Abstract
North Africa (NAF) is composed of six countries located in the African Sahara, namely the Western Sahara, Morocco, Algeria, Tunisia, Libya, and Egypt. Common features between these countries include similar climate, ecology, population genetics, and the socioeconomic environment. This commonality reflects on the chronic kidney disease (CKD) profile in these countries. While there are some estimates on the epidemiology of end-stage kidney disease, that of earlier stages is unknown. Several national screening programs are currently addressing this issue, such as the EGIPT-CKD project in Egypt and the MAREMAR study in Morocco. Preliminary results from the former suggest a prevalence of proteinuria in 10.6% of the relatives of patients on regular dialysis treatment. Despite the lack of reliable registries, it was possible to gather information on the etiology of CKD by direct contact with leading nephrologists in those countries. It turns out that glomerulonephritis (GN) accounts for 9–20%, diabetes 11–18%, hypertensive nephrosclerosis 10–35%, chronic interstitial nephritis 7–17%, and polycystic disease 2–3%. Compared to two decades earlier, diabetes has become more common at the expense of GN, proliferative GN, and amyloidosis regressed in favor of IgA and membranous nephropathies in Tunisian adults. Conventional schistosomal nephropathies are regressing in favor of hepatitis C viral (HCV) nephropathy in Egyptians. Focal segmental glomerulosclerosis is increasing at the expense of proliferative GNs in the region at large. Access to regular dialysis has been optimized during the past decade, with favorable outcomes despite the high incidence of HCV infection, tuberculosis, and protein-calorie malnutrition. Kidney transplantation is available in all NAF countries except the Western Sahara. About 650 transplants are performed annually from live donors, the majority in Egypt, where data from the largest center in Mansoura display a 10-year graft survival of 62%. Many transplants are performed from living unrelated donors, particularly in Egypt, which creates an ethical debate. Legislation for deceased-donor transplantation has been passed successively over the past two decades in Tunisia, Morocco, Algeria, and Egypt, which is expected to reflect quantitatively and qualitatively on the transplantation activity in the near future.
Keywords: CKC burden, CKD screening, developing world, glomerulonephritis, tropical nephrology
INTRODUCTION
North Africa (NAF), broadly defined as the ‘Sahara', is bound by the Mediterranean to the North, the Atlas Mountain range to the South, the Atlantic Ocean to the west, and the Red Sea to the East. It hosts six countries: The Western Desert Republic, Morocco, Algeria, Tunisia, Libya, and Egypt, from west to east. Some definitions also include Mauritania and/or the Sudan, but these are not universally adopted, and will not be included in this article. The term ‘Maghreb' countries is even more loosely defined, yet it has been restricted to Morocco, Algeria, and Tunisia since the nineteenth century.
There is much in common among these countries with regard to the environment, population genetics, and socioeconomic standards, which are the three main factors that seem to define the burden and pattern of kidney disease.1 The environment is generally warm and humid near the cost, gradually merging into the hot and dry desert conditions in the deeper inland. This profile reflects on many disease vectors as well as the patient's predisposition and response to kidney injury.
Genetic studies confirm that there are two distinct ancestors in the region, namely the Berbers in the West and the Egyptians in the East. Both have mingled with each other as well as with immigrants from neighboring regions, most significantly sub-Saharan Africa and the Arabian Peninsula. Not surprisingly, this has amplified certain genetic polymorphisms that are known to predispose to chronic kidney disease, determine its pattern, and influence its progression, such as the ACE, MYD9 NPHS1, 2, ACTN4 variation, and others.1
Despite a significant discrepancy in the gross national income (GNI) in these countries, variation in health-related expenditure has buffered the universally known impact of wealth on primary care, and subsequently on the prevalence of CKD (Table 1). This striking dissociation of CKD prevalence from GNI in NAF can be traced to differences in the prevailing historic and current political systems in individual countries.2
Table 1. Burden of CKD in North Africa.
| Egypt | Libya | Tunisia | Algeria | Morocco | |
|---|---|---|---|---|---|
| Population (millions)a | 83 | 7 | 11 | 35 | 32 |
| GNI (US$ as PPP)b | 2070 | 12,020 | 3720 | 4420 | 2770 |
| Incidence (pmp) | 192 | 90 | 159 | 120 | 125 |
| Prevalence (pmp) | 650 | 323 | 734 | 475 | 300 |
Abbreviations: CKD, chronic kidney disease; GNI, gross national income; PPP, purchasing power parity.
Most recent census data.
World Bank data.
SOURCES OF INFORMATION
As in many developing countries, it is difficult to collect reliable data on CKD from NAF. Most of the available information is provided through personal communication with leading nephrologists, which can be quite biased by obvious factors. For the sake of this review, such information was cross checked against other resources, including national and regional registries (Egypt, Tunisia, Morocco), periodicals, and indexed international journals. The latter are strikingly few, amounting to about 1500 articles over the past 10 years, two-thirds of which come from Egypt and 22% from Tunisia. Articles relevant to the epidemiology of CKD constitute only 6% of the total indexed medical publications from the region (computed from Pub Med data, March 2011).
