Abstract
Progressive loss of kidney function leads to reduced production of calcitriol (1,25-dihydroxyvitamin D; active vitamin D) and an imbalance in serum calcium (Ca) and phosphorus (P) levels, which are associated with progression of renal failure as well as increased rates of cardiovascular (CV) events and mortality. In addition, multifactorial hypocalcemia and resistance to parathyroid hormone (PTH) can lead to prolonged and excessive synthesis and secretion of PTH, eventually leading to development of secondary hyperparathyroidism and renal osteodystrophy. These changes associated with chronic kidney disease (CKD), extending beyond bone and related biochemical abnormalities, have prompted the development of the term CKD–mineral and bone disorder to describe its systemic nature. Excessive P loading, among other factors, will promote vascular calcification (VC), and PTH production will affect bone remodeling. Although administration of calcitriol increases serum Ca levels and decreases PTH, it is also associated with elevated Ca × P product. Therefore, compounds that selectively activate vitamin D receptors (VDR activators), potentially reducing Ca–P toxicity and distinctly affecting pathogenic mechanisms of VC, might enhance CV and renal protection, increase the vitamin D therapeutic window, and thus provide a significant clinical benefit. Moreover, selective VDR activators have been associated with improvement in survival, at least among dialysis patients. Thus, selective VDR activators should be considered a novel and interesting approach to enhance the standard of care in CKD patients.
Keywords: chronic kidney disease, CKD–MBD, paricalcitol, vascular calcification, VDR, vitamin D
Progressive loss of kidney function in chronic kidney disease (CKD) leads to reduced production of 1-α-(OH)2-D3 (1,25-dihydroxyvitamin D; calcitriol) and abnormal mineral homeostasis reflected mainly by an imbalance in both serum calcium (Ca) and phosphorus (P) levels, as well as increasing levels of parathyroid hormone (PTH).1, 2, 3 Prevention of evolving secondary hyperparathyroidism (SHPT) and renal osteodystrophy was classically considered the main focus of nephrologists in the past. However, the relative importance of these CKD-associated mineral disturbances has been increasingly acknowledged and greatly associated with progression of renal failure, cardiovascular (CV) events, and mortality. Among all these mineral disturbances, the activation of vitamin D receptors (VDRs) seems to have independent beneficial pleiotropic extraskeletal effects.4, 5
CHRONIC KIDNEY DISEASE–MINERAL AND BONE DISORDER
Multifactorial hypocalcemia, calcitriol and vitamin D deficiency, P retention and fibroblast growth factor (FGF)-23 dysregulation, resistance to the action of PTH and vitamin D, and decreased activation, decreased number, or downregulation of several related receptors (VDR, Ca-sensing receptor, FGF-23/Klotho), among other factors, can lead to prolonged and excessive synthesis and secretion of PTH, eventually leading to the development of SHPT and different forms of renal osteodystrophy.1, 6, 7 Bone is currently considered an active ‘endocrine' organ, as demonstrated by the osteocyte product FGF-23 regulating P balance, vitamin D metabolism, and PTH production.8, 9 Furthermore, the effect of PTH on FGF-23 has been recently described, completing a bone–parathyroid endocrine feedback loop in which SHPT has been shown to be essential for the high FGF-23 levels in early CKD.10 The role of FGF-23 and Klotho in CKD is widely covered by Vervloet and Larsson in this issue.11
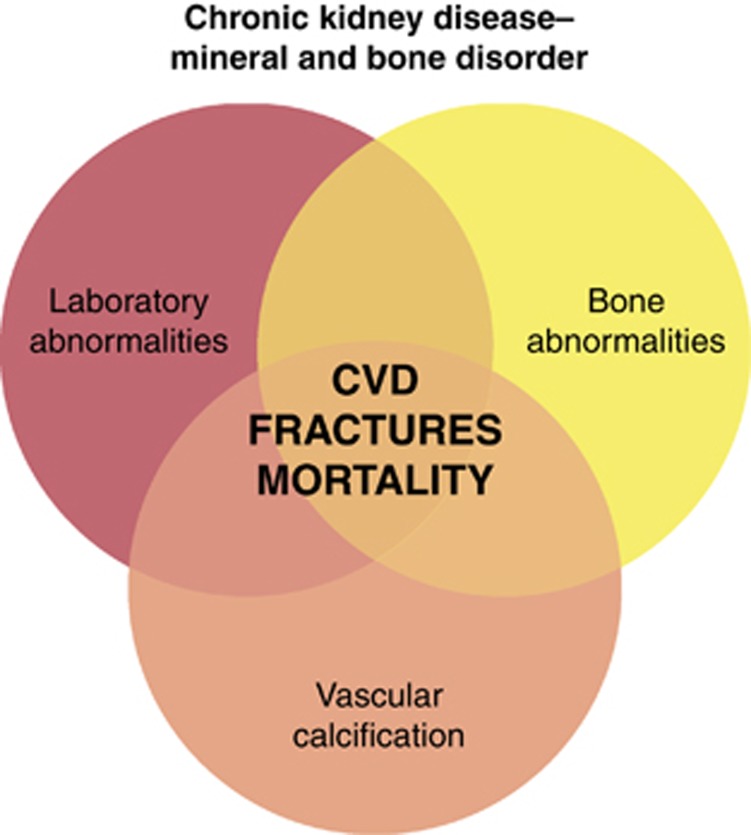
Beyond parathyroid and bone diseases, numerous studies have shown clearly the association between disorders of mineral metabolism (and the therapies used to correct these abnormalities) and fractures, extraskeletal and vascular calcification (VC), progression of renal failure, CV disease, and mortality.12, 13 These studies have prompted the development of the new term CKD–mineral and bone disorder (MBD) to acknowledge its systemic consequences and damage to organs beyond bone, primarily VC. Thus, CKD–MBD is a real syndrome, currently defined by one or a combination of the following: (1) abnormalities of Ca, P, PTH, or vitamin D metabolism; (2) abnormalities in bone turnover, mineralization, volume, linear growth, or strength; and/or (3) vascular or other soft-tissue calcifications12, 13, 14 (Figure 1).
