Abstract
Bidirectional mechanisms exist that link diseases affecting the heart and kidney. This link is complex and remains poorly understood; therefore, charting the shared territory of cardiovascular (CV) and renal medicine poses major problems. Until now, no convincing rationale for delineating new syndromes existed. The multiple connections of the arterial system and the heart and kidney with other systems, from energy and protein balance to the musculoskeletal, clearly require special focus and rigorous framing. Nephrologists have yet to fully understand why the application of dialysis has had only limited success in halting the parallel burdens of CV and non-CV death in patients with end-stage renal disease. Cardiologists, intensivists, and nephrologists alike should settle whether and when extracorporeal ultrafiltration benefits patients with decompensated heart failure. These sparse but interconnected themes spanning from the basic science–clinical transition phase to clinical science, epidemiology, and medical technology already form the basis for the young discipline of ‘CV and renal medicine'.
Keywords: cardio-renal, cardiovascular risk, CKD, death, ESRD, progression of CKD
Chronic kidney disease (CKD) is now recognized as a public health priority in economically developed and developing countries alike.1, 2 Concern about CKD goes far beyond mere loss of renal function because the risk of fatal and non-fatal atherosclerotic complications in these patients is much higher than that of progressing to end-stage renal disease.3 From a diachronic perspective, CKD is a fundamental element of transitional epidemiology, an epochal change whereby chronic diseases have replaced communicable diseases as leading causes of death and disability.4 Because of the numerous functional relationships of the kidney with blood pressure and fluid volume control and with cardiovascular (CV) function in general, loss of renal function is one of the strongest triggers of high CV risk. Although the epidemiological burden of CKD at the population level is now well framed,5 the mechanistic nature of the relationship between CV and renal disease remains a complex, hitherto largely unresolved, issue.
THE COMPLEXITY OF THE CV–RENAL LINK
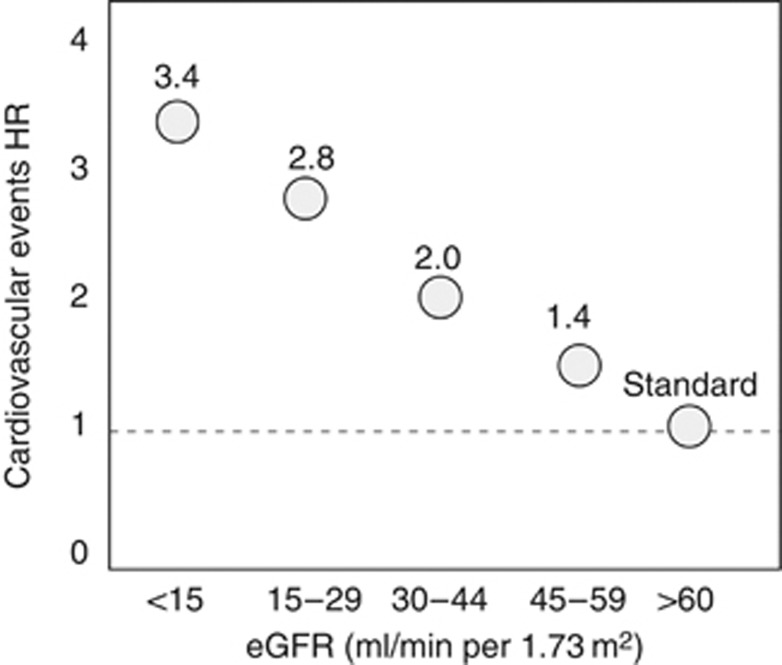
Independently of age, sex, background CV damage, and Framingham factors, the risk for CV death increases gradually as renal function deteriorates to be 3.4 times higher in patients with kidney failure than in individuals with normal glomerular filtration rate (GFR; >60 ml/min per 1.73 m2) (Figure 1).6 This relationship is generally explained by assuming a causality chain starting from the kidney. Yet, the CV–renal link underlies complex reciprocal interorgan influences, whereby kidney disease may adversely affect the heart and the arterial system and vice versa.
Figure 1.
Relationship between glomerular filtration rate (GFR) and the risk for cardiovascular events. Data are adjusted for age, sex, and other Framingham risk factors. HR, hazard rate; eGFR, estimated GFR.
Severe CV disease can be triggered in experimental models by renal mass ablation.7 This intervention triggers left ventricular hypertrophy, fibrosis, and defective capillarization in the rat, as well as severe arterial damage with extensive inflammation, plaque formation, and a propensity to calcified lesions8, 9 in the APO E−/E− mouse. These models support the hypothesis that left ventricular hypertrophy, defective myocardial oxygen supply, and severe arterial lesions commonly observed in CKD are the consequence of reduced renal mass. On the other hand, the reverse possibility—that cardiac disease may engender the other—is well documented. In uninephrectomized rats, myocardial infarction triggers a marked rise in albumin excretion rate and a parallel increase in focal glomerulosclerosis, and both alterations are proportional to the myocardial necrosis area.10 Myocardial infarction in patients with coronary heart disease prompts a decline in the GFR of the same order observed in patients with primary renal diseases.11 In the earlier stages (NYHA I–II) of heart failure, patients exhibit an early, reversible, but ultimately permanent loss of the renal vasodilatory response to nitric oxide (NO)-generating agents.12 As heart failure intensifies in its severity into the congestive phase (NYHA III–IV), GFR becomes cardiac output13 and central venous pressure14 dependent such that a moderate-to-severe reduction in GFR is typical in these patients.
