Chapter 2.1: Definition and classification of AKI
INTRODUCTION
AKI is one of a number of conditions that affect kidney structure and function. AKI is defined by an abrupt decrease in kidney function that includes, but is not limited to, ARF. It is a broad clinical syndrome encompassing various etiologies, including specific kidney diseases (e.g., acute interstitial nephritis, acute glomerular and vasculitic renal diseases); non-specific conditions (e.g, ischemia, toxic injury); as well as extrarenal pathology (e.g., prerenal azotemia, and acute postrenal obstructive nephropathy)—see Chapters 2.2 and 2.3 for further discussion. More than one of these conditions may coexist in the same patient and, more importantly, epidemiological evidence supports the notion that even mild, reversible AKI has important clinical consequences, including increased risk of death.2, 5 Thus, AKI can be thought of more like acute lung injury or acute coronary syndrome. Furthermore, because the manifestations and clinical consequences of AKI can be quite similar (even indistinguishable) regardless of whether the etiology is predominantly within the kidney or predominantly from outside stresses on the kidney, the syndrome of AKI encompasses both direct injury to the kidney as well as acute impairment of function. Since treatments of AKI are dependent to a large degree on the underlying etiology, this guideline will focus on specific diagnostic approaches. However, since general therapeutic and monitoring recommendations can be made regarding all forms of AKI, our approach will be to begin with general measures.
Definition and staging of AKI
AKI is common, harmful, and potentially treatable. Even a minor acute reduction in kidney function has an adverse prognosis. Early detection and treatment of AKI may improve outcomes. Two similar definitions based on SCr and urine output (RIFLE and AKIN) have been proposed and validated. There is a need for a single definition for practice, research, and public health.
- 2.1.1: AKI is defined as any of the following (Not Graded):
- Increase in SCr by ⩾0.3 mg/dl (⩾26.5 μmol/l) within 48 hours; or
- Increase in SCr to ⩾1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or
- Urine volume <0.5 ml/kg/h for 6 hours.
2.1.2: AKI is staged for severity according to the following criteria (Table 2). (Not Graded)
2.1.3: The cause of AKI should be determined whenever possible. (Not Graded)
Table 2. Staging of AKI.
| Stage | Serum creatinine | Urine output |
|---|---|---|
| 1 | 1.5–1.9 times baseline OR ⩾0.3 mg/dl (⩾26.5 μmol/l) increase | <0.5 ml/kg/h for 6–12 hours |
| 2 | 2.0–2.9 times baseline | <0.5 ml/kg/h for ⩾12 hours |
| 3 | 3.0 times baseline OR Increase in serum creatinine to ⩾4.0 mg/dl (⩾353.6 μmol/l) OR Initiation of renal replacement therapy OR, In patients <18 years, decrease in eGFR to <35 ml/min per 1.73 m2 | <0.3 ml/kg/h for ⩾24 hours OR Anuria for ⩾12 hours |
RATIONALE
Conditions affecting kidney structure and function can be considered acute or chronic, depending on their duration. AKI is one of a number of acute kidney diseases and disorders (AKD), and can occur with or without other acute or chronic kidney diseases and disorders (Figure 2). Whereas CKD has a well-established conceptual model and definition that has been useful in clinical medicine, research, and public health,42, 43, 44 the definition for AKI is evolving, and the concept of AKD is relatively new. An operational definition of AKD for use in the diagnostic approach to alterations in kidney function and structure is included in Chapter 2.5, with further description in Appendix B.
Figure 2.
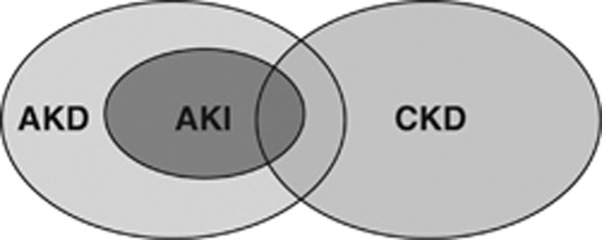
Overview of AKI, CKD, and AKD. Overlapping ovals show the relationships among AKI, AKD, and CKD. AKI is a subset of AKD. Both AKI and AKD without AKI can be superimposed upon CKD. Individuals without AKI, AKD, or CKD have no known kidney disease (NKD), not shown here. AKD, acute kidney diseases and disorders; AKI, acute kidney injury; CKD, chronic kidney disease.
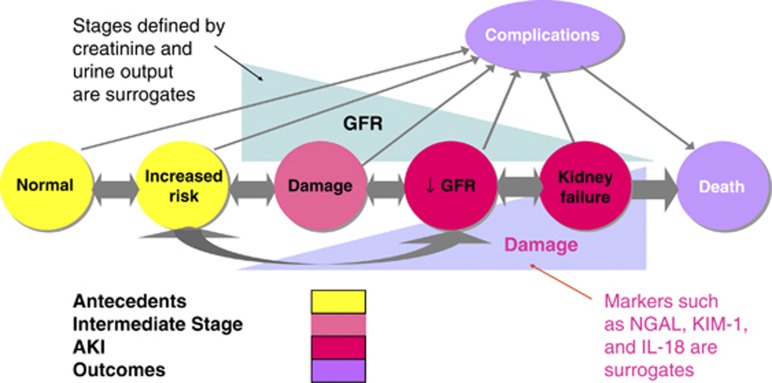
The conceptual model of AKI (Figure 3) is analogous to the conceptual model of CKD, and is also applicable to AKD.42, 45 Circles on the horizontal axis depict stages in the development (left to right) and recovery (right to left) of AKI. AKI (in red) is defined as reduction in kidney function, including decreased GFR and kidney failure. The criteria for the diagnosis of AKI and the stage of severity of AKI are based on changes in SCr and urine output as depicted in the triangle above the circles. Kidney failure is a stage of AKI highlighted here because of its clinical importance. Kidney failure is defined as a GFR <15 ml/min per 1.73 m2 body surface area, or requirement for RRT, although it is recognized that RRT may be required earlier in the evolution of AKI. Further description is included in Chapter 2.5 and Appendix A.
Figure 3.
Conceptual model for AKI. Red circles represent stages of AKI. Yellow circles represent potential antecedents of AKI, and the pink circle represents an intermediate stage (not yet defined). Thick arrows between circles represent risk factors associated with the initiation and progression of disease that can be affected or detected by interventions. Purple circles represent outcomes of AKI. “Complications” refers to all complications of AKI, including efforts at prevention and treatment, and complications in other organ systems. AKI, acute kidney injury; GFR, glomerular filtration rate. Adapted from Murray PT, Devarajan P, Levey AS, et al. A framework and key research questions in AKI diagnosis and staging in different environments. Clin J Am Soc Nephrol 2008; 3: 864–868 with permission from American Society of Nephrology45 conveyed through Copyright Clearance Center, Inc.; accessed http://cjasn.asnjournals.org/content/3/3/864.full
It is widely accepted that GFR is the most useful overall index of kidney function in health and disease, and changes in SCr and urine output are surrogates for changes in GFR. In clinical practice, an abrupt decline in GFR is assessed from an increase in SCr or oliguria. Recognizing the limitations of the use of a decrease in kidney function for the early detection and accurate estimation of renal injury (see below), there is a broad consensus that, while more sensitive and specific biomarkers are needed, changes in SCr and/or urine output form the basis of all diagnostic criteria for AKI. The first international interdisciplinary consensus criteria for diagnosis of AKI were the RIFLE criteria32 proposed by the ADQI. Modifications to these criteria have been proposed in order to better account for pediatric populations (pRIFLE)32 and for small changes in SCr not captured by RIFLE (AKIN criteria).23 Recommendations 2.1.1 and 2.1.2 represent the combination of RIFLE and AKIN criteria (Table 3).
Table 3. Comparison of RIFLE and AKIN criteria for diagnosis and classification of AKI.
|
AKI staging |
Urine output |
RIFLE |
|
|---|---|---|---|
| Serum creatinine | (common to both) | Class | Serum creatinine or GFR |
| Stage 1 Increase of more than or equal to 0.3 mg/dl (⩾26.5 μmol/l) or increase to more than or equal to 150% to 200% (1.5- to 2-fold) from baseline | Less than 0.5 ml/kg/h for more than 6 hours | Risk | Increase in serum creatinine × 1.5 or GFR decrease >25% |
| Stage 2 Increased to more than 200% to 300% (>2- to 3-fold) from baseline | Less than 0.5 ml/kg per hour for more than 12 hours | Injury | Serum creatinine × 2 or GFR decreased >50% |
| Stage 3 Increased to more than 300% (>3-fold) from baseline, or more than or equal to 4.0 mg/dl (⩾354 μmol/l) with an acute increase of at least 0.5 mg/dl (44 μmol/l) or on RRT | Less than 0.3 ml/kg/h for 24 hours or anuria for 12 hours | Failure | Serum creatinine × 3, or serum creatinine >4 mg/dl (>354 μmol/l) with an acute rise >0.5 mg/dl (>44 μmol/l) or GFR decreased >75% |
| Loss | Persistent acute renal failure=complete loss of kidney function >4 weeks | ||
| End-stage kidney disease | ESRD >3 months | ||
Note: For conversion of creatinine expressed in SI units to mg/dl, divide by 88.4. For both AKIN stage and RIFLE criteria, only one criterion (creatinine rise or urine output decline) needs to be fulfilled. Class is based on the worst of either GFR or urine output criteria. GFR decrease is calculated from the increase in serum creatinine above baseline. For AKIN, the increase in creatinine must occur in <48 hours. For RIFLE, AKI should be both abrupt (within 1–7 days) and sustained (more than 24 hours). When baseline creatinine is elevated, an abrupt rise of at least 0.5 mg/dl (44 μmol/l) to >4 mg/dl (>354 μmol/l) is sufficient for RIFLE class Failure (modified from Mehta et al.23 and the report of the Acute Dialysis Quality Initiative consortium22).
AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; ESRD, end-stage renal disease; GFR, glomerular filtration rate; RIFLE, risk, injury, failure, loss, and end stage; RRT, renal replacement therapy. Reprinted from Endre ZH. Acute kidney injury: definitions and new paradigms. Adv Chronic Kidney Dis 2008; 15: 213–221 with permission from National Kidney Foundation46; accessed http://www.ackdjournal.org/article/S1548-5595(08)00049-9/fulltext
Existing evidence supports the validity of both RIFLE and AKIN criteria to identify groups of hospitalized patients with increased risk of death and/or need for RRT.2, 5, 25, 28, 29, 30 Epidemiological studies, many multicentered, collectively enrolling more than 500 000 subjects have been used to establish RIFLE and/or AKIN criteria as valid methods to diagnose and stage AKI. Recently, Joannidis et al.29 directly compared RIFLE criteria with and without the AKIN modification. While AKI classified by either criteria were associated with a similarly increased hospital mortality, the two criteria identified somewhat different patients. The original RIFLE criteria failed to detect 9% of cases that were detected by AKIN criteria. However, the AKIN criteria missed 26.9% of cases detected by RIFLE. Examination of the cases missed by either criteria (Table 4) shows that cases identified by AKIN but missed by RIFLE were almost exclusively Stage 1 (90.7%), while cases missed by AKIN but identified by RIFLE included 30% with RIFLE-I and 18% RIFLE-F; furthermore, these cases had hospital mortality similar to cases identified by both criteria (37% for I and 41% for F). However, cases missed by RIFLE but identified as Stage 1 by AKIN also had hospital mortality rates nearly twice that of patients who had no evidence of AKI by either criteria (25% vs. 13%). These data provide strong rationale for use of both RIFLE and AKIN criteria to identify patients with AKI.
Table 4. Cross-tabulation of patients classified by RIFLE vs. AKIN.
|
RIFLE |
||||||
|---|---|---|---|---|---|---|
| AKIN | Non-AKI | Risk | Injury | Failure | Total (AKIN) | |
| Non-AKI | n* | 8759 (12.9%) | 781 (27.7%) | 452 (37.4%) | 271 (41.3%) | 10 263 (15.9%) |
| Stage1 | n* | 457 (25.2%) | 282 (33.0%) | 243 (44.0%) | 95 (60.0%) | 1077 (34.5%) |
| Stage 2 | n* | 36 (30.6%) | 21 (47.6%) | 885 (25.9%) | 91 (54.9) | 1033 (29.0%) |
| Stage 3 | n* | 11 (18.2%) | 8 (12.5%) | 16 (62.5%) | 1948 (41.3) | 1983 (41.2%) |
| Total (RIFLE) | n* | 9263 (13.6%) | 1092 (29.2%) | 1596 (32.3%) | 2405 (42.6%) | 14 356 (21.7%) |
*Number of patients classified into the respective stages of AKI by AKIN or RIFLE are cross-tabulated against each other. Hospital mortality of each group is given in parentheses. Shaded fields denote patients assigned to the same degree of AKI by both classification systems.
AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; RIFLE, risk, injury, failure, loss, and end stage. With kind permission from Springer Science+Business Media: Intensive Care Med. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. 35 (2009): 1692–1702. Joannidis M, Metnitz B, Bauer P et al.29; accessed http://www.springerlink.com/content/r177337030550120/
Staging of AKI (Recommendation 2.1.2) is appropriate because, with increased stage of AKI, the risk for death and need for RRT increases.2, 5, 25, 28, 29, 30, 31 Furthermore, there is now accumulating evidence of long-term risk of subsequent development of cardiovascular disease or CKD and mortality, even after apparent resolution of AKI.47, 48, 49
For staging purposes, patients should be staged according to the criteria that give them the highest stage. Thus when creatinine and urine output map to different stages, the patient is staged according to the highest (worst) stage. The changes in GFR that were published with the original RIFLE criteria do not correspond precisely to changes in SCr. As SCr is measured and GFR can only be estimated, creatinine criteria should be used along with urine output for the diagnosis (and staging) of AKI. One additional change in the criteria was made for the sake of clarity and simplicity. For patients reaching Stage 3 by SCr >4.0 mg/dl (>354 μmol/l), rather than require an acute increase of ⩾0.5 mg/dl (⩾44 μmol/l) over an unspecified time period, we instead require that the patient first achieve the creatinine-based change specified in the definition (either ⩾0.3 mg/dl [⩾26.5 μmol/l] within a 48-hour time window or an increase of ⩾1.5 times baseline). This change brings the definition and staging criteria to greater parity and simplifies the criteria.
Recommendation 2.1.2 is based on the RIFLE and AKIN criteria that were developed for average-sized adults. The creatinine change–based definitions include an automatic Stage 3 classification for patients who develop SCr >4.0 mg/dl (>354 μmol/l) (provided that they first satisfy the definition of AKI in Recommendation 2.1.1). This is problematic for smaller pediatric patients, including infants and children with low muscle mass who may not be able to achieve a SCr of 4.0 mg/dl (354 μmol/l). Thus, the pediatric-modified RIFLE AKI criteria32 were developed using a change in estimated creatinine clearance (eCrCl) based on the Schwartz formula. In pRIFLE, patients automatically reach Stage 3 if they develop an eCrCl <35 ml/min per 1.73 m2. However, with this automatic pRIFLE threshold, the SCr change based AKI definition (recommendation 2.1.1) is applicable to pediatric patients, including an increase of 0.3 mg/dl (26.5 μmol/l) SCr.32
There are important limitations to these recommendations, including imprecise determination of risk (see Chapter 2.2) and incomplete epidemiology of AKI, especially outside the ICU. Clinical judgment is required in order to determine if patients seeming to meet criteria do, in fact, have disease, as well as to determine if patients are likely to have AKI even if incomplete clinical data are available to apply the diagnostic criteria. The application of the diagnostic and staging criteria is discussed in greater detail, along with specific examples in Chapter 2.4.
The use of urine output criteria for diagnosis and staging has been less well validated and in individual patients the need for clinical judgment regarding the effects of drugs (e.g., angiotensin-converting enzyme inhibitors [ACE-I]), fluid balance, and other factors must be included. For very obese patients, urine output criteria for AKI may include some patients with normal urine output. However, these recommendations serve as the starting point for further evaluation, possibly involving subspecialists, for a group of patients recognized to be at increased risk.
Finally, it is axiomatic that patients always be managed according to the cause of their disease, and thus it is important to determine the cause of AKI whenever possible. In particular, patients with decreased kidney perfusion, acute glomerulonephritis, vasculitis, interstitial nephritis, thrombotic microangiopathy, and urinary tract obstruction require immediate diagnosis and specific therapeutic intervention, in addition to the general recommendations for AKI in the remainder of this guideline (Table 5).
Table 5. Causes of AKI and diagnostic tests.
| Selected causes of AKI requiring immediate diagnosis and specific therapies | Recommended diagnostic tests |
|---|---|
| Decreased kidney perfusion | Volume status and urinary diagnostic indices |
| Acute glomerulonephritis, vasculitis, interstitial nephritis, thrombotic microangiopathy | Urine sediment examination, serologic testing and hematologic testing |
| Urinary tract obstruction | Kidney ultrasound |
AKI, acute kidney injury.
It is recognized that it is frequently not possible to determine the cause, and often the exact cause does not dictate a specific therapy. However, the syndrome of AKI includes some patients with specific kidney diseases (e.g., glomerulonephritis) for which a specific treatment is available. As such, it is always necessary to search for the underlying cause of AKI (see Chapter 2.3).
Research Recommendations
- The role of biomarkers other than SCr in the early diagnosis, differential diagnosis, and prognosis of AKI patients should be explored. Some important areas in which to focus include:
- Early detection where the gold standard is AKI by clinical diagnosis after the fact and the biomarker is compared to existing markers (SCr and urine output) at the time of presentation.
- Prognosis where a biomarker is used to predict risk for AKI or risk for progression of AKI.
- Prognosis where a biomarker is used to predict recovery after AKI vs. death or need for long-term RRT.
The influence of urinary output criteria on AKI staging needs to be further investigated. Influence of fluid balance, percent volume overload, diuretic use, and differing weights (actual, ideal body weight, lean body mass) should be considered. Also, it is currently not known how urine volume criteria should be applied (e.g., average vs. persistent reduction for the period specified).
The influence of SCr or eGFR criteria on AKI staging needs to be further investigated. The use of different relative and absolute SCr increments or eGFR decrements at different time points and with differently ascertained baseline values requires further exploration and validation in various populations.
Chapter 2.2: Risk assessment
The kidney is a fairly robust organ that can tolerate exposure to several insults without suffering significant structural or functional change. For this reason, any acute change in kidney function often indicates severe systemic derangement and predicts a poor prognosis. Risk for AKI is increased by exposure to factors that cause AKI or the presence of factors that increase susceptibility to AKI. Factors that determine susceptibility of the kidneys to injury include dehydration, certain demographic characteristics and genetic predispositions, acute and chronic comorbidities, and treatments. It is the interaction between susceptibility and the type and extent of exposure to insults that determines the risk of occurrence of AKI.
Understanding individual “risk factors” may help in preventing AKI. This is particularly gratifying in the hospital setting, where the patient's susceptibility can be assessed before certain exposures as surgery or administration of potentially nephrotoxic agents. Accordingly, some susceptibility factors may be modified, and contemplated exposures avoided or tailored to reduce the risk of AKI.