Figure 1.
The interrelated nature of biochemical abnormalities, bone disease, and extraskeletal calcification in chronic kidney disease–mineral and bone disorder. Reprinted from Moe et al.,14 copyright 2011, with permission from Elsevier. CVD, cardiovascular disease.
RENAL OSTEODYSTROPHIES AND VITAMIN D
Renal osteodystrophy
The old general term of renal osteodystrophy is currently restricted to an alteration of bone morphology in patients with CKD, representing one measure of the skeletal component of CKD–MBD that is quantifiable by histomorphometry of bone biopsy.12, 13 Although bone biopsy is invasive and thus cannot be performed easily, it is the gold standard for the diagnosis of renal osteodystrophy, as biochemical parameters do not adequately predict the underlying bone histology.13 This is a field with a particular need for improvement, especially taking into account the great variability of PTH assays15, 16 and the current scarcity of information available on bone behavior and bone disease beyond the so-called intact PTH ‘extremes of risk'.13 Thus, a significant proportion of patients with intermediate PTH levels (that is, two to nine times the upper limit of normality for a particular assay) present quite different rates of bone formation, from adynamic bone disease (ABD) to normality and hyperparathyroid bone disease.17, 18
It is well known that the traditional types of renal osteodystrophy have been defined on the basis of turnover and mineralization, with substantial differences in pathophysiology and treatment. A recent Kidney Disease: Improving Global Outcomes (KDIGO) report has suggested that bone biopsies in patients with CKD should be characterized by determining bone turnover (T), mineralization (M), and volume (V) (the TMV system). Six types of bone disorder are distinguished in the CKD–MBD complex:12 hyperparathyroid bone disease (high turnover, normal mineralization, and any bone volume); mixed bone disease (high turnover with mineralization defect and normal bone volume); osteomalacia (low turnover with abnormal mineralization and low-to-medium bone volume); ABD (low turnover with normal mineralization and low or normal bone volume); and two distinct disorders due to specific causative agents, namely, amyloid bone disease and aluminum bone disease.12, 17
According to old studies, bone abnormalities are found almost universally in patients with CKD requiring dialysis (stage 5D) and in the majority of patients with CKD stages 3–5.1, 18, 19, 20, 21, 22, 23 Moreover, the distribution of histological types in patients with CKD stage 5 has been changing over the years.13, 24 ABD is increasing in prevalence and in many CKD populations now represents the most frequent type of bone lesion.18, 25, 26 Steadily growing proportions of elderly, diabetic, and malnourished patients may, at least partially, explain this finding.
The unsolved problem of ABD
The term ‘aplastic' or ‘adynamic' bone disease was introduced in the early 1980s. This type of disorder is characterized by a low bone turnover with normal mineralization represented by an absence of osteoid accumulation. In adynamic bone, the number of osteoblasts, the bone formation rate, and the activation frequency are markedly diminished. Bone histomorphometry cutoff levels in different studies are inconsistent, and the reported prevalence of ABD varies from 5 to 49% in CKD stages 3–4 (refs. 17, 21, 27, 28, 29) and from 10 to 71% in CKD stage 5D.17, 18, 21, 27, 29, 30 The regulation of osteoblast apoptosis is now recognized to be a major mechanism in determining rates of bone formation.29 Increased osteoblast apoptosis and decreased osteoblastogenesis are important determinants of decreased bone formation rate and activation frequency. A variant of ABD (so-called ABD-V), characterized by high osteoclastic resorption, has also been described.31
In the past, aluminum overload was the predominant cause of low-turnover bone disease in dialysis patients. A chronic low-dose exposure with concomitant high doses of vitamin D—probably favoring mineralization—was said to preferentially lead to ABD rather than osteomalacia.32 Thus, it is important to distinguish between aluminum-induced and non-aluminum-induced forms of ABD.17, 21, 28, 29 Several other conditions decrease bone turnover and bone remodeling activity. Independently of CKD, advanced age and diabetes (through elevated levels of advanced glycation end-products), glucocorticoid-induced osteoporosis, and hypoparathyroidism are associated with low bone turnover. Growth hormone resistance or insulin-like growth factor-I insufficiency, excess of proinflammatory cytokines, and low serum calcitriol levels likely have a role in suppressing bone formation by inhibiting the number of osteoblasts that can form bone.29 Low PTH levels or bone resistance to the action of PTH in CKD (relative hypoparathyroidism) are also regarded as important risk factors for ABD,1, 17, 21, 26, 29, 33 and many factors from P loading, decreased calcitriol, or uremia itself to antagonistic PTH fragments, downregulation of PTH receptors, or decreased pulsatility of PTH have been related to skeletal or calcemic resistance to PTH.2, 34, 35, 36, 37 Recently, the bone formation rate has also been shown to be associated with resistance to PTH.38 Several other factors have been linked to ABD, such as Ca loading (via P binders or Ca in the dialysate), vitamin D treatment (children with high doses, high dialysate Ca content, or high incidences of hypercalcemia), peritoneal dialysis (dialysate Ca, increased glucose, low albumin), acidosis, hypogonadism, inflammation, or the use of bisphosphonates in CKD patients.17, 21, 29, 39
The importance of ABD is that it diminishes the ability to repair microdamage, resulting in an increased fracture risk, according to observational evidence using bone markers but not bone biopsies.40, 41 Curiously enough, patients with idiopathic hypoparathyroidism have preserved or increased bone mass during therapy with Ca and vitamin D, and patients with total parathyroidectomy do not have an associated increase in the risk of fracture.29 Low PTH is also associated with low protein intake and may be a risk factor for malnutrition.29 As will be mentioned later, of more importance is the existence of a relationship between ABD, abnormal Ca balance, calcific arteriolopathy, and VC,29 and the fact that a U-shaped curve depicts PTH levels and mortality.42 Thus, the causal relationship between ABD and vascular disease may, at least in part, explain why not only high but also low intact PTH plasma levels, as well as low PTH and high serum Ca levels (a combination typical for ABD), are associated with increased mortality.42, 43, 44 This finding is not, however, uniform across studies.45
VDR activation is essential for bone formation
Multiple circulating factors contribute to bone activity by interfering with PTH receptor, FGF-23/Klotho, or VDR pathways. Deficiency in vitamin D and its metabolites leads to a failure in bone formation primarily caused by dysfunctional mineralization. It is also well known that vitamin D not only indirectly decreases bone formation rate in hyperparathyroid bone disease, but also directly increases both bone turnover and bone growth.36, 46 Recent studies in VDR-knockout mice have identified the VDR as being crucial in maintaining normal bone formation and bone mineralization by directly enhancing osteoblast differentiation.47, 48 In this model, Ca and vitamin D had both independent and interdependent effects on skeletal and mineral homeostasis. Mineralization of bone reflected ambient Ca levels rather than the calcitriol/VDR system, whereas increased Ca absorption and optimal osteoblastogenesis and osteoclastogenesis were modulated by calcitriol/VDR.
