Abstract
Chronic prostatitis/Chronic pelvic pain syndrome (CP/CPPS) affects up to 15% of the male population and is characterized by pelvic pain. Mast cells are implicated in the murine experimental autoimmune prostatitis (EAP) model as key to chronic pelvic pain development. The mast cell mediator tryptase-β and its cognate receptor protease-activated receptor 2 (PAR2) are involved in mediating pain in other visceral disease models. Prostatic secretions and urines from CP/CPPS patients were examined for the presence of mast cell degranulation products. Tryptase-β and PAR2 expression were examined in murine experimental autoimmune prostatitis (EAP). Pelvic pain and inflammation were assessed in the presence or absence of PAR2 expression and upon PAR2 neutralization. Tryptase-β and carboxypeptidase A3 were elevated in CP/CPPS compared to healthy volunteers. Tryptase-β was capable of inducing pelvic pain and was increased in EAP along with its receptor PAR2. PAR2 was required for the development of chronic pelvic pain in EAP. PAR2 signaling in dorsal root ganglia lead to ERK1/2 phosphorylation and calcium influx. PAR2 neutralization using antibodies attenuated chronic pelvic pain in EAP. The tryptase-PAR2 axis is an important mediator of pelvic pain in EAP and may play a role in the pathogenesis of CP/CPPS.
Keywords: Mast cells, prostatitis, pelvic pain, CPPS
1. Introduction
Chronic prostatitis/Chronic pelvic pain syndrome (CP/CPPS) is a clinical problem affecting up to 15% of men, with an uncertain etiology and few if any, therapeutic options [8; 12]. While the immune and nervous systems are postulated to play a role in the chronic pain that is characteristic of CP/CPPS, the interplay between the two remains understudied. This stands in contrast to the well-appreciated role of proinflammatory products in long-term neuronal changes in visceral inflammatory conditions such as Crohn’s and ulcerative colitis [21]. Work in our laboratory has demonstrated that mast cells are crucial mediators in inducing experimental autoimmune prostatitis (EAP), an animal model to study pelvic pain associated with CP/CPPS [15]. Degranulation of mast cells releases tryptase, histamine,and nerve growth factors that are known to drive proinflammatory pathways and influence neuronal signaling. Patients that suffer from CP/CPPS show increased levels of mast cell tryptase in expressed prostatic secretion (EPS) [15]. Increased amounts of tryptase-β are also found in intestinal tissue from ulcerative colitis and Crohn’s disease patients [21]. Interestingly, there is significant correlation between the distance of mast cells to nerve terminals and the reported magnitude of abdominal pain in patients with ulcerative colitis [48]. Mouse mast cell protease (mMCP-6), an ortholog of human tryptase-β, contributes to an increase in proinflammatory cytokines and colon hypersensitivity in animal models of inflammatory bowel disease (IBD) [22].
Protease-activated receptors (PARs) are G-protein coupled receptors that have multiple roles in inflammation, proliferation, and pain transmission [1; 13]. In particular, the PAR2 receptor is activated by tryptase that cleaves the N-terminal domain of the receptor [33; 34] and initiates an intracellular cascade that involves changes in calcium uptake and signaling through the MAPK/ERK pathway [39]. Previous studies have shown that the PAR2 receptor is directly linked to visceral pain in IBD and colitis models due to its important role in inflammation and its presence on epithelial cells, immune cells, and the terminals of afferent nerves [18; 32]. In the colon, PAR2 activation has been shown to increase levels of T Helper Type I cytokines and results in increased recruitment of inflammatory cells [7]. Pharmacological targeting of PAR2 with an antagonist mitigates inflammation in a colitis model [30]. However, most studies on the role of the PAR2 receptor to date have been limited to acute pain or inflammation models of the colon [6; 7].
In this study we postulated a role for the tryptase-PAR2 axis in the pathogenesis of CP/CPPS. We examined its role in the EAP model by focusing on its functional role in pain behavior and neuronal activity in dorsal root ganglia (DRG). Our study implicates activation of PAR2 in the development of chronic pelvic pain. Furthermore, we demonstrate for the first time that clinical patients with CP/CPPS have elevated levels of tryptase and carboxypeptidase A (CPA3), markers of mast cell activity. We show that inhibition of the PAR2 receptor ameliorates pain in the animal model, thereby suggesting an attractive new target for novel treatments in CP/CPPS.
2. Materials and Methods
2.1. Urine and EPS Collected From Patients
Northwestern University approved the human research protocol and written consent was obtained from healthy controls and CP/CPPS patients prior to enrollment in the study. Patients with a history of CP/CPPS symptoms and diagnosed by the Northwestern University Department of Urology were approached to volunteer in the study (Clinical trials identifier NCT01676857). The mean age for the healthy volunteer group was 38.16 with a range between 22–68. The mean age for the patient group was 47.22 with a range between 25–76. There was no statistical difference (t-test) with regards to age between the two groups. Mean total score for the NIH-CPSI was 1.4 (control) and 21.6 (CPPS) respectively. Pain, urinary, QOL and total scores but not age were statistically different between the CPPS and control groups. Expressed prostatic secretions (EPS) and fresh urines (VB1–3) were collected using the Meares-Stamey 4-glass urine test as previously described [41] and promptly placed in cryostat tubes and frozen at −80°C until ELISAs or activity assays were performed. The manufacturers instructions were followed for quantitation of caboxypeptidase A (CPA3) activity (Sigma; CS1130) or Mast cell degranulation kit for tryptase (Millipore; IMM001) and NGF (Promega; G7630). Sample size limitations precluded the detection of all analytes exclusively in the EPS sample. Protein concentrations in EPS samples and urines were used for normalization and in the case of urines the VB3-VB2 CPA3 activity and NGF concentrations were compared to derive prostate specific expression levels of these proteins.
2.2. Mice and Treatments
5–7 week old NOD/ShiLtj (NOD), C57BL/6J (B6), and PAR2 deficient mice (B6.Cg-F2rl1tm1mslb/J) (PAR2 KO) mice from Jackson Laboratory (Bar Harbor, ME) were used for experiments. Animal experiments and surgical procedures were approved by the Northwestern University Animal Care and Use Committee. Experiments were conducted on adult male mice that were age matched littermates. Rat prostate antigen (rat prostate lysates) were diluted (10mg/ml) with adjuvant and subcutaneously injected in the shoulder of mice to induce EAP as previously described by Rudick et al [40]. In therapeutic experiments, mice were injected intraperitoneally with either rat IgG (1µg; R&D systems; MAB006) or PAR2-antibody (SAM11; 1µg; Santa Cruz; sc-13504) at day 20; doses and procedure were previously described by Ferrell et al [16]. Mice were anesthesized with isoflurane for intraurethral instillation. Briefly, a 10µl Hamilton syringe with a sterile 30-gauge needle (Beckton Dickinson) attached to a sterile polyethylene catheter (PE-10) tube (length: 1.5 cm) was carefully inserted intraurethrally and either mMCP-6 (100ng/ml; 10µl) or saline (10µl) was slowly (15 second instillation) delivered into each mouse. To ensure that the solution(s) did not reach the bladder, the volume delivered and the PE-10 tube was verified in preliminary studies.
2.3 Mice Behavioral Assays
Mice were tested for pelvic pain by a blinded tester as previously described by our laboratory [37; 38; 40]. Briefly, mice were habituated for one hour by placing them in individual plexiglass chambers (6×10×12 cm) on top of a stainless steel wire grid floor and suspended 2 feet above a flat surface. Calibrated von Frey filaments with forces of 0.04, 0.16, 0.4, 1, and 4 g were used to determine the development of pelvic allodynia. The filaments were applied in increasing force order with a brief resting period between filaments (approximately 5 seconds) and filaments were applied 10 times. The filaments were applied to the pelvic area considered located adjacent to the prostate. The following were interpreted as positive response to the filament: 1) licking and scratching (hindpaws) of the pelvic area immediately after stimulation, 2) retraction of the abdomen, and 3) immediate avoidance (jumping) [37].
