Abstract
Articular cartilage is classified into permanent hyaline cartilage and has significant differences in structure, extracelluar matrix components, gene expression profile, and mechanical property from transient hyaline cartilage found in growth plate. In the process of synovial joint development, articular cartilage is originated from the interzone, developing at the edge of the cartilaginous anlagen, it establishes zonal structure over time and supports smooth movement of the synovial joint through life. The cascade actions of key regulators such as Wnts, GDF5, Erg, and PTHLH coordinate sequential steps of articular cartilage formation. Articular chondrocytes are restrictedly controlled not to differentiate into a hypertrophic stage by autocrine and paracrine factors and extracerllular matrix microenvironment, but retain potential to undergo hypertrophy. The basal calcified zone of articular cartilage is connected with subchondral bone, but not invaded by blood vessels nor replaced by bone, which is highly contrasted with the growth plate. Articular cartilage has limited regenerative capacity, but likely possesses and potentially uses intrinsic stem cell source in the superficial layer, Ranvier’s groove, the intra-articular tissues such as synovium and fat pad, and marrow below the subchondral bone. Considering the biological views on articular cartilage, several important points are raised for regeneration of articular cartilage. We should evaluate the nature of regenerated cartilage as permanent hyaline cartilage and not just hyaline cartilage. We should study how a hypertrophic phenotype of transplanted cells can be lastingly suppressed in regenerating tissue. Further, we should develop the methods and reagents to activate recruitment of intrinsic stem/progenitor cells into the damaged site.
Keywords: cartilage repair, permanent cartilage, transient cartilage, articular cartilage, growth-plate, chondrocyte, PRG4, mesenchymal stem cell
INTRODUCTION
Articular cartilage has very limited reparative capacity. Once articular cartilage is damaged, full recovery of its structure, function and biomechanical property is hardly expected in most cases, and the damaged articular cartilage can proceed toward degeneration. Because articular cartilage contributes to smooth movement of synovial joints through its low friction and high resistance to compressive force, dysfunction of articular cartilage greatly disturbs motion of the limbs and trunk and may cause pain leading to a decrease in quality of life. Restoration of articular cartilage function often relies on joint replacement by surgery (arthroplasty). Such surgical treatment has risks for complications and side effects, and gives a big economic burden to society. Thus stimulation of articular cartilage repair and regeneration of articular cartilage are strong demands.
Current approaches for articular cartilage regeneration that include drug treatment, cell-based therapies -autologous chondrocyte implantation (ACI) and ACI with biomaterials (MACI)-, osteo-articular autografts and surgical marrow stimulation of intrinsic repair potential show appreciable outcomes in traumatic injury for patients of young ages (Mastbergen et al., 2013; Minas, 2012; Schindler, 2011), especially when the lesion is limited in size and severity. Next generation of cell-based therapy is to use stem cells instead of chondrocytes (Oldershaw, 2012; Tuan et al., 2013). The stem cell sources can be adult mesenchymal stem cells isolated from bone marrow, fat, synovium and periosteal and perichondrium membranes, embryonic stem cells and possibly iPS cells. The preclinical and clinical trials using adult mesenchymal stem cells have demonstrated encouraging results although more cases and long-term observation are required for reaching a conclusion about the efficacy (Pastides et al., 2013). Much effort will be continued to evolve surgical procedures and devices, to explore cell sources and tissue engineering materials for cell-based therapies, and to improve imaging techniques for observation of affected and repaired lesions.
However, there are piles of problems unsolved at this moment. Regenerated cartilage often undergoes degeneration and reorganization into fibrocartilaginous tissue and may eventually disappear, leading to delamination and exposure of subchondral bone (Niemeyer et al., 2008). Complete integration of regenerated cartilage with existing cartilage and subchondral bone is difficult (Hollander et al., 2010). The extensive defects in size and depth are incurable. Furthermore, repair of articular cartilage is expected to be poor in the elderly unlike younger generations. To solve these problems, we face a fundamental problem that regenerative therapy of articular cartilage requires induction of permanent hyaline cartilage. Articular cartilage, together with growth plate cartilage in long bones and vertebrates, is hyaline cartilage (Hall, 2005). The former is permanent cartilage that maintains its function throughout life while the later is transient cartilage that transitionally exists serving skeletal templates in the process of endochondral ossification. If the inductive cartilage represents transient cartilage characteristics, chondrocytes undergo hypertrophy and may eventually be replaced by bone. Thus we must have a deep understanding of the nature and function of articular cartilage and how it is specified and regulated as permanent hyaline cartilage. In this review, we will review how articular cartilage is distinct from transient growth plate cartilage, how articular cartilage develops and matures its structure, which cells can support self-renewal and repair, and how articular chondrocyte phenotype is specified and maintained. Through summarizing previous findings and considering their significance, we will seek biological ideas toward regeneration of articular cartilage.
How Is Articular Cartilage Different from the Growth Plate?
Articular cartilage is rich in extracellular matrix and is distributed as solitary chondrocytes or chondrocytes that make up small colonies consisting of a few cells while the growth plate contains more cellular components and has a columnar structure of chondrocytes which shape, alignment and size dramatically change toward the chondroosseous border. Gross appearance of articular cartilage as well as resting zone of epiphyseal cartilage or costal cartilage is whitish and opaque while the growth plate cartilage containing proliferating, prehypertrophic and hypertrophic zones is much more translucent (Williams et al., 2010). Considering the histological view that articular cartilage has less cellular components, the opaqueness of articular cartilage may reflect less water content compared to the growth plate. Previous studies have reported that articular cartilage contains a larger amount of collagenous proteins and a higher level of collagen cross-link than the growth plate (Wardale and Duance, 1994). Such differences likely lead to a difference in the biomechanical property of the two types of cartilage. Cohen et al. (Cohen et al., 1998) have reported that the growth plate and the chondroepiphysis consisting of the resting zone of cartilage show significant differences in both elastic module and permeability coefficients: the growth plate is half as stiff as the chondroepiphysis and twice as permeable. Thus it is indicated that permanent hyaline cartilage and transient hyaline cartilage have differences in mechanical property as well as longevity: the former is more stiff and more resistant to compressive force while the latter is more fragile and short lived. We should not only focus on long-term existence of regenerated cartilaginous tissue but also their mechanical properties, such as compressive strength, shear strength, tensile strength and coefficient of friction. It is important to investigate how histological and biochemical characteristics of regenerated cartilage relate with their mechanical properties. Utilization of atomic force microscopy (AFM) (Leijten et al., 2012; Plodinec et al., 2010; Wen et al., 2012) and near infrared (NIR) spectroscopy (Afara et al., 2012) would enhance such studies.
The microarray analysis results have revealed that articular chondrocytes have a distinct profile of gene expression from that of growth plate chondrocytes (Hissnauer et al., 2010; Leijten et al., 2012). The genes expressed in articular cartilage at higher levels include PRG4 (proteoglycan 4), TNC (tenascin C), GREM1 (Gremlin 1), DKK1 (Dickkopf 1), FRZB (Frizzled-related protein 3), ABI3BP (Abl interactor family member), THBS4 (Thrombospondin-4) and SIX1 (sine-oculis-related homeobox transcription factor), while the genes expressed at much lower levels are hypertrophy related genes such as ALPL (tissue-nonspecific alkaline phosphatase), COL10A1 (collagen 10a1) and PTH1R (PTH/PTHLH receptor). Furthermore, Yamane et al. (Yamane et al., 2007) have reported a list of the genes that are differentially expressed in articular cartilage surface comparing to resting zone chondrocytes of growth plate. Expression of articular cartilage-specific/dominant genes could be used as a hallmark for articular cartilage regeneration.