VDR activation of the osteoblast also functions to prevent apoptosis, similarly to the effect of estrogen and androgen receptors in preventing apoptosis of osteoblasts and osteocytes. Intermittent injections of PTH also serve an antiapoptotic function to increase bone formation and bone mass in contrast to continuous PTH infusions, which cause bone loss.49, 50 In addition, osteoblasts contain 1α-hydroxylase, presumably to function in an autocrine/paracrine manner to maintain bone formation. It is necessary to remember that vitamin D upregulates its own receptor, whereas PTH decreases VDR and blocks homologous upregulation.29, 51 As expected, it has been demonstrated that the actions of VDR activators on bone are more important than those expected just for the inhibition of PTH, and thus the effects of active vitamin D compounds and calcimimetics on bone differ under certain experimental conditions in vivo.52 Moreover, some of the improvement in Ca and skeletal homeostasis seen in VDR-knockout mice may occur through alternative vitamin D signaling pathways.53 Interestingly, other actions of vitamin D are explained by mitochondrial non-genomic activity of VDR,54 making way for challenging future studies on VDR physiology.
Different VDR activators may also differentially affect bone. In an in vitro study, Nakane et al.55 found that paricalcitol caused less Ca release from mouse calvariae than did calcitriol and doxercalciferol; furthermore, paricalcitol was the most effective preparation in stimulating collagen synthesis. The authors concluded that this bone effect of paricalcitol may contribute to its lower calcemic profile and that paricalcitol may be more effective in stimulating bone formation. Importantly, opposite effects of calcitriol and paricalcitol on the PTH ratio (whole PTH(1–84)/carboxy-terminal PTH) have been described in patients with CKD stage 5D.56 The relatively higher PTH 1–84 levels with paricalcitol is in agreement with data obtained in a rat model of renal failure showing that paricalcitol can prevent and treat SHPT without a major decrease in bone turnover.57 There are no data on the effects of new activators of the VDR on human bone.
Finally, with regard to renal osteodystrophy, it is necessary to consider that an early diagnosis of CKD, through the generalized use of estimated glomerular filtration rate laboratory reports, will allow earlier diagnosis of biochemical SHPT, abnormalities in vitamin D metabolism, and CKD–MBD.58, 59, 60 Efforts should be made to improve our knowledge of bone histology and its relationship with new biochemical parameters. Earlier reports suggesting that the use of the PTH ratio improves the prediction of bone histology56, 61 have not been corroborated in subsequent studies. Bone alkaline phosphatase as well as new parameters may improve the predictive value of intact PTH or the PTH ratio.62, 63 It is also of particular importance to learn the effect of different treatments on bone outcomes, particularly the normalization of bone histomorphometry (as stated in the recent KDIGO Clinical Practice Guidelines) and bone buffering capacity, as it is becoming increasingly clear that there is a link between abnormal vascular processes (that is, VC) and bone metabolism (the so-called bone–vascular axis), and this may also have an impact on survival.8, 13, 64, 65
VC, BONE ACTIVITY, AND VDR ACTIVATION
VC is a common feature in both prevalent and incident dialysis patients, as well as before entering dialysis.66, 67, 68 VC has been classically associated with both atheromatosis and atherosclerosis, as well as poor outcomes.69, 70 Uremia is a calcifying environment and CKD has been described as the ‘perfect storm' for CV calcification.71 The presence of VC in patients with CKD is multifactorial, and several biochemical abnormalities (excessive P loading, hyperphosphatemia, hypercalcemia, high Ca × P product, FGF-23, and high and low PTH levels) have been related to clinically significant VC. This topic is widely reviewed by Brandenburg et al. in this issue.72
It is not clear which aspect of bone pathology is most closely associated with VC. Not only severe forms of SHPT but also low levels of PTH are associated with VC. As mentioned previously, low PTH levels seem to increase the prevalence of both ABD and VC,17, 21, 69, 73 likely by preventing the Ca-buffering ability of bone and the handling of any extra-Ca load.74, 75 Using bone biopsies, Tomiyama et al.76 found an association of VC with bone density, volume, and formation rate in CKD patients who are not yet in dialysis treatment. Similarly, London et al.73 found an association between VC and indices of low bone turnover in the aorta and systemic arteries of dialysis patients. In an expanded cohort, London et al.69 reported a significant interaction between the dose of Ca-containing P binders, bone activity, VC, and stiffening. Of relevance was the fact that Ca load had a significant impact on VC and stiffness in patients with ABD when compared with patients with active bone. On the other hand, Barreto et al.77 did not observe an association between the type of bone disease and coronary calcification on cross-sectional analysis. In a prospective study, the same authors found a negative correlation between coronary calcifications and trabecular bone volume, and low-turnover bone status at the 12-month bone biopsy was the only independent predictor for progression of coronary artery calcification.78 Adragao et al.79 completed these results by describing an association between low bone volume, but not bone turnover, and coronary calcifications. This effect was not observed in patients who were on dialysis for more than 6 years. Finally, Asci et al.30 recently reported that bone formation rate is positively correlated with calcification scores as well as lower bone volume. When only patients with coronary calcifications were included, there was a U-shaped relationship between coronary calcifications and bone turnover, whereas the association with bone volume was lost.30 The discrepancies among these different studies may be due to the different vascular zones analyzed, different aluminum exposures in the past, number of parathyroidectomized patients, dialysis vintage, age, gender, diabetes, or other factors. Adragao et al.80, 81 have also shown that femoral bone density reflects histologically determined cortical bone volume, as well as associations between measurement of bone mineral density and VC or arterial stiffness. In any case, bone is currently considered an important variable when evaluating predisposing factors for VC. Frequent associations of osteoporosis and atherosclerotic VC or CV disease have also been observed in postmenopausal women,82 as have associations between the progression of VC and bone demineralization, underlining the importance of this relationship beyond CKD.
