Abstract
Erectile dysfunction (ED) is a major adverse effect of radical prostatectomy (RP). We conducted a randomized controlled trial to examine the efficacy of aerobic training (AT) compared with usual care (UC) on ED prevalence in 50 men (n = 25 per group) after RP. AT consisted of five walking sessions per week at 55– 100% of peak oxygen uptake (VO2peak) for 30–60 min per session following a nonlinear prescription. The primary outcome was change in the prevalence of ED, as measured by the International Index of Erectile Function (IIEF), from baseline to 6 mo. Secondary outcomes were brachial artery flow–mediated dilation (FMD), VO2peak, cardiovascular (CV) risk profile (eg, lipid profile, body composition), and patient-reported outcomes (PROs). The prevalence of ED (IIEF score ≤21) decreased by 20% in the AT group and by 24% in the UC group (difference: p = 0.406). There were no significant between-group differences in any erectile function subscale (p > 0.05). Significant between-group differences were observed for changes in FMD and VO2peak, favoring AT. There were no group differences in other markers of CV risk profile or PROs. In summary, nonlinear AT does not improve ED in men with localized prostate cancer in the acute period following RP.
Keywords: Exercise training, Prostate cancer, Efficacy, Exercise capacity, Endothelial function
Radical prostatectomy (RP) is associated with a broad spectrum of adverse toxicities, of which erectile dysfunction (ED) is the most common, with a prevalence as high as 80% [1]. The pathophysiology of ED following RP involves both neuronal and vascular endothelial cell dysfunction, which, in combination, lead to impaired penile tissue oxygenation resulting in smooth muscle apoptosis, fibrosis, and veno-occlusion dysfunction [2]. Aerobic training (AT) leads to a multitude of vascular adaptations, including marked improvements in peripheral artery flow–mediated dilation (FMD) and exercise tolerance (peak oxygen uptake [VO2peak]) [3]. FMD provides a robust measure of vascular endothelial function, whereas VO2peak evaluates the integrative capacity of the cardiopulmonary and musculoskeletal system to deliver and use oxygen to resynthesize ATP; both measures are strong independent predictors of cardiovascular (CV) events [4,5]. AT-induced improvements in FMD and VO2peak occur in conjunction with improved erectile function [6], with FMD being the strongest predictor of improvement in erectile function [6]. Whether AT improves ED following RP has not been investigated. We conducted a two-arm randomized controlled trial to examine the effects of AT compared with recommended usual care (UC) on these outcomes in men with prostate cancer (PCa) following RP.
Full study methods are described in the supplementary online content. In a single-center randomized controlled trial, 50 men with localized (stage I–II) prostate adenocarcinoma following bilateral nerve-sparing RP were randomly allocated to the following groups (n = 25 per group): (1) AT or (2) UC. AT consisted of five supervised walking sessions per week, 30–45 min per session, at 55–100% of VO2peak for 6 mo, following a nonlinear prescription approach. Specifically, in nonlinear prescriptions, AT sessions are sequenced in such a fashion that training-induced physiologic stress is continually altered in terms of intensity and duration in conjunction with appropriate rest and recovery sessions to optimize VO2peak adaptation (Supplemental Fig. 1). UC participants were instructed to maintain their usual exercise levels. The primary end point was the prevalence of ED assessed by the International Index of Erectile Function (IIEF). The IIEF contains five subscales that are summed to obtain the total IIEF score; a score ≤21 indicates ED. Peripheral artery FMD was evaluated using high-resolution B-mode ultrasound, as previously described [7]. VO2peak was assessed using maximal cardiopulmonary exercise testing (CPET) on a motorized treadmill with expired gas analysis as recommended [8]. The Cardiovascular Risk Profile included fasting glucose and lipid profile, while body composition was assessed using air-displacement plethysmography; all assessments were conducted according to established procedures. Patient-reported outcomes (PROs) were assessed using the Functional Assessment of Cancer Therapy–Prostate (to assess quality of life), FACT-fatigue (to assess fatigue), the Center for Epidemiological Studies Depression Scale (to assess depression), the Pittsburgh Sleep Inventory (to assess sleep quality), and the Brief Pain Inventory (to assess pain). Safety was evaluated according to the frequency and severity of adverse events (AEs) observed during CPET procedures and during each supervised AT session.