How does Articular Cartilage Develop?
Articular cartilage development is tightly synchronized with the development of other synovial joint structures. There are still many issues that remain unclear in understanding the entire mechanisms by which the synovial joint, including articular cartilage, is originated, organized, and maintained. However, the accumulation of findings over the decades from the histological analyses and cell tracing and cell transplantation experiments provide a clear outline of the mode of synovial joint development (Archer et al., 2003; Khan et al., 2007; Pacifici et al., 2006). Synovial joint formation generally starts with the appearance of a specific population of mesenchymal cells, called interzone cells, at the future joint site between two cartilaginous skeletal elements (Archer et al., 2003; Khan et al., 2007). The interzone cells are characterized by their histological view, a mass packed with elongated cells sandwiched by cartilaginous anlagen and their gene expression profile that is up-regulation of unique molecules such as Wnt-9a, GDF-5, Erg, Gli-3, CD-44, collagen 2A and collagen 1 (Iwamoto et al., 2007; Koyama et al., 2008; Pacifici et al., 2006). Cavitation then occurs within the interzone (Archer et al., 2003; Ito and Kida, 2000) while the joint capsule, consisting of the outer ligaments and the inner synovial membrane, develops connecting the two cartilaginous elements (Merida-Velasco et al., 1997). Our previous studies have shown that synovial joint components including articular cartilage are originated from the interzone. In this study, we generated the compound transgenic mice of Gdf5-Cre (Rountree et al., 2004) with RosaR26R-lacZ reporter mice and monitored the resulting lacZ-positive cells at successive prenatal and postnatal stages (Koyama et al., 2008). The lacZ-positive cells first constituted the entire interzone at early stages, gave rise to the articular cartilage and synovial capsule at later stages but were absent in flanking the secondary ossification center and the growth plate at every stage examined (Koyama et al., 2008), indicating that the interzone is one origin of articular cartilage as well as other synovial joint components. Hyde et al. (Hyde et al., 2007) have demonstrated results consistent with our findings by the cell lineage tracing experiments using the transgenic mice that encode Cre-recombinase in the Matrillin 1 allele in RosaR26R-LacZ background. The LacZ-positive chondrocytes appear in the growth plate, but not in articular cartilage in these mice, leading to the conclusion that articular chondrocytes and the remainder of the chondrocytes in the cartilage anlagen are derived from different cell sources.
Articular cartilage development progresses simultaneously with the formation of the secondary ossification center. In the beginning of the formation of the secondary ossification center, the canals are invaginated from the perichondrium and generate small ossification nuclei on the edge of cartilage entities (Blumer et al., 2008; Blumer et al., 2007). The chondrocytes at this site increase their cell size with reduction of gene expression of collagen 2B and aggrecan and up-regulation of expression of hypertrophic markers such as collagen 10, and start expressing various matrix degradation enzymes (Davoli et al., 2001). Finally this region is turned into a bone marrow cavity with supply of osteoprogenitors and blood vessels from the canals (Blumer et al., 2008). In contrast, the cells at the edge/periphery of the cartilage anlagen participate in formation of articular cartilage and exhibit a unique profile of gene expression, histology, function and mechanical property as described in the previous section. The developing articular cartilage contains 3–4 layers that show a distinct cell shape and size (Figure 1A). The cell size increases toward the bottom facing the secondary ossification center and the bottom hypertrophic cells are calcified and invaded by the vascular system (Hunziker et al., 2007) (Figure 1A, up down arrow). The articular cartilage matures over time and rearranges its structure, including becoming lined by subchondral bone (Figure 1B). The mature articular cartilage constitutes zonal organization that are divided into the superficial layer, the transition or mid layer, the deep or radial layer and the calcified layer in order from the surface of articular cartilage (Figure 1B) (Becerra et al., 2010; Las Heras et al., 2012; Poole, 2003).
Figure 1.
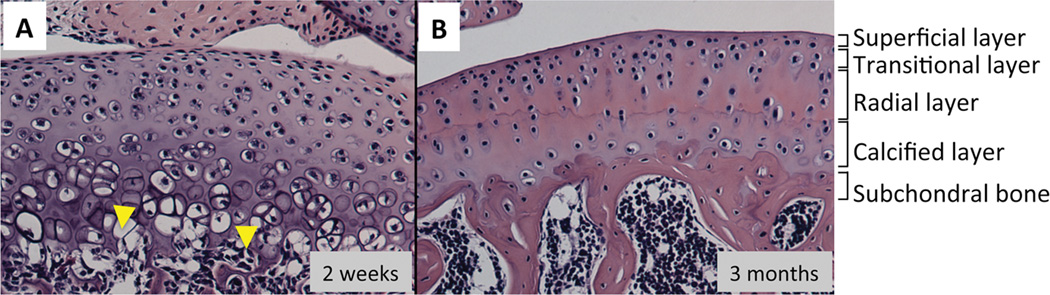
Comparison of histology between developing and mature articular cartilage. The longitudinal section of tibial plateau prepared from 3-weeks old (A) or 3-months old (B) C57BL6 mouse were stained with hematoxylin and eosin. A, The developing articular cartilage show vascular invasion at the bottom (arrow). B, The mature articular cartilage has superficial, tangential, radial and calcified layers, and subchondral bone lies below the calcified zone.
We can point out several important thoughts from the previous studies on articular cartilage development. First, the superficial layer would be a key for appositional growth of articular cartilage. This layer has been demonstrated to contain stem/progenitor cells that have slow-cell cycle (Hayes et al., 2001; Hunziker et al., 2007; Ohlsson et al., 1992) and express stem cell markers (Dowthwaite et al., 2004; Hattori et al., 2007) while the cells in the transitional layer as well as the upper radial zone show a rapid proliferating activity (Hunziker et al., 2007). Thus it is suggested that the superficial layer is a pool of stem cells and supports longitudinal and lateral growth of articular cartilage.
Second, articular chondrocytes are rigorously controlled not to differentiate into a hypertrophic stage by autocrine and paracrine factors and extracerllular matrix microenvironment. These regulatory factors include PTHrp, TGFβ superfamily, Indian hedgehog, FGFs and Wnts. Dysregulation of their signaling pathways causes abnormal articular cartilage development (Chen et al., 2008; Kobayashi et al., 2005; Koyama et al., 2008; Rountree et al., 2004; Storm and Kingsley, 1999; Wang et al., 2001; Yuasa et al., 2009) and also leads to degenerative changes in articular cartilage in animal models (Macica et al., 2011; Maeda et al., 2007; Masuya et al., 2007; Rountree et al., 2004; Serra et al., 1997; Zhu et al., 2008). Various growth factors including transforming growth factor β(TGFβ) superfamily, fibroblast growth factor (FGF) family and insuline-like growth factor (IGF) family proteins have been tested as a drug for articular cartilage repair in animal studies (Chubinskaya et al., 2007; Ellman et al., 2013; Oldershaw, 2012; Tuan et al., 2013). Further, TGFβ1-expressing chondrocytes, recombinant human FGF18 and osteogenic protein-1 (OP-1) proteins have been tested for osteoarthritis or cartilage repair in phase I or II trials. These growth factors have been selected mostly based on their anabolic actions on cultured chondrocytes or explants of articular cartilage, but just as importantly, their anti-maturation activites should be examined.