Other multiple risk factors, passive and active regulated processes, have also been associated with the occurrence and/or progression of VC and, as mentioned before, they are comprehensively reviewed elsewhere in this Supplement.
It is important to emphasize that different vitamin D derivatives functioning on the same VDR (selective VDR activators such as paricalcitol or maxacalcitol) seem to have different effects on Ca and P intestinal absorption at the same degree of PTH suppression.83, 84, 85 Thus, the affinity of paricalcitol for the VDR is three times less than that of calcitriol, but its calcemic and phosphatemic effects have been shown to be 10 times lower.86 The affinity of paricalcitol for vitamin D-binding protein is also three times less than that of calcitriol. Beyond the effect on Ca and P levels, selective VDR activators have uniformly shown distinct independent effects on VC. For instance, paricalcitol or maxacalcitol appear to have fewer in vivo experimental CV or renal procalcifying effects than doxercalciferol or equivalent doses of calcitriol despite comparable serum Ca × P products.87, 88, 89, 90 Whether the described differential effects on bone, such as relatively lower bone suppression, contribute to this observation is not known.55 Considering all the previous observations on biochemical parameters and the striking distinct effects on VC, compounds that selectively activate VDR might enhance CV and renal protection,91, 92, 93 providing a significant clinical benefit due to the known relationship between Ca and P levels and VC and mortality.45, 66, 67
VDR ACTIVATION AND VASCULAR DYSFUNCTION
The key discoveries regarding the conversion of vitamin D to its hormonal form and its regulation, as well as the evolving picture of its molecular mechanisms of action, have been described elsewhere.5, 85 Nevertheless, the recognition of its role beyond regulation of Ca homeostasis and bone metabolism represents a recent development. Vitamin D deficiency is an increasingly recognized public health problem in the general population and in chronic diseases such as CKD.5 Impaired VDR activation and signaling results in cellular dysfunction in several organs and biological systems, leading to an increased risk for disturbances of immunity, cancer, cell differentiation, infection, diabetes, and, in CKD, for arteriosclerosis, arterial dysfunction, and VC.4, 5, 94 The relationship between vitamin D deficiency and heart disease has been extensively reviewed by Pilz et al. in this issue.95
VDR is ubiquitous and both VDR and 1α-hydroxylase (CYP27B1) are present in the CV system.94 The action of vitamin D in these tissues is implicated in the regulation of endothelial, VSMC, heart function, inflammatory, and fibrotic pathways and immune responses. Failure of CV VDR activation is associated with many CV problems, as well as the progression of kidney and CV disease.4, 5, 94, 95
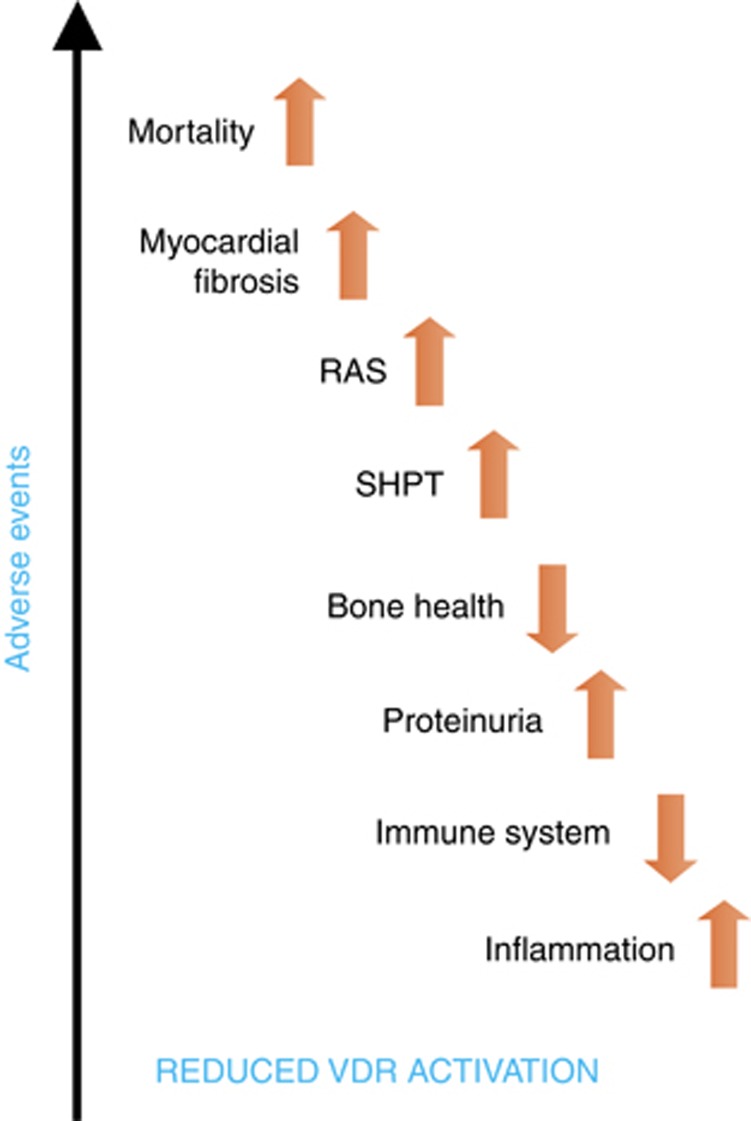
In view of the foregoing, it seems likely that the relationship between VDR activation and hard outcomes, both in CKD and the general population, extends at least partially beyond VC and bone activity, as discussed previously. Furthermore, recent epidemiological evidence suggests that there is a narrow range of vitamin D levels at which vascular function is optimized.96 Levels above or below this range seem to confer a significant increase in risk for CV disease,96 and thus a U-shaped curve or a reversed J curve may describe quite well the relationship between adverse systemic or vascular effects and the vitamin D status.97 In Figure 2, we depict the relationship between reduced VDR activation and outcomes. In addition to Pilz et al. in this issue,95 the narrow relationship of the heart, CV system, and kidney with the vitamin D system has recently been reviewed.94 In both dialysis and non-dialysis patients, vitamin D deficiency affects survival, and activation of VDR is associated with improved survival.98, 99, 100, 101 This observation may not be surprising, considering that SHPT is associated with higher mortality even in moderate non-dialysis CKD.102 Nevertheless, these survival benefits are observed even beyond mineral metabolism control as they are significant over a wide range of biochemical mineral metabolism parameters (that is, higher and lower quartiles of Ca, P, and PTH serum levels), VC scores, and measures of stiffness.103 Thus, pleiotropic effects of vitamin D likely have a role.