Risk assessment in community-acquired AKI is different from hospital-acquired AKI, for two main reasons: i) Available evidence on risk factors is largely derived from hospital data and extrapolation to the community setting is questionable. ii) The opportunity to intervene, prior to exposure, is quite limited. Most patients are seen only after having suffered an exposure (trauma, infection, poisonous plant, or animal). However, there is still room to assess such patients, albeit after exposure, in order to identify those who are more likely to develop AKI, thereby requiring closer monitoring and general supportive measures. It may also be helpful to identify such patients in order to avoid additional injury. A more complete discussion of the approach to identification and management of risk for AKI is provided in Appendices C and D.
2.2.1: We recommend that patients be stratified for risk of AKI according to their susceptibilities and exposures. (1B)
2.2.2: Manage patients according to their susceptibilities and exposures to reduce the risk of AKI (see relevant guideline sections). (Not Graded)
2.2.3: Test patients at increased risk for AKI with measurements of SCr and urine output to detect AKI. (Not Graded) Individualize frequency and duration of monitoring based on patient risk and clinical course. (Not Graded)
RATIONALE
There are many types of exposures that may cause AKI (Table 6) and these are discussed in detail in Appendix C. However, the chances of developing AKI after exposure to the same insult differ among different individuals. This is attributed to a number of susceptibility factors which vary widely from individual to individual. Our understanding of susceptibility factors (Table 6) is based on many observational studies that address different settings with regards to the type, severity, duration, and multiplicity of insults. While this heterogeneity provides insight into some susceptibility factors that are common across various populations, the generalizability of results from one particular setting to the next is uncertain.
Table 6. Causes of AKI: exposures and susceptibilities for non-specific AKI.
| Exposures | Susceptibilities |
|---|---|
| Sepsis | Dehydration or volume depletion |
| Critical illness | Advanced age |
| Circulatory shock | Female gender |
| Burns | Black race |
| Trauma | CKD |
| Cardiac surgery (especially with CPB) | Chronic diseases (heart, lung, liver) |
| Major noncardiac surgery | Diabetes mellitus |
| Nephrotoxic drugs | Cancer |
| Radiocontrast agents | Anemia |
| Poisonous plants and animals |
CKD, chronic kidney disease; CPB, cardiopulmonary bypass.
The course and outcome of AKI are modified by other factors, but since these are manifested within the context of actual disease, they must be categorized as “prognostic” rather than “risk” factors, hence being discussed separately in Appendix D. Lastly, the fact that some 30% of patients who recover from AKI remain at increased risk of CKD, cardiovascular disease, and death calls for the identification of the risk factors that can identify such patients in the hopes of providing them with timely preventive measures.50, 51, 52
Finally, it is important to screen patients who have undergone an exposure (e.g., sepsis, trauma) and to continue monitor high-risk patients until the risk has subsided. Exact intervals for checking SCr and in which individuals to monitor urine output remain matters of clinical judgment; however, as a general rule, high risk in-patients should have SCr measured at least daily and more frequently after an exposure, and critically ill patients should have urine output monitoring. This will necessitate urinary bladder catheterization in many cases, and the risks of infection should also be considered in the monitoring plan.
A recent clinical practice assessment in the UK concluded that only 50% of patients with AKI were considered to have received a “good” overall standard of care. This figure fell to just over 30% if AKI developed during a hospital admission rather than being diagnosed before admission.53 The authors also felt that there was an unacceptable delay in recognizing AKI in 43% of those that developed the condition after admission, and that in a fifth of such patients its development was predictable and avoidable. Their recommendations were simple: risk assessment for AKI as part of the initial evaluation of emergency admissions, along with appropriate serum biochemistry on admission and at frequent intervals thereafter.53
RESEARCH RECOMMENDATIONS
Better delineation of risk for hospital- and community-acquired AKI is needed.
Better delineation of the effects of age on the risk for AKI is needed.
Studies are needed to develop and validate scoring systems for AKI risk prediction in various settings, in addition to cardiac surgery and exposure to radiocontrast material.
Genome-wide association studies are needed to determine risk of AKI in different hospital settings and with respect to long-term outcomes.
Studies are needed on risk factors for the development of, recovery from, and long-term outcomes of community-acquired AKI, including sepsis, trauma, tropical infections, snake bites, and ingestion of toxic plants, etc.
Chapter 2.3: Evaluation and general management of patients with and at risk for AKI
Given that AKI is associated with significant morbidity and mortality, and because no specific treatment is available to reverse AKI, early recognition and management is paramount. Indeed, recognition of patients at risk for AKI, or with possible AKI but prior to clinical manifestations, is likely to result in better outcomes than treating only established AKI. Chapter 2.2 introduced the approach to risk assessment with further detail provided in Appendix C. This chapter will concern itself with the evaluation and general management of patients with, or even at risk for, AKI. Further detail is provided in Appendix D. We highlight the importance of beginning management at the earliest point in the development of AKI—in patients with suspected AKI or even in those at increased risk who have been exposed to the various factors discussed in Chapters 2.2 and Appendix C.
Although much of the remaining chapters in this guideline pertain to management of specific aspects of AKI, there are general management principles that are common to all patients and these will be discussed here and further expounded upon in Appendix D. Treatment goals in patients with AKI include both reducing kidney injury and complications related to decreased kidney function.
2.3.1: Evaluate patients with AKI promptly to determine the cause, with special attention to reversible causes. (Not Graded)
2.3.2: Monitor patients with AKI with measurements of SCr and urine output to stage the severity, according to Recommendation 2.1.2. (Not Graded)
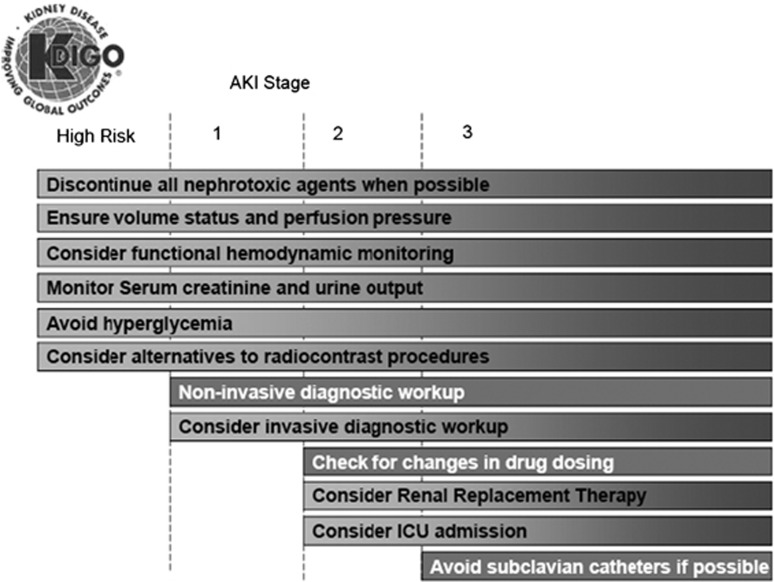
2.3.3: Manage patients with AKI according to the stage (see Figure 4) and cause. (Not Graded)
- 2.3.4: Evaluate patients 3 months after AKI for resolution, new onset, or worsening of pre-existing CKD. (Not Graded)
- If patients have CKD, manage these patients as detailed in the KDOQI CKD Guideline (Guidelines 7–15). (Not Graded)
- If patients do not have CKD, consider them to be at increased risk for CKD and care for them as detailed in the KDOQI CKD Guideline 3 for patients at increased risk for CKD. (Not Graded)
Figure 4.
Stage-based management of AKI. Shading of boxes indicates priority of action—solid shading indicates actions that are equally appropriate at all stages whereas graded shading indicates increasing priority as intensity increases. AKI, acute kidney injury; ICU, intensive-care unit.
RATIONALE
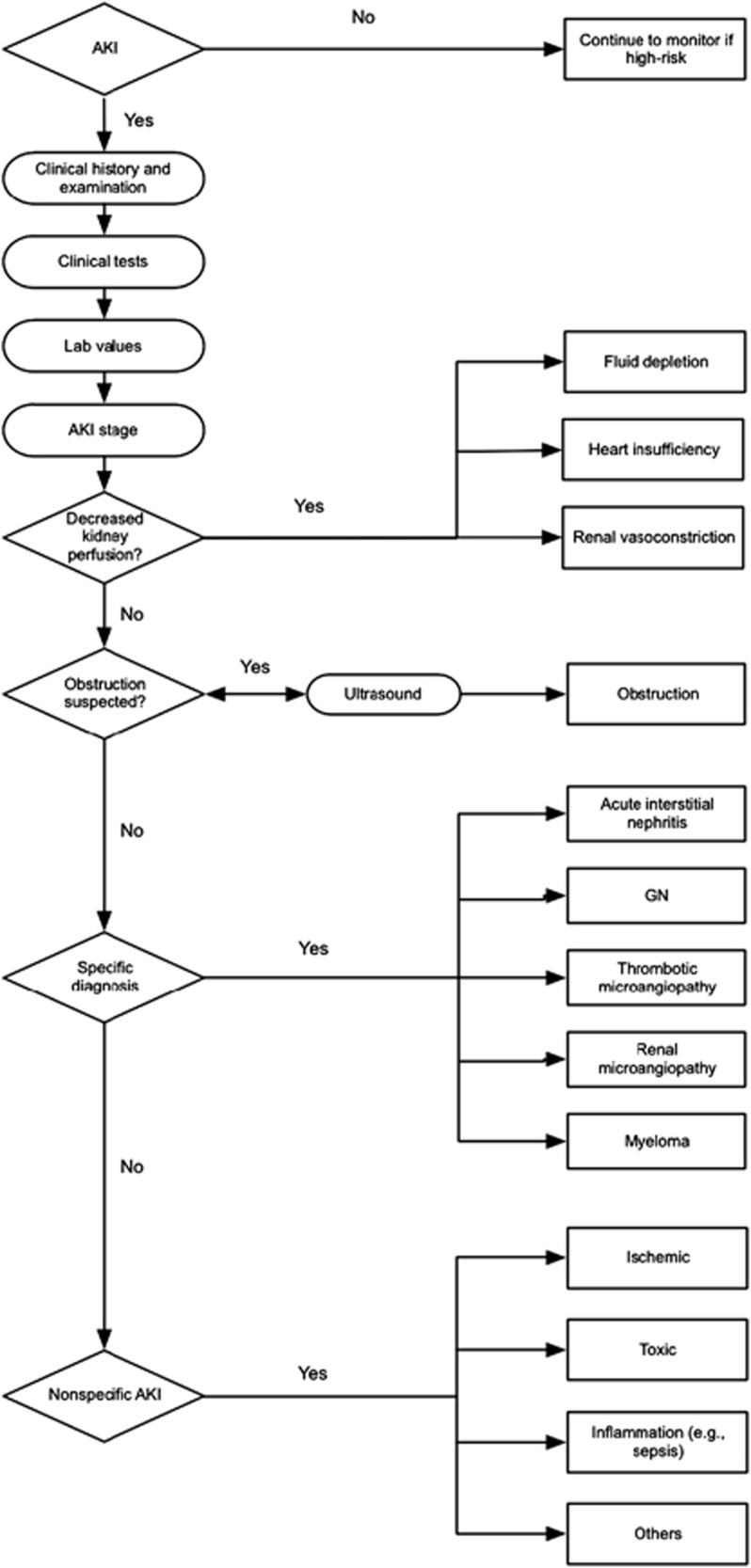
As emphasized in Chapter 2.2, AKI is not a disease but rather a clinical syndrome with multiple etiologies. While much of the literature examining epidemiology and clinical consequences of AKI appear to treat this syndrome as a homogeneous disorder, the reality is that AKI is heterogeneous and often is the result of multiple insults. Figure 5 illustrates an approach to evaluation of AKI. Further discussion of evaluation in clinical practice is provided in Appendix D.
