Abstract
Background
Reduced regional cerebral blood flow (rCBF) is a well-established finding in Alzheimer's disease (AD), although fewer studies have examined the role of increased regional cerebrovascular resistance. By calculating the ratio of mean arterial pressure to rCBF, it is possible to estimate an index of regional cerebrovascular resistance (CVRi) that may be a sensitive measure of occult cerebrovascular disease.
Objective
To compare probable AD patients to mild cognitive impairment (MCI) and normal control (NC) participants on CVRi, the ratio of mean arterial pressure to rCBF.
Methods
Eighty-one participants (12 AD, 23 MCI, 46 NC) were compared on CVRi using voxel-wise analyses. Region-of-interest analyses examined correlations between subcortical CVRi and both cognition and white matter lesion (WML) volume.
Results
Voxel-wise analyses revealed CVRi elevation in AD relative to NCs (subcortical, medial temporal, posterior cingulate, precuneus, inferior parietal, superior temporal) and MCI (subcortical, posterior cingulate). MCI participants exhibited intermediate CVRi values within cortical and medial temporal areas. Significant CVRi clusters were larger and more widespread than those of parallel CBF analyses. Among MCI and AD participants, subcortical CVRi elevation was associated with lower Dementia Rating Scale score (r = −0.52, p = 0.001, for both thalamus and caudate), and caudate CVRi correlated with WML volume (r = 0.45, p = 0.001).
Conclusions
Cortical and subcortical CVRi is elevated in AD, particularly within the caudate and thalamus, where it is associated with decreased cognitive performance and increased WMLs. Findings suggest CVRi may play a role in cognitive decline and cerebrovascular disease in MCI and AD.
Keywords: Alzheimer's disease, cerebral blood flow, cerebrovascular resistance, mild cognitive impairment
INTRODUCTION
Neuropathological studies suggest that up to 30% of probable Alzheimer's disease (AD) is accounted for by a mix of both AD pathology (plaques and tangles) and vascular pathology (ischemic and hemorrhagic strokes) [1]. Cumulative evidence indicates that this vascular contribution to the clinical manifestation of AD is not limited to the effects of clinical stroke, and that occult cerebrovascular pathology, including atherosclerosis of the circle of Willis [2] and sub-cortical small vessel disease [3], is present in nearly 80% of AD cases [3]. These atherosclerotic and arteriosclerotic lesions may develop slowly in parallel with AD pathologies, adding to cognitive decline and lowering the threshold for the clinical manifestation of AD. Although there have been significant advances in the early detection of preclinical biomarkers for AD pathology, considerably less attention has been paid to preclinical biomarkers for vascular lesions in AD. Current methods for imaging subclinical vascular lesions rely on signal changes that are associated with irreversible parenchymal damage, including incidental stroke, lacunar infarction, hemorrhage, and ischemic white matter lesions (WMLs). Development of methodologies capable of detecting cerebrovascular dysfunction prior to the manifestation of these frank lesions would represent a major advancement in early detection and treatment.
Reduced regional cerebral blood flow (rCBF) may be one such earlier marker of cerebrovascular dysfunction, and studies consistently find that AD is associated with decreased rCBF within specific brain areas [4]. Interestingly, the rCBF reductions found in AD tend to be within areas prone to AD pathology, such as the posterior cingulate/precuneus and medial temporal lobes, in addition to areas prone to cerebrovascular disease, such as the subcortical grey matter (e.g., cau-date and thalamus) [4]. This proximity may be due to the fact that rCBF has multiple determinants, including neural activity, neurovascular signaling, local vascular tissue function, and autonomic function, any number of which may be impacted by AD and/or vascular disease processes [5].
From a broader physiologic perspective, the most important determinant of CBF is blood pressure (BP). Hypertension in midlife, and both high and low BP in latelife, are associated with increased risk of AD [6]. In addition, BP elevation is associated with reduced CBF in older adults. Together these data suggest that BP values should be taken into account when measuring CBF in AD and in those at risk of AD. The relationship between arterial pressure and CBF is defined by the degree of cerebrovascular resistance (CVR), expressed as the ratio of arterial pressure (Pα) to CBF (CVR = Pα/CBF) [7]. In normal adults at steady state, cerebrovascular autoregulatory mechanisms modulate CVR in order to maintain CBF at approximately 45 to 54 mL 100 g−1 min−1 across a broad BP range (mean arterial pressure of approximately 50 to 150 mmHg) [8]. However, recent studies of acute changes in BP and CBF in AD patients have found that the disease is associated with CVR elevation and autoregulatory dysfunction [9], which is in turn associated with atherosclerosis within the Circle of Willis [10]. These studies have used transcranial doppler ultrasound, which provides a valuable physiological measure of acute changes in global CVR. Although the strength of transcranial doppler ultra-sound lies in its high temporal resolution, allowing for investigation of acute variation in CVR, it is limited by very low spatial resolution. Thus, the question of which specific brain areas may be particularly prone to elevated CVR remains unanswered.