Figure 2.
Reduced VDR activation and outcome. The reduction of VDR activation is associated with multiple deleterious effects in different systems leading to an increase in mortality. RAS, renin–angiotensin system; SHPT, secondary hyperparathyroidism; VDR, vitamin D receptor.
In dialysis patients, a huge retrospective study suggested that intravenous paricalcitol may provide additional survival benefits as compared with intravenous calcitriol;104 however, although consistency in the beneficial effects of vitamin D in CKD patients is important, differences among the specific vitamin D compounds are still a matter of controversy.105 It also has to be admitted that the overall exposure to vitamin D over time may be higher with selective VDR activators, as intermittent drug withdrawal due to hypercalcemia or hyperphosphatemia is less frequent owing to their wider therapeutic window.4 As therapy with VDR activators appears to be useful in preventing bone loss and in decreasing morbidity and mortality, across different biochemical mineral metabolism parameters, some consider vitamin D and/or selective VDR activation to be essential therapy in CKD regardless of bone turnover status.29, 106 It is conceivable that other beneficial effects of activation of the VDR beyond bone result in some benefit even in the presence of ABD.17 However, the current Kidney Disease Outcomes Quality Initiative and KDIGO recommendations limit the administration of VDR activators for the treatment of hyperparathyroidism only.107 Moreover, if vitamin D therapy is planned in patients with CKD for reasons beyond CKD–MBD, such as reduction of albuminuria in patients with type 2 diabetes mellitus,108 only a selective VDR activator has shown this ability in a recent prospective short-term study. In this setting, selectivity is probably desirable considering the long-term potential consequences, such as the impact on Ca levels, the development of ABD, or the potential effects on VC of different VDR activators.
In conclusion, VDR can be activated by vitamin D or selective VDR activators. Selective VDR activation is defined by different effects at the tissue and molecular (gene) levels. A selective effect of paricalcitol in mineral metabolism is identified by its low calcemic, low phosphatemic profile. On the basis of gene expression profiles, there are other non-mineral metabolism effects and tissue-specific differences between vitamin D and selective VDR activation. Thus, treating individual components of CKD–MBD is no longer recommended, and a wider perspective encompassing skeletal, renal, and CV effects is required. In this regard, selective VDR activation has been associated with survival improvement at least in dialysis patients, and new VDR activators should be considered a novel and interesting approach to enhance the standard of care in these high-risk patients.
This issue was organized by the VDR Expert Centers and was funded by Abbott Laboratories with a collaborative effort to advance and support the science and the improvement of quality of life for renal patients. JB is part of the Spanish advisory boards for Abbott and Amgen, and he collaborated in advisory boards for Genzyme. JB has given national and international lectures with the sponsorship of Abbott, Amgen, Genzyme, and Shire. MC has given national and international lectures with the sponsorship of Abbott, Amgen, Genzyme, Shire, and Roche.
Footnotes
TO CITE THIS ARTICLE: Bover J, Cozzolino M. Mineral and bone disorders in chronic kidney disease and end-stage renal disease patients: new insights into vitamin D receptor activation. Kidney inter., Suppl. 2011; 1: 122–129.