DISEASE BURDEN
The reported frequency of end-stage kidney disease (CKD Stage V on dialysis) is shown in Table 1.
There are no respective reports, or even rough estimates, on the earlier stages of CKD. Several studies addressing this issue are underway in Egypt, Morocco, and Tunisia. Perhaps the largest screening programs are the ‘EGIPT-CKD' (Egypt Information, Prevention and Treatment of CKD) in Egypt and the ‘MAREMAR' (Maladie Rénale chronique au Maroc) in Morocco. The former targets relatives of patients on regular hemodialysis in a northern governorate. It includes a subjective questionnaire, blood pressure measurement, and testing for urinary protein/creatinine ratio and blood hemoglobin, sugar, cholesterol, and creatinine with calculation of eGFR. Of the first 900 screened subjects, 10.6% had microalbuminuria.3
The MAREMAR study4 is a joint venture including the local health authorities, International Society of Nephrology, and World Health Organization. It aims at estimating the prevalence of CKD, hypertension, and diabetes, identifying subjects at risk to develop CKD and establishing an intervention program for a follow-up period of 5 years. The project targets a random sample of 10,000 individuals in two villages, the costal El-Jadida and the inland Khemisset. No data have yet been published from this study.
CAUSES OF CKD
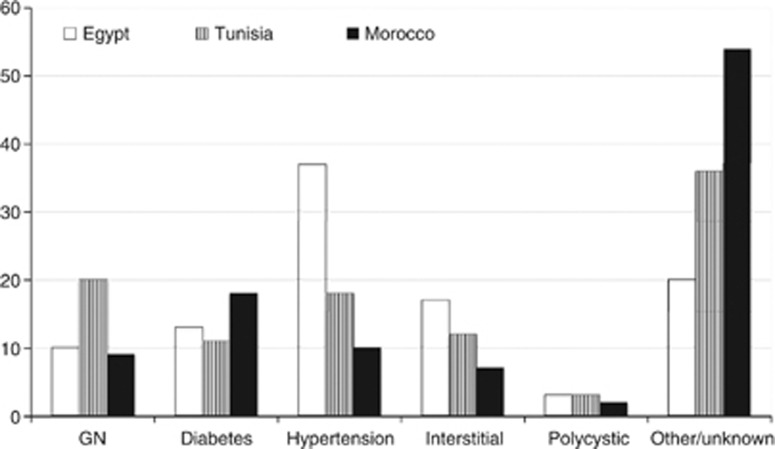
Figure 1 shows the prevalent causes of CKD-V where such information is available. Compared to the previous decade,5 diabetes has become more common at the expense of glomerulonephritis (GN). The displayed discrepancy of primary hypertensive nephrosclerosis may be related to differences in adopted definition.
Figure 1.
Current proportional contribution of the most common causes of end-stage renal disease in three North African countries. GN, glomerulonephritis.
A similar trend is observed in CKD-III/IV. In addition, several published cohorts highlight the relative significance of regional etiological factors such as schistosomiasis (Egypt, Algeria), echinococcosis (Libya, Tunisia), hepatitis C viral (HCV) infection (Egypt), familial Mediterranean fever (Egypt, Libya), and exposure to ochratoxins (Tunisia, Morocco, Egypt) or leaded gasoline fumes (Egypt), to name a few.
In a study from Tunisia comparing the histopathological types of GN across two decades (1975–1985 vs 1995–2005), it was shown that proliferative GNs and amyloidosis have regressed in favor of IgA nephropathy, lupus nephritis, and membranous nephropathy in adults.6 This may be explained by the improved community hygiene, leading to suppressed Th1 in favor of Th2 response.7 Along the same line, membranous nephropathy has regressed in children, which can be attributed to decline of hepatitis B viral infection. Similar observations have been made in Egypt, where the incidence of focal segmental glomerulosclerosis is increasing (unpublished data) for unclear reasons.
These observations are in line with the decreasing incidence of schistosomal nephropathy in Egypt in parallel with successful massive control programs, in contrast to hepatitis C-associated nephropathies, which remain highly prevalent.
DIALYSIS
Regular dialysis treatment (RDT) has been available at a national level in Egypt since 1964, Tunisia since 1969, Algeria since 1977, and Libya and Morocco since 2004.2 There does not seem to be any regular dialysis activity in the Western Sahara. As expected, the dialysis population accounts for the main part of prevalent CKD, over 95% on hemodialysis. The logistics, outcomes, and complications of RDT in NAF countries are broadly similar to those of other developing countries.5 Interesting issues specific to the region include an average of 52.1% prevalence of HCV infection in Egypt,8 up to 6% of tuberculosis in Tunisia, Egypt, and Morocco,9 and the high incidence of protein-calorie malnutrition in the lower socioeconomic classes. Fortunately, HIV infection is infrequent in NAF dialysis units for unclear reasons.