2.4. Tissue Extraction and Western Blots
Mice were euthanized by cervical dislocation and DRG (S1–S4) were excised, lysed, and homogenized with a tissue grinder. Protein concentrations were determined with a Bradford protein kit assay (Thermo Scientific; 23225). Protein (20 µg) was loaded onto 10% SDS-PAGE gel, and immunoblotted using standard procedures. Membranes were incubated with primary rabbit polyclonal anti-p-ERK 1/2 (1:1,000; Cell signaling; AB1504) or rat monoclonal mast cell protease-6 (1:1000; R&D systems; MAB3736) overnight at 4°C followed by either goat anti-rabbit or goat anti-rat IgG-HRP (1:3,000 dilution; Cell signaling) secondary antibody and detection using chemiluminescence. PVDF membranes were rinsed with stripping buffer for 5 minutes, washed (TBST), and placed in blocking buffer at room temperature for 1 hour. The membranes were relabeled with rabbit polyclonal anti-tubulin (1:1,000; Cell Signaling) and exposed to the secondary antibody goat anti-rabbit IgG with horseradish peroxidase (1:3,000 dilution; Bio-Rad; cat.170–6515). Image J software was used to determine optical density of protein bands in the immunoblot.
2.5. Immunofluorescence and Immunohistochemistry Assays
Mouse tissues were fixed by transcardial perfusion with 4% paraformaldehyde in cold PBS. The sacral DRG (S1–S4) were excised and prepared for frozen sectioning. DRG were sectioned at 15µm. Sections were placed in blocking solution for 1 hour at room temperature. Then sections were incubated with either rabbit anti-p-ERK1/2 (1:50; Cell Signaling), rabbit anti-PAR2 (1:50; Santa Cruz; sc-13504), or anti-goat isotype (1:50; Santa Cruz; sc-2028) at 4°C overnight. The sections were washed 3 times in PBS for 5 min and placed in Alexa 488 anti-rabbit secondary antibody (1:400; Invitrogen; A11034) for 1–2hrs at room temperature in the dark. Sections were washed 3 times 5 min each in PBS. Slides were covered with mounting medium containing DAPI (Invitrogen; 1319493) and sealed with a coverslip (Corning). Immunohistochemical tissues were sectioned and processed at the Northwestern pathology core. Briefly, tissue were fixed in 10% formalin and embedded in paraffin and stained for either toluidine blue or hematoxylin & eosin (H&E). Images were viewed with a Leica DMI 6000B inverted microscope and z stacks were deconvolved using AutoQuant Deconvolution algorithms by Media Cybernetics.
2.6. RT-PCR of Prostate and Neuronal cell lines
Tissue and cell lines were prepared for mRNA isolation by using the SV Total RNA Isolation System (Promega; Z3100) per the manufacturer’s protocol. Purified mRNA were reverse transcribed into cDNA for real time PCR analysis using Super Script II reverse transcriptase system (Invitrogen). The following conditions were set for cDNA amplification: initial denaturation at 94°C for 5 min followed by 40 cycles of denaturation, annealing for 30 sec at 60°C and extension for 60 sec at 72°C. Threshold cycles (Ct) values were normalized to L19 and expressed relative to controls. Primer sequences used (IDT, Inc): 5′-caa ctc ccg cca gca gat, 3′-ccg gga atg gac agt cac a (L19); 5′-tgg gtt gtg gag tga gtg ttc, 3′-gct cgc tca gcc aga tgc aat (CCL2); 5′- atg aca cct ggc tgg gag caa a, 3′-act gcc tgc tgc ttc tcc tac a (CCL3); 5′-cct cca cag cta cct ctt cta t, 3′-cga tca cag ccc tga aca aa (TNF-α); 5′-gtg tgt gac gtt ccc att aga, 3′-tta gaa aca gtc cag ccc ata c (IL-1β); 5′-gcg ccc aga cag aag tca tag, 3′-ggc aaa ctt ttt gac cgc c (CXCL2) and 5′-tta ctg cat ctg cct acg tgc tca, 3′-tgc act acg agc aga agg ttg cta (PAR2).
2.7. Measurement of Intracellular Calcium in DRG and Prostate Cells
Tissues were prepared as previously described by Malin et al. [31] with modifications. Briefly, DRG (S1-S4) were excised from mice and placed in 8 chamber coverslides (Lab-Tek; 155411) containing 200µl of neurobasal medium (Gibco) at 37°C overnight. Cells were placed in 180µl of loading buffer (10mM Hepes, 11mM glucose, 2.5mM calcium chloride, 1.2mM magnesium chloride) containing Fura-2 AM (.Invitrogen; F1221) and incubated at 37°C for 15 minutes. The coverslides were washed twice with the loading buffer and a final volume of 180µl was placed in each chamber. Stimulation of cells occurred by adding 1nM capsaicin to wells. Ratiometric readings at 340nm and 380nm were recorded for 45–60 seconds prior to the addition of stimulant (baseline) and for 150 seconds after stimulation. For experiments involving prostate cells RWPE-1 (cultured in Keratinocyte-SFM 1X; Gibco; 10724-011), BPH (stromal primary) (cultured in RPMI 1640 1X in 10%FBS; Corning; 10–040-CV), and B35 (cultured in DMEM 1x in 10%FBS; Corning; 10–013-CV), cells were incubated with (10 µ g/ml) ENMD-1068 (abcam; ab141699) for 1 hour and washed twice with loading buffer before recording as described for DRG. Cell lines were stimulated with (10µg/ml) human recombinant tryptase-P/TPSB2 (R&D systems; 3796-SE). All experiments were conducted in triplicates. Images of cells were obtained with a Leica DMI 6000B microscope with a 40× objective.
2.8. Statistical Analysis
Quantitative data were expressed as mean ± SEM. Prism software was used to apply two tailed t-tests to experiments comparing two groups. Two-way or one-way ANOVA followed by a Newmans-Keuhls multiple comparison post-hoc test was used for behavioral experiments. Data were considered statistically significant different if p<0.05.
2.9 Study approval
The collection of clinical samples and clinical and pathological information was approved by Northwestern University Institutional Review Board. Criteria for inclusion and exclusion were reported in Clinicaltrials.gov (Clinical trials identifier NCT01676857). All animal studies were approved by Northwestern University IACUC.
3. Results
3.1. CP/CPPS is associated with elevated levels of mast cell mediators in EPS and urine
Mast cells have been postulated to play an important role in the pathogenesis of CP/CPPS in clinical patients and in animal models [15; 36; 45]. We have previously demonstrated that mast cell tryptase, a product of mast cell degranulation, is elevated in patients with CP/CPPS [15]. In this study, we examined expressed prostatic secretions (EPS) or urine samples obtained immediately prior (VB2) and immediately after prostatic massage (VB3) from patients and healthy volunteers for the presence of the mast cell degranulation products, tryptase, CPA3 and NGF. VB2 urines were considered to be representative of mast cell products released from the bladder and urethra and VB3 as representative of products secreted from the prostate, in addition to those from the bladder and urethral of men. We compared the EPS and VB2 subtracted VB3 (VB3-VB2, prostate specific release) levels of tryptase-P, CPA3 and NGF between CP/CPPS and healthy volunteers. We observed significantly elevated levels of tryptase in the EPS of CP/CPPS patients (Fig. 1A) compared to healthy controls (p=0.0060). CPA3, a mast cell carboxypeptidase that acts with tryptase-β and is released upon mast cell activation and degranulation was significantly elevated in CP/CPPS (Fig. 1B) urines (VB3-VB2) compared to healthy volunteers (p=0.0444). We also examined NGF, a secreted protein from mast cells that is known to be elevated in similar pain conditions such as interstitial cystitis [27]. We observed no significant difference between CP/CPPS and control urines for NGF levels (Fig. 1C). These data demonstrate that degranulation products of mast cells, CPA3 and tryptase-β are associated with CP/CPPS.