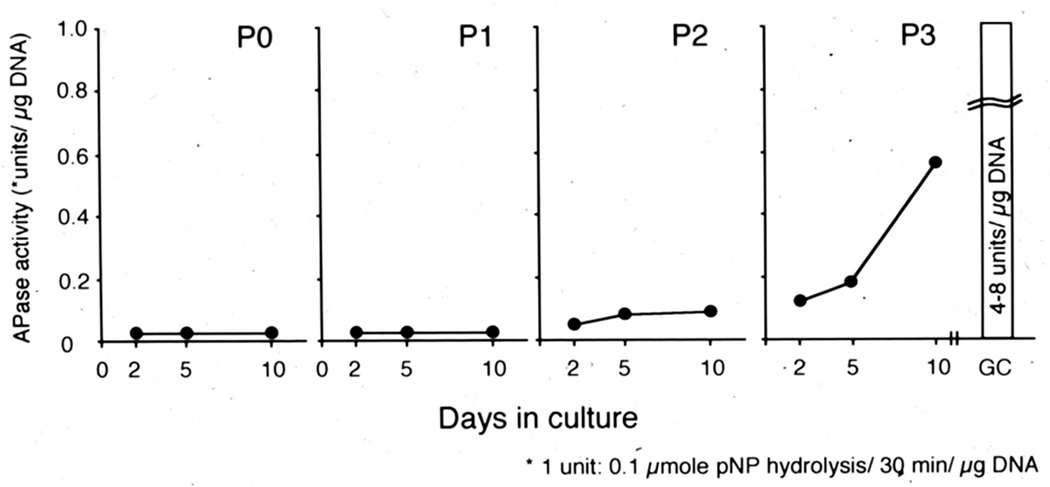
When articular chondrocytes are liberated from the matrix and maintained in culture, they start to display a hypertrophic phenotype such as an increase in alkaline phosphatase activity (Fig. 2). Furthermore, chodrocytes isolated from other permanent cartilages, such as the free-end of costal cartilage of 4-weeks old rabbit and lower tip of sternum cartilage (xiphoid process) of chick, also expressed maturation markers in culture (Castagnola et al., 1987; Iwamoto et al., 1989), These findings indicate that chondrocytes in permanent cartilage, including articular chondrocytes, retain the potential to proceed toward hypertrophy and that its potential is negatively regulated under the control of the microenvironment that is established and maintained by a fine balance of extracellular matrix components and structure, contents, and activities of various signaling factors (Becerra et al., 2010; Enomoto-Iwamoto et al., 2001; Pitsillides and Beier, 2011; Poole, 2003). Ectopic hypertrophy of articular chondrocytes is found together with up-regulation of cartilage matrix metabolism in osteoarthritic cartilage (Pitsillides and Beier, 2011; Shlopov et al., 1997; van der Kraan and van den Berg, 2012; von der Mark et al., 1992), indicating that hypertrophic phenotype is associated with and would be caused by an abnormal increase in matrix turn-over in articular cartilage. Expression of a hypertrophic phenotype can also happen when the articular chondrocytes are expanded in vitro for AIC or after the mesenchymal stem cells are stimulated to have a chondrogenic phenotype for cell-based articular regenerative therapies. Even though the cultured cells do not exhibit a hypertrophic phenotype at transplantation, possibly under control of exogenous factors, the transplanted cells would start expressing such phenotype after release from the restraint.
Figure 2.
Hypertrophic phenotype is induced in articular chondrocytes over time. Articular chondrocytes were isolated from femoral and tibial articular cartilage of 4-weeks old New Zealand rabbits and cultured at high density (25,000 cells/cm2) on the collagen-coated dish. Ten days after the plating, the cells were detached with trypsin/collagenase digestion and re-plated under the same condition. The passage was repeated three times (P1-P3). Primary (P0) and P1-P3 passaged cultures were subjected to measurement of alkaline phosphatase (APase) activity on Day 2, 5 and 10. The articular chondrocytes did not show APase activity in P0 and P1 cultures, increased it in P2 cultures and strongly induced in P3 cultures.
Third, the articular cartilage has the calcified zone at the bottom, but this zone is protected from transition to bone. This is highly contrasted with the calcified hypertrophic zone of the growth plate. The calcified zone of articular cartilage does not contact the vascular system, but faces the subchondral bone while the bottom of the calcified zone of the growth plate is invaded by blood vessels and osteoclasts, connecting to the primary spongiosa. Stempel et al. (Stempel et al., 2011) have shown that expression of anti- and pro-angiogenic molecules and osteoclast formation differ between the calcified zone of articular cartilage and the chondro-osseous junction of the growth plate. Recent studies have indicated that changes in the boundary between articular cartilage and subchondral bone, such as sclerosis and remodeling of subchondral bone, neovascularization and activation of osteoclast formation/activity are critical in pathogenesis and treatment of osteoarthritis (Burr and Gallant, 2012; Lories and Luyten, 2011). Thus there must be a unique mechanism that controls stability/longevity of the calcified zone of articular cartilage and subchondral bone. The knowledge on its regulatory mechanism is very little at this moment, but is required for regeneration of articular cartilage to generate proper integration with the subchondral bone.
Which Factors Control Articular Chondrocyte Phenotype?