Beyond acute autoregulatory effects, aging is also associated with a steady decline in resting rCBF and a steady increase in resting BP, both of which are in turn associated with increased risk of cognitive decline and dementia. Furthermore, a recent study by Muller and colleagues found a significant interaction between resting BP and resting CBF in predicting brain atrophy, such that older adults with both elevated BP and reduced global CBF were at particularly high risk of atrophy [11]. This finding suggests that a chronic, insidious change in the ratio of resting BP to resting rCBF could represent a biomarker for vascular dys-function in an evolving Alzheimer's dementia. Many prior studies have examined CBF reductions in AD, although few have taken BP into account. We sought to compare patients with probable AD to those with mild cognitive impairment (MCI) and to normally aging control (NC) participants on a calculated ratio of resting mean arterial pressure (MAP) to rCBF in order to estimate an index of regional CVR (CVRi). We chose to focus on MAP as our estimate of Pα since it represents an averaged estimate of arterial pressure, rather than any specific feature of the BP curve such as the peak (i.e., systolic) or trough (i.e., diastolic) BP components. We hypothesized that this estimate of CVRi (i.e., MAP/CBF) would be elevated in AD, particularly within subcortical structures prone to subclinical cerebrovascular disease. In addition, we predicted that subcortical CVR would be correlated with measures of subclinical cerebrovascular disease (WMLs) and the degree of cognitive impairment. Although not the primary focus of the present study, we also explored the use of other BP measures to obtain alternative estimates of vascular resistance, particularly pulse pressure (PP), by calculating a PP-to-CBF ratio (i.e., PP/CBF).
MATERIALS AND METHODS
Participants
Eighty-one participants (12 with probable AD, 23 with MCI, and 46 NC) were recruited from community and University of California San Diego (UCSD) Alzheimer's Disease Research Center resources. Participants underwent neurologic and neuropsychologic examinations [12]. Exclusions included history of neurologic illness, clinical stroke, head injury, learning disability, or major psychiatric illness. All procedures were approved by the UCSD IRB, and all participants or their caregivers were provided informed consent prior to enrollment.
Participant groups
Participants in the AD group met NINCDS-ADRDA criteria for probable AD and had a modified Hachinski score ≤3. Diagnosis of MCI was made using criteria delineated by Jak et al. [12]. Briefly, participants scoring more than one standard deviation below normative expectations on two or more tests within a single cognitive domain or three or more tests across multiple domains were diagnosed with MCI. All MCI participants scored within normal limits on an objective measure of independent living skills [13].
Demographic characteristics and clinical data
Global cognition was assessed by the Mattis Dementia Rating Scale (DRS) [14]. Patient BP was recorded prior to neuropsychological testing and within one week of brain imaging. Two brachial artery BP recordings were obtained from each arm while the participant was seated and resting comfortably. These measures were averaged to obtain estimates of systolic BP, diastolic BP, and PP (systolic – diastolic BP). Genetic material was obtained by buccal swab. Apolipoprotein E (APOE) genotype was determined using a polymerase chain reaction-based method [15]. Participants were grouped into those with and without a copy of the APOE [H9255]4 allele for data analysis. The presence or absence of the following vascular risk factors derived from the Framingham Stroke Risk Profile [16] were identified during patient interview, chart review, and physical exam: history of cardiovascular disease [coronary artery disease (myocardial infarction, angina pectoris, coronary insufficiency), intermittent claudication, cardiac failure], use of antihypertensive medications, diabetes, atrial fibrillation, left ventricular hypertrophy, and transient ischemic attack or minor stroke.
Image acquisition
Data were acquired on a GE Signa Excite 3-T whole body MR scanner using an 8-channel receive-only head coil. Structural images were acquired with high-resolution T1-weighted scans with a 3D MPRAGE sequence (26 cm FOV, 256 × 256 matrix, TR = 7 ms, TE = min full, flip angle = 8°, inversion time = 900 ms, bandwidth = 31.25 kHz, and 170 1.2 mm sagittal slices) or a 3D FSPGR sequence (identical parameters to MPRAGE except 25 cm FOV, 256 × 192 matrix, TR = 8.1 ms, inversion time = 600 ms, 172 1 mm sagittal slices), and for a subset of 48 participants (10 AD, 13 MCI, 25 NC), T2-weighted fluid attenuated inversion recovery (FLAIR) images (20 cm FOV, 256 × 256 matrix, flip angle = 90°, TE = 142 ms, TR = 10000 ms, 5 mm axial slices with no inter-slice gap) were also acquired. Resting CBF images were acquired using pulsed arterial spin-labeling with a modified flow-sensitive alternating inversion recovery sequence (post-saturation and inversion times of TI1 = 600 ms and TI2 = 1600 ms, TR = 2.5 s, TE = minimum, FOV = 22 × 22 cm, 64 × 64 matrix, 20 5 mm axial slices, 40 volumes [20 tag+control image pairs]). This sequence utilized presaturation pulses and PICORE QUIPPS 2 post-inversion saturating pulses and a spiral read out with four interleaves [17]. Additionally, a scan with the inversion pulses turned off was acquired to obtain an estimate of the magnetization of cerebrospinal fluid (CSF) and a minimum contrast scan was acquired to adjust for coil inhomogeneities [18].