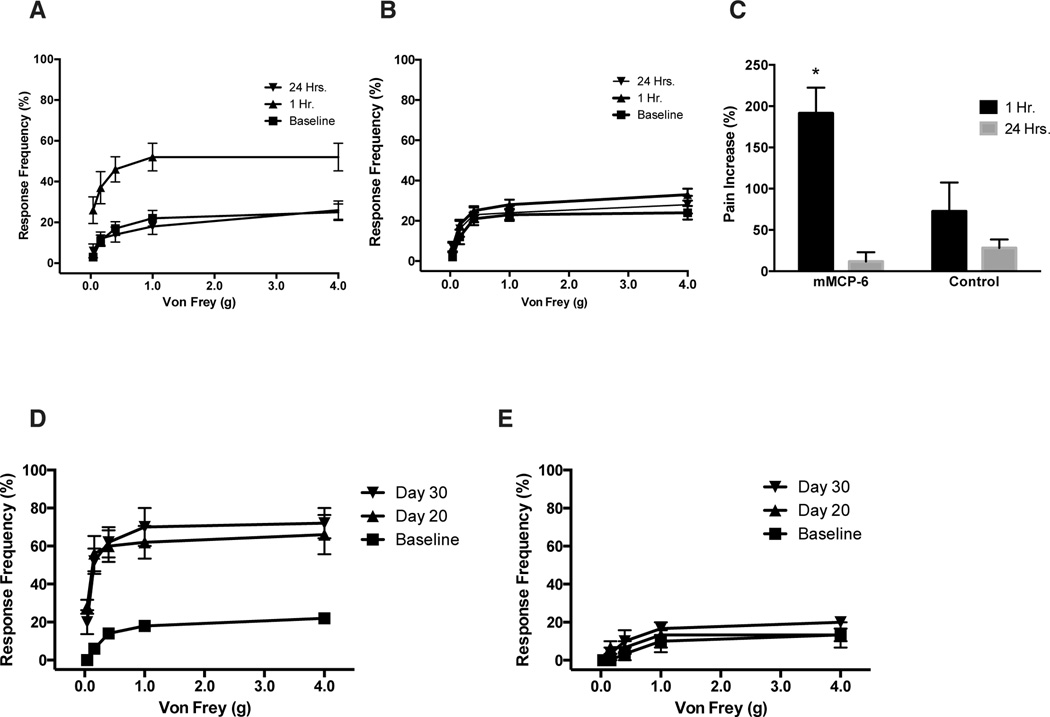
Fig. 1. Tryptase and carboxypeptidase A are increased in CP/CPPS patients.
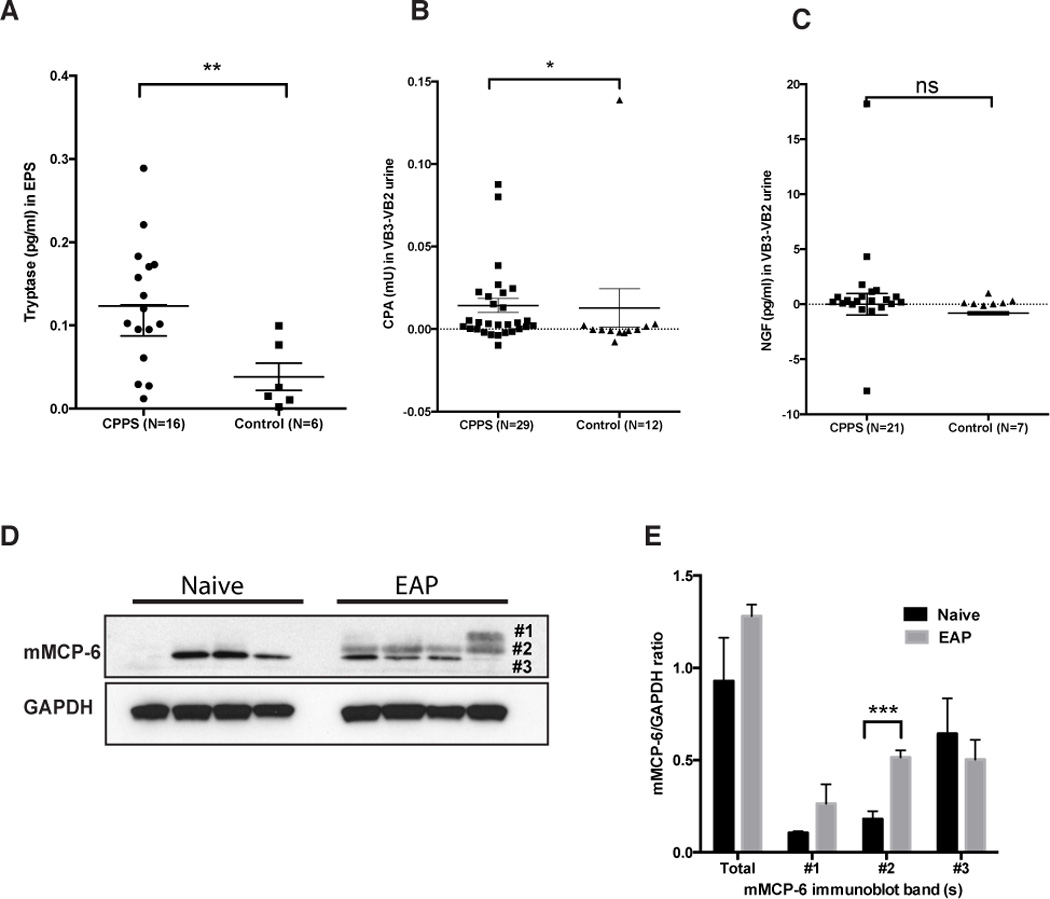
(A) Mast cell tryptase was significantly increased in EPS from CP/CPPS (n=16) patients compared to healthy volunteers (n=6). (B) Urine samples (V3 and V2) collected from CP/CPPS (n=29) patients showed increased presence of carboxypeptidase A (CPA3) compared to control (n=12); (C) however, NGF levels did not change compared to control group. (D) Western blots were performed with 20µg/sample of mouse prostate from NOD mice (n=4) with EAP. (E) Densitometry analysis of immunoblot demonstrated higher molecular weight forms of mMCP-6 in the prostate of EAP-NOD mice compared to naive. Data represent the mean ± SEM. (*) denotes p<0.05, ** p<0.01 and ***p<0.001 compared with control.
3.2. EAP increases mMCP-6 and its cognate receptor PAR2 expression in the prostate
The EAP murine model of CP/CPPS was used to analyze the role of the murine ortholog of tryptase - mMCP-6 and its cognate receptor PAR2. Although previous studies have demonstrated the involvement of mMCP-6 in inflammatory responses [43], the role of mMCP-6 in prostatitis is unknown. NOD mice were induced to develop EAP by subcutaneous injection of rat prostate antigen and the expression of mMCP-6 and PAR2 were examined in whole prostate lysates, tissue sections and microdissected epithelial and stromal cells. mMCP-6 expression was increased in EAP with the appearance of additional molecular weight forms of mMCP-6 in EAP compared to control (Fig. 1D). Interestingly, statistically significant increase in a higher molecular weight form (band #2, Fig. 1E) was observed in EAP compared to control. PAR2 receptor expression was also significantly elevated in the prostates of NOD mice with EAP compared to naive mice (Fig. 2B and C). Immunofluorescence staining demonstrated that PAR2 expression in the prostate was predominantly localized to the stroma of the prostate (Fig. 2A compare insets) and was also elevated in mice induced with EAP (Fig. 2A). Using microdissected epithelial and stromal cells from naive and EAP prostates we analyzed the location of PAR2 expression using real-time PCR. Our results show that PAR2 expression is increased 3–4 fold in the epithelium and greater than 4 fold in the stroma of the prostate in EAP mice compared to naive mice (Fig. 2E).
Fig. 2. PAR2 is elevated in the prostate of EAP mice.