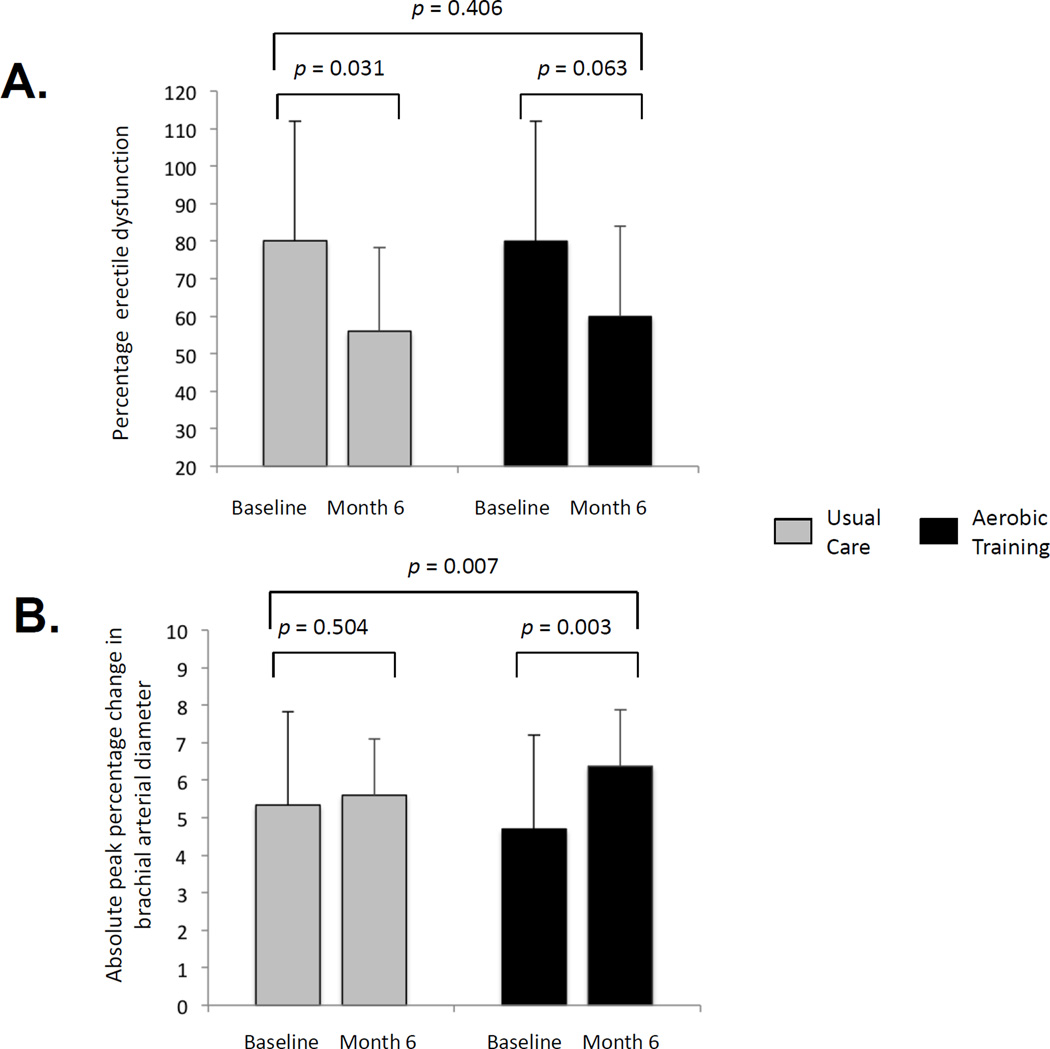
Full study results are presented in the supplementary online content. Participant characteristics were balanced at baseline (Supplemental Table 1). Of the 50 randomized patients, 46 (92%) and 35 (70%) completed study end point assessments at 6 mo and 12 mo, respectively (Supplemental Fig. 2). No serious AEs were observed during CPET or AT sessions. Mean adherence to supervised sessions and home-based sessions was 83% and 72%, respectively. Thirty-six percent of UC patients were exercising regularly at month 6, compared with 24% at baseline. The ED prevalence decreased in both groups from baseline to 6 mo (Table 1, Fig. 1a) and from baseline to 12 mo (Supplemental Table 2), with no significant differences between groups (p > 0.05).