References
- Llach F, Bover J.Renal osteodystrophiesIn: Brenner BM (ed)The Kidney6th edn, vol 2.WB Saunders Company: Philadelphia; 20002103–2186. [Google Scholar]
- Bover J, Rodriguez M, Trinidad P, et al. Factors in the development of secondary hyperparathyroidism during graded renal failure in the rat. Kidney Int. 1994;45:953–961. doi: 10.1038/ki.1994.129. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Pasho S, Fallabrino G, et al. Pathogenesis of secondary hyperparathyroidism. Int J Artif Organs. 2009;32:75–80. doi: 10.1177/039139880903200203. [DOI] [PubMed] [Google Scholar]
- Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- Rojas-Rivera J, De La PC, Ramos A, et al. The expanding spectrum of biological actions of vitamin D. Nephrol Dial Transplant. 2010;25:2850–2865. doi: 10.1093/ndt/gfq313. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Ciceri P, Volpi EM, et al. Pathophysiology of calcium and phosphate metabolism impairment in chronic kidney disease. Blood Purif. 2009;27:338–344. doi: 10.1159/000209246. [DOI] [PubMed] [Google Scholar]
- Krajisnik T, Olauson H, Mirza MA, et al. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010;78:1024–1032. doi: 10.1038/ki.2010.260. [DOI] [PubMed] [Google Scholar]
- London GM. Bone-vascular axis in chronic kidney disease: a reality. Clin J Am Soc Nephrol. 2009;4:254–257. doi: 10.2215/CJN.06661208. [DOI] [PubMed] [Google Scholar]
- Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi-Moshayoff V, Wasserman G, Meir T, et al. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- Vervloet MG, Larsson TE. Fibroblast growth factor 23 and Klotho in chronic kidney disease. Kidney inter., Suppl. 2011;1:130–135. [Google Scholar]
- Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- Moe SM, Drüeke T, Lameire N, et al. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Souberbielle JC, Boutten A, Carlier MC, et al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int. 2006;70:345–350. doi: 10.1038/sj.ki.5001606. [DOI] [PubMed] [Google Scholar]
- Souberbielle JC, Roth H, Fouque DP. Parathyroid hormone measurement in CKD. Kidney Int. 2010;77:93–100. doi: 10.1038/ki.2009.374. [DOI] [PubMed] [Google Scholar]
- Frazao JM, Martins P. Adynamic bone disease: clinical and therapeutic implications. Curr Opin Nephrol Hypertens. 2009;18:303–307. doi: 10.1097/MNH.0b013e32832c4df0. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Frazao JM, Monier-Faugere MC, et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19:405–412. doi: 10.1681/ASN.2006101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen G, Ballanti P, Bonucci E, et al. Renal osteodystrophy in predialysis and hemodialysis patients: comparison of histologic patterns and diagnostic predictivity of intact PTH. Nephron. 2002;91:103–111. doi: 10.1159/000057611. [DOI] [PubMed] [Google Scholar]
- Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310:358–363. doi: 10.1136/bmj.310.6976.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg V, Floege J. Adynamic bone disease-bone and beyond. NDT Plus. 2008;3:135–147. doi: 10.1093/ndtplus/sfn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hercz G, Sherrard DJ, et al. Relationship between intact 1–84 parathyroid hormone and bone histomorphometric parameters in dialysis patients without aluminum toxicity. Am J Kidney Dis. 1995;26:836–844. doi: 10.1016/0272-6386(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Nordal KP, Dahl E. Low dose calcitriol versus placebo in patients with predialysis chronic renal failure. J Clin Endocrinol Metab. 1988;67:929–936. doi: 10.1210/jcem-67-5-929. [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Malluche HH. Trends in renal osteodystrophy: a survey from 1983 to 1995 in a total of 2248 patients. Nephrol Dial Transplant. 1996;11 (Suppl 3:111–120. doi: 10.1093/ndt/11.supp3.111. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Mawad H, Monier-Faugere MC. The importance of bone health in end-stage renal disease: out of the frying pan, into the fire. Nephrol Dial Transplant. 2004;19 (Suppl 1:i9–i13. doi: 10.1093/ndt/gfh1002. [DOI] [PubMed] [Google Scholar]
- Sherrard DJ, Hercz G, Pei Y, et al. The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int. 1993;43:436–442. doi: 10.1038/ki.1993.64. [DOI] [PubMed] [Google Scholar]
- Torres A, Lorenzo V, Hernandez D, et al. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int. 1995;47:1434–1442. doi: 10.1038/ki.1995.201. [DOI] [PubMed] [Google Scholar]
- Spasovski G. Low turn-over bone disease in patients with chronic renal disease. Med Pregl. 2007;60 (Suppl 2:21–24. [PubMed] [Google Scholar]
- Andress DL. Adynamic bone in patients with chronic kidney disease. Kidney Int. 2008;73:1345–1354. doi: 10.1038/ki.2008.60. [DOI] [PubMed] [Google Scholar]
- Asci G, Ok E, Savas R, et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol Dial Transplant. 2010;26:1010–1015. doi: 10.1093/ndt/gfq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha LA, Higa A, Barreto FC, et al. Variant of adynamic bone disease in hemodialysis patients: fact or fiction. Am J Kidney Dis. 2006;48:430–436. doi: 10.1053/j.ajkd.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Cannata-Andia JB. Hypokinetic azotemic osteodystrophy. Kidney Int. 1998;54:1000–1016. doi: 10.1046/j.1523-1755.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Bover J, Jara A, Trinidad P, et al. The calcemic response to PTH in the rat: effect of elevated PTH levels and uremia. Kidney Int. 1994;46:310–317. doi: 10.1038/ki.1994.276. [DOI] [PubMed] [Google Scholar]
- Bover J, Jara A, Trinidad P, et al. Dynamics of skeletal resistance to parathyroid hormone in the rat: effect of renal failure and dietary phosphorus. Bone. 1999;25:279–285. doi: 10.1016/s8756-3282(99)00169-6. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E, Finch J, Clay P, et al. A novel mechanism for skeletal resistance in uremia. Kidney Int. 2000;58:753–761. doi: 10.1046/j.1523-1755.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Mathew S, Davies M, Lund R, et al. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone–vascular links in chronic kidney disease. Eur J Clin Invest. 2006;36 (Suppl 2:43–50. doi: 10.1111/j.1365-2362.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- Andress DL, Howard GA, Birnbaum RS. Identification of a low molecular weight inhibitor of osteoblast mitogenesis in uremic plasma. Kidney Int. 1991;39:942–945. doi: 10.1038/ki.1991.118. [DOI] [PubMed] [Google Scholar]