Data processing
Data were processed using Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/), FMRIB Software Library (FSL, Oxford, United Kingdom), and locally created Matlab scripts (The Mathworks Inc., Natick, Massachusetts, 2010).
Structural images
Skull-stripping was completed using Brain Surface Extractor and manual editing to remove residual non-brain material [19]. Whole brain images were segmented into gray matter (GM), white matter (WM), and CSF using FSL's FAST (FMRIB's Automated Segmentation Tool). The high-resolution T1-weighted images were registered to ASL space and the partial volume segmentations were registered and down-sampled to the ASL data resolution. For quantification of WML volume, a semiautomated volumetric approach was applied to T2-FLAIR images using a method previously described [20]. Briefly, WMLs were manually traced in 17 to 21 image slices in the axial plane using AFNI. Total WML volume was quantified as the total voxel counts (mm3).
ASL images
Each ASL dataset was reconstructed using the SENSE algorithm [21]. The ASL time series was co-registered to the middle time-point to minimize effects of participant motion. A mean ASL image was formed for each participant from the average difference of control and tag images using surround subtraction, and slice timing delays were accounted for to ensure the inversion time (TI2) was slice specific [17]. The mean ASL image was converted to absolute units of CBF (mL 100 g tissue−1 min−1) using information on the relationship between the intensities of CSF and water [21]. The CBF data were corrected for partial volume effects to ensure accurate estimations of GM CBF that were not influenced by known decreased perfusion in WM or increased CSF volume [21, 22]. The partial volume corrected (PVE-corrected) CBF signal intensities were calculated using the following formula: CBFcorr = CBFuncorr/(GM + 0.4 * WM), which assumed that CSF has zero CBF and that CBF in GM is 2.5 times greater than in WM [22]. GM and WM are GM and WM partial volume fractions, respectively, and were computed based on the tissue content of each perfusion voxel as determined in FSL's FAST. The CBFcorr data were spatially smoothed using a 4.0 mm full-width, half-maximum Gaussian filter. CBF voxels with negative intensities were replaced with zero [22] and these data were used in voxel-wise analyses. A conservative threshold that removed CBF values outside of the expected physiological range of CBF (below 10 or greater than 150) was applied for use in the ROI analysis. Voxel-wise comparisons utilized non-thresholded values. CBF data were normalized to standard Talairach space [23] and re-sampled to a 4 × 4 × 4 mm resolution grid. To limit analyses to supratentorial GM, individual masks that retained voxels with at least a 90% probability of containing GM and excluded brainstem and cerebellar regions, were generated and merged with each participant's CBFcorr data.
Cerebrovascular resistance
CVRi data were computed using each participant's MAP as an estimate of Pα, and their CBFcorr data. Participant MAP was calculated from brachial artery resting systolic and diastolic BP using the following formula: MAP = diastolic BP + (PP/3). PP-to-CBF ratio was also calculated (PP/CBF). Similarly, CVRi and PP-to-CBF ratio voxels represented participant BP estimates (MAP or PP, respectively) divided by voxel CBF value.
Statistical analyses
All statistical tests were two-tailed with a significance cutoff of p < 0.05.
Demographic characteristics and clinical data
One-way ANOVAs with Tukey post-hoc tests were used to compare groups on age, education, and MAP. Chi-square was used to compare groups on gender, APOE genotype ratios, antihypertensive medication use, and the presence of one or more vascular risk factor(s). Data were screened for outliers (± 3 standard deviations [SD]) and departures from normality. One participant exhibited extremely elevated WML volume, i.e., >3 SDs above the mean. All WML analyses were conducted both with and without the outlier to ensure that findings were not due solely to this one influential value.
Voxel-wise analyses
Although our a priori hypothesis predicted group differences within subcortical structures, we conducted voxel-wise analyses to explore additional ROIs [4]. Whole-brain voxel-wise t-tests were conducted in AFNI on the quantified and PVE-corrected CVRi data. The t-tests compared all three groups (NC versus AD, NC versus MCI, AD versus MCI) on CVRi, with age and gender included as covariates. AFNI's AlphaSim program corrected for multiple comparisons to control for Type I error with a voxel-wise alpha level of 0.05. Clusters exceeding the minimum volume threshold of 1280 μl or 20 contiguous voxels and a statistical threshold of t = 2.0 were considered statistically significant. This threshold/volume combination protected a whole-brain false positives probability of p < 0.05.