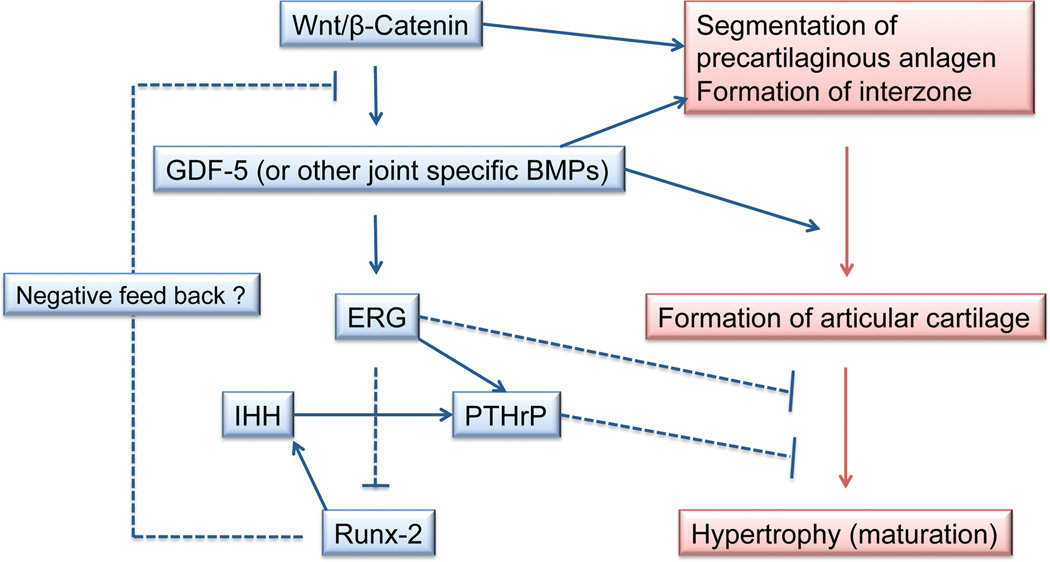
Although molecular mechanisms of articular cartilage formation remain unclear, previous studies have identified several key molecules essential for articular cartilage formation. Figure 3 summarizes the interaction of these key factors in regulation of articular chondrocyte phenotype. Several Wnt family members such as Wnt-9a, -4, and -16 are expressed in a presumptive joint forming region (Guo et al., 2004). Ectopic expression of Wnt9a or activation of its canonical signaling pathway (Wnt/β-catenin signaling pathway) induces ectopic joint-like structure and expression of joint/articular cartilage markers including GDF5 (Guo et al., 2004; Hartmann and Tabin, 2001; Tamamura et al., 2005). GDF5 is expressed in joint forming regions and induces downstream targets including Erg (Iwamoto et al., 2007) and thought to play an essential role in regulating the organization of articular cartilage and joint morphogenesis because GDF5 stimulates chondrogenesis in vitro and loss of function or dominant negative mutation of GDF5 results in joint fusion and/or degeneration of articular cartilage (Masuya et al., 2007). We have previously generated the transgenic mice harboring Erg under the control of Col2a1 protmoter/enahancer and demonstrated that overexpression of Erg makes growth plate chondrocytes to express articular cartilage markers such as tenascin C (TNC) and suppresses their maturation simultaneously (Iwamoto et al., 2007). In these mice mice, the epiphyseal cartilage consisted of homogenous chondrocytes in size and shape and did not exhibit typical growth plate structure (Fig. 4B). The long bone elements have bony tissue in the metaphysis, but the junction between epiphyseal cartilage and bone does not have blood vessel invasion and the epiphyseal catilage just connects to bone (Fig. 4). This histological feature is very different from that normally seen in the chondro-osseous junction and rather resembles the interaction of articular cartilage and subchondral bone (Fig. 1 and 4). It is indicated that Erg may have an ability to make epiphyseal cartilage to form an articular cartilage-like structure. Our recent study suggests that Erg directly stimulates PTHLH gene transcription through the conserved ets binding sites in the proximal promoter of PTHLH (Okabe et al., 2011). Because expression of PTHLH in articular cartilage requires Indian hedgehog (Ihh)-derived signaling from diaphysis, Erg may enhance Ihh-mediated PTHLH gene expression in articular chondrocytes and its anti-maturation activity could be partially mediated by PTHLH. Runt-related transcription factor 2 (Runx2) is a potent stimulator of chondrocyte maturation (Enomoto et al., 2000; Enomoto-Iwamoto et al., 2001) and negatively regulates GDF5 expression (Ueta et al., 2001). Runx2-deficieny in cartilage causes inhibition of chondrocyte hypertrophy and endochondral ossification, and also induces up-regulation of tenascin C (Ueta et al., 2001). The actions of Erg and PTHLH surpass pro-maturation activity of Runx2 in chondrocytes (Iwamoto et al., 2003).
Figure 3.
Induction and interplay of soluble and transcription factors in articular cartilage formation.
Figure 4.
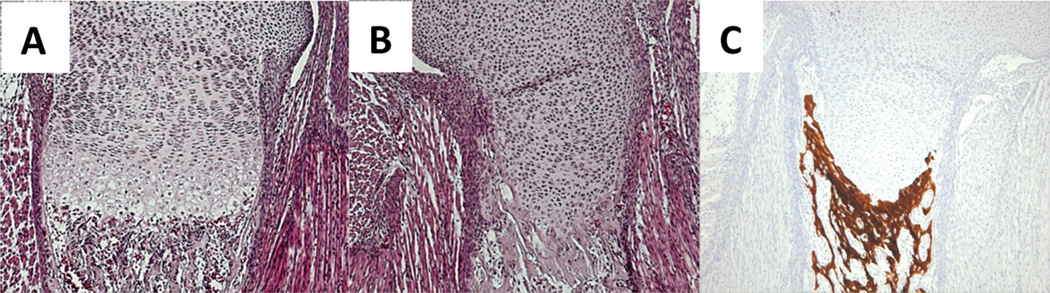
Growth-plate of wild type and transgenic mouse that expresses human Erg under the control of Col2a1 promoter/enhancer. Humeruses obtained from E18.5 wild type (A) and transgenic mice (B and C) were subjected to histological analysis. A and B are HE staining sections. C is a Von Kossa staining to detect calcified tissue.
Sox9 is a key transcription factor for chondrogenesis. It associates with enhancer elements of major cartilage matrix genes such as Col2, Col9, Col11 and aggrecan and activates gene expressions (Lefebvre and Smits, 2005). It is also necessary for chondrocyte survival (Ikegami et al., 2011). In the growth plate, sox9 expression decreases with chondrocytes hypertrophy. In the late stage osteoarthritis, cartilage matrix synthesis and sox9 are both decreased (Haag et al., 2008). Continuous expression of Sox 9 suppresses chondrocyte hypertrophy while deletion of sox9 in chondrocytes causes precautious endochondral ossification (Kim et al., 2011). Further, forced-expression of Sox-5, -6 and -9 together induces chondrogenic differentiation of mesencymal stem cells and these cells do not undergo hypertrophy, indicating that these transcription factors refrain the cells from undergoing hypertrophy (Ikeda et al., 2004). It is important to investigate whether the induced chondrocytes are permanent or transient cells before hypertrophy.
The cascade actions of these and other key regulators coordinates sequential steps of articular cartilage formation synchronized with synovial joint development (Fig. 3). Further studies will indentify more soluble factors, transcription factors and cofactors regulating articular cartilage formation and expression of articular chondrocyte phenotype, and also uncover how they mutually interact and harmonize to support sophisticated biological process of articular cartilage formation.
Possible Resident Stem/Progenitor Cells that can Participate in Regeneration of Articular Cartilage
Which cells are responsible for articular cartilage renewal and how can these cells be utilized to stimulate articular cartilage regeneration? In this section we discuss the intrinsic cells that can possibly participate in regeneration of articular cartilage. Utilization of extrinsic cells such as adult mesenchymal cells, embryonic stem cells or iPS cells have been intensively studied in combination with various scaffold materials and growth factors. These topics have been discussed elsewhere(Freyria and Mallein-Gerin, 2012; Liu et al., 2012; Mastbergen et al., 2013; Noth et al., 2008; Oldershaw, 2012).
Superficial Layer Cells
The importance of the superficial layer for maintenance of articular cartilage has already been noted based on histopathological, biomechanical and biochemical findings that the superficial layer has unique structure, composition, and mechanical property and decreases in depth over time and that osteoarthritic change starts with loss of the superficial layer and the lamina splendens, an acellular film covering the articular cartilage surface (Chan et al., 2010; Crockett et al., 2006; Korhonen et al., 2002; Noth et al., 2008; Silver et al., 2001). The cell labeling studies with tritium thymidine or bromodeoxyuridine have been performed in the developing synovial joints in animals such as rats, opossums and rabbits (Hayes et al., 2001; Hunziker et al., 2007; Ohlsson et al., 1992). The results have revealed that the superficial layer contains the cells labeled with these thymidine derivatives over a long period, indicating that this layer contains the pool of cells with a very slow rate of proliferation, a characteristic of stem cells. Recent studies have provided further functional evidence for the presence of stem cells in the superficial layer of articular cartilage. These studies have demonstrated that the cells isolated from the superficial layer of postnatal bovine articular cartilage have progenitor characteristics including high colony formation capacity and expression of mesenchymal stem cell markers and can acquire and express a chondrogenic phenotype over passage number (Dowthwaite et al., 2004; Hattori et al., 2007). Furthermore, presence of stem/progenitors cells in the superficial layer has been demonstrated in human articular cartilage (Muinos-Lopez et al., 2012; Tallheden et al., 2006). In spite of these accumulative findings, we still do not know if this layer supports articular cartilage renewal or can provide the cells for repair of articular cartilage.
