqui watershed, Northern Chile: industrial (gold mining at El Indio-Tambo district) vs. geologic processes. J Geochem Explor. 2004;84:53–64. [Google Scholar]
- 25.Borba RP, Figueiredo BR, Rawlins B, Matschullat J. Geochemical distribution of arsenic in waters, sediments and weathered gold mineralized rocks from Iron Quadrangle. Brazil Environ Geol. 2003;44:39–52. [Google Scholar]
- 26.Sahoo NR, Pandalai HS, Subramanyam A. Secondary geochemical dispersion in the Precambrian auriferous Hutti-Maski schist belt, Raichur district, Karnataka, India. Part II. Application of factorial design in the analysis of secondary dispersion of As. J Geochem Explor. 2000;71:291–303. [Google Scholar]
- 27.DGML, Deccan Gold Mines Limited. Letter of Offer to Shareholders. 2004:20. http://www.sebi.gov.in/dp/deccan.pdf - Government of India, Securities and Exchange Board of India.
- 28.GOK. Test Report of water samples of drinking water supply schemes in Shorapur Taluka district. Gulbarga, Government of Karnataka, Zilla Panchayet Gulbarga. Senior Geologist. Ground water Division, Zilla Panchayet; Gulbarga: 2008. [Google Scholar]
- 29.GOK. The detailed study on the presence of arsenic in ground water in Raichur district. Report prepared by Department of Mines and Geology, Govt of Karnataka with collaboration of UNICEF. 2010 Jan; [Google Scholar]
- 30.Kozhisseri D. Now arsenic in Karnataka, Study suspects gold mines to be the culprit. Down to Earth. 2008;14:1–15. [Google Scholar]
- 31.Sivanandan TV. Rare skin cancer breaks out in hamlet due to arsenic poisoning. The Hindu; Jun 13, 2009. http://www.thehindu.com/2009/06/13/stories/2009061358580100.htm. [Google Scholar]
- 32.Sivanandan TV. Analysis shows arsenic high in water at Kiradalli Tanda. The Hindu; Jun 14, 2009. http://www.thehindu.com/2009/06/14/stories/2009061450040100.htm. [Google Scholar]
- 33.Tenginkai SG, Ugarkar AG. Gold-bearing rocks of Mangalur greenstone belt, Gulbarga District, Karnataka. Proceedings of the Indian Academy of Sciences. Earth and Planetary Sci. 1987;96:103–117. [Google Scholar]
- 34.Pal A, Nayak B, Das B, Hossain MA, Ahamed S, Chakraborti D. Additional danger of arsenic exposure through inhalation from burning of cow dung cakes laced with arsenic as a fuel in arsenic affected villages in Ganga–Meghna–Brahmaputra plain. J Environ Monitor. 2007;9:1067–1070. doi: 10.1039/b709339j. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee A, Das D, Mandal BK, Chowdhury TR, Samanta G, Chakraborti D. Arsenic in ground water in six districts of West Bengal, India: the biggest arsenic calamity in the world. Part I. Arsenic species in drinking water and urine of the affected people. Analyst. 1995;120:643–650. doi: 10.1039/an9952000917. [DOI] [PubMed] [Google Scholar]
- 36.Das D, Chatterjee A, Mandal BK, Samanta G, Chakraborti D. Arsenic in ground water in six districts of West Bengal, India: the biggest arsenic calamity in the world. Part II. Arsenic concentration in drinking water, hair, nails, urine, skin-scale and liver tissue (biopsy) of the affected people. Analyst. 1995;120:917–924. doi: 10.1039/an9952000917. [DOI] [PubMed] [Google Scholar]
- 37.Samanta G, Chakraborti D. Flow injection atomic absorption spectrometry for the standardization of arsenic, lead and mercury in environmental and biological standard reference materials. Fresnius J Anal Chem. 1997;357:827–832. [Google Scholar]
- 38.Samanta G, Chowdhury TR, Mandal BK, Biswas BK, Chowdhury UK, Basu GK, Chanda CR, Lodh D, Charkraborti D. Flow injection hydride generation atomic absorption spectrometry for determination of arsenic in water and biological samples from arsenic affected districts of West Bengal, India and Bangladesh. Microchem J. 1999;62:174–191. [Google Scholar]
- 39.Rahman MM, Mukherjee DP, Sengupta MK, Chowdhury UK, Lodh D, Chanda CR, Roy S, Selim M, Zaman Q, Milton AH, Sahidulla SM, Rahman MT, Chakraborti D. Effectiveness and mobility of arsenic field testing kits: are the million dollar screening projects effective or not. Environ Sci Technol. 2002;36:5385–5394. doi: 10.1021/es020591o. [DOI] [PubMed] [Google Scholar]
- 40.Nadim A, Karam M, Hajar M. Eradication of Dracunculiasis in India – Report of the International Certification Team. World Health Organization; Nov, 1999. [Google Scholar]
- 41.Radhakrishna BP. Mineral Resources of Karnataka. Geological Society of India; Bangalore: 1996. pp. 259–260. [Google Scholar]
- 42.World Bank. India-Upper Krishna Irrigation Project (Phase II) - Implementation Completion Report (Report No 18091) World Bank - Rural Development Sector Unit, South Asia Region; Jun 29, 1998. [Google Scholar]
- 43.National Food Authority. Australian food standard code. Canberra: Australian Government Publication Service; Mar, 1993. [Google Scholar]
- 44.Aelion CM, Davis HT, Lawson AB, Cai B, McDermott S. Associations of estimated residential soil arsenic and lead concentrations and community-level environmental measures with mother–child health conditions in South Carolina. Health & Place. 2012;18:774–781. doi: 10.1016/j.healthplace.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearce DC, Dowling K, Sim MR. Cancer incidence and soil arsenic exposure in a historical gold mining area in Victoria, Australia: a geospatial analysis. J Expo Sci Env Epid. 2012;22:248–257. doi: 10.1038/jes.2012.15. [DOI] [PubMed] [Google Scholar]
- 46.Putila JJ, Guo NL. Association of arsenic exposure with lung cancer incidence rates in the United States. PLoS One. 2011;6:e25886. doi: 10.1371/journal.pone.0025886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakraborti D, Das B, Nayak B, Pal A, Rahman MM, Sengupta MK, Hossain MA, Ahamed S, Biswas KC, Sahu M, Saha KC, Mukherjee SC, Pati S, Dutta RN, Quamruzzman Q. Groundwater arsenic contamination and its adverse health effects in the Ganga-Meghna-Brahmaputra plain. In: Roy K, editor. Book: Arsenic calamity of groundwater in Bangladesh: contamination in water, soil and plants. Nihon University; Japan: 2008. pp. 13–52. [Google Scholar]
- 48.Sivanandan TV. Mangalur mines likely to strike gold again. The Hindu; Dec 19, 2008. http://www.thehindu.com/2008/12/19/stories/2008121952380600.htm. [Google Scholar]
- 49.DGML. Status of applications (as on 21 April 2010) Deccan Gold Mining Limited; 2010. [Accessed on 24 August 2011.]. http://www.deccangoldmines.com/pdf/DGML-Website-updated-dt.21.4.2010.doc. [Google Scholar]
- 50.ICMR. Ethical guidelines for biomedical research on human participants. Chapter III. Indian Council of Medical Research; New Delhi: 2006. pp. 21–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