Region-of-Interest (ROI) analyses
For regression analyses a priori anatomical regions were selected from subcortical structures: thalamus and caudate nucleus. ROI masks were created in AFNI based on the Talairach-Tourneaux atlas and applied to each participant's quantified and PVE-corrected CVRi data. Planned correlational analyses (Pearson product moment correlations) examined the relationship between subcortical CVRi (thalamus and caudate) and both global cognition and WML volume. Multiple linear regression was used to examine these relationships after controlling for all clinical and demographic variables (see above).
RESULTS
Demographic and clinical comparisons
Analyses indicated a significant group difference in MAP, F(2, 78) = 3.19, p = 0.047, such that NC participants exhibited lower MAP than AD patients, p = 0.04. Participant groups were well matched on all other clinical and demographic variables (see Table 1). By definition, the groups differed on DRS total score, therefore, these were not statistically analyzed (see Table 1 for demographic characteristics and clinical data means and SDs of the matched sample).
Table 1.
Clinical and demographic dataa
| NC (n = 46) | MCI (n= 23) | AD (n= 12) | |
|---|---|---|---|
| Age (y) | 73.8 (7.7) | 74.3 (8.5) | 75.8 (9.9) |
| Education (y) | 16.3 (2.1) | 15.7 (2.7) | 15.5 (2.4) |
| Gender (% male) | 34.8% | 56.5% | 58.3% |
| ApoE4 genotype (%)b | 28.3% | 47.8% | 50.0% |
| Vascular Risk (%)c | 21.7% | 34.8% | 25.0% |
| Taking BP Med (%) | 50.0% | 56.5% | 58.3% |
| DRS total score | 141.8 (1.8) | 136.7 (4.4) | 123.0 (7.6) |
| MAP (mmHg) | 92.2(9.1) | 95.2(11.7) | 100.4 (11.3)* |
Mean or % (Standard Deviation)
ApoE4 genotype (%), percent with one or more copies of ApoE4 allele; cVascular Risk (%), percent with one or more vascular risk factors. NC, normal control; MCI, mild cognitive impairment; AD, Alzheimer's disease; BP, blood pressure; DRS, Mattis Dementia Rating Scale; MAP, mean arterial pressure.
p < 0.05 for AD versus NC.
Voxel-wise analysis
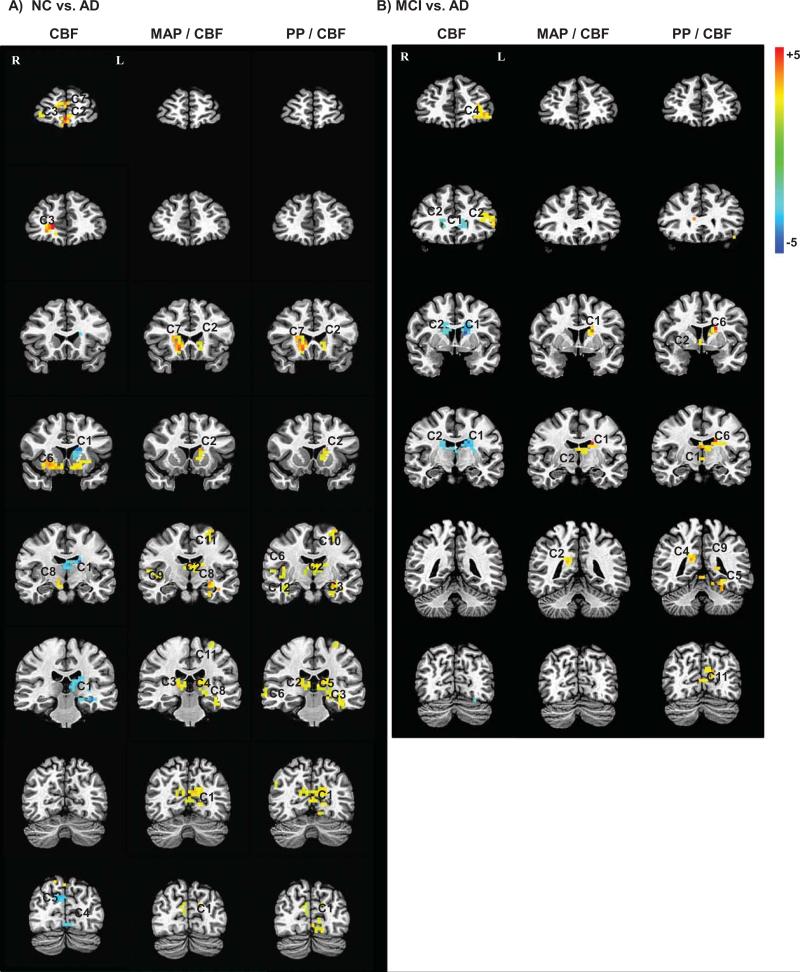
Voxel-wise analyses comparing the NC and AD groups, controlling for age and gender, indicated 11 significant clusters within subcortical (thalamus, cau-date) and cortical (posterior cingulate, medial temporal lobe (MTL)) brain regions (Fig. 1A; Table 2). The AD group exhibited elevated CVRi in all clusters relative to the NC group. AD patients also showed two sub-cortical (thalamus, caudate) and cortical (precuneus) clusters of significantly elevated CVRi in comparison with the MCI group (Fig. 1B; Table 2). No significant clusters differentiated the NC and MCI groups.