Table 1.
Effects on erectile function
| Variable | Usual care | Aerobic training | Difference between groups |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 |
p value† |
Baseline | Month 6 |
p value† |
Mean change | 95% CI |
p value§ |
|
| Prevalence of ED*, % | 80 | 56 | 0.031 | 80 | 60 | 0.063 | +4 | −20 to 28 | 0.406 |
| Total IIEF score, mean (SD) | 14 (12) | 20 (13) | 0.041 | 12 (9) | 20 (13) | 0.002 | +3 | −4 to 10 | 0.384 |
| Erectile function, mean (SD) | 4 (4) | 6 (5) | 0.081 | 3 (3) | 6 (6) | 0.006 | +2 | −2 to 5 | 0.290 |
| Orgasmic function, mean (SD) | 3 (3) | 4 (3) | 0.029 | 2 (3) | 4 (3) | 0.012 | 0 | −2 to 2 | 0.962 |
| Sexual desire, mean (SD) | 4 (2) | 4 (2) | 0.914 | 4 (2) | 4 (2) | 0.025 | +1 | 0–2 | 0.145 |
| Intercourse satisfaction, mean (SD) | 2 (3) | 3 (3) | 0.019 | 2 (3) | 3 (4) | 0.013 | 0 | −2 to 2 | 1.000 |
| Overall satisfaction, mean (SD) | 2 (3) | 2 (2) | 0.538 | 2 (2) | 3 (2) | 0.113 | 0 | 0–1 | 0.480 |
CI = confidence interval; ED = erectile dysfunction; IIEF = International Index of Erectile Function; SD = standard deviation.
Paired t test p value for continuous variables; McNemar test p value for categorical variables for change within group from baseline to month 6.
The p value for δ change between groups from baseline to month 6 using repeated measures ANOVA for continuous variables and the Fisher exact test for categorical variables.
A total IIEF score <21 indicates ED.
Fig. 1.
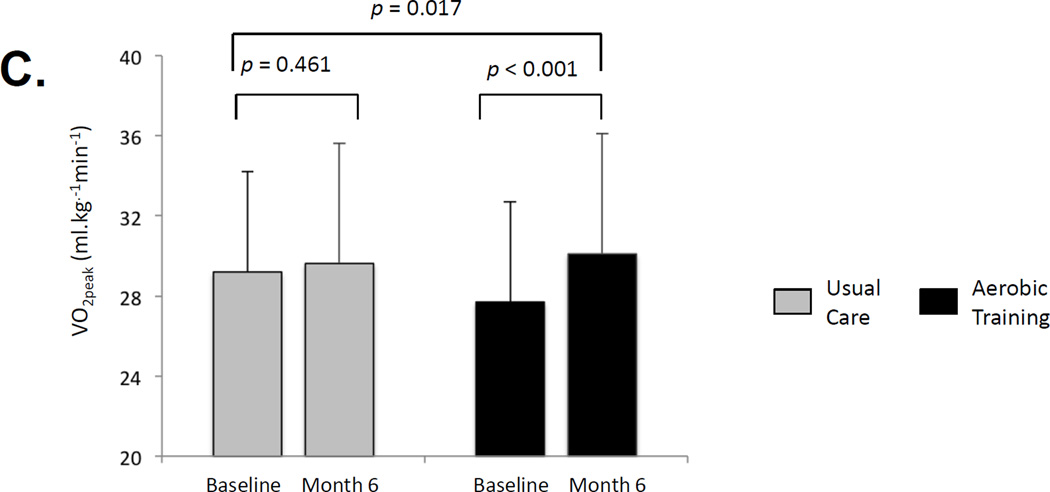
Effect on erectile dysfunction and cardiovascular mechanisms: effect of aerobic training compared with usual care on (a) mean change in prevalence of erectile dysfunction, as assessed by the International Index of Erectile Function; (b) mean absolute change in peripheral arterial flow–mediated dilation (percentage change to peak brachial artery diameter); and (c) mean absolute change in peak oxygen uptake (VO2peak).