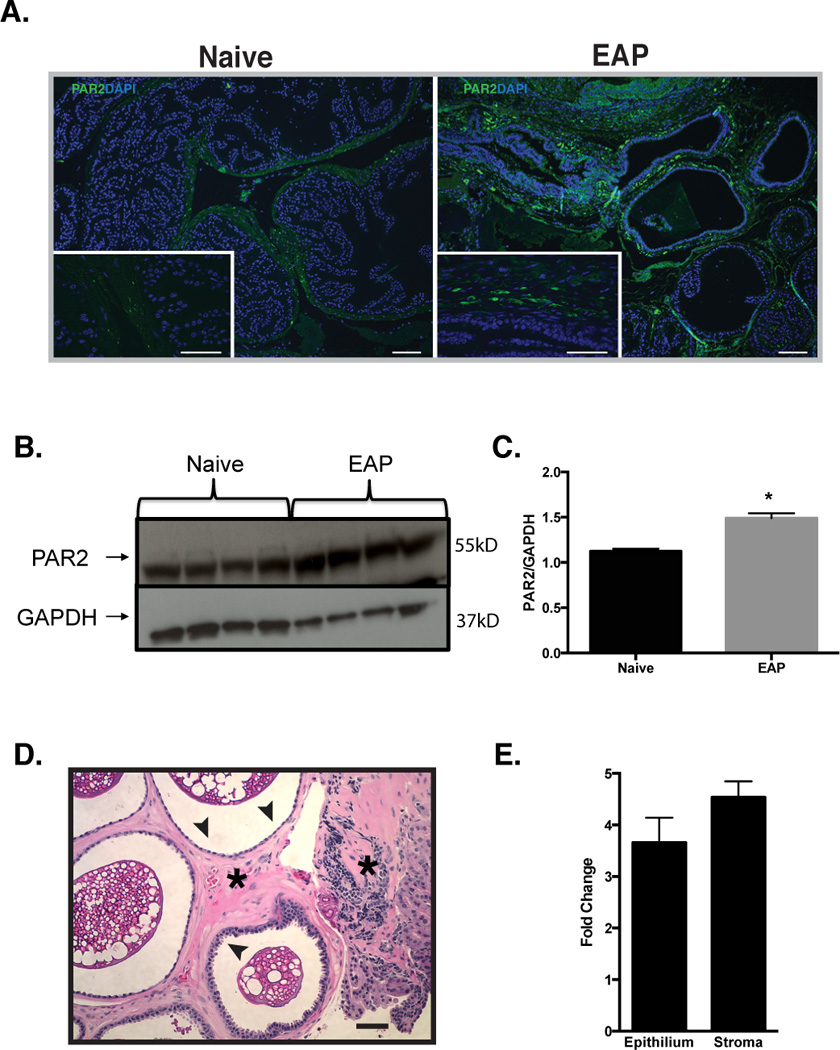
(A) Representative immunofluorescence images of the prostate showed that NOD mice with EAP have elevated PAR2 expression (green) in the stroma (insets) compared to naive cohorts. DAPI is labeled blue and bars represent 50 microns. (B) Immunoblot and (C) densitometry data confirmed that the prostate from EAP mice (n=4) up-regulate PAR2 expression, compared to naive (n=4). (D) Representative H&E stained section of a prostate from a NOD mouse with EAP were used to microdissect epithelium (arrows) and stroma (asterisks) separately. (E) Real-time PCR analysis showed increased in mRNA for PAR2 in the stroma and epithelial layers of mice with EAP (pooled). Data was normalized to L-19 and expressed as fold change respective to naive. (*) denotes p<0.05. Real-time PCR experiments were performed three times.
3.3. Tryptase-PAR2 interaction leads to calcium elevation and proinflammatory gene expression in prostate and neuronal cells in vitro
Previous studies have demonstrated that mMCP-6 induces calcium uptake in neuronal cells [25]. We therefore examined the ability of various prostate cells and a neuronal cell line to respond to tryptase-p/mMCP-6 in the presence or absence of a selective PAR2 antagonist (ENMD-1068). mMCP-6 elicited intracellular calcium influx in the B35 rat neuroblastoma cell line and Tryptase-βin the human prostate epithelial RWPE-1 and primary prostate stromal cells (BPH) to varying degrees, with B35 cells responding most robustly to stimulation (Fig. 3A–C). The tryptase-βinduced calcium elevation was significantly inhibited in the presence of a PAR2 pharmacological inhibitor in the B35 (Fig. 3A) and RWPE-1 cell lines (Fig. 3C) by 75% and 31%, respectively but not in BPH (stromal) cells (Fig. 3B). These results show that tryptase-PAR2 signaling is intact in cell types that are representative of the prostate.
Fig. 3. Tryptase induces calcium influx located in prostate and neuronal cell lines.
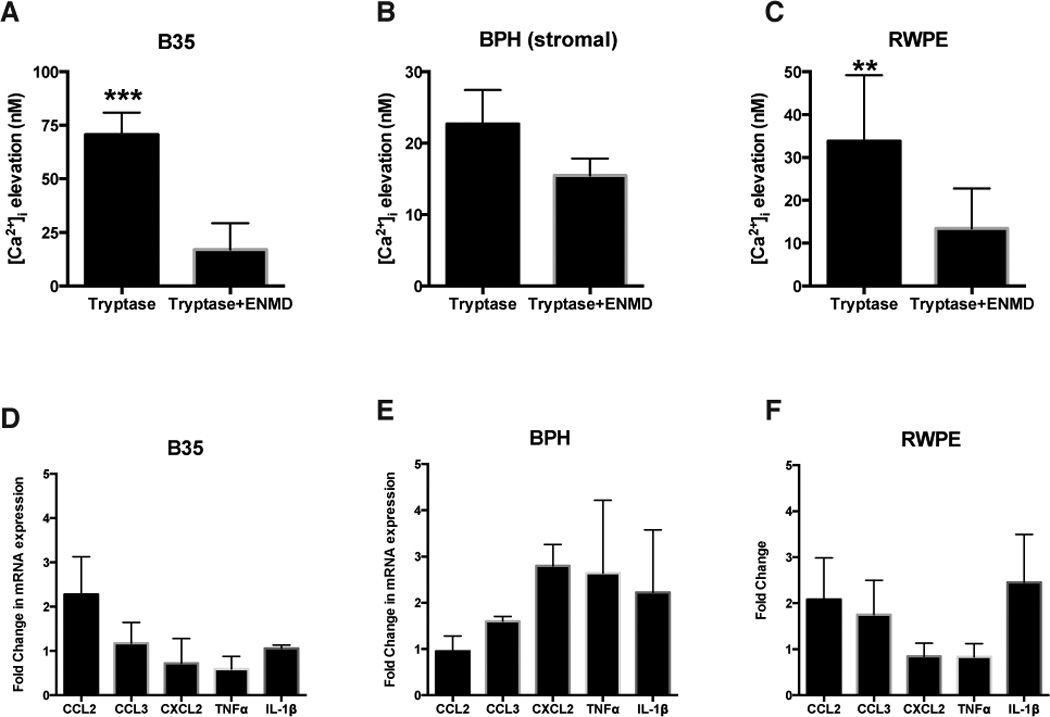
(A) Calcium elevation [Ca2+]i in RWPE-1 (p<0.001; n=4), BPH (stromal) (p=0.119; n=3), and B35 cells (p<0.01; n=4) was recorded at baseline and after the addition of recombinant human-tryptase before or after 1-hour ENMD (PAR2 antagonist) incubation. (B) Real-time PCR was conducted to determine the expression of proinflammatory markers CCL2, CCL3, CXCL2, TNF-α, and IL-1β in cell cultures. Data represent the mean ± SEM. **p<0.01 and ***p<0.001 compared with respective control. Real-time PCR experiments were performed three or more times.