Figure 5.
Evaluation of AKI according to the stage and cause.
The clinical evaluation of AKI includes a careful history and physical examination. Drug history should include over-the-counter formulations and herbal remedies or recreational drugs. The social history should include exposure to tropical diseases (e.g., malaria), waterways or sewage systems, and exposure to rodents (e.g., leptospirosis, hantavirus). Physical examination should include evaluation of fluid status, signs for acute and chronic heart failure, infection, and sepsis.
Measurement of cardiac output, preload, preload responsiveness, and intra-abdominal pressure should be considered in the appropriate clinical context. Laboratory parameters—including SCr, blood urea nitrogen (BUN), and electrolytes, complete blood count and differential—should be obtained. Urine analysis and microscopic examination as well as urinary chemistries may be helpful in determining the underlying cause of AKI. Imaging tests, especially ultrasound, are important components of the evaluation for patients with AKI. Finally, a number of biomarkers of functional change and cellular damage are under evaluation for early diagnosis, risk assessment for, and prognosis of AKI (see Appendix D for detailed discussion).
Individualize frequency and duration of monitoring based on patient risk, exposure and clinical course. Stage is a predictor of the risk for mortality and decreased kidney function (see Chapter 2.4). Dependent on the stage, the intensity of future preventive measures and therapy should be performed.
Because the stage of AKI has clearly been shown to correlate with short-term2, 5, 27, 29 and even longer-term outcomes,31 it is advisable to tailor management to AKI stage. Figure 4 lists a set of actions that should be considered for patients with AKI. Note that for patients at increased risk (see Chapters 2.2 and 2.4), these actions actually begin even before AKI is diagnosed.
Note that management and diagnostic steps are both included in Figure 4. This is because response to therapy is an important part of the diagnostic approach. There are few specific tests to establish the etiology of AKI. However, a patient's response to treatment (e.g., discontinuation of a possible nephrotoxic agent) provides important information as to the diagnosis.
Nephrotoxic drugs account for some part of AKI in 20–30% of patients. Often, agents like antimicrobials (e.g., aminoglycosides, amphotericin) and radiocontrast are used in patients that are already at high risk for AKI (e.g., critically ill patients with sepsis). Thus, it is often difficult to discern exactly what contribution these agents have on the overall course of AKI. Nevertheless, it seems prudent to limit exposure to these agents whenever possible and to weigh the risk of developing or worsening AKI against the risk associated with not using the agent. For example, when alternative therapies or diagnostic approaches are available they should be considered.
In order to ensure adequate circulating blood volume, it is sometimes necessary to obtain hemodynamic variables. Static variables like central venous pressure are not nearly as useful as dynamic variables, such as pulse-pressure variation, inferior vena cava filling by ultrasound and echocardiographic appearance of the heart (see also Appendix D).
Note that while the actions listed in Figure 4 provide an overall starting point for stage-based evaluation and management, they are neither complete not mandatory for an individual patient. For example, the measurement of urine output does not imply that the urinary bladder catheterization is mandatory for all patients, and clinicians should balance the risks of any procedures with the benefits. Furthermore, clinicians must individualize care decisions based on the totality of the clinical situation. However, it is advisable to include AKI stage in these decisions.
The evaluation and management of patients with AKI requires attention to cause and stage of AKI, as well as factors that relate to further injury to the kidney, or complications from decreased kidney function. Since AKI is a risk factor for CKD, it is important to evaluate patients with AKI for new onset or worsening of pre-existing CKD. If patients have CKD, manage patients as detailed in the KDOQI CKD Guideline (Guidelines 7–15). If patients do not have CKD, consider them to be at increased risk for CKD and care for them as detailed in the KDOQI CKD Guideline 3 for patients at increased risk for CKD.
RESEARCH RECOMMENDATIONS
Clinical research aimed at testing early management strategies is urgently needed. Such trials should also address the risks and benefits of commonly used fluid-management strategies, including intravenous (i.v.) fluids and diuretics.
Methods to better assess fluid status in critically ill and other hospitalized patients at risk for AKI are needed.
Research is needed, with follow-up beyond hospital stay, to better understand the clinical consequences of AKI in patients with and without underlying CKD.
Chapter 2.4: Clinical applications
This chapter provides a detailed application of the AKI definition and staging for clinical diagnosis and management. The definitions and classification system discussed in Chapter 2.1 can be used easily in many patients and requires little clinical interpretation. However, in real time, clinicians do not always have a complete dataset to work with and individual patients present with unique histories. As discussed in the previous chapter, it is difficult to distinguish AKI from CKD in many cases. In addition, as many as two-thirds of all cases of AKI begin prior to hospitalization (community-acquired AKI). Therefore, clinicians may be faced with patients in whom kidney function is already decreased and, during the hospitalization, improves rather than worsens. Finally, many patients do not have a prior measurement of kidney function available for comparison. This chapter provides detailed examples of the application of these definitions to the clinical setting.
Examples of application of AKI definitions
Table 7 illustrates a number of examples whereby patients presenting with possible AKI can be diagnosed. Cases A-F have a measurement of baseline SCr. To simplify decision-making, baseline estimated glomerular filtration rate (eGFR) exceeds 60 ml/min per 1.73 m2 in these patients, so none has pre-existing CKD. Cases A-F can all be diagnosed with AKI by applying the first two criteria in Recommendation 2.1.1. (a documented increase of at least 0.3 mg/dl (>26.5 μmol/l) [within 48 hours or a 50% increase from presumed baseline). Note that a patient can be diagnosed with AKI by fulfilling either criterion 1 or 2 (or 3, urine output) and thus cases B,C,D, and F all fulfill the definition of AKI. Note also that patients may be diagnosed earlier using criterion 1 or 2. Early diagnosis may improve outcome so it is advantageous to diagnose patients as rapidly as possible. For example, case A can be diagnosed with AKI on day 2 by the first criterion, whereas the second criterion is not satisfied until day 3 (increase from 1.3 to 1.9). However, this is only true because the episode of AKI began prior to medical attention, and thus the day 1 SCr level was already increased. If creatinine measurements had available with 48 hours prior to day 1 and if this level had been at baseline (1.0 mg/dl [88.4 μmol/l]), it would have been possible to diagnose AKI on day 1 using the second criterion.
Table 7. AKI diagnosis.
|
Serum creatinine mg/dl (μmol/l) |
Diagnosis AKI? |
||||||
|---|---|---|---|---|---|---|---|
| Criterion 1 | Criterion 2 | ||||||
| Case | Baseline | Day 1 | Day 2 | Day 3 | Day 7 | 50% from baseline | ⩾0.3 mg/dl (⩾26.5 μmol/l) rise in ⩽48 hours |
| A | 1.0 (88) | 1.3 (115) | 1.5 (133) | 2.0 (177) | 1.0 (88) | Yes | Yes |
| B | 1.0 (88) | 1.1 (97) | 1.2 (106) | 1.4 (124) | 1.0 (88) | No | Yes |
| C | 0.4 (35) | 0.5 (44) | 0.6 (53) | 0.7 (62) | 0.4 (35) | Yes | No |
| D | 1.0 (88) | 1.1 (97) | 1.2 (106) | 1.3 (115) | 1.5 (133) | Yes | No |
| E | 1.0 (88) | 1.3 (115) | 1.5 (133) | 1.8 (159) | 2.2 (195) | Yes | Yes |
| F | ? | 3.0 (265) | 2.6 (230) | 2.2 (195) | 1.0 (88) | Yes | No |
| G | ? | 1.8 (159) | 2.0 (177) | 2.2 (195) | 1.6 (141) | ? | Yes |
| H | ? | 3.0 (265) | 3.1 (274) | 3.0 (265) | 2.9 (256) | ? | No |
Cases F-H do not have a baseline measurement of SCr available. Elevated SCr (reduced eGFR) on day 1 of the hospitalization is consistent with either CKD or AKD without AKI. In Case F, baseline SCr can be inferred to be below the day 1 value because of the subsequent clinical course; thus, we can infer the patient has had an episode of AKI. In case G, AKI can be diagnosed by application of criterion 2, but the patient may have underlying CKD. Case H does not fulfill the definition for AKI based on either criteria, and has either CKD or AKD without AKI.