Similarly, there were no significant between-group differences in any erectile function subscale (Table 1, Supplemental Table 2). However, in comparison with UC, AT was associated with significant improvements in FMD, expressed as percentage change in peak artery diameter (Fig. 1b, Supplemental Table 3) and VO2peak (Fig. 1c, Supplemental Fig. 3, Supplemental Table 3). There were no significant group differences in changes of other CV risk profile outcomes (p > 0.05) (Supplemental Table 4) or PROs (p > 0.05) (Supplemental Table 5). There were significant correlations between AT adherence and change in FMD (r = 0.38; p = 0.081) and VO2peak (r = 0.57; p = 0.003) but not between AT adherence and any erectile function end points (p > 0.05; data not presented).
Our principal finding was that despite robust effects on CV mechanisms, AT did not differentially improve erectile function in the short term or long term after RP compared with recommended UC. This finding is in direct contrast to prior work showing that AT-induced improvements in FMD and VO2peak were associated with a twofold improvement in erectile function in stable heart failure [6]. Given similar mechanistic effects, consideration of potential explanations for our observed null effects is appropriate.
First and most important, in the setting of heart failure, endothelial-derived nitric oxide (NO) release is the principal contributor to ED, whereas in the post-RP setting, surgery-induced neuronal injury is a major contributor [9]. The robust AT-induced improvements in peripheral FMD suggest that NO bioactivity may have also been improved in the cavernous arteries, although such effects did not sufficiently compensate for surgery-induced neuronal injury. Second, our AT prescription (ie, “dose”) may have been inadequate to affect erectile function, particularly given the considerable exercise contamination in the UC group. Contrary to this notion, our nonlinear AT prescription, incorporating high-intensity AT sessions, caused a robust VO2peak improvement, demonstrating that AT was successfully delivered and dosed (VO2peak was not significantly improved in UC). Exploratory analyses indicated that AT adherence (dose) significantly correlated with VO2peak change (and FMD) but not with erectile function. Taken together, these findings indicate that the AT prescription (and UC contamination) was not responsible for the observed null findings.
Third, 6 mo of AT may be too short to affect erectile function. Exploratory analyses at 12 mo (approximately 16 mo after RP) indicated further reductions in ED prevalence with no differences between AT and UC, indicating that immediate post-RP AT does not affect long-term erectile function recovery.
An important limitation of this study is the small sample size, which may have increased the chance of a type 1 or 2 error because of limited power based on an optimistic a priori between-group ED prevalence δ. In addition, the eligibility requirement to attend supervised AT sessions limits the generalizability of findings. In conclusion, AT improves CV function but not ED in men with localized PCa following RP.
Supplementary Material
Take-home message.
This proof-of-concept trial demonstrated that aerobic training following a novel nonlinear prescription approach incorporating high-intensity training is safe and feasible and improves cardiovascular function but not erectile dysfunction in men with localized prostate cancer in the acute period following radical prostatectomy.
Acknowledgments
Funding support
This study was supported by a research grant from the National Cancer Institute (R21-CA133895) awarded to Lee W. Jones.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have nothing to disclose.
References
- 1.Sivarajan G, Prabhu V, Taksler GB, et al. Ten-year outcomes of sexual function after radical prostatectomy: results of a prospective longitudinal study. Eur Urol. doi: 10.1016/j.eururo.2013.08.019. In press. [DOI] [PubMed] [Google Scholar]
- 2.Watts GF, Chew KK, Stuckey BG. The erectile-endothelial dysfunction nexus: new opportunities for cardiovascular risk prevention. Nat Clin Pract Cardiovasc Med. 2007;4:263–273. doi: 10.1038/ncpcardio0861. [DOI] [PubMed] [Google Scholar]
- 3.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587:5551–5558. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 6.Belardinelli R, Lacalaprice F, Faccenda E, et al. Effects of short-term moderate exercise training on sexual function in male patients with chronic stable heart failure. Int J Cardiol. 2005;101:83–90. doi: 10.1016/j.ijcard.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flowmediated dilation. Med Sci Sports Exerc. 2002;34:960–965. doi: 10.1097/00005768-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 9.Fode M, Ohl DA, Ralph D, et al. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. 2013;112:998–1008. doi: 10.1111/bju.12228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.