We next examined the functional consequence of tryptase-PAR2 activation in vitro. Previous studies suggest that cytokines and chemokines are altered in patients with CP/CPPS and have been targeted as biomarkers. For example, studies suggest that patients with CP/CPPS have higher levels of IL-1β, TNF-α, IL-6, and IL-8 in the seminal plasma [2] and increased chemokine CCL2 and CCL3 expression [14; 35]. Similarly, published work from our lab has shown elevated levels of CCL2, CCL3 and CXCL2 in the prostate during the development of EAP [38]. A recent study demonstrated that the cytokines TNF-α, IL17A, IFN-γ, and IL-1β were increased in the prostate of mice with EAP [3]. We therefore examined the ability of tryptase- β/mMCP-6 to increase mRNA expression levels of CCL2, CCL3, CXCL2 and the proinflammatory cytokines TNF-α and IL-1β in RWPE-1, BPH and B35 cells by real-time PCR. BPH (stromal) cells showed a 2–3 fold induction of CXCL2, TNF-α and IL-1β (Fig. 3E) while RWPE-1 cells showed a 2–4 fold induction of CCL2, CCL3 and IL-1β in response to tryptase treatment (Fig. 3F). B35 cells in contrast, did not exhibit a strong proinflammatory response with no changes in CCL3, CXCL2, TNF-α and IL-1β mRNA but a 2–3 fold induction of CCL2 (Fig. 3D). These results demonstrate that prostate-derived cell lines that express PAR2 are capable of signaling in response to tryptase-βresulting in transcriptional activation of proinflammatory cytokines and chemokines.
3.4. Intra-urethral mMCP-6 instillation induces tactile allodynia in male mice
Previous studies have shown that EAP induction reduces the response threshold to pelvic tactile behavioral assays [40]. To determine whether mMCP-6 causes enhanced tactile allodynia, a surrogate metric for pelvic pain, recombinant mMCP-6 was instilled intraurethrally in NOD mice and tactile allodynia was tested at 1-hour and 24-hours. Mice that received recombinant mMCP-6 showed an increased (Fig. 4A and C) response frequency (>175% pain increase from baseline) at 1-hour compared to naive (Fig. 4B and C); and the response frequency returned to baseline at 24-hours post-instillation (Fig. 4A and C).
Fig. 4. Tryptase/mMCP-6 induced pelvic pain via PAR2.
NOD mice were assessed for pelvic tactile allodynia/hyperalgesia with von Frey filaments before (baseline) and after intraurethral instillation of (A) mMCP-6 (n= 5) or (B) saline (n=5) at 1hr and 24hrs. (C) The pain increase (%) was generated from the total response frequency at 1hr or 24 hrs divided by respective baseline x100. Pelvic pain response was evaluated in (D) C57BL/6J (B6) (n=5) and (E) PAR2 deficient mice (n=4) with EAP (PAR2 KO-EAP) at day 0, 20, and 30. The following von Frey filaments were used to evaluate each mouse: 2.44, 3.22, 3.61, 4.08 and 4.56. Data represent the mean ± SEM. (*) denotes p<0.05. In vivo experiments were performed two or more independent times.
In order to determine the role of the PAR2 receptor in pelvic pain, we next induced EAP in PAR2 KO mice and tested for tactile allodynia at days 0, 20, and 30. PAR2 KO-EAP mice did not demonstrate enhanced tactile referred hyperalgesia of the pelvic region at day 20 and 30 (Fig. 4E) compared to B6 control mice with EAP (Fig. 4D). This result indicates that PAR2 is involved in the development of chronic pain in the EAP model of prostatitis.
3.5. EAP-induced dorsal root ganglia activation is abrogated in the absence of PAR2
PAR2 activation elicits functional effects through ERK1/2 phosphorylation in neuronal tissues [52] and changes in intracellular calcium levels [19]. ERK1/2 phosphorylation is an important signaling event in multiple visceral pain models and is linked to modulation of intracellular calcium levels [Ca2+]i [9; 23; 46]. Here, we examined DRG from mice with EAP for enhanced p-ERK1/2 expression and whether the absence of PAR2 abrogates ERK1/2 phosphorylation. Mice were euthanized 30 days after EAP and p-ERK1/2 expression in sacral DRG (S1–S4) was assessed by immunoblot analysis. Mice with EAP showed enhanced p-ERK1/2 expression (153%) compared to naive (Fig. 5A and B). These results were confirmed using immunofluorescence of DRG (Fig. 5D). Despite an elevated baseline p-ERK1/2 expression in DRG from PAR2 KO-Naive mice, no change was observed in PAR2 KO with EAP at day 30 (Fig. 5A and C).
Fig. 5. PAR2 is involved in ERK signaling and calcium influx.
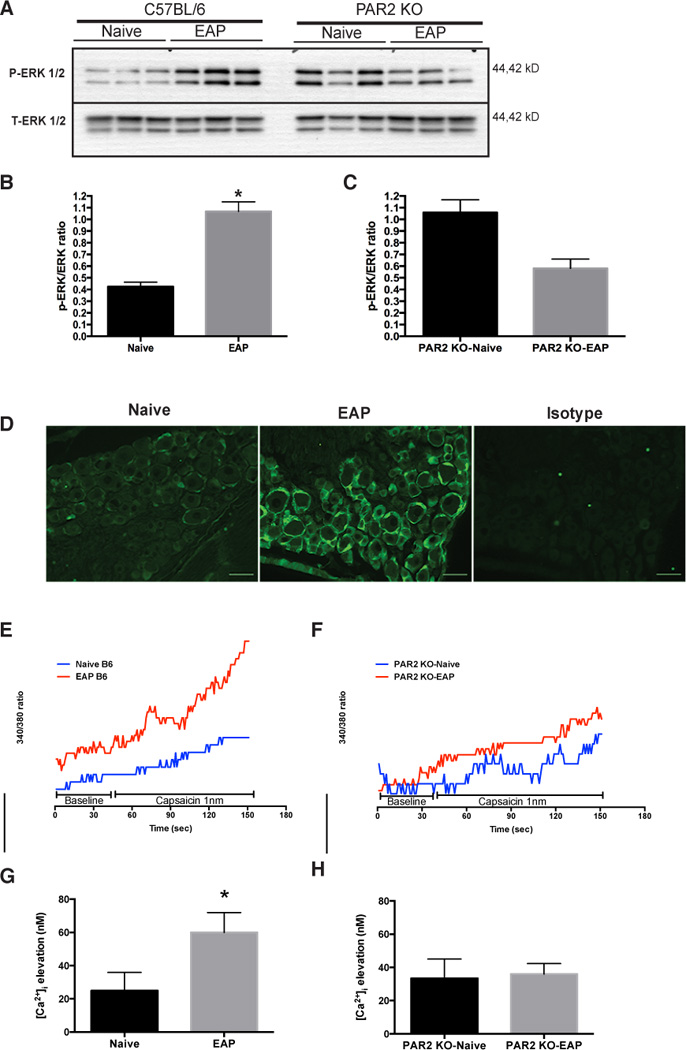
(A) Western blot shows DRG (S1–S4) p-ERK1/2 expression at day 30 from mice with EAP (n=3) compared to naive (n=3) and PAR2 deficient mice with EAP (PAR2 KO-EAP; n=3) compared to control (PAR2 KO-Naive; n=3). (B) Densitometry data showed a significant increased in p-ERK1/2 expression in mice (B6) with EAP compared to naive cohorts and (C) no difference in p-ERK1/2 expression was observed between PAR2 KO-Naive and PAR2 KO-EAP. (D) Representative immunofluorescence of p-ERK 1/2 (green) in DRG of mice with EAP and naive at day 30 showed increased p-ERK1/2. (E and G) Changes in [Ca2+]i were recorded before (45 seconds) and after (150 seconds) delivery of 1nm capsaicin to DRG extracted from mice with EAP and compared to naive cohorts. (G) EAP treated animals showed a significant increase in [Ca2+]i compared to control. (F and H) However, [Ca2+]i remained the same in DRG from PAR2 KO-EAP compared to PAR2 KO-Naive at day 30. Calcium experiments were repeated independently at least three times. Scale bars represent 50µm and (*) denotes p<0.05.