Which signaling molecules control survival, proliferation and differentiation of stem/progenitor cells in the superficial layer? TGFβsignaling is one of the candidates. The superficial layer cells express TGFβs, TGFβ1,2 and 3 (Hayes et al., 2001), their receptors, and regulatory proteins (Yamane et al., 2007). Furthermore, the mechanical forces produced by joint motion mediate activation of the latent form of TGFβ1 in synovial fluid (Albro et al., 2012) and the active TGFβ1 can be trapped in the superficial layer (Albro et al., 2013). TGFβs strongly enhance synthesis of proteoglycan 4 proteins (also called superficial zone proteins or lubricin) and stimulate expression of cartilage matrix such as aggrecan and collagen 2B in the superficial layer cells in micromass culture (Dowthwaite et al., 2004; Hattori et al., 2007). The superficial cell cultures used in these studies represent stem/progenitor characteristics (Dowthwaite et al., 2004; Hattori et al., 2007). Thus TGFβs would play a role as a stimulator of differentiation of the stem/progenitor cells into articular chondrocytes. Embree et al. (Embree et al., 2010) have demonstrated that biglycan and fibromodulin double knock out mice exhibited temporomandibular joint osteoarthritis and that deficiency of these extracellular matrix molecules enhances the response to TGFβ1 in condyle chondrocytes. Interestingly, this mutant exhibits an increase in the articular zone of condyle cartilage at the early stage, but stronger osteoarthritic phenotype with aging. The authors explained the mouse phenotype by reasoning that overactive TGFβ1 induces abnormal and accelerated chondrogenesis and excess of matrix turnover. Over-activation of TGFβ1 signaling might also accelerate chondrogenic differentiation of stem/progenitor cells in condyle articular cartilage and induce depletion of this cell population. Wnt/β-catenin signaling would maintain stem/progenitor cells in superficial layer, preventing from chondrogenic differentiation. We have previously found that acute activation of Wnt/β-catenin signaling increases thickness of the superficial layer (Fig. 5), while conditional ablation of b-catenin causes the opposite (Yasuhara et al., 2011; Yuasa et al., 2009). Treatment of Wnt3a maintained expression of Prg4 and Erg in the superficial layer cells in culture, while ablation of β-catenin strongly impaired proliferation and expression of these genes in the cultured cells and stimulates chondrogenesis in the transplants (Yasuhara et al., 2011). These findings have indicated that Wnt/β-catenin signaling is a key regulator of proliferation and differentiation of the superficial cells and may be important for articular cartilage long-term function.
Figure 5.
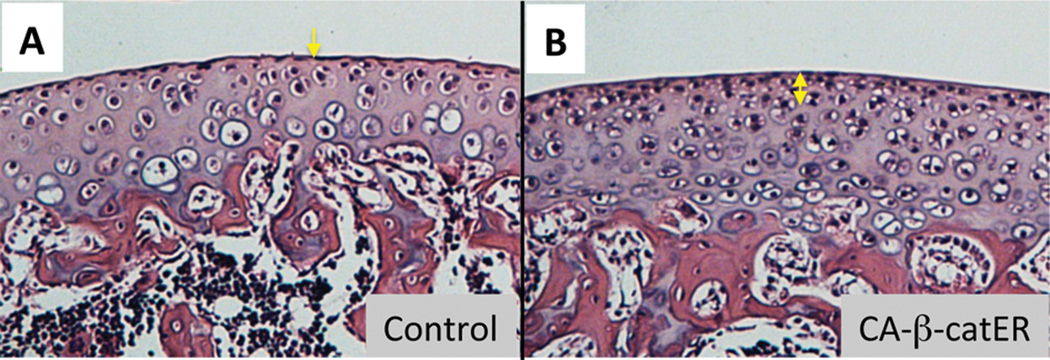
Transient activation of Wnt/β-catenin signaling increases the depth of superficial layer of articular cartilage.
Transgenic mice (CA-β-catER) that harbor a constitutive-active form of β-catenin fused to a modified estrogen receptor ligand-binding domain (CA-β-catER) to promoter/enhancer sequences from the Collagen 11a2 gene were generated. Two-week-old CA-β-catER transgenic mice (B) and their wild-type littermates (A) received seven daily peritoneal injections of tamoxifen (200 μg/20 μl/mouse) and were sacrificed at 5 weeks. Longitudinal sections of proximal tibial articular cartilage were stained with hematoxyline and eosin. The superficial layer was thickened in the mutant (B, double arrowed line) compared to that of the wild type mouse (A, arrow).
Other Intrinsic Cell Sources
The cell labeling studies have demonstrated that the ossification groove of Ranvier, a wedge-shaped groove adjacent to the growth plate, would be a pool of stem cells. Karlsson et al (Karlsson et al., 2009) have examined localization of the labeled cells with bromodeoxyuridine (BrdU) in the knee joint of 3-month-old rabbits that had been exposed to the BrdU for 12 consecutive days. They have found that BrdU-labeled cells are present in the groove of Ranvier up to 56 days after initiation of the BrdU administration. Together with the finding that this region shows immunoreactivity of the antibodies against several progenitor cell markers, they conclude that the groove of Ranvier has the poperies of a stem cell niche and that the groove of Ranvier would directly or indirectly support renewal of articular cartilage.
The intra-articular tissues such as synovium and infrapatellar fat pad have been demonstrated to contain mesenchymal stem cells in humans (De Bari et al., 2001.; Dragoo et al., 2003; Wickham et al., 2003) as well as in animals (Buckley et al., 2010; Futami et al., 2012; Jones et al., 2008; Koga et al., 2008; Yoshimura et al., 2007)and that these cells have some distinct phenotype and superiority to express a chondrogenic phenotype compared to the mesenchymal stem cells derived from other tissues such as bone marrow and muscles (Futami et al., 2012; Segawa et al., 2009; Yoshimura et al., 2007). Contribution of these cells to renew and repair articular cartilage has yet to be investigated further. Mesenchymal stem cells have also been found in synovial fluid in the normal knee joint and their number increase in the affected knee joints (Jones et al., 2008; Morito et al., 2008), indicating that these cells potentially participate in articular cartilage homeostasis or repair. The source of these mesenchymal stem cells may be synovium or infrapattelar fat pad on the basis of the findings that their nature resembles that of the mesenchymal cells isolated from these tissues (Futami et al., 2012; Segawa et al., 2009; Yoshimura et al., 2007) and that slow-cycle cells in the synovium rapidly and strongly respond to the joint injury (Kurth et al., 2011).