Fig. 1.
Images from voxel-wise CVRi comparisons: Representative sections are displayed from significant clusters (see Table 2) in voxel-wise comparisons of CBF, MAP/CBF (CVRi), and PP/CBF (PP-to-CBF ratio) between NC and AD groups (A), as well as MCI and AD groups (B). Clusters are overlayed in radiologic orientation onto a high resolution anatomical image for reference. Color scale bar indicates t-values. Blue clusters indicate significantly lower values and yellow-red clusters indicate significantly higher values in the AD group versus the comparison group (NC for panel A; MCI for panel B).
Table 2.
Summary of significant clusters from voxel-wise CVRi comparisons
| Cluster | Anatomic label | Voxels | Max intensity x, y, z (mm) | Maximum t-score |
|---|---|---|---|---|
| NC versus AD | ||||
| 1 | Precuneus | 7744 | —6, 61, 20 | 5.42 |
| 2 | Thalamus | 3648 | 14,—7, 16 | 4.97 |
| 3 | Medial temporal lobe | 2048 | 26, 5, —24 | 3.82 |
| 4 | Thalamus | 2048 | 14,21, 16 | 4.97 |
| 5 | Thalamus | 1792 | —10, 37, 20 | 4.18 |
| 6 | Caudate | 1664 | —14, —19,4 | 4.96 |
| 7 | Inferior parietal | 1664 | 34, 41, 40 | 3.50 |
| 8 | Medial temporal lobe | 1536 | 26, 13, —8 | 3.83 |
| 9 | Superior temporal | 1536 | —50, 17, 8 | 4.18 |
| 10 | Posterior cingulate | 1344 | 10,41,44 | 3.68 |
| 11 | Parietal | 1344 | 30, 13, 60 | 3.62 |
| MCI versus AD | ||||
| 1 | Thalamus/Caudate | 1984 | 18, 9, 24 | 5.1697 |
| 2 | Thalamus/Precuneus | 1536 | —10, 37, 20 | 3.4937 |
(r = 0.45, p = 0.001) (Fig. 2C), which remained significant after controlling for all covariates, ΔR2 = 0.09, β = 0.37, p = 0.02.
For visual comparison, corresponding representative sections from CBF and PP-to-CBF ratio analyses are displayed in Fig. 1. Results of CBF analyses indicated 8 significant clusters differentiating AD from NC participants and 4 significant clusters differentiating AD from MCI participants. Participants with AD exhibited areas of both high (frontal, lentiform, insular) and low (subcortical) CBF relative to the NC and MCI groups (Fig. 1A–B). In contrast, CVRi analyses indicate only regions of elevated CVRi in AD, with a clear absence of any reduction in CVR within frontal and lentiform regions. The brain regions showing elevated CVRi in AD participants were predominately within subcortical, MTL, and posterior cingulate/precuneus areas, but also included other cortical regions with smaller clusters (e.g., inferior parietal, superior temporal) (Fig. 1A). Results of PP-to-CBF ratio analyses indicated 14 significant clusters differentiating NC from AD participants and 11 significant clusters differentiating MCI from AD participants. The pattern of findings from the PP-to-CBF ratio analysis was quite similar to that of CVRi, but exhibited slightly larger clusters that were more widespread and bilateral throughout cortical regions (Fig. 1A–B).
ROI analysis
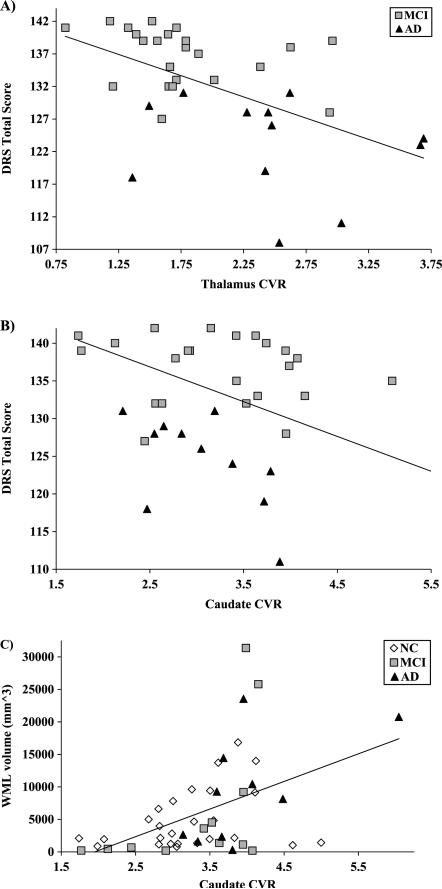
Correlational analyses across all three groups revealed significant inverse relationships between our test of global cognition and CVRi within the thalamus (r = −0.42, p<0.00008) and caudate (r = −0.34, p = 0.002), which remained significant after controlling for all covariates: thalamus (ΔR2 = 0.10, β = −0.36, p = 0.002) and caudate (ΔR2 = 0.08, β = −0.32, p = 0.007). Inverse correlations were even more pronounced when the analysis was restricted to the MCI and AD subgroup (n = 35; r = −0.52, p = 0.001, for both caudate and thalamus), indicating that these were not merely due to normal versus non-normal differences in cognition. These associations remained significant after controlling for all covariates: thalamus (ΔR2 = 0.16, β = 0.48, p = 0.01) (Fig. 2A) and caudate (ΔR2 = 0.17, β = −0.53, p = 0.008) (Fig. 2B).