TRANSPLANTATION
Renal transplantation from living related donors was started in Egypt in 1976, Tunisia and Algeria in 1985, and Libya and Morocco in 1989. The Libyan program stumbled in the early nineties and resumed in 2004. Living unrelated donors were accepted in Egypt in 1979, regulated by the Egyptian Medical Association in 1982 and by the Ministry of Health in 2010.10 Deceased-donor laws were passed in Tunisia in 1991, Morocco in 2000, Algeria in 2002, and Egypt in 2010. Nevertheless, very few such transplants have been performed in these countries. About 600–650 live donor transplants are performed in NAF each year, of which 500 are in Egypt. In addition, an unknown number of patients from Algeria, Tunisia, and Morocco receive their transplants in France.
The surgical and immunosuppression protocols are similar to those in the rest of the world. Since the national registries in most NAF countries are not very reliable, the transplant outcomes in the largest center in Mansoura, Egypt, may be taken as a rough indicator. In its latest published report, the overall graft survival was 76.1% and 49.5% at 5 and 10 years, respectively. Patient survival was 87.1% and 71.5%.11 These figures are broadly similar to the pooled data from 400 centers in 45 countries participating in the Collaborative Transplant Study. Over 10% improvement of graft outcomes has even been achieved in 2011 (unpublished data).
Among the unique aspects of renal transplantation in the region are problems related to the high frequency of infection, particularly HCV and tuberculosis. The economic burden of an expanding pool of patients is already encroaching on other items of health care. Even more pressing is the moral and social debate on paid donation of kidneys, which will probably be resolved only with the predominant use of deceased-donor kidneys.
Acknowledgments
I am indebted to many colleagues in different NAF countries who have graciously provided information that would otherwise be impossible to obtain. I would like to specifically mention and thank Professors Aziz El-Matri of Tunisia, Mohammed Benghanem of Morocco, Salah Hottman of Algeria, and Abdulhafid Ali Shebani of Libya. Publication of this article was supported in part by the National Health and Medical Research Council of Australia through an Australia Fellowship Award (#511081: theme Chronic Disease in High Risk Populations) to Dr Wendy Hoy, School of Medicine, the University of Queensland, and the National Institutes of Health—NIDDK DK079709, NCRR RR026138, and NIMHD MD000182.
The author declared no competing interests.
References
- Barsoum RS. Glomerulonephritis in disadvantaged populations. Clin Nephrol. 2010;74 (Suppl 1:S44–S50. doi: 10.5414/cnp74s044. [DOI] [PubMed] [Google Scholar]
- Barsoum R.History of dialysis in AfricaIn: Ing T, Rahman MA, Kjellstrand C (eds).Dialysis: History, Development and PromiseVol. 7B.Imperial College Press: London; 2012,599–610. [Google Scholar]
- Gouda Z, Mashaal G, Bello AK, et al. Egypt information, prevention, and treatment of chronic kidney disease (EGIPT-CKD) programme: prevalence and risk factors for microalbuminuria among the relatives of patients with CKD in Egypt. Saudi J Kidney Dis Transplant. 2011;22:1055–1063. [PubMed] [Google Scholar]
- Persy VP, Remuzzi G, Perico N, et al. Prevention and transplantation in chronic kidney disease: what is achievable in emerging countries. Nephron Clin Pract. 2010;115:c122–c132. doi: 10.1159/000312875. [DOI] [PubMed] [Google Scholar]
- Barsoum RS. End-stage renal disease in the developing world. Artif Organs. 2002;26:735–736. doi: 10.1046/j.1525-1594.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- Ben Maïz H, Abderrahim E, Ben Moussa F, et al. Epidemiology of glomerular diseases in Tunisia from 1975 to 2005. Influence of changes in healthcare and society. Bull Acad Natl Med. 2006;190:403–416. [PubMed] [Google Scholar]
- Hurtado A, Johnson RJ. Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int Suppl. 2005;97:S62–S67. doi: 10.1111/j.1523-1755.2005.09711.x. [DOI] [PubMed] [Google Scholar]
- Daw MA, Dau AA.Hepatitis C virus in Arab world: a state of concern Scientific World J 20122012published online 2 May 2012. doi: 10.1100/2012/719494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kabbaj D, Bahadi A, Oualim Z. Prevalence of tuberculosis in hemodialysis patients. Saudi J Kidney Dis Transplant. 2010;21:164–167. [PubMed] [Google Scholar]
- Barsoum R. Trends in unrelated-donor kidney transplantation in the developing world. Pediatr Nephrol. 2008;23:1925–1929. doi: 10.1007/s00467-008-0858-2. [DOI] [PubMed] [Google Scholar]
- Bakr MA, Ghoneim MA. Living donor renal transplantation, 1976–2003: the Mansoura experience. Saudi J Kidney Dis Transplant. 2005;16:573–583. [PubMed] [Google Scholar]