Recent studies have shown that the bone marrow cavity underneath the subchondral bone would provide mesenchymal stromal cells to damaged articular cartilage and support its repair (Johnson et al., 2012; Koelling et al., 2009). This cell source has been accounted for stimulation of intrinsic repair by marrow stimulation (Schindler, 2011). In addition, articular cartilage by itself may contain more mesenchymal progenitor cells than we expected. Pretzel et al. (Pretzel et al., 2011) have shown that normal and osteoarthritic articular cartilage contain fairy high number of mesenchymal progenitor cells (CD105+/CD166+) in the radial zone and that the cell population containing CD166-positive cells has strong chondrogenic potential, suggesting that these cells can be utilized for its regeneration. The technique of autologous chondrocyte implantation (ACI) (Mastbergen et al., 2013; Schindler, 2011) could rely on chondrogenic differentiation of such cell population as well as proliferation of articular chondrocytes.
Perspective
One of clear directions of articular cartilage regenerative therapy is to pursuit construction of regenerative tissue that closely mimics the zonal structure and histology and biochemical components of the original articular cartilage. This will provide recovery of synovial joint function since the regenerative tissue is expected to have similar nature and function of the original articular cartilage. We now have the methods to induce chondrogenic phenotype in mesenchymal stem cells and to expand chondrocytes in vitro. However, when such engineered cells are transplanted into the damaged site, they do not reconstruct articular cartilage-like structures in terms of the cell-matrix ratio, histological feature and longevity. We should examine which changes occur after the transplantation and find the method and factor(s) that trigger a cascade of the signaling activation in the transplanted cells that leads sequential steps of articular cartilage formation that mimic its developmental stages. Continuous investigation on development of articular cartilage and synovial joint may uncover novel factors that we could use for this purpose.
At this moment, our practical goal is to generate the biological or artificial tissue that has the similar mechanical property to the natural articular cartilage. From the point of view of biological methods, we should carefully analyze quality and quantity of the extracellular matrix synthesized by the cells after the transplantation. The transplanted cells should produce cartilage collagens and proteoglycan at a proper ratio and proper rate. Half-life of articular cartilage matrix proteins, especially collagens is very long (over 20 years), indicating that low anabolic and catabolic activities of matrix proteins. An increase in matrix synthesis and intense accumulation of the synthesized matrix surrounding the cells is early characteristic of osteoarthritis. Given these findings, we assume that the matrix synthesis rate of the transplanted cells should be controlled not to be high, rather than low, which would lead the synthesized matrix scatteredly distributed and interstitial growth of the regenerated tissue. A high rate of matrix synthesis may results in a high rate of matrix degradation. Furthermore, the transplanted cells should be protected from the external invasion and provide optimal microenvironment: covering the transplanted cells with natural or artificial materials would be effects; hypoxia or mimicking of hypoxia state such as expression of HIF1-α would be also alternative. Alternative idea is to make the tissue that has similar mechanical property but is not necessary to have nature of histological and biochemical contents. For revival of low friction property, induction of proteoglycan 4 (superficial zone protein or lubricin) or hyaluronic acid in the regenerated cartilage must be the first choice. We could also resort the adjacent tissues such as synovium and existing articular cartilage as the source since they are secreted proteins and can be accumulated into the regenerated tissue. TGFβ superfamily proteins strongly stimulate production of proteoglycan 4 in synovial fibroblasts, superficial layer cells of articular cartilage and mesenchymal stem cells (Neu et al., 2007). Interestingly alternative spliced variants of proteoglycan 4 are synthesized in response to load bearing (Andrades et al., 2012). Although functional difference among the variant has not been clarified yet, we should consider induction of an appropriate isoform of paroteoglycan 4.
Seamless integrity of the regenerated cartilage with the neighboring tissues is essential to complete articular cartilage regeneration. The matrix turn over of the surrounding cartilage is slow and therefore integration of newly made articular cartilage will take long time. How can we facilitate tissue integration? One possibility is to use biocompatible and adhesive scaffold (Benders et al., 2013; Tuan et al., 2013). The material is also expected to penetrate surrounding tissue, support chondrogenesis and recruit progenitor cells into it. Candidates of such materials have been developed and will be further improved In addition we should probably consider the way to activate chondrocytes at the donor site. Fibrillar collagen in articular cartilage is heavily cross-linked by lysyl oxidase mediated reaction (Eyre et al., 1984) and non-enzymatic glycation (Jandeleit-Dahm et al., 2005). While cross-link of matrix increases tissue stiffness, it could probably disturb integration of regenerated cartilage to the surrounding tissue. Variety of cross-link breakers and inhibitors has been developed and some are under clinical trial for other pathology (Susic et al., 2004; Vasan et al., 2001). These drugs might be useful for effective induction of the integration.
Although still limited number of works has been done, joint distraction seems effectively facilitate cartilage regeneration by itself or in combination with a bone marrow stimulation (Deie et al., 2007; Mastbergen et al., 2013). Installation of current joint distraction devices requires surgery. Those devices are often extensive in size and hamper patient's daily life during treatment. Smaller and less invasive devices are hopefully developed soon (Kamei et al. 2013). Regeneration of the perfect articular cartilage may still be a dream. With the knowledge obtained so far and ongoing efforts, the goal is, however, much closer than we thought before.
Acknowledgements
We thank Ms. Leslie Cantley for editorial assistance. Grant Sponsor: National Institute of Health; Grant numbers AR46000 and AG025868.