Tryptase activated PAR2 may mediate pain by sensitizing the TRPV1 receptor found in peripheral afferents [51], leading to altered calcium uptake and neuronal hyperexcitability. We hypothesized that increased neuronal excitability will be reflected in enhanced responsiveness of DRG neurons to low concentrations of the TRPV1 ligand, capsaicin. To explore whether mice with EAP exhibited sensitized DRG, we measured [Ca2+]i levels at day 30. DRG from mice with EAP showed an enhanced responsiveness to capsaicin that resulted in an elevation of [Ca2+]i that was significantly greater than controls (Fig. 5E and G). In contrast, elevation of [Ca2+]i in response to capsaicin was absent in DRG from PAR2 KO-Naive and PARK2 KO-EAP mice suggesting that EAP induced neuronal excitability is dependent on PAR2.
3.6. Absence of PAR2 does not abrogate mast cell and leukocyte recruitment to the prostate
We have previously reported that EAP is characterized by the presence of inflammation in the prostate and the recruitment/proliferation of mast cells [15; 38]. To determine whether PAR2 is required to establish inflammation and/or recruit mast cells, prostate tissues were analyzed from B6 and PAR2 deficient mice that received EAP. Inflammation scoring of H&E stained prostate sections from B6 mice with EAP (Fig. 6A panel a) and PAR2 KO-EAP mice (Fig. 6A panel b) at day 30 reveal similar levels of inflammation (Fig. 6B). Mast cell-specific toluidine blue staining revealed mast cells in the stroma of the prostate in B6 and PAR2 KO-EAP mice with EAP (Fig. 6A panels c & d). Quantification of mast cell numbers in the two groups showed similar trends in mast cell increase as well as activation status of mast cells in B6 and PAR2 KO-EAP mice with EAP at day 30 (Fig. 6C).
Fig. 6. PAR2 loss does not inhibit inflammation and mast cell activation in EAP mice.
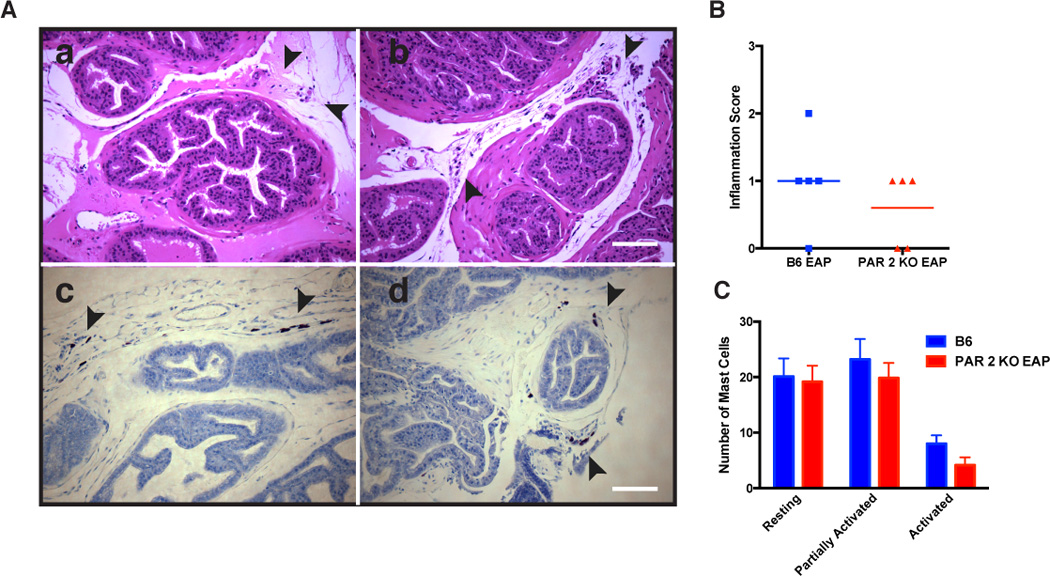
(A) Representative H&E staining of the prostate excised from (panel a) EAP mice and (panel b) PAR2 KO with EAP at day 30 show leukocytic influx at day 30. Also, mouse prostate sections were stained with acidified toluidine blue and showed the same number of mast cells in (panel c) B6 mice with EAP and (panel d) PAR2 KO with EAP. (B) Inflammation score was assessed from H&E staining sections of the prostates obtained from B6 EAP and PAR2 KO with EAP at day 30 and were quantified by a blinded observer. (C) Resting mast cells, partially activated, and activated mast cells were also quantified and show no difference between groups. Data reflect mean ± SEM for 3 non-serial sections from 3 animals.
3.7 Neutralizing PAR2 mitigates EAP-induced pelvic pain
Studies conducted on acute cancer pain show that blocking PAR2 activation may have protective properties [26]. We therefore assessed inhibition of PAR2 in abrogating pelvic pain induced by EAP. A PAR2 neutralizing antibody (SAM11) or an isotype control antibody (IgG) were administered intraperitoneally at 20 days post-EAP induction and pelvic tactile allodynia was assessed at 3, 7 and 10 days after treatment. Our results show that the neutralizing antibody resulted in a significant reduction of pelvic pain at days 7 and 10 days after administration compared to the isotype control (Fig. 7A, B and C). These results suggest that PAR2 is important for the development and maintenance of visceral nociception originating from the prostate and may be a potential target for therapeutic intervention.
Fig. 7. Anti-PAR2 antibody (SAM11) reduced pelvic pain.
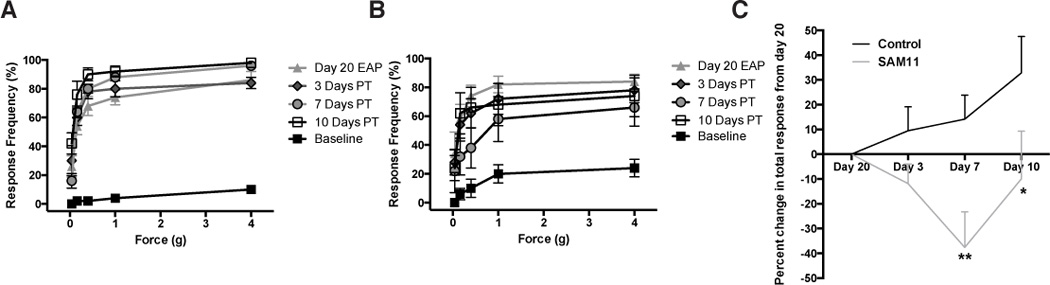
NOD mice (5 per group) were induced with EAP for 20 days and pain development was confirmed at day 20 followed by intraperitoneal administration of saline or a PAR2 neutralizing antibody (SAM11). Mice assessed for tactile hyperalgesia/allodynia with von Frey filaments at day 0 (baseline) day 20 and after injection with (A) saline or (B) SAM11 at day 3, 7, and 10. (C) Graph depicts percent change in total responses from day 20 EAP at day 3, 7, and 10. Data represent the mean ± SEM. (*) and (**) denotes p<0.05 and p<0.01, respectively. Behavioral experiments were performed independently two times.
4. Discussion
Despite the growing number of studies on chronic pain of visceral organs, few studies focus on the neuroimmune crosstalk in CP/CPPS. Unfortunately, the lack of research in CP/CPPS has limited successful long-term treatment of symptoms, as reveal by a recent study conducted on patients after a 2-year follow-up [47]. This study attempts to elucidate the pathophysiology of CP/CPPS by demonstrating that chronic prostatitis in a murine model of CP/CPPS activates a PAR2-dependent mechanism that is important for peripheral neuronal activation and the development of chronic pelvic pain.
Clinical CP/CPPS in this study was associated with increased levels of mast cell tryptase in EPS with no change in NGF levels in postprostatic massage urines. These results confirm earlier observations from our group with regards to tryptase [15] in a new, albeit small, clinical population of CP/CPPS patients and reinforces the potential role of mast cells in CP/CPPS. In contrast to our earlier report of an elevated NGF in EPS from CP/CPPS patients, the present study did not observe this in the prostate-specific fraction of urines (VB3-VB2). In addition to the significant dilution of secreted NGF in urine compared to EPS, differences in patient population and assay methodology (ELISA versus immunoblot analysis) may have contributed to the difference in results between these studies. The limited quantity of EPS obtained from each subject limited the number of analytes that could be examined, preventing this study from using the same clinical specimen for all analyses. In addition to tryptase, we demonstrate that clinical CP/CPPS is associated with elevated levels of CPA3 in post-prostatic massage urines (VB3-VB2). CPA3 levels displayed significant heterogeneity within the patient population that may be explained by the biological heterogeneity of the population or additional underlying mast cell mediated pathology of the bladder, which could lead to elevated VB2 levels of CPA3 and a corresponding absence of elevations in CPA3 levels in the VB3-VB2 urine fraction. The increased levels of tryptase and CPA3, two markers of mast cell degranulation, in EPS and urines of patients with CP/CPPS strongly suggests a role for the mast cell in mediating CP/CPPS pathophysiology.