Fig. 2.
Subcortical CVR correlates with cognition and WML volume: The association between DRS score and CVR in both (A) thalamus and (B) caudate is displayed for the MCI and AD subgroup; (C) caudate CVR is also correlated with WML volume across all three groups.
Among the subset of participants with WML (n = 48), there was a significant positive association between WML volume and CVRi within the caudate (r = 0.45, p = 0.001) (Fig. 2C), which remained significant after controlling for all covariates, ΔR2 = 0.09, β = 0.37, p = 0.02.
DISCUSSION
As predicted, subcortical CVRi was elevated in AD patients relative to both NC participants and those with MCI. CVRi was particularly elevated within the thalamus and caudate nucleus, where CVRi values were also inversely related to our test of global cognition across the entire sample and within the cognitively impaired (MCI and AD) subgroups. These subcortical regions play important roles in cognition and frequently exhibit small vessel disease [24]. Caudate CVRi elevation was also associated with greater WML volume, further suggesting a relationship with small vessel disease. Together these findings suggest that subcortical CVRi may be a useful marker of vascular dysfunction and cerebrovascular disease, which could play a role in the onset and progression of AD.
In addition to small vessel disease, elevated subcortical CVRi may be related to atherosclerosis within the Circle of Willis, which is common in AD and could disrupt CBF to the lenticulostriate and both posterior and anterior cerebral arteries [2, 3]. In support of this hypothesis, prior studies have found that AD patients exhibit reduced large vessel blood flow velocity (elevated CVR), measured by transcranial doppler ultrasound, which correlates with atherosclerosis within the circle of Willis [9, 10, 24]. However, smaller arteriolar networks are the principal source of vascular resistance in the brain [25], and recent research indicates the importance of resistance within even smaller capillary beds [26]. For this reason, we chose a measure more representative of capillary and/or arteriolar flow within specific brain regions (i.e., ASL MRI). Subcortical small vessel disease and changes in capillary microstructure have both been associated with AD and may be responsible for CVRi elevation, particularly within the caudate nucleus, where CVRi elevation was associated with WML volume [5].
It is also possible that Aβ-mediated vasoconstriction may play a role in subcortical CVRi elevation. In this case, however, it is not immediately clear why CVRi elevation would be most prominent in subcortical structures since cortical and MTL regions are traditionally thought to contain the most extensive amyloid plaque pathology [27]. Interestingly, recent studies utilizing Pittsburgh compound B PET imaging of brain amyloid in both familial and sporadic AD have found that there is extensive Aβ deposition within subcortical regions, even during the preclinical stages of the disease [28–30]. Additionally, neuropathologic studies have described extensive amyloid deposition in the caudate and thalamus of nearly all AD patients [27, 28], where it has been correlated with the extent of cognitive impairment [28] and neurofibrillary pathology [29]. Together with the present study, these findings suggest a major role for subcortical amyloid deposition and cerebrovascular dysfunction in AD. Whether the elevated CVRi within subcortical regions is due to small vessel disease, Aβ deposition, or some combination of these factors awaits future studies.
Voxel-wise analysis also indicated more subtle CVRi elevation in specific cortical regions (precuneus, inferior parietal, superior temporal) in AD relative to NC participants, with MCI patients not differing from either group these cortical areas. This differential pattern of results for subcortical (NC and MCI < AD) versus cortical (NC < MCI < AD) CVRi may provide a clue to its pathological significance. We speculate that the subcortical vasculature, comprised of terminal arteries without extensive anastomoses, may be selectively vulnerable to the effects of small vessel disease and possibly Aβ-mediated vasoconstriction. Regardless of etiology, the contrasting pattern of find ings between cortical and subcortical CVRi may prove useful in predicting clinical outcomes in MCI.