References
- Afara I, Prasadam I, Crawford R, Xiao Y, Oloyede A. Non-destructive evaluation of articular cartilage defects using near-infrared (NIR) spectroscopy in osteoarthritic rat models and its direct relation to Mankin score. Osteoarthritis Cartilage. 2012;20:1367–1373. doi: 10.1016/j.joca.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Albro MB, Cigan AD, Nims RJ, Yeroushalmi KJ, Oungoulian SR, Hung CT, Ateshian GA. Shearing of synovial fluid activates latent TGF-beta. Osteoarthritis Cartilage. 2012;20:1374–1382. doi: 10.1016/j.joca.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Nims RJ, Cigan AD, Yeroushalmi KJ, Alliston T, Hung CT, Ateshian GA. Accumulation of exogenous activated TGF-beta in the superficial zone of articular cartilage. Biophys J. 2013;104:1794–1804. doi: 10.1016/j.bpj.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrades JA, Motaung SC, Jimenez-Palomo P, Claros S, Lopez-Puerta JM, Becerra J, Schmid TM, Reddi AH. Induction of superficial zone protein (SZP)/lubricin/PRG 4 in muscle-derived mesenchymal stem/progenitor cells by transforming growth factor-beta1 and bone morphogenetic protein-7. Arthritis Res Ther. 2012;14:R72. doi: 10.1186/ar3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat. 2008;190:305–315. doi: 10.1016/j.aanat.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Blumer MJ, Longato S, Schwarzer C, Fritsch H. Bone development in the femoral epiphysis of mice: the role of cartilage canals and the fate of resting chondrocytes. Dev Dyn. 2007;236:2077–2088. doi: 10.1002/dvdy.21228. [DOI] [PubMed] [Google Scholar]
- Buckley CT, Vinardell T, Thorpe SD, Haugh MG, Jones E, McGonagle D, Kelly DJ. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J Biomech. 2010;43:920–926. doi: 10.1016/j.jbiomech.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- Castagnola P, Torella G, Cancedda R. Type X collagen synthesis by cultured chondrocytes derived from the permanent cartilaginous region of chick embryo sternum. Dev Biol. 1987;123:332–337. doi: 10.1016/0012-1606(87)90391-5. [DOI] [PubMed] [Google Scholar]
- Chan SM, Neu CP, Duraine G, Komvopoulos K, Reddi AH. Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage. Osteoarthritis Cartilage. 2010;18:956–963. doi: 10.1016/j.joca.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Chen X, Macica CM, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Lai WM, Mow VC. A transversely isotropic biphasic model for unconfined compression of growth plate and chondroepiphysis. J Biomech Eng. 1998;120:491–496. doi: 10.1115/1.2798019. [DOI] [PubMed] [Google Scholar]
- Crockett R, Grubelnik A, Roos S, Dora C, Born W, Troxler H. Biochemical composition of the superficial layer of articular cartilage. J Biomed Master Res. 2006;82:958–964. doi: 10.1002/jbm.a.31248. [DOI] [PubMed] [Google Scholar]
- Davoli MA, Lamplugh L, Beauchemin A, Chan K, Mordier S, Mort JS, Murphy G, Docherty AJ, Leblond CP, Lee ER. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats aged 0-21 days: II. Two proteinases, gelatinase B and collagenase-3, are implicated in the lysis of collagen fibrils. Dev Dyn. 2001;222:71–88. doi: 10.1002/dvdy.1160. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Deie M, Ochi M, Adachi N, Kajiwara R, Kanaya A. A new articulated distraction arthroplasty device for treatment of the osteoarthritic knee joint: a preliminary report. Arthroscopy. 2007;23:833–838. doi: 10.1016/j.arthro.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Samimi B, Zhu M, Hame SL, Thomas BJ, Lieberman JR, Hedrick MH, Benhaim P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740–747. [PubMed] [Google Scholar]
- Ellman MB, Yan D, Ahmadinia K, Chen D, An HS, Im HJ. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem. 2013;114:735–742. doi: 10.1002/jcb.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree MC, Kilts TM, Ono M, Inkson CA, Syed-Picard F, Karsdal MA, Oldberg A, Bi Y, Young MF. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol. 2010;176:812–826. doi: 10.2353/ajpath.2010.090450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Enomoto H, Komori T, Iwamoto M. Participation of Cbfa1 in regulation of chondrocyte maturation. Osteoarthritis Cartilage. 2001;9(Suppl A):S76–S84. doi: 10.1053/joca.2001.0448. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Freyria AM, Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury. 2012;43:259–265. doi: 10.1016/j.injury.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, Muneta T, Arikawa-Hirasawa E, Sekiya I, Kaneko K. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS ONE. 2012;7:e45517. doi: 10.1371/journal.pone.0045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag J, Gebhard PM, Aigner T. SOX gene expression in human osteoarthritic cartilage. Pathobiology. 2008;75:195–199. doi: 10.1159/000124980. [DOI] [PubMed] [Google Scholar]
- Hall BK. Bones and cartilage: Devlopmental and evolutionary skeletal biology. San Diego: Elsevier (USA) Ltd.); 2005. Cartilage; pp. 33–45. [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocytes stem/progenitor cells. Biochem Biophys Res Commun. 2007;358:99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- Hissnauer TN, Baranowsky A, Pestka JM, Streichert T, Wiegandt K, Goepfert C, Beil FT, Albers J, Schulze J, Ueblacker P, et al. Identification of molecular markers for articular cartilage. Osteoarthritis Cartilage. 2010;18:1630–1638. doi: 10.1016/j.joca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Dickinson SC, Kafienah W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cells. 2010;28:1992–1996. doi: 10.1002/stem.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304:825–833. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50:3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- Ikegami D, Akiyama H, Suzuki A, Nakamura T, Nakano T, Yoshikawa H, Tsumaki N. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development. 2011;138:1507–1519. doi: 10.1242/dev.057802. [DOI] [PubMed] [Google Scholar]
- Ito MM, Kida MY. Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J Anat 197 Pt. 2000;4:659–679. doi: 10.1046/j.1469-7580.2000.19740659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Kitagaki J, Tamamura Y, Gentili C, Koyama E, Enomoto H, Komori T, Pacifici M, Enomoto-Iwamoto M. Runx2 expression and action in chondrocytes are regulated by retinoid signaling and parathyroid hormone-related peptide (PTHrP) Osteoarthritis Cartilage. 2003;11:6–15. doi: 10.1053/joca.2002.0860. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Sato K, Nakashima K, Shimazu A, Kato Y. Hypertrophy and calcification of rabbit permanent chondrocytes in pelleted cultures: synthesis of alkaline phosphatase and 1,25-dihydroxycholecalciferol receptor. Dev Biol. 1989;136:500–507. doi: 10.1016/0012-1606(89)90275-3. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, Williams JA, Nakamura T, Enomoto-Iwamoto M, Pacifici M. Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol. 2007;305:40–51. doi: 10.1016/j.ydbio.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandeleit-Dahm KA, Lassila M, Allen TJ. Advanced glycation end products in diabetes-associated atherosclerosis and renal disease: interventional studies. Ann N Y Acad Sci. 2005;1043:759–766. doi: 10.1196/annals.1333.088. [DOI] [PubMed] [Google Scholar]
- Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, Schultz PG. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, Chapman T, Emery P, Hatton P, McGonagle D. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008;58:1731–1740. doi: 10.1002/art.23485. [DOI] [PubMed] [Google Scholar]
- Kamei G, Ochi M, Okuhara A, Fujimiya M, Deie M, Adachi N, Nakamae A, Nakasa T, Ohkawa S, Takazawa K, et al. A new distraction arthroplasty device using magnetic force; a cadaveric study. Clin Biomech (Bristol, Avon) 2013;28:423–428. doi: 10.1016/j.clinbiomech.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Thornemo M, Henriksson HB, Lindahl A. Identification of a stem cell niche in the zone of Ranvier within the knee joint. J Anat. 2009;215:355–363. doi: 10.1111/j.1469-7580.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IM, Redman SN, Williams R, Dowthwaite GP, Oldfield SF, Archer CW. The development of synovial joints. Curr Top Dev Biol. 2007;79:1–36. doi: 10.1016/S0070-2153(06)79001-9. [DOI] [PubMed] [Google Scholar]
- Kim Y, Murao H, Yamamoto K, Deng JM, Behringer RR, Nakamura T, Akiyama H. Generation of transgenic mice for conditional overexpression of Sox9. J Bone Miner Metab. 2011;29:123–129. doi: 10.1007/s00774-010-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115:1734–1742. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- Korhonen RK, Wong M, Arokoski J, Lindgren HJ, Hunziker EB, Jurvelin JS. Importance of the superficial tissue layer for the indenation stifness of articular cartialge. Med Eng Phys. 2002;24:99–108. doi: 10.1016/s1350-4533(01)00123-0. [DOI] [PubMed] [Google Scholar]
- Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth TB, Dell'accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- Las Heras F, Gahunia HK, Pritzker KP. Articular cartilage development: a molecular perspective. Orthop Clin North Am. 2012;43:155–171. doi: 10.1016/j.ocl.2012.01.003. v. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Leijten JC, Emons J, Sticht C, van Gool S, Decker E, Uitterlinden A, Rappold G, Hofman A, Rivadeneira F, Scherjon S, et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302–3312. doi: 10.1002/art.34535. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu J, Zhu Y, Han J. Therapeutic application of mesenchymal stem cells in bone and joint diseases. Clin Exp Med. 2012 doi: 10.1007/s10238-012-0218-1. [DOI] [PubMed] [Google Scholar]
- Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–49. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- Macica C, Liang G, Nasiri A, Broadus AE. Genetic evidence of the regulatory role of parathyroid hormone-related protein in articular chondrocyte maintenance in an experimental mouse model. Arthritis Rheum. 2011;63:3333–3343. doi: 10.1002/art.30515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS, Mackem S, Lanske B. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci U S A. 2007;104:6382–6387. doi: 10.1073/pnas.0608449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastbergen SC, Saris DB, Lafeber FP. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol. 2013;9:277–290. doi: 10.1038/nrrheum.2013.29. [DOI] [PubMed] [Google Scholar]
- Masuya H, Nishida K, Furuichi T, Toki H, Nishimura G, Kawabata H, Yokoyama H, Yoshida A, Tominaga S, Nagano J, et al. A novel dominant-negative mutation in Gdf5 generated by ENU mutagenesis impairs joint formation and causes osteoarthritis in mice. Hum Mol Genet. 2007;16:2366–2375. doi: 10.1093/hmg/ddm195. [DOI] [PubMed] [Google Scholar]
- Merida-Velasco JA, Sanchez-Montesinos I, Espin-Ferra J, Merida-Velasco JR, Rodriguez-Vazquez JF, Jimenez-Collado J. Development of the human knee joint ligaments. Anat Rec. 1997;248:259–268. doi: 10.1002/(SICI)1097-0185(199706)248:2<259::AID-AR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Minas T. A primer in cartilage repair. J Bone Joint Surg Br. 2012;94:141–146. doi: 10.1302/0301-620X.94B11.30679. [DOI] [PubMed] [Google Scholar]
- Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford) 2008;47:1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- Muinos-Lopez E, Rendal-Vazquez ME, Hermida-Gomez T, Fuentes-Boquete I, Diaz-Prado S, Blanco FJ. Cryopreservation effect on proliferative and chondrogenic potential of human chondrocytes isolated from superficial and deep cartilage. Open Orthop J. 2012;6:150–159. doi: 10.2174/1874325001206010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu CP, Khalafi A, Komvopoulos K, Schmid TM, Reddi AH. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56:3706–3714. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, Steinwachs M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36:2091–2099. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- Noth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson O, Lindahl A. Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci U S A. 1992;89:9826–9830. doi: 10.1073/pnas.89.20.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe T, Ohta Y, Asai S, Wakitani S, Enomoto-Iwamoto M, Pacifici M, Iwamoto M. Erg and PTHrp genetically cooperate to maintain articular cartilage long-term function. Osteoarthritis Cartilage. 2011;19(Suppl 1):S38–S39. [Google Scholar]
- Oldershaw RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol. 2012;93:389–400. doi: 10.1111/j.1365-2613.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci. 2006;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastides P, Chimutengwende-Gordon M, Maffulli N, Khan W. Stem cell therapy for human cartilage defects: a systematic review. Osteoarthritis Cartilage. 2013;21:646–654. doi: 10.1016/j.joca.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Beier F. Cartilage biology in osteoarthritis--lessons from developmental biology. Nat Rev Rheumatol. 2011;7:654–663. doi: 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- Plodinec M, Loparic M, Aebi U. Imaging articular cartilage tissue using atomic force microscopy (AFM) Cold Spring Harb Protoc. 2010;2010:pdb prot5499. doi: 10.1101/pdb.prot5499. [DOI] [PubMed] [Google Scholar]
- Poole AR. What type of cartilage repair are we attempting to attain? J Bone Joint Surg Am 85-A Suppl. 2003;2:40–44. doi: 10.2106/00004623-200300002-00006. [DOI] [PubMed] [Google Scholar]
- Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, Kinne RW. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011;13:R64. doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler OS. Current concepts of articular cartilage repair. Acta Orthop Belg. 2011;77:709–726. [PubMed] [Google Scholar]
- Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40:2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- Silver FH, Bradica G, Tria A. Relationship among biomechanical, biochemical, and cellular changes associated with osteoarthritis. Crit Rev Biomed Eng. 2001;29:373–391. doi: 10.1615/critrevbiomedeng.v29.i4.10. [DOI] [PubMed] [Google Scholar]
- Stempel J, Fritsch H, Pfaller K, Blumer MJ. Development of articular cartilage and the metaphyseal growth plate: the localization of TRAP cells, VEGF, and endostatin. J Anat. 2011;218:608–618. doi: 10.1111/j.1469-7580.2011.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999;209:11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- Susic D, Varagic J, Ahn J, Frohlich ED. Collagen cross-link breakers: a beginning of a new era in the treatment of cardiovascular changes associated with aging, diabetes, and hypertension. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:97–101. doi: 10.2174/1568006043481347. [DOI] [PubMed] [Google Scholar]
- Tallheden T, Brittberg M, Peterson L, Lindahl A. Human articular chondrocytes--plasticity and differentiation potential. Cells Tissues Organs. 2006;184:55–67. doi: 10.1159/000098947. [DOI] [PubMed] [Google Scholar]
- Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303–311. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta C, Iwamoto M, Kanatani N, Yoshida C, Liu Y, Enomoto-Iwamoto M, Ohmori T, Enomoto H, Nakata K, Takada K, et al. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J Cell Biol. 2001;153:87–100. doi: 10.1083/jcb.153.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Vasan S, Foiles PG, Founds HW. Therapeutic potential of AGE inhibitors and breakers of AGE protein cross-links. Expert Opin Investig Drugs. 2001;10:1977–1987. doi: 10.1517/13543784.10.11.1977. [DOI] [PubMed] [Google Scholar]
- von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K, Stoss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35:806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- Wang Q, Green RP, Zhao G, Ornitz DM. Differential regulation of endochondral bone growth and joint development by FGFR1 and FGFR3 tyrosine kinase domains. Development. 2001;128:3867–3876. doi: 10.1242/dev.128.19.3867. [DOI] [PubMed] [Google Scholar]
- Wardale RJ, Duance VC. Characterisation of articular and growth plate cartilage collagens in porcine osteochondrosis. J Cell Sci. 1994;107(Pt 1):47–59. doi: 10.1242/jcs.107.1.47. [DOI] [PubMed] [Google Scholar]
- Wen CY, Wu CB, Tang B, Wang T, Yan CH, Lu WW, Pan H, Hu Y, Chiu KY. Collagen fibril stiffening in osteoarthritic cartilage of human beings revealed by atomic force microscopy. Osteoarthritis Cartilage. 2012;20:916–922. doi: 10.1016/j.joca.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- Williams JA, Kane M, Okabe T, Enomoto-Iwamoto M, Napoli JL, Pacifici M, Iwamoto M. Endogenous retinoids in mammalian growth plate cartilage: analysis and roles in matrix homeostasis and turnover. J Biol Chem. 2010;285:36674–36681. doi: 10.1074/jbc.M110.151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane S, Cheng E, You Z, Reddi AH. Gene expression profiling of mouse articular and growth plate cartilage. Tissue Eng. 2007;13:2163–2173. doi: 10.1089/ten.2006.0431. [DOI] [PubMed] [Google Scholar]
- Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, Fortina P, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91:1739–1752. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- Yuasa T, Kondo N, Yasuhara R, Shimono K, Mackem S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, O'Keefe RJ, Chen D. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]