The example of Case A raises several important issues. First, frequent monitoring of SCr in patients at increased risk of AKI will significantly improve diagnostic time and accuracy. If Case A had not presented to medical attention (or if SCr had not been checked) until day 7, the case of AKI would likely have been missed. Frequent measurement of SCr in high-risk patients, or in patients in which AKI is suspected, is therefore encouraged—see Chapter 2.3. The second issue highlighted by Case A is the importance of baseline SCr measurements. Had no baseline been available it would still have been possible to diagnose AKI on day 3 (by either using criterion 2 or by using criterion 1 and accepting the baseline SCr as 1.3); however, not only would this have resulted in a delay in diagnosis, it would have resulted in a delay in staging (see Table 7). On day 7, it can be inferred that the patient's baseline was no higher than 1.0 mg/dl (88 μmol/l) and thus correct staging of Case A as Stage 2 (two-fold increase from the reference SCr, see below and Table 7) on day 3 could have been determined in retrospect. However, if a baseline SCr was available to use as the reference, the correct stage could be determined on day 3.
Case B illustrates why criterion 2 can detect cases of AKI missed by criterion 1. It also clarifies why these cases are unusual. Had the SCr increased to 1.5 mg/dl (132.6 μmol/l) as opposed to peaking at 1.4 mg/dl (123.8 μmol/l), it would have been picked up by criterion 1 as well. By contrast Cases C, D, and even F illustrate how criterion 2 may miss cases identified by criterion 1. Note that Case F can only be diagnosed by inference. By day 7, it can be inferred that the baseline was no higher than 1.0 mg/dl (88 μmol/l) and thus it can be determined that the patient presented with AKI. However, if the baseline SCr could be estimated it would be possible to make this inference as early as day 1.
Estimating baseline SCr
Many patients will present with AKI without a reliable baseline SCr on record. Baseline SCr can be estimated using the Modification of Diet in Renal Disease (MDRD) Study equation assuming that baseline eGFR is 75 ml/min per 1.73 m2 (Table 9).22 This approach has been used in many, but not all, studies of AKI epidemiology using RIFLE2, 5, 25, 30, 31, 32, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63 (see Table 8) and has recently been validated.64 Hence, most current data concerning AKI defined by RIFLE criteria are based on estimated baseline SCr for a large proportion of patients.
Table 8. Overview of the approaches to determine baseline SCr in the application of RIFLE classification in previous studies.
| Study | No. of pts analyzed | Multi-/ single-center | Criteria used | Method to determine baseline SCr | % recorded | % estimated |
|---|---|---|---|---|---|---|
| Bagshaw25 | 120123 | multi | cr+uo | estimated by MDRD formula | 0 | 100 |
| Ostermann30 | 41972 | multi | cr | estimated by MDRD formula | 0 | 100 |
| Uchino5 | 20126 | single | cr | retrieved from hospital database, or estimated by MDRD formula | N/A | N/A |
| Bell54 | 8152 | single | cr+uo | retrieved from hospital database, or estimated by MDRD formula | N/A | N/A |
| Hoste2 | 5383 | single | cr+uo | estimated by MDRD formula, or admission creatinine value, whatever was lower | N/A | N/A |
| Ali31 | 5321 | multi | cr | retrieved from hospital database, or admission creatinine value | 100 | 0 |
| Cruz55 | 2164 | multi | cr+uo | retrieved from hospital database, or estimated by MDRD formula | 78 | 22 |
| Perez-Valdivieso56 | 1008 | single | cr | estimated by MDRD formula | 0 | 100 |
| Kuitunen57 | 813 | single | cr+uo | preoperative value | 100 | 0 |
| Coca58 | 304 | single | cr | the lowest s-creatinine value in the first 5 hospital days | 100 | 0 |
| Arnaoutakis59 | 267 | single | N/A | N/A | N/A | N/A |
| Abosaif60 | 247 | single | cr+uo | retrieved from hospital database, or admission creatinine value | 100 | 0 |
| Maccariello61 | 214 | multi | cr+uo | retrieved from hospital database, or estimated by MDRD formula | N/A | N/A |
| Jenq62 | 134 | single | cr+uo | admission creatinine value, or estimated by MDRD formula | 90 | 10 |
cr, creatinine criteria; MDRD, Modification of Diet in Renal Disease; N/A, not available; pts, patients; SCr, serum creatinine; uo, urine output criteria.
Reprinted from Zavada J, Hoste E, Cartin-Ceba R et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant 2010; 25(12): 3911–3918 (Ref. 64) by permission from The European Renal Association-European Dialysis and Transplant Association; accessed http://ndt.oxfordjournals.org/content/25/12/3911.long
Table 9 shows the range of estimated SCr obtained by back-calculation for various age, sex, and race categories. When the baseline SCr is unknown, an estimated SCr can be used provided there is no evidence of CKD (see Appendix B). Fortunately, when there is a history of CKD, a baseline SCr is usually available. Unfortunately, many cases of CKD are not identified, and thus estimating the baseline SCr may risk labeling a patient with AKI when in reality the diagnosis was unidentified CKD. As discussed further in Appendix B, it is essential to evaluate a patient with presumed AKI for presence of CKD. Furthermore, CKD and AKI may coexist. By using all available clinical data (laboratory, imaging, history, and physical exam) it should be possible to arrive at both an accurate diagnosis as well as an accurate estimate of baseline SCr. Importantly, excluding some cases of hemodilution secondary to massive fluid resuscitation (discussed below), the lowest SCr obtained during a hospitalization is usually equal to or greater than the baseline. This SCr should be used to diagnose (and stage) AKI. For example, if no baseline SCr was available in Case A, diagnosis of AKI could be made using the MDRD estimated SCr (Table 9). If Case A were a 70-year-old white female with no evidence or history of CKD, the baseline SCr would be 0.8 mg/dl (71 μmol/l) and a diagnosis of AKI would be possible even on day 1 (criterion 1, ⩾50% increase from baseline). However, if the patient was a 20-year-old black male, his baseline SCr would be estimated at 1.5 mg/dl (133 μmol/l). Since his admission SCr is lower, this is assumed to be the baseline SCr until day 7 when he returns to his true baseline, and this value can be taken as the baseline. These dynamic changes in interpretation are not seen in epidemiologic studies, which are conducted when all the data are present, but are common in clinical medicine. Note that the only way to diagnose AKI (by SCr criteria) in Case H is to use an estimated SCr.
Table 9. Estimated baseline SCr.
| Age (years) | Black males mg/dl (μmol/l) | Other males mg/dl (μmol/l) | Black females mg/dl (μmol/l) | Other females mg/dl (μmol/l) |
|---|---|---|---|---|
| 20–24 | 1.5 (133) | 1.3 (115) | 1.2 (106) | 1.0 (88) |
| 25–29 | 1.5 (133) | 1.2 (106) | 1.1 (97) | 1.0 (88) |
| 30–39 | 1.4 (124) | 1.2 (106) | 1.1 (97) | 0.9 (80) |
| 40–54 | 1.3 (115) | 1.1 (97) | 1.0 (88) | 0.9 (80) |
| 55–65 | 1.3 (115) | 1.1 (97) | 1.0 (88) | 0.8 (71) |
| >65 | 1.2 (106) | 1.0 (88) | 0.9 (80) | 0.8 (71) |
Estimated glomerular filtration rate=75 (ml/min per 1.73 m2)=186 × (serum creatinine [SCr]) − 1.154 × (age) − 0.203 × (0.742 if female) × (1.210 if black)=exp(5.228 − 1.154 × In [SCr]) − 0.203 × In(age) − (0.299 if female) + (0.192 if black).
Reprinted from Bellomo R, Ronco C, Kellum JA et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204-212 with permission from Bellomo R et al.22; accessed http://ccforum.com/content/8/4/R204
Examples of application of AKI stages
Once a diagnosis of AKI has been made, the next step is to stage it (Recommendation 2.1.2). Like diagnosis, staging requires reference to a baseline SCr when SCr criteria are used. This baseline becomes the reference SCr for staging purposes. Table 10 shows the maximum stage for each Case described in Table 7. Staging for Case A was already mentioned. The maximum stage is 2 because reference SCr is 1.0 mg/dl (88 μmol/l) and the maximum SCr is 2.0 mg/dl (177 μmol/l). Had the reference SCr been 0.6 mg/dl (53 μmol/l), the maximum stage would have been 3. Case F was staged by using the lowest SCr (1.0 mg/dl [88 μmol/l]) as the reference. Of course, the actual baseline for this case might have been lower but this would not affect the stage, since it is already Stage 3. Note that if this patient was a 35-year-old white male, his MDRD estimated baseline SCr would be 1.2 mg/dl (106 μmol/l) (Table 9) and his initial stage on admission (day 1) would be assumed to be 2. However, once his SCr recovered to 1.0 mg/dl (88 μmol/l) on day 7, it would be possible to restage him as having had Stage 3. Once he has recovered, there may be no difference between Stage 2 or 3 in terms of his care plan. On the other hand, accurately staging the severity of AKI may be important for intensity of follow-up and future risk.