Prior studies comparing CBF across AD patients and normally aging adults have reported reductions in “global CBF”, which has frequently included significantly reduced CBF to subcortical structures, such as the caudate and thalamus [4]. Despite these findings and other studies indicating increased subcortical Aβ deposition [30], most studies have focused on CBF reductions in cortical regions, specifically within the posterior cingulate and precuneus, and have not taken into account BP variation within or between groups [4]. In a voxel-wise CBF analysis, we confirmed the cortical findings of prior studies [4], indicating increased anterior (frontal lobe, anterior cingulate, lentiform) and reduced posterior cortical (posterior cingulate, precuneus, MTL) CBF in AD, a pattern suggestive of default mode network dysfunction [31]. In addition, we found reduced subcortical (thalamus and caudate) CBF in AD relative to NC and MCI participants. A side-by-side comparison of these findings with those of CVRi indicated that the elevated CBF within anterior regions (frontal lobe, anterior cingulate, lentiform) in AD patients was not reflected by reduced CVRi. In fact, there were no significant clusters of reduced CVRi in any of the comparisons. Additionally, clusters of elevated CVRi were larger and more widespread than those of reduced CBF, involving bilateral subcortical and medial temporal regions, as well as unilateral superior temporal and inferior parietal areas. Together these findings suggest that BP modulates the resting CBF abnormalities found in AD patients, and CVRi calculations may provide more useful information beyond CBF alone in the study of vascular function in AD, MCI, and normal aging. Future longitudinal studies may reveal whether the addition of BP-corrected CBF values (i.e., CVRi or PP-to-CBF ratio) could improve diagnostic and prognostic accuracy in MCI and dementia.
The present study was primarily focused on estimating CVR through the use of an averaged BP estimate, which is why MAP was chosen to create our CVR index (CVRi). It is also possible that measures of the pulsatile component of BP could be potentially useful in estimating CVR in the elderly. In support of this notion, we have previously shown that PP elevation is associated with cognitive impairment in nondemented adults [32], and that antemortem PP elevation is associated with postmortem cerebrovascular disease in autopsy-confirmed AD patients [33]. Thus, we examined PP-to-CBF ratio as another potentially useful index of CVR. This ratio produced results that were similar to those of CVRi, but with more widespread cortical involvement and generally larger, more bilateral clusters, suggesting that the pulsatile component of BP may play a significant role in CBF abnormalities found in AD patients. These findings suggest that increased arterial stiffness, leading to PP elevation, may play a role in the CBF and CVR abnormalities observed in AD and MCI.
Study limitations include the relatively small sample size and lack of neuropathologic characterization in our AD group. Our estimate of CVRi was simple and utilized clinically available measures of seated, resting BP that were not obtained simultaneously with the supine CBF assessment. A more physiologically accurate estimate of acute changes in CVR would require synchronized measurement of BP and CBF with MRI-compatible BP measurement technology; thus, the estimate used in the current study requires validation alongside a synchronized assessment of CVR. Additionally, it remains unclear how brachial artery (or any other systemic artery) BP estimate relates to vascular pressures within the cerebral microvasculature. The extent to which ASL MRI provides an index of capillary and/or arteriolar blood flow is also uncertain, which may have bearing on the question of what microvascular pressure estimate would be most relevant to the ASL MRI signal. Given these technological limits on noninvasive CBF and BP measurement, we have opted to use a simple averaged estimate of BP (i.e., MAP). Despite these limitations, the current study represents one of the only attempts to examine the influence of arterial pressure on regional CBF in MCI and AD, making the findings a potentially useful first step towards understanding the contribution of arterial pressure to the CBF abnormalities observed in these patients.
Finally, a strength of the current study is the fact that significant CVRi elevation was found even in a sample with relatively low Hachinski scores and the clinical diagnosis of probable AD, suggesting that CVRi may be sensitive to occult cerebrovascular disease. Given the high prevalence of occult cerebrovascular lesions in AD, investigation of preclinical markers of cerebrovascular disease in conjunction with those of early AD pathology may improve our ability to detect and predict dementia during the earliest stages of disease when treatments are more likely to be efficacious.
ACKNOWLEDGMENTS
The current study was supported by the Alzheimer's Association (IIRG 07-59343 to M.W.B., NIRG 09- 131856 to C.E.W.), VA CSR&D (CDA-2-022-08S to C.E.W.), and the National Institute on Aging (R01 AG012674 to M.W.B., K24 AG026431 to M.W.B., and P50 AG05131 to D.P.S.). The authors thank the staff and participants of the UCSD Shiley-Marcos Alzheimer's Disease Research Center for their important contributions.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1755).