We have previously demonstrated that mast cells are required for the development of chronic pelvic pain in EAP. Mast cells lie adjacent to epithelial cells, vessels and peripheral nerves [24], making their cell products available to a variety of cell types. Neurogenic inflammation and altered mast cell function have been hypothesized to be important patients with chronic pain. There is accumulating evidence that inflammatory cells, such as mast cells, can influence nociceptive signaling via tryptase, a PAR2 agonist [5; 6; 15]. In this study we demonstrate that instillation of mMCP-6 intraurethrally induces tactile allodynia, a characteristic feature of EAP. Here however, tactile allodynia is transient with pain responses decreasing to baseline by 24 hours, suggesting a continued requirement for mMCP-6 release from mast cells for the maintenance of pelvic pain. EAP induction is also associated with increased mMCP-6, in particular higher molecular weight forms of mMCP-6 are found to be present in significantly higher levels than controls. Multiple molecular forms of tryptase have been previously reported in human sgnclrtding up to five major and minor forms [10; 42] and have been demonstrated to be enzymatically active with the different sizes explained by dimerization and N-linked glycosylation [10]. The increased levels of higher molecular weight forms of mMCP-6 in EAP mice compared to controls suggest that glycosylated forms are preferentially increased in in the EAP prostate.
PAR2 is expressed on a variety of cell types including epithelial, endothelial, neutrophils mast cells, macrophages and neurons. Loss of PAR2 expression has different effects on a variety of animal models of disease. While deficiency can protect from colitis, lung fibrosis and kidney inflammation, PAR2 absence can exacerbate lung infections and brain ischaemia [49]. PAR2 activation is implicated in mast cell degranulation-induced hyperalgesia, as well as in formalin-induced hyperalgesia [50]. In agreement with these reports, our studies in EAP-induced PAR2 KO mice demonstrate that pelvic pain is abrogated, suggesting that the mast cell mediated pain mechanisms in EAP are induced in a PAR2 dependent manner. In chronic inflammation models, parameters of inflammation are reduced in PAR2 KO mice [17]. In contrast to these reports, our studies suggest a lack of significant reduction in inflammation in the prostate upon PAR2 deficiency. These results, however, parallel our earlier observation that mast cell deficient KitW-sh / KitW-sh (Sash) mice have attenuated pain responses upon induction of EAP but showed no difference in the level of inflammatory infiltrate [15]. These results suggest that PAR2 mediated pain mechanisms may be distinct from antigen-induced inflammation in the EAP model of CP/CPPS.
PAR2 on sensory neurons is functional, as PAR2 agonists (selective peptides, trypsin or tryptase) are able to induce calcium mobilization [44]. Liu et al. (2013) reported that plantar injection of sarcoma cells induced PAR2 up-regulation and increased intracellular calcium in DRG [28]. PAR2 activation on sensory neurons potentiates the response of those cells to agonists of TRPV1 [4; 11] in DRG and this process is mediated by PAR2. Similarly, the present study showed that DRGs from mice with EAP demonstrate increased calcium uptake in response to the TRPV1 agonist - capsaicin that was absent in PAR2 KO-EAP treated mice. In acute visceral pain models mast cells are known to increase ERK 1/2 phosphorylation in neurons [20]. Here we report that chronic inflammation accompanied by chronic visceral pain contributes to increased neuronal excitability and maintenance of ERK signaling.
PAR2 small molecule antagonists have been found to be efficacious in experimental models of colitis and arthritis [29; 30] and neutralizing antibodies for the receptor have been used in inhibiting joint inflammation [25]. In this study we demonstrate that systemic administration of a neutralizing antibody to PAR2 can therapeutically inhibit pelvic pain in the EAP model of CP/CPPS. These effects are evident after a single administration of the antibody and suggest that blockade of PAR2 may be a potential strategy for treating pelvic pain in CP/CPPS. These results are in agreement with earlier reports from our laboratory, which demonstrated that inhibition of mast cell degranulation reduced tactile allodynia in the EAP model, and suggest that mast cells mediate these events via the PAR2 receptor.
Overall, our results identify mast cell tryptase and CPA3 as clinically relevant molecules in CP/CPPS and show that tryptase-fi is important for mediating chronic pelvic pain in the EAP animal model. Furthermore, the tryptase-PAR2 axis is functional in the prostate and can mediate signaling and activation of dorsal root ganglia. Inhibition of this pathway using PAR2 neutralizing antibodies is of therapeutic benefit in blocking pelvic pain. Finally, our results suggest that mast cells and more specifically, the tryptase-PAR2 axis plays an important role in the pathogenesis of CP/CPPS and that these may serve as novel targets for therapeutic intervention.
Acknowledgements
This project was supported by award number R01DK083609 and K01DK079019 from the National Institute Of Diabetes And Digestive And Kidney Diseases. We wish to thank Ashlee Bell-Cohn and Larry Wong, PhD for help and critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflict of interest to report with regard to the study reported in this manuscript.
The tryptase-PAR2 axis is a crucial mediator of pelvic pain in EAP and may play an important role in the pathogenesis of CP/CPPS.
References
- 1.Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD. Structure, function and pathophysiology of protease activated receptors. Pharmacology & therapeutics. 2011;130(3):248–282. doi: 10.1016/j.pharmthera.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998;52(5):744–749. doi: 10.1016/s0090-4295(98)00390-2. [DOI] [PubMed] [Google Scholar]
- 3.Altuntas CZ, Daneshgari F, Veizi E, Izgi K, Bicer F, Ozer A, Grimberg KO, Bakhautdin B, Sakalar C, Tasdemir C, Tuohy VK. A novel murine model of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) induced by immunization with a spermine binding protein (p25) peptide. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304(6):R415–R422. doi: 10.1152/ajpregu.00147.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(18):4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. The Journal of clinical investigation. 2007;117(3):636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. The American journal of pathology. 2002;161(5):1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. The Journal of urology. 2008;180(4):1378–1382. doi: 10.1016/j.juro.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in bladder afferent pathways with cyclophosphamide-induced cystitis. Neuroscience. 2009;163(4):1353–1362. doi: 10.1016/j.neuroscience.2009.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cromlish JA, Seidah NG, Marcinkiewicz M, Hamelin J, Johnson DA, Chretien M. Human pituitary tryptase: molecular forms, NH2-terminal sequence, immunocytochemical localization, and specificity with prohormone and fluorogenic substrates. The Journal of biological chemistry. 1987;262(3):1363–1373. [PubMed] [Google Scholar]
- 11.Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(18):4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels NA, Link CL, Barry MJ, McKinlay JB. Association between past urinary tract infections and current symptoms suggestive of chronic prostatitis/chronic pelvic pain syndrome. Journal of the National Medical Association. 2007;99(5):509–516. [PMC free article] [PubMed] [Google Scholar]
- 13.Dery O, Corvera CU, Steinhoff M, Bunnett NW. TProteinase-activated receptors: novel mechanisms of signaling by serine proteases. The American journal of physiology. 1998;274(6 Pt 1):C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 14.Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, Thumbikat P, Pope RM, Landis JR, Koch AE, Schaeffer AJ. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 alpha as possible biomarkers for the chronic pelvic pain syndrome. The Journal of urology. 2008;179(5):1857–1861. doi: 10.1016/j.juro.2008.01.028. discussion 1861−1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. The Journal of urology. 2012;187(4):1473–1482. doi: 10.1016/j.juro.2011.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrell WR, Kelso EB, Lockhart JC, Plevin R, McInnes IB. Protease-activated receptor 2: a novel pathogenic pathway in a murine model of osteoarthritis. Annals of the rheumatic diseases. 2010;69(11):2051–2054. doi: 10.1136/ard.2010.130336. [DOI] [PubMed] [Google Scholar]