Table 10. AKI staging.
|
Serum creatinine mg/dl (μmol/l) |
|||||||
|---|---|---|---|---|---|---|---|
| Case | Baseline | Day 1 | Day 2 | Day 3 | Day 7 | Reference creatinine | Max AKI stage |
| A | 1.0 (88) | 1.3 (115) | 1.5 (133) | 2.0 (177) | 1.0 (88) | 1.0 (88) | 2 |
| B | 1.0 (88) | 1.1 (97) | 1.2 (106) | 1.4 (124) | 1.0 (88) | 1.0 (88) | 1 |
| C | 0.4 (35) | 0.5 (44) | 0.6 (53) | 0.7 (62) | 0.4 (35) | 0.4 (35) | 1 |
| D | 1.0 (88) | 1.1 (97) | 1.2 (106) | 1.3 (115) | 1.5 (133) | 1.0 (88) | 1 |
| E | 1.0 (88) | 1.3 (115) | 1.5 (133) | 1.8 (159) | 2.2 (195) | 1.0 (88) | 2 |
| F | ? | 3.0 (265) | 2.6 (230) | 2.2 (195) | 1.0 (88) | 1.0 (88) | 3 |
| G | ? | 1.8 (159) | 2.0 (177) | 2.2 (195) | 1.6 (141) | ? | ⩾1 |
| H | ? | 3.0 (265) | 3.1 (274) | 3.0 (265) | 2.9 (256) | ? | ? |
AKI, acute kidney injury.
Note that Cases G and H can only be staged if the reference SCr can be inferred. Case G may be as mild as stage 1 if the baseline is equal to the nadir SCr on day 7. On the other hand, if this case were a 70-year-old white female with no known evidence or history of CKD, the reference SCr would be 0.8 mg/dl (71 μmol/l) based on an estimated baseline (Table 9). In this case, the severity on day 1 would already be stage 2.
Urine output vs. SCr
Both urine output and SCr are used as measures of an acute change in GFR. The theoretical advantage of urine output over SCr is the speed of the response. For example, if GFR were to suddenly fall to zero, a rise in SCr would not be detectable for several hours. On the other hand, urine output would be affected immediately. Less is known about the use of urine output for diagnosis and staging compared to SCr, since administrative databases usually do not capture urine output (and frequently it is not even measured, especially outside the ICU). However, studies using both SCr and urine output to diagnose AKI show increased incidence, suggesting that the use of SCr alone may miss many patients. The use of urine output criteria (criterion 3) will also reduce the number of cases where criterion 1 and criterion 2 are discordant (cases B,C,D, and F in Table 7), as many of these cases will be picked up by urine output criteria.
Timeframe for diagnosis and staging
The purpose of setting a timeframe for diagnosis of AKI is to clarify the meaning of the word “acute”. A disease process that results in a change in SCr over many weeks is not AKI (though it may still be an important clinical entity: see Appendix B). For the purpose of this guideline, AKI is defined in terms of a process that results in a 50% increase in SCr within 1 week or a 0.3 mg/dl (26.5 μmol/l) increase within 48 hours (Recommendation 2.1.1). Importantly, there is no stipulation as to when the 1-week or 48-hour time periods can occur. It is stated unequivocally that it does not need to be the first week or 48 hours of a hospital or ICU stay. Neither does the time window refer to duration of the inciting event. For example, a patient may have a 2-week course of sepsis but only develop AKI in the second week. Importantly, the 1-week or 48-hour timeframe is for diagnosis of AKI, not staging. A patient can be staged over the entire episode of AKI such that, if a patient develops a 50% increase in SCr in 5 days but ultimately has a three-fold increase over 3 weeks, he or she would be diagnosed with AKI and ultimately staged as Stage 3.
As with any clinical criteria, the timeframe for AKI is somewhat arbitrary. For example, a disease process that results in a 50% increase in SCr over 2 weeks would not fulfill diagnostic criteria for AKI even if it ultimately resulted in complete loss of kidney function. Similarly, a slow process that resulted in a steady rise in SCr over 2 weeks, and then a sudden increase of 0.3 mg/dl (26.5 μmol/l) in a 48-hour period, would be classified as AKI. Such are the inevitable vagaries of any disease classification. However, one scenario deserves specific mention, and that is the case of the patient with an increased SCr at presentation. As already discussed, the diagnosis of AKI requires a second SCr value for comparison. This SCr could be a second measured SCr obtained within 48 hours, and if it is ⩾0.3 mg/dl (⩾26.5 μmol/l) greater than the first SCr, AKI can be diagnosed. Alternatively, the second SCr can be a baseline value that was obtained previously or estimated from the MDRD equation (see Table 9). However, this poses two dilemmas. First, how far back can a baseline value be retrieved and still expected to be “valid” second, how can we infer acuity when we are seeing the patient for the first time?
Both of these problems will require an integrated approach as well as clinical judgment. In general, it is reasonable in patients without CKD to assume that SCr will be stable over several months or even years, so that a SCr obtained 6 months or even 1 year previously would reasonable reflect the patient's premorbid baseline. However, in a patient with CKD and a slow increasing SCr over several months, it may be necessary to extrapolate the baseline SCr based on prior data. In terms of inferring acuity it is most reasonable to determine the course of the disease process thought to be causing the episode of AKI. For example, for a patient with a 5-day history of fever and cough, and chest radiograph showing an infiltrate, it would be reasonable to infer that the clinical condition is acute. If SCr is found to be ⩾50% increased from baseline, this fits the definition of AKI. Conversely, a patient presenting with an increased SCr in the absence of any acute disease or nephrotoxic exposure will require evidence of an acute process before a diagnosis can be made. Evidence that the SCr is changing is helpful in establishing acuity.
Clinical judgment
While the definitions and classification system discussed in Chapter 2.1 provide a framework for the clinical diagnosis of AKI, they should not be interpreted to replace or to exclude clinical judgment. While the vast majority of cases will fit both AKI diagnostic criteria as well as clinical judgment, AKI is still a clinical diagnosis—not all cases of AKI will fit within the proposed definition and not all cases fitting the definition should be diagnosed as AKI. However, exceptions should be very rare.
Pseudo-AKI
As with other clinical diagnoses defined by laboratory results (e.g., hyponatremia), the clinician must be cautious to interpret laboratory data in the clinical context. The most obvious example is with laboratory errors or errors in reporting. Erroneous laboratory values should obviously not be used to diagnose disease and suspicious lab results should always be repeated. Another example is when two SCr measurements are obtained by different laboratories. While the coefficient of variation for SCr is very small (<5%) by various clinical testing methods, variation (bias) from one laboratory to the next may be considerably higher, although it is unlikely to approach 50%. Given that the SCr definition of AKI always uses at least two values, the variation and bias between each measure is further magnified—the coefficient of variation for comparison of two lab tests is equal to the square root of the sum of each coefficient squared. Although the international standardization of SCr measurements will largely eliminate interlaboratory bias in the future, care is needed in interpreting lab values obtained from different labs. Furthermore, daily variation in SCr due to differences in diet and activity may be as great as 10%. Finally, endogenous chromogens (e.g., bilirubin, ascorbic acid, uric acid) and exogenous chromogens and drugs (e.g., cephalosporins, trimethoprim, cimetidine) may interfere with the creatinine assay. The cumulative effect of these various factors influencing precision, bias, and biological variation may approach the level at which it could impact the diagnosis of AKI. A similar problem exists with urine output. Particularly outside the ICU, urine output is not often reported and urine collections may be inaccurate, especially in noncatheterized patients. Finally, as discussed in Chapter 2.1, a weight-based criterion for urine output will mean that some very obese patients will fulfill the definition of AKI without any kidney abnormality. Clinical judgment should always be exercised in interpreting such data.
Atypical AKI
A complementary problem to pseudo-AKI is the situation where a case of AKI fails to meet the definition. These cases should be distinguished from conditions in which data are simply missing (discussed above) and refer to situations in which existing data are unreliable. For example, a patient might receive very large quantities of intravascular fluids such that SCr is falsely lowered.65 Similarly, massive blood transfusions will result in the SCr more closely reflecting the kidney function of the blood donors than the patient. It is unusual for these cases not to result in oliguria and, thus, most patients will be diagnosed with AKI even if SCr is not increased. Nevertheless, the clinician should be cognizant of possibility that SCr may be falsely lowered by large-volume fluid resuscitation or transfusion; thus, a normal value may not rule out AKI. Changes in creatinine production are also well known in conditions such as muscle breakdown where production increases and in muscle wasting (including advanced liver disease) where production is decreased. Creatinine production may also be decreased in sepsis66 possibly due to decreased muscle perfusion.
Chapter 2.5: Diagnostic approach to alterations in kidney function and structure
Definitions of AKI, CKD and AKD
AKI and CKD were defined by separate Work Groups according to different criteria. The definition for each is based on alterations in kidney function or structure. AKI and CKD have many causes which may lead to alterations of kidney function and structure that do not meet the criteria for the definition of either AKI or CKD, yet patients with these diseases and disorders may need medical attention to restore kidney function and reverse damage to kidney structure to avoid adverse outcomes. A uniform and systematic nomenclature could enhance understanding and communication about these diseases and disorders, and lead to improved medical care, research, and public health. For these reasons, the Work Group proposed an operational definition for AKD to provide an integrated clinical approach to patients with abnormalities of kidney function and structure.
Table 11 compares the definitions for AKI, CKD, and AKD. We have also included an operational definition of “no known kidney disease” (NKD) for those who do not meet these criteria, with the understanding that clinical judgment is required to determine the extent of the evaluation that is necessary to assess kidney function and structure. In the following sections, we will elaborate on each component of these definitions.