- Wesseling-Perry K, Harkins GC, Wang HJ, et al. The calcemic response to continuous parathyroid hormone (PTH)(1–34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH(7–84) J Clin Endocrinol Metab. 2010;95:2772–2780. doi: 10.1210/jc.2009-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WG, Ramirez JA, Belin TR, et al. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int. 1994;46:1160–1166. doi: 10.1038/ki.1994.380. [DOI] [PubMed] [Google Scholar]
- Atsumi K, Kushida K, Yamazaki K, et al. Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis. 1999;33:287–293. doi: 10.1016/s0272-6386(99)70302-1. [DOI] [PubMed] [Google Scholar]
- Danese MD, Kim J, Doan QV, et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47:149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Djurdjev O, Cardew S, et al. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15:770–779. doi: 10.1097/01.asn.0000113243.24155.2f. [DOI] [PubMed] [Google Scholar]
- Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO4, Ca x PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2213. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Dardenne O, Prud'homme J, Glorieux FH, et al. Rescue of the phenotype of CYP27B1 (1alpha-hydroxylase)-deficient mice. J Steroid Biochem Mol Biol. 89–2004;90:327–330. doi: 10.1016/j.jsbmb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, et al. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- van Driel M, Koedam M, Buurman CJ, et al. Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006;99:922–935. doi: 10.1002/jcb.20875. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolik CA, Black EC, Cain RL, et al. Anabolic and catabolic bone effects of human parathyroid hormone (1–34) are predicted by duration of hormone exposure. Bone. 2003;33:372–379. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- Reinhardt TA, Horst RL. Parathyroid hormone down-regulates 1,25-dihydroxyvitamin D receptors (VDR) and VDR messenger ribonucleic acid in vitro and blocks homologous up-regulation of VDR in vivo. Endocrinology. 1990;127:942–948. doi: 10.1210/endo-127-2-942. [DOI] [PubMed] [Google Scholar]
- Nguyen-Yamamoto L, Bolivar I, Strugnell SA, et al. Comparison of active vitamin D compounds and a calcimimetic in mineral homeostasis. J Am Soc Nephrol. 2010;21:1713–1723. doi: 10.1681/ASN.2009050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiizaki K, Hatamura I, Imazeki I, et al. Improvement of impaired calcium and skeletal homeostasis in vitamin D receptor knockout mice by a high dose of calcitriol and maxacalcitol. Bone. 2009;45:964–971. doi: 10.1016/j.bone.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Silvagno F, De VE, Attanasio A, et al. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS One. 2010;5:e8670. doi: 10.1371/journal.pone.0008670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane M, Fey TA, Dixon DB, et al. Differential effects of Vitamin D analogs on bone formation and resorption. J Steroid Biochem Mol Biol. 2006;98:72–77. doi: 10.1016/j.jsbmb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Geng Z, Mawad H, et al. Improved assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001;60:1460–1468. doi: 10.1046/j.1523-1755.2001.00949.x. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E, Cozzolino M, Lu Y, et al. Efficacy of 19-Nor-1,25-(OH)2D2 in the prevention and treatment of hyperparathyroid bone disease in experimental uremia. Kidney Int. 2003;63:2020–2027. doi: 10.1046/j.1523-1755.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- Bover J, Gorriz JL, Martin de Francisco AL, et al. Unawareness of the K/DOQI guidelines for bone and mineral metabolism in predialysis chronic kidney disease: results of the OSERCE Spanish multicenter-study survey. Nefrologia. 2008;28:637–643. [PubMed] [Google Scholar]
- Craver L, Marco MP, Martinez I, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1–5—achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Mawad H, Malluche HH. Opposite effects of calcitriol and paricalcitol on the parathyroid hormone-(1–84)/large carboxy-terminal-parathyroid hormone fragments ratio in patients with stage 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1255–1260. doi: 10.2215/CJN.03461006. [DOI] [PubMed] [Google Scholar]
- Urena P, Hruby M, Ferreira A, et al. Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol. 1996;7:506–512. doi: 10.1681/ASN.V73506. [DOI] [PubMed] [Google Scholar]
- Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6:877–882. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer LL, Tintut Y, Parhami F. Novel mechanisms in accelerated vascular calcification in renal disease patients. Curr Opin Nephrol Hypertens. 2002;11:437–443. doi: 10.1097/00041552-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Demer L, Tintut Y. The bone–vascular axis in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:349–353. doi: 10.1097/MNH.0b013e32833a3d67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- Russo D, Miranda I, Ruocco C, et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255–1261. doi: 10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- London GM, Marchais SJ, Guerin AP, et al. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol. 2008;19:1827–1835. doi: 10.1681/ASN.2007050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- Towler DA. Vascular calcification in ESRD: Another cloud appears in the perfect storm—but highlights a silver lining. Kidney Int. 2004;66:2467–2468. doi: 10.1111/j.1523-1755.2004.66095.x. [DOI] [PubMed] [Google Scholar]
- Brandenburg VM, Ketteler M, Rodriguez M. Ten years of progress in our understanding of uremic vascular calcification and disease: a decade summarized in 20 steps. Kidney inter., Suppl. 2011;1:116–121. [Google Scholar]
