Abstract
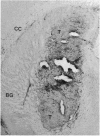
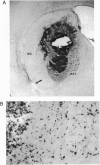
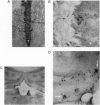
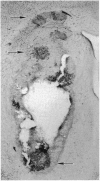
The delivery of viral vectors to the brain for treatment of intracerebral tumors is most commonly accomplished by stereotaxic inoculation directly into the tumor. However, the small volume of distribution by inoculation may limit the efficacy of viral therapy of large or disseminated tumors. We have investigated mechanisms to increase vector delivery to intracerebral xenografts of human LX-1 small-cell lung carcinoma tumors in the nude rat. The distribution of Escherichia coli lacZ transgene expression from primary viral infection was assessed after delivery of recombinant virus by intratumor inoculation or intracarotid infusion with or without osmotic disruption of the blood-brain barrier (BBB). These studies used replication-compromised herpes simplex virus type 1 (HSV; vector RH105) and replication-defective adenovirus (AdRSVlacZ), which represent two of the most commonly proposed viral vectors for tumor therapy. Transvascular delivery of both viruses to intracerebral tumor was demonstrated when administered intraarterially (i.a.) after osmotic BBB disruption (n = 9 for adenovirus; n = 7 for HSV), while no virus infection was apparent after i.a. administration without BBB modification (n = 8 for adenovirus; n = 4 for HSV). The thymidine kinase-negative HSV vector infected clumps of tumor cells as a result of its ability to replicate selectively in dividing cells. Osmotic BBB disruption in combination with i.a. administration of viral vectors may offer a method of global delivery to treat disseminated brain tumors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barba D., Hardin J., Sadelain M., Gage F. H. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4348–4352. doi: 10.1073/pnas.91.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett P. A., Roman-Goldstein S., Ramsey F., McCormick C. I., Sexton G., Szumowski J., Neuwelt E. A. Differential permeability and quantitative MR imaging of a human lung carcinoma brain xenograft in the nude rat. Am J Pathol. 1995 Feb;146(2):436–449. [PMC free article] [PubMed] [Google Scholar]
- Boviatsis E. J., Park J. S., Sena-Esteves M., Kramm C. M., Chase M., Efird J. T., Wei M. X., Breakefield X. O., Chiocca E. A. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994 Nov 15;54(22):5745–5751. [PubMed] [Google Scholar]
- Cameron J. M., McDougall I., Marsden H. S., Preston V. G., Ryan D. M., Subak-Sharpe J. H. Ribonucleotide reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J Gen Virol. 1988 Oct;69(Pt 10):2607–2612. doi: 10.1099/0022-1317-69-10-2607. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Shine H. D., Goodman J. C., Grossman R. G., Woo S. L. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Allen E. D., Kozarsky K. F., Wilson J. M., Roessler B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Doran S. E., Ren X. D., Betz A. L., Pagel M. A., Neuwelt E. A., Roessler B. J., Davidson B. L. Gene expression from recombinant viral vectors in the central nervous system after blood-brain barrier disruption. Neurosurgery. 1995 May;36(5):965–970. doi: 10.1227/00006123-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Freeman S. M., Abboud C. N., Whartenby K. A., Packman C. H., Koeplin D. S., Moolten F. L., Abraham G. N. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993 Nov 1;53(21):5274–5283. [PubMed] [Google Scholar]
- Ho D. Y., Mocarski E. S. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988 Nov;167(1):279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Huang Q., Vonsattel J. P., Schaffer P. A., Martuza R. L., Breakefield X. O., DiFiglia M. Introduction of a foreign gene (Escherichia coli lacZ) into rat neostriatal neurons using herpes simplex virus mutants: a light and electron microscopic study. Exp Neurol. 1992 Mar;115(3):303–316. doi: 10.1016/0014-4886(92)90196-w. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Miyanohara A., Levine F., Cahill T., Friedmann T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992 May;66(5):2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E. A., Goldman D. L., Dahlborg S. A., Crossen J., Ramsey F., Roman-Goldstein S., Braziel R., Dana B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991 Sep;9(9):1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Pagel M. A., Dix R. D. Delivery of ultraviolet-inactivated 35S-herpesvirus across an osmotically modified blood-brain barrier. J Neurosurg. 1991 Mar;74(3):475–479. doi: 10.3171/jns.1991.74.3.0475. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Weissleder R., Nilaver G., Kroll R. A., Roman-Goldstein S., Szumowski J., Pagel M. A., Jones R. S., Remsen L. G., McCormick C. I. Delivery of virus-sized iron oxide particles to rodent CNS neurons. Neurosurgery. 1994 Apr;34(4):777–784. doi: 10.1227/00006123-199404000-00048. [DOI] [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Blaese R. M., Oldfield E. H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993 Jan 1;53(1):83–88. [PubMed] [Google Scholar]
- Ross B. D., Kim B., Davidson B. L. Assessment of ganciclovir toxicity to experimental intracranial gliomas following recombinant adenoviral-mediated transfer of the herpes simplex virus thymidine kinase gene by magnetic resonance imaging and proton magnetic resonance spectroscopy. Clin Cancer Res. 1995 Jun;1(6):651–657. [PubMed] [Google Scholar]
- Vile R. G., Nelson J. A., Castleden S., Chong H., Hart I. R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994 Dec 1;54(23):6228–6234. [PubMed] [Google Scholar]