REFERENCES
- 1.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 3.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135(Pt 12):3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer's disease. J Alzheimers Dis. 2010;20:871–880. doi: 10.3233/JAD-2010-091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 7.Buxton R. Introduction to Functional Magnetic Resonance Imaging. Cambridge University Press; New York, NY.: 2002. [Google Scholar]
- 8.Panerai RB. Assessment of cerebral pressure autoregulation in humans–a review of measurement methods. Physiol Meas. 1998;19:305–338. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- 9.Gommer ED, Martens EG, Aalten P, Shijaku E, Verhey FR, Mess WH, Ramakers IH, Reulen JP. Dynamic cerebral autoregulation in subjects with Alzheimer's disease, mild cognitive impairment, and controls: Evidence for increased peripheral vascular resistance with possible predictive value. J Alzheimers Dis. 2012;30:805–813. doi: 10.3233/JAD-2012-111628. [DOI] [PubMed] [Google Scholar]
- 10.Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, Garami Z, Belohlavek M, Sabbagh MN, Sue LI, Beach TG. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimers Dement. 2011;7:436–444. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI. Blood pressure, cerebral blood flow, and brain volumes. The SMART-MR study. J Hypertens. 2010;28:1498–1505. doi: 10.1097/HJH.0b013e32833951ef. [DOI] [PubMed] [Google Scholar]
- 12.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeb P. Independent living scales manual. Psychology Corp, Harcourt Brace Jovanovich; San Antonio, TX.: 1996. [Google Scholar]
- 14.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Karasu TB, editors. Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. Grune & Stratton; New York: 1976. pp. 77–121. [Google Scholar]
- 15.Saunders NB, Zollinger WD, Rao VB. A rapid and sensitive PCR strategy employed for amplification and sequencing of porA from a single colony-forming unit of Neisseria meningitidis. Gene. 1993;137:153–162. doi: 10.1016/0378-1119(93)90001-j. [DOI] [PubMed] [Google Scholar]
- 16.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. NeuroImage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Brumm KP, Perthen JE, Liu TT, Haist F, Ayalon L, Love T. An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. NeuroImage. 2010;51:995–1005. doi: 10.1016/j.neuroimage.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fennema-Notestine C, Ozyurt IB, Clark CP, Morris S, Bischoff-Grethe A, Bondi MW, Jernigan TL, Fischl B, Segonne F, Shattuck DW, Leahy RM, Rex DE, Toga AW, Zou KH, Brown GG. Quantitative evaluation of automated skull-stripping methods applied to contemporary and legacy images: Effects of diagnosis, bias correction, and slice location. Hum Brain Mapp. 2006;27:99–113. doi: 10.1002/hbm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delano-Wood L, Abeles N, Sacco JM, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in mild cognitive impairment: Differential influence of lesion type on neuropsychological functioning. Stroke. 2008;39:794–799. doi: 10.1161/STROKEAHA.107.502534. [DOI] [PubMed] [Google Scholar]
- 21.Weiger M, Pruessmann KP, Osterbauer R, Bornert P, Boesiger P, Jezzard P. Sensitivity-encoded single-shot spiral imaging for reduced susceptibility artifacts in BOLD fMRI. Magn Reson Med. 2002;48:860–866. doi: 10.1002/mrm.10286. [DOI] [PubMed] [Google Scholar]
- 22.Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: Initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talarach J, Tournoux P. Co-Planar stereotaxic atlas of the human brain. Thiem Medical Publishers; New York: 1988. [Google Scholar]
- 24.Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119:277–290. doi: 10.1007/s00401-010-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols W, O'Rourke M. Theoretical, Experimental and Clinical Principles. 4th edition Edward Arnold; London, UK.: 2006. McDonald's Blood Flow in Arteries. [Google Scholar]
- 26.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 28.Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: Findings from the Nun study. Alzheimer Dis Assoc Disord. 1999;13:226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Beach TG, Sue LI, Walker DG, Sabbagh MN, Serrano G, Dugger BN, Mariner N, Yantos K, Henry-Watson J, Chiarolanza G, Hidalgo JA, Souders L. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinico-pathological Alzheimer's disease: Implications for amyloid imaging. J Alzheimers Dis. 2012;28(4):266–273. doi: 10.3233/JAD-2011-111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, Bi W, Hoge JA, Cohen AD, Ikonomovic MD, Saxton JA, Snitz BE, Pollen DA, Moonis M, Lippa CF, Swearer JM, Johnson KA, Rentz DM, Fischman AJ, Aizenstein HJ, DeKosky ST. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi M, Hirosawa T, Yokokura M, Yagi S, Mori N, Yoshikawa E, Yoshihara Y, Sugihara G, Takebayashi K, Iwata Y, Suzuki K, Nakamura K, Ueki T, Minabe Y, Ouchi Y. Effects of brain amyloid deposition and reduced glucose metabolism on the default mode of brain function in normal aging. J Neurosci. 2011;31:11193–11199. doi: 10.1523/JNEUROSCI.2535-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nation DA, Wierenga CE, Delano-Wood L, Jak AJ, Delis DC, Salmon DP, Bondi MW. Elevated pulse pressure is associated with age-related decline in language ability. J Int Neuropsychol Soc. 2010;16:933–938. doi: 10.1017/S1355617710000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nation DA, Delano-Wood L, Bangen KJ, Wierenga CE, Jak AJ, Hansen LA, Galasko DR, Salmon DP, Bondi MW. Antemortem pulse pressure elevation predicts cerebrovascular disease in autopsy-confirmed Alzheimer's disease. J Alzheimers Dis. 2012;30:595–603. doi: 10.3233/JAD-2012-111697. [DOI] [PMC free article] [PubMed] [Google Scholar]