Table 11. Definitions of AKI, CKD, and AKD.
| Functional criteria | Structural criteria | |
|---|---|---|
| AKI | Increase in SCr by 50% within 7 days, OR Increase in SCr by 0.3 mg/dl (26.5 μmol/l) within 2 days, OR Oliguria | No criteria |
| CKD | GFR <60 ml/min per 1.73 m2 for >3 months | Kidney damage for >3 months |
| AKD | AKI, OR GFR <60 ml/min per 1.73 m2 for <3 months, OR Decrease in GFR by ⩾35% or increase in SCr by >50% for <3 months | Kidney damage for <3 months |
| NKD | GFR ⩾60 ml/min per 1.73 m2 Stable SCr | No damage |
GFR assessed from measured or estimated GFR. Estimated GFR does not reflect measured GFR in AKI as accurately as in CKD. Kidney damage assessed by pathology, urine or blood markers, imaging, and—for CKD—presence of a kidney transplant. NKD indicates no functional or structural criteria according to the definitions for AKI, AKD, or CKD. Clinical judgment is required for individual patient decision-making regarding the extent of evaluation that is necessary to assess kidney function and structure.
AKD, acute kidney diseases and disorders; AKI, acute kidney injury; CKD, chronic kidney disease; GFR, glomerular filtration rate; NKD, no known kidney disease; SCr, serum creatinine.
GFR and SCr
CKD, AKD, and AKI are defined by parameters expressing the level of kidney function. Table 12 gives examples of each condition based on GFR and different magnitudes of increase in SCr.
Table 12. Examples of AKI, CKD, and AKD based on GFR and increases in SCr.
| Baseline GFR (ml/min per 1.73 m2) | Increase in SCr during 7 consecutive days | GFR during next 3 months | Diagnosis |
|---|---|---|---|
| >60 | >1.5 × | NA | AKI |
| >60 | <1.5 × | <60 | AKD without AKI |
| >60 | <1.5 × | >60 | NKD |
| Baseline GFR (ml/min per 1.73 m2) | Change in SCr during next 7 days | GFR during next 3 months | Diagnosis |
|---|---|---|---|
| <60 | >1.5 × | NA | AKI + CKD |
| <60 | <1.5 × | >35% decrease | AKD without AKI + CKD |
| <60 | <1.5 × | <35% decrease | CKD |
GFR assessed from measured or estimated GFR. Estimated GFR does not reflect measured GFR in AKI as accurately as in CKD.
AKD, acute kidney diseases and disorders; AKI, acute kidney injury; CKD, chronic kidney disease; GFR, glomerular filtration rate; NKD, no known kidney disease; SCr, serum creatinine.
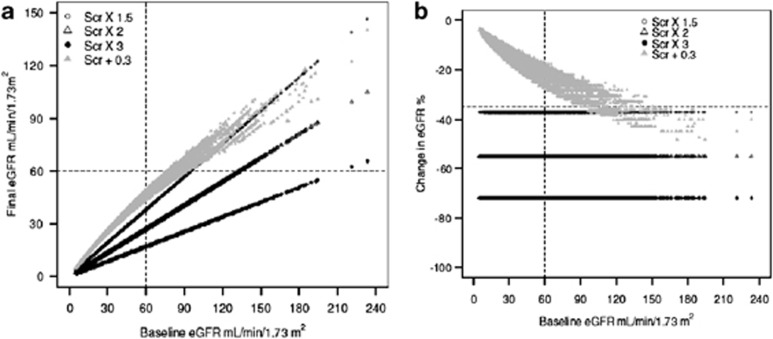
To illustrate the relationship of changes in SCr to changes in eGFR, we simulated changes in eGFR that would result from changes in SCr corresponding to the KDIGO definition of AKI in the Chronic Kidney Disease Epidemiology Collaboration cohort.67, 68 Figure 6 shows the relationship of these changes in eGFR to the definition and stages of AKI. Not all patients with AKI would meet the eGFR criteria for the definition of AKD.
Figure 6.
Chronic Kidney Disease Epidemiology Collaboration cohort changes in eGFR and final eGFR corresponding to KDIGO definition and stages of AKI. Panels (a) and (b) show the final eGFR and the percent changes in eGFR, respectively, corresponding to the KDIGO definition and stages of AKI. The horizontal line in panel a and b indicates the threshold value for AKD (<60 ml/min per 1.73 m2 and >35% reduction in initial GFR, respectively). Points above the horizontal line indicate subjects who meet the SCr criteria for the definition of AKI but do not meet eGFR criteria for the definition of AKD. AKD, acute kidney disorder/disease; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; SCr, serum creatinine. (Lesley Inker, personal communication.)
GFR/SCr algorithm
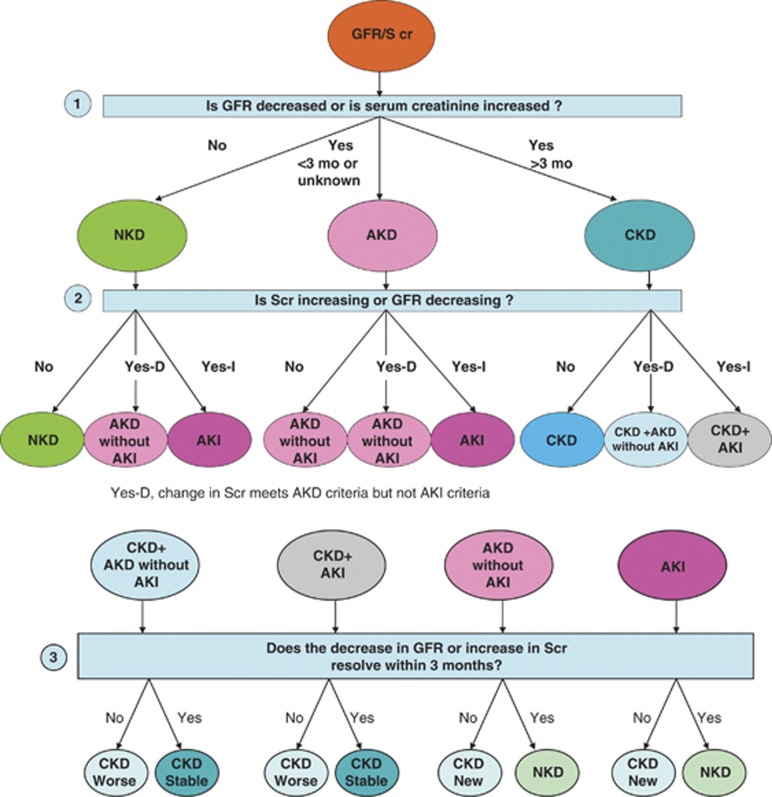
Figure 7 provides a diagnostic algorithm based on a sequential approach through three questions: i) Is GFR decreased or is SCr increased (according to the criteria in Table 12)?; ii) Is SCr increasing or GFR decreasing (according to the criteria in Table 12)?; and iii) Does the decrease in GFR or increase in SCr resolve within 3 months? Based on a “yes” or “no” response to these three sequential questions, all combinations of AKI, AKD, and CKD can be identified. In this section, we review the algorithm and illustrate its use for classification of patients with acute and chronic kidney disease in two previously reported cohorts.
Figure 7.
GFR/SCr algorithm. See text for description. AKD, acute kidney disease/disorder; AKI, acute kidney injury; CKD, chronic kidney disease; GFR, glomerular filtration rate; NKD, no known kidney disease; SCr, serum creatinine.
The answer to Question 1 requires ascertainment of an index GFR/SCr as well during the prior 3 months. The index GFR/SCr can be assigned as any of the GFR/SCr measures during the interval of observation. The answer classifies patients into three categories: NKD, AKD, and CKD. Question 2 requires repeat ascertainment of kidney function after the index measure. “No” indicates that the increase in SCr or decrease in GFR after the index measure does not meet AKI or AKD criteria; “Yes-D” indicates that increase in SCr and decrease in GFR meets the AKD criteria but not AKI criteria; and “Yes-I” indicates that increase in SCr meets AKI criteria. Question 3 requires repeat ascertainment of GFR/SCr 3 months after the index measure. “Yes” indicates GFR >60, indicating NKD. No indicates GFR <60, and based on prior level of GFR, may indicate stable, new, or worse CKD.
Oliguria as a measure of kidney function
Although urine flow rate is a poor measure of kidney function, oliguria generally reflects a decreased GFR. If GFR is normal (approximately 125 ml/min, corresponding to approximately 107 ml/kg/h for a 70-kg adult), then reduction in urine volume to <0.5 ml/kg/h would reflect reabsorption of more than 99.5% of glomerular filtrate. Such profound stimulation of tubular reabsorption usually accompanies circulatory disturbances associated with decreased GFR. Oliguria is unusual in the presence of a normal GFR and is usually associated with the non–steady state of solute balance and rising SCr sufficient to achieve the criteria for AKI. As a corollary, if GFR and SCr are normal and stable over an interval of 24 hours, it is generally not necessary to measure urine flow rate in order to assess kidney function.
In principle, oliguria (as defined by the criteria for AKI) can occur without a decrease in GFR. For example, low intake of fluid and solute could lead to urine volume of less than 0.5 ml/kg/h for 6 hours or 0.3 ml/kg/h for 24 hours. On the other hand, severe GFR reduction in CKD usually does not lead to oliguria until after the initiation of dialysis.
As described in Chapter 2.1, the thresholds for urine flow for the definition of AKI have been derived empirically and are less well substantiated than the thresholds for increase in SCr. Urinary diagnostic indices, such as the urinary concentrations of sodium and creatinine and the fractional reabsorption of sodium and urea, remain helpful to distinguish among causes of AKI, but are not used in the definition (see Appendix D).
Kidney damage
Table 13 describes measures of kidney damage in AKD and CKD. Kidney damage is most commonly ascertained by urinary markers and imaging studies. Most markers and abnormal images can indicate AKD or CKD, based on the duration of abnormality. One notable exception is small kidneys, either bilateral or unilateral, indicating CKD, which are discussed separately below. Kidney damage is not a criterion for AKI; however, it may be present. Renal tubular epithelial cells and coarse granular casts, often pigmented and described as “muddy brown”, remain helpful in distinguishing the cause of AKI, but are not part of the definition.