- 17.Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, Smith AJ, Hunter GD, McLean JS, McGarry F, Ramage R, Jiang L, Kanke T, Kawagoe J. Essential role for proteinase-activated receptor-2 in arthritis. The Journal of clinical investigation. 2003;111(1):35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan J, Greenwood SM, Cobb SR, Bushell TJ. Indirect modulation of neuronal excitability and synaptic transmission in the hippocampus by activation of proteinase-activated receptor-2. British journal of pharmacology. 2011;163(5):984–994. doi: 10.1111/j.1476-5381.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao G, Ouyang A, Kaufman MP, Yu S. ERK1/2 signaling pathway in mast cell activation-induced sensitization of esophageal nodose C-fiber neurons. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2011;24(3):194–203. doi: 10.1111/j.1442-2050.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- 21.Gelbmann CM, Mestermann S, Gross V, Kollinger M, Scholmerich J, Falk W. Strictures in Crohn’s disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut. 1999;45(2):210–217. doi: 10.1136/gut.45.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton MJ, Sinnamon MJ, Lyng GD, Glickman JN, Wang X, Xing W, Krilis SA, Blumberg RS, Adachi R, Lee DM, Stevens RL. Essential role for mast cell tryptase in acute experimental colitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):290–295. doi: 10.1073/pnas.1005758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152(5):1182–1191. doi: 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keith IM, Jin J, Saban R. Nerve-mast cell interaction in normal guinea pig urinary bladder. The Journal of comparative neurology. 1995;363(1):28–36. doi: 10.1002/cne.903630104. [DOI] [PubMed] [Google Scholar]
- 25.Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, Sommerhoff CP, McLean JS, Ferrell WR. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. The Journal of pharmacology and experimental therapeutics. 2006;316(3):1017–1024. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- 26.Lam DK, Dang D, Zhang J, Dolan JC, Schmidt BL. Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(41):14178–14183. doi: 10.1523/JNEUROSCI.2399-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu HT, Kuo HC. Increased urine and serum nerve growth factor levels in interstitial cystitis suggest chronic inflammation is involved in the pathogenesis of disease. PloS one. 2012;7(9):e4687. doi: 10.1371/journal.pone.0044687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Liu YP, Yue DM, Liu GJ. Protease-activated receptor 2 in dorsal root ganglion contributes to peripheral sensitization of bone cancer pain. Eur J Pain. 2013 doi: 10.1002/j.1532-2149.2013.00372.x. [DOI] [PubMed] [Google Scholar]
- 29.Lohman RJ, Cotterell AJ, Barry GD, Liu L, Suen JY, Vesey DA, Fairlie DP. An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(7):2877–2887. doi: 10.1096/fj.11-201004. [DOI] [PubMed] [Google Scholar]
- 30.Lohman RJ, Cotterell AJ, Suen J, Liu L, Do AT, Vesey DA, Fairlie DP. Antagonism of protease-activated receptor 2 protects against experimental colitis. The Journal of pharmacology and experimental therapeutics. 2012;340(2):256–265. doi: 10.1124/jpet.111.187062. [DOI] [PubMed] [Google Scholar]
- 31.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nature protocols. 2007;2(1):152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen C, Coelho AM, Grady E, Compton SJ, Wallace JL, Hollenberg MD, Cenac N, Garcia-Villar R, Bueno L, Steinhoff M, Bunnett NW, Vergnolle N. Colitis induced by proteinase-activated receptor-2 agonists is mediated by a neurogenic mechanism. Canadian journal of physiology and pharmacology. 2003;81(9):920–927. doi: 10.1139/y03-080. [DOI] [PubMed] [Google Scholar]
- 33.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(20):9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nystedt S, Larsson AK, Aberg H, Sundelin J. The mouse proteinase-activated receptor-2 cDNA and gene. Molecular cloning and functional expression. The Journal of biological chemistry. 1995;270(11):5950–5955. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- 35.Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. European urology. 2007;51(2):524–533. doi: 10.1016/j.eururo.2006.07.016. discussion 533. [DOI] [PubMed] [Google Scholar]
- 36.Pontari MA, Ruggieri MR. TMechanisms in prostatitis/chronic pelvic pain syndrome. The Journal of urology. 2008;179(5 Suppl):S61–S67. doi: 10.1016/j.juro.2008.03.139. [DOI] [PubMed] [Google Scholar]
- 37.Quick ML, Done JD, Thumbikat P. Measurement of tactile allodynia in a murine model of bacterial prostatitis. Journal of visualized experiments : JoVE. 2013;(71):e50158. doi: 10.3791/50158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quick ML, Mukherjee S, Rudick CN, Done JD, Schaeffer AJ, Thumbikat P. CCL2 and CCL3 are essential mediators of pelvic pain in experimental autoimmune prostatitis. American journal of physiology Regulatory, integrative and comparative physiology. 2012;303(6):R580–R589. doi: 10.1152/ajpregu.00240.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramachandran R, Mihara K, Chung H, Renaux B, Lau CS, Muruve DA, DeFea KA, Bouvier M, Hollenberg MD. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2) The Journal of biological chemistry. 2011;286(28):24638–24648. doi: 10.1074/jbc.M110.201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudick CN, Schaeffer AJ, Thumbikat P. Experimental autoimmune prostatitis induces chronic pelvic pain. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294(4):R1268–R1275. doi: 10.1152/ajpregu.00836.2007. [DOI] [PubMed] [Google Scholar]
- 41.Schaeffer AJ. Clinical practice. Chronic prostatitis and the chronic pelvic pain syndrome. The New England journal of medicine. 2006;355(16):1690–1698. doi: 10.1056/NEJMcp060423. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cellsPurification and characterization. The Journal of biological chemistry. 1981;256(22):11939–11943. [PubMed] [Google Scholar]
- 43.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182(1):647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nature medicine. 2000;6(2):151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 45.Thumbikat P, Shahrara S, Sobkoviak R, Done J, Pope RM, Schaeffer AJ. Prostate secretions from men with chronic pelvic pain syndrome inhibit proinflammatory mediators. The Journal of urology. 2010;184(4):1536–1542. doi: 10.1016/j.juro.2010.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;49(3):393–403. doi: 10.1016/0304-3959(92)90247-9. [DOI] [PubMed] [Google Scholar]
- 47.Tripp DA, Nickel JC, Shoskes D, Koljuskov A. A 2-year follow-up of quality of life, pain, and psychosocial factors in patients with chronic prostatitis/chronic pelvic pain syndrome and their spouses. World journal of urology. 2013;31(4):733–739. doi: 10.1007/s00345-013-1067-6. [DOI] [PubMed] [Google Scholar]
- 48.van Hoboken EA, Thijssen AY, Verhaaren R, van der Veek PP, Prins FA, Verspaget HW, Masclee AA. Symptoms in patients with ulcerative colitis in remission are associated with visceral hypersensitivity and mast cell activity. Scandinavian journal of gastroenterology. 2011;46(7-8):981–987. doi: 10.3109/00365521.2011.579156. [DOI] [PubMed] [Google Scholar]
- 49.Vergnolle N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacology & therapeutics. 2009;123(3):292–309. doi: 10.1016/j.pharmthera.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Proteinaseactivated receptor-2 and hyperalgesia: A novel pain pathway. Nature medicine. 2001;7(7):821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 51.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Dai Y, Kobayashi K, Zhu W, Kogure Y, Yamanaka H, Wan Y, Zhang W, Noguchi K. Potentiation of the P2×3 ATP receptor by PAR-2 in rat dorsal root ganglia neurons, through protein kinase-dependent mechanisms, contributes to inflammatory pain. The European journal of neuroscience. 2012;36(3):2293–2301. doi: 10.1111/j.1460-9568.2012.08142.x. [DOI] [PubMed] [Google Scholar]