- London GM, Marty C, Marchais SJ, et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- Kurz P, Monier-Faugere MC, Bognar B, et al. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int. 1994;46:855–861. doi: 10.1038/ki.1994.342. [DOI] [PubMed] [Google Scholar]
- Hercz G, Pei Y, Greenwood C, et al. Aplastic osteodystrophy without aluminum: the role of ‘suppressed' parathyroid function. Kidney Int. 1993;44:860–866. doi: 10.1038/ki.1993.323. [DOI] [PubMed] [Google Scholar]
- Tomiyama C, Carvalho AB, Higa A, et al. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J Bone Miner Res. 2010;25:499–504. doi: 10.1359/jbmr.090735. [DOI] [PubMed] [Google Scholar]
- Barreto DV, Barreto FC, Carvalho AB, et al. Coronary calcification in hemodialysis patients: the contribution of traditional and uremia-related risk factors. Kidney Int. 2005;67:1576–1582. doi: 10.1111/j.1523-1755.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- Barreto DV, Barreto FC, Carvalho AB, et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis. 2008;52:1139–1150. doi: 10.1053/j.ajkd.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Adragao T, Herberth J, Monier-Faugere MC, et al. Low bone volume—a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:450–455. doi: 10.2215/CJN.01870408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adragao T, Branco P, Birne R, et al. Bone mineral density, vascular calcifications, and arterial stiffness in peritoneal dialysis patients. Perit Dial Int. 2008;28:668–672. [PubMed] [Google Scholar]
- Adragao T, Herberth J, Monier-Faugere MC, et al. Femoral bone mineral density reflects histologically determined cortical bone volume in hemodialysis patients. Osteoporos Int. 2010;21:619–625. doi: 10.1007/s00198-009-0988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanko LB, Christiansen C, Cox DA, et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E, Cozzolino M, Finch JL. Differential effects of 19-nor-1,25-(OH)2D2 and 1alpha-hydroxyvitamin D(2) on calcium and phosphorus in normal and uremic rats. Kidney Int. 2002;62:1277–1284. doi: 10.1111/j.1523-1755.2002.kid573.x. [DOI] [PubMed] [Google Scholar]
- Coyne DW, Grieff M, Ahya SN, et al. Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. Am J Kidney Dis. 2002;40:1283–1288. doi: 10.1053/ajkd.2002.36899. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. Evolution of our understanding of vitamin D. Nutr Rev. 2008;66:S73–S87. doi: 10.1111/j.1753-4887.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E, Finch J, Ritter C, et al. A new analog of calcitriol, 19-nor-1,25-(OH)2D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hypercalcemia. Am J Kidney Dis. 1995;26:852–860. doi: 10.1016/0272-6386(95)90455-7. [DOI] [PubMed] [Google Scholar]
- Hirata M, Katsumata K, Endo K, et al. In subtotally nephrectomized rats 22-oxacalcitriol suppresses parathyroid hormone with less risk of cardiovascular calcification or deterioration of residual renal function than 1,25(OH)2 vitamin D3. Nephrol Dial Transplant. 2003;18:1770–1776. doi: 10.1093/ndt/gfg296. [DOI] [PubMed] [Google Scholar]
- Mizobuchi M, Finch JL, Martin DR, et al. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- Cardus A, Panizo S, Parisi E, et al. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22:860–866. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- Lopez I, Mendoza FJ, Guilera-Tejero E, et al. The effect of calcitriol, paricalcitol, and a calcimimetic on extraosseous calcifications in uremic rats. Kidney Int. 2008;73:300–307. doi: 10.1038/sj.ki.5002675. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Fallabrino G, Pasho S, et al. Importance of vitamin D receptor activation in clinical practice. Contrib Nephrol. 2009;163:213–218. doi: 10.1159/000223801. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Galassi A, Gallieni M, et al. Pathogenesis and treatment of secondary hyperparathyroidism in dialysis patients: the role of paricalcitol. Curr Vasc Pharmacol. 2008;6:148–153. doi: 10.2174/157016108783955310. [DOI] [PubMed] [Google Scholar]
- Bover J, Farre N, Andres E, et al. Update on the treatment of chronic kidney disease–mineral and bone disorder. J Ren Care. 2009;35 (Suppl 1:19–27. doi: 10.1111/j.1755-6686.2009.00049.x. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Ketteler M. The link between bone and cardiovascular disease in CKD: new insights into pathogenesis and treatment. Int J Artif Organs. 2009;32:65–66. doi: 10.1177/039139880903200201. [DOI] [PubMed] [Google Scholar]
- Pilz S, Tomaschitz A, Dreschsler C, et al. Vitamin D deficiency and heart disease. Kidney inter., Suppl. 2011;1:111–115. [Google Scholar]
- Hsu JJ, Tintut Y, Demer LL. Vitamin D and osteogenic differentiation in the artery wall. Clin J Am Soc Nephrol. 2008;3:1542–1547. doi: 10.2215/CJN.01220308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino M, Brandenburg V. Paricalcitol and outcome: a manual on how a vitamin D receptor activator (VDRA) can help us to get down the ‘U'. Clin Nephrol. 2009;71:593–601. doi: 10.5414/cnp71593. [DOI] [PubMed] [Google Scholar]
- Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008;73:1355–1363. doi: 10.1038/ki.2008.35. [DOI] [PubMed] [Google Scholar]
- Marco MP, Craver L, Betriu A, et al. Influence of vitamin D receptor gene polymorphisms on mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:965–974. doi: 10.1053/ajkd.2001.28582. [DOI] [PubMed] [Google Scholar]
- Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- Barreto DV, Barreto FC, Liabeuf S, et al. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1128–1135. doi: 10.2215/CJN.00260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- Tentori F, Albert JM, Young EW, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2009;24:963–972. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]
- Bover J, Andres E, Lloret MJ, et al. Dietary and pharmacological control of calcium and phosphate metabolism in dialysis patients. Blood Purif. 2009;27:369–386. doi: 10.1159/000209250. [DOI] [PubMed] [Google Scholar]
- Gal-Moscovici A, Sprague SM. Use of vitamin D in chronic kidney disease patients. Kidney Int. 2010;78:146–151. doi: 10.1038/ki.2010.113. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]