Table 13. Markers of kidney damage in AKD and CKD.
| Markers | AKD | CKD |
|---|---|---|
| Pathology | X | X |
| Urinary markers | ||
| RBC/casts | X | X |
| WBC/casts | X | X |
| RTE/casts | X | X |
| Fine and coarse granular casts | X | X |
| Proteinuria | X | X |
| Blood markers (tubular syndromes) | X | X |
| Imaging | ||
| Large kidneys | X | X |
| Small kidneys | — | X |
| Size discrepancy | — | X |
| Hydronephrosis | X | X |
| Cysts | X | X |
| Stones | X | X |
| History of kidney transplantation | — | X |
Kidney damage is not required for diagnosis of AKI. In the presence of AKI, findings of kidney damage do not indicate a separate diagnosis of AKD.
AKD, acute kidney diseases and disorders; CKD, chronic kidney disease; RBC, red blood cells; RTE, renal tubular epithelial cells; WBC, white blood cells.
Small kidneys as a marker of kidney damage
Loss of renal cortex is considered a feature of CKD, and is often sought as a specific diagnostic sign of CKD. Kidney size is most often evaluated by ultrasound. In a study of 665 normal volunteers,69 median renal lengths were 11.2 cm on the left side and 10.9 cm on the right side. Renal size decreased with age, almost entirely because of parenchymal reduction. The lowest 10th percentiles for length of the left and right kidney were approximately 10.5 and 10.0 cm, respectively, at age 30 years, and 9.5 and 9.0 cm, respectively, at age 70 years.
Integrated approach to AKI, AKD, and CKD
Clinical evaluation is necessary for all patients with alterations in kidney function or structure. The expectation of the Work Group is that the diagnostic approach will usually begin with assessment of GFR and SCr. However, evaluation of kidney function and structure is not complete unless markers of kidney damage—including urinalysis, examination of the urinary sediment, and imaging studies—have been performed. Table 14 shows a summary of the diagnostic approach using measures for kidney function and structure. Based on interpretation of each measure separately, the clinical diagnosis indicated by an “X” can be reached.
Table 14. Integrated approach to interpret measures of kidney function and structure for diagnosis of AKI, AKD, and CKD.
|
Measures |
||||
|---|---|---|---|---|
| Diagnosis | GFR/SCr | Oliguria | Kidney damage | Small kidneys |
| AKI | X | X | ||
| AKD | X | X | ||
| CKD | X | X | X | X |
X indicates that the measures can contribute to the diagnosis indicated.
AKD, acute kidney diseases and disorders; AKI, acute kidney injury; CKD, chronic kidney disease.
SPONSORSHIP
KDIGO gratefully acknowledges the following sponsors that make our initiatives possible: Abbott, Amgen, Belo Foundation, Coca-Cola Company, Dole Food Company, Genzyme, Hoffmann-LaRoche, JC Penney, NATCO—The Organization for Transplant Professionals, NKF—Board of Directors, Novartis, Robert and Jane Cizik Foundation, Shire, Transwestern Commercial Services, and Wyeth. KDIGO is supported by a consortium of sponsors and no funding is accepted for the development of specific guidelines.
DISCLAIMER
While every effort is made by the publishers, editorial board, and ISN to see that no inaccurate or misleading data, opinion or statement appears in this Journal, they wish to make it clear that the data and opinions appearing in the articles and advertisements herein are the responsibility of the contributor, copyright holder, or advertiser concerned. Accordingly, the publishers and the ISN, the editorial board and their respective employers, office and agents accept no liability whatsoever for the consequences of any such inaccurate or misleading data, opinion or statement. While every effort is made to ensure that drug doses and other quantities are presented accurately, readers are advised that new methods and techniques involving drug usage, and described within this Journal, should only be followed in conjunction with the drug manufacturer's own published literature.
Footnotes
SUPPLEMENTARY MATERIAL
Appendix A: Background.
Appendix B: Diagnostic Approach to Alterations in Kidney Function and Structure.
SUPPLEMENTARY MATERIAL
Appendix C: Risk Determination.
Appendix D: Evaluation and General Management Guidelines for Patients with AKI.
SUPPLEMENTARY MATERIAL
Appendix D: Evaluation and General Management Guidelines for Patients with AKI.
SUPPLEMENTARY MATERIAL
Appendix B: Diagnostic Approach to Alterations in Kidney Function and Structure.
Supplementary material is linked to the online version of the paper at http://www.kdigo.org/clinical_practice_guidelines/AKI.php
References
- Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Goldsmith D, et al. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204 –R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31 . doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. 2005;33:2194 –2201. doi: 10.1097/01.ccm.0000182798.39709.84. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, George C, Dinu I, et al. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203 –1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Bellomo R, Ronco C. Classification of acute kidney injury using RIFLE: What's the purpose. Crit Care Med. 2007;35:1983 –1984. doi: 10.1097/01.CCM.0000277518.67114.F8. [DOI] [PubMed] [Google Scholar]
- Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538 –546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- Thakar CV, Christianson A, Freyberg R, et al. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37:2552 –2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692 –1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837 –1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292 –1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLe criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028 –1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- Hackworth LA, Wen X, Clermont G, et al. Hospital versus community-acquired acute kidney injury in the critically ill: differences in epidemiology (abstr) J Am Soc Nephrol. 2009;20:115A . [Google Scholar]
- Cerda J, Bagga A, Kher V, et al. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138 –153. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- Cerda J, Lameire N, Eggers P, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881 –886. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. National Academy Press: Washington, DC; 2001. [PubMed] [Google Scholar]
- Eknoyan G. Are global nephrology guidelines feasible. Nat Clin Pract Nephrol. 2008;4:521 . doi: 10.1038/ncpneph0925. [DOI] [PubMed] [Google Scholar]
- Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310 –1314. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- Levin A, Stevens LA. Executing change in the management of chronic kidney disease: perspectives on guidelines and practice. Med Clin North Am. 2005;89:701 –709. doi: 10.1016/j.mcna.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490 . doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig K, Macleod A, Craig J, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;70:2058 –2065. doi: 10.1038/sj.ki.5001875. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39 (2 Suppl 1) :S1 –266. [PubMed] [Google Scholar]
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089 –2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17 –28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- Murray PT, Devarajan P, Levey AS, et al. A framework and key research questions in AKI diagnosis and staging in different environments. Clin J Am Soc Nephrol. 2008;3:864 –868. doi: 10.2215/CJN.04851107. [DOI] [PubMed] [Google Scholar]
- Endre ZH. Acute kidney injury: definitions and new paradigms. Adv Chronic Kidney Dis. 2008;15:213 –221. doi: 10.1053/j.ackd.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Amdur RL, Chawla LS, Amodeo S, et al. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089 –1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961 –973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179 –1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- Harel Z, Chan CT. Predicting and preventing acute kidney injury after cardiac surgery. Curr Opin Nephrol Hypertens. 2008;17:624 –628. doi: 10.1097/MNH.0b013e32830f4590. [DOI] [PubMed] [Google Scholar]
- Reddy VG. Prevention of postoperative acute renal failure. J Postgrad Med. 2002;48:64 –70. [PubMed] [Google Scholar]
- Venkataraman R. Can we prevent acute kidney injury. Crit Care Med. 2008;36:S166 –S171. doi: 10.1097/CCM.0b013e318168c74a. [DOI] [PubMed] [Google Scholar]
- Stewart J, Findlay G, Smith N, et al. Adding Insult to Injury: A review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure) National Confidential Enquiry into Patient Outcome and Death: London, UK; 2009. [Google Scholar]
- Bell M, Liljestam E, Granath F, et al. Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20:354 –360. doi: 10.1093/ndt/gfh581. [DOI] [PubMed] [Google Scholar]
- Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418 –425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- Perez-Valdivieso JR, Bes-Rastrollo M, Monedero P, et al. Prognosis and serum creatinine levels in acute renal failure at the time of nephrology consultation: an observational cohort study. BMC Nephrol. 2007;8:14 . doi: 10.1186/1471-2369-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: evaluation of the RIFLe classification. Ann Thorac Surg. 2006;81:542 –546. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Coca SG, Bauling P, Schifftner T, et al. Contribution of acute kidney injury toward morbidity and mortality in burns: a contemporary analysis. Am J Kidney Dis. 2007;49:517 –523. doi: 10.1053/j.ajkd.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Arnaoutakis GJ, Bihorac A, Martin TD, et al. RIFLE criteria for acute kidney injury in aortic arch surgery J Thorac Cardiovasc Surg 20071341554 –1560.discussion 1560–1551. [DOI] [PubMed] [Google Scholar]
- Abosaif NY, Tolba YA, Heap M, et al. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis. 2005;46:1038 –1048. doi: 10.1053/j.ajkd.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Maccariello E, Soares M, Valente C, et al. RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med. 2007;33:597 –605. doi: 10.1007/s00134-007-0535-0. [DOI] [PubMed] [Google Scholar]
- Jenq CC, Tsai MH, Tian YC, et al. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med. 2007;33:1921 –1930. doi: 10.1007/s00134-007-0760-6. [DOI] [PubMed] [Google Scholar]
- Tallgren M, Niemi T, Poyhia R, et al. Acute renal injury and dysfunction following elective abdominal aortic surgery. Eur J Vasc Endovasc Surg. 2007;33:550 –555. doi: 10.1016/j.ejvs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Zavada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911 –3918. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82 . doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217 –1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247 –254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749 –2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- Emamian SA, Nielsen MB, Pedersen JF, et al. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol. 1993;160:83 –86. doi: 10.2214/ajr.160.1.8416654. [DOI] [PubMed] [Google Scholar]