Bidirectional mechanisms exist that link diseases affecting the heart and the kidney. Activation of the sympathetic and renin–angiotensin–aldosterone systems, alterations in nitric oxide bioavailability, inflammation and overproduction of reactive oxygen species are established common pathophysiological pathways mediating clinical outcomes in cardiac and renal insufficiency. These pathways are causally implicated in the high risk of these conditions because therapies targeting the same pathways produce unquestionable beneficial effects in patients with either renal or CV diseases.15, 16 Parallel aging of the CV system and of the kidney and the challenge of maintaining energy balance in the face of anorexic stimuli, inflammation, and oxidative stress is the obvious common soil of advanced cardiac and renal failure. Beyond exemplary clinical phenotypes, identifying the precise time sequence and the directionality of these links is a very difficult undertaking that is bound to be complicated by confounders of various kinds. Thus, in heuristic terms, a systematic approach considering the continuum of the CV–renal connections rather than a reductionist approach seems to be a better grounded method for the study of the risk associated with the CV–renal link in human diseases.
TAXONOMY, SYNDROMES, AND THE CV–RENAL LINK
The term ‘cardiorenal' as applied to human diseases was probably used for the first time in 1913 by Thomas Lewis.17 From that time on, this term has been increasingly used in the medical literature to describe problems of various kinds related to the heart and/or the arterial system and the kidney. More recently, it was used to designate a new syndrome, the cardio–renal syndrome (CRS).18 A description of this syndrome, contemplating five subtypes, was proposed in 2008 (see ref. 19). Charting the shared territory of CV and renal medicine at the present stage of knowledge poses major problems. To illustrate the current difficulties, we briefly discuss the CRS, which is an apparently mature construct, specifically.
Establishing a new syndrome requires well-defined underpinning conceptual frameworks related to (1) the definition of criteria for diagnosis and to stages for prognosis; (2) the synergism of the syndrome components in determining clinical outcomes; and (3) the uniqueness of treatment decisions.
Definition
The formidable problems related to the definition of the CRS were well recognized by an expert panel of the AHA in 2004 (see ref. 20), and these difficulties have been recently reiterated in a narrative review by Bock and Gottlieb.18 In essence, no accepted definition has been established to date. The proposal of distinguishing five separate subtypes of CRS19 is based on a simplistic interpretation of the cardio-renal link in the clinical setting. However, this praiseworthy attempt at better framing the CRS fails to provide precise criteria for the general definition of the syndrome or for the identification of the proposed underlying subtypes. Furthermore, scarce knowledge on the implication for survival of renal function decline during treatment of decompensated heart failure until now has precluded the development of well-established and convincing treatment recommendations. Excessive hemoconcentration secondary to volume removal predicts renal function worsening in heart failure but this effect is associated with reduced rather than augmented mortality.21 Beyond CRS, the increasing use of the term cardio–renal in much broader contexts encompassing anemia22 and diseases with less severe degrees of cardiac and renal involvement23 further compounds the definition issue.
Synergism of components
Renal dysfunction in decompensated heart failure unquestionably signals a high-risk condition.18 Yet, the notion that CRS is a risk marker above and beyond the risk associated with its individual components, a criterion that would support the construct of this syndrome, remains largely unsupported. Indeed, there is no proof that such a coexistence has a synergistic effect on clinical outcomes, that is, an effect greater than that expected from the mere combination of the two risk factors. For example, proof that a synergism exists between inflammation and wasting in end-stage renal disease has been established recently,24 which supports the construct of the Malnutrition–Inflammation syndrome. Renal function does not add predictive value to clinical scores aimed at assisting prognosis in patients with heart failure25, 26 and there is no evidence of biological and/or statistical interaction between renal function and other components of these scores. It is undisputable that a decline in renal function renders fluid volume control in decompensated heart failure problematic. However, antinatriuretic mechanisms are already operative in early heart failure12 when the GFR is still modestly or not affected, which points to a continuously operating interdependent process that allows for reciprocal fine-tuning of cardiac and renal function in heart diseases rather than to a later, threshold-exceeding event.
Treatment decisions
As to treatment decisions, in line with the general diktat that clinical monitoring should be comprehensive in severe systemic diseases, renal function measurement is recommended by current guidelines of heart failure.27 However, treatment of advanced heart failure remains guided by cardiorespiratory symptom, exercise tolerance, and hemodynamic considerations,27 rather than tailored on the basis of associated clinical conditions and renal failure.
Overall, the lack of robust anchoring of this syndrome to a precise clinical or pathophysiological rationale is reminiscent of the problems that emerged after the general proposition of the ‘metabolic syndrome', an entity initially recognized and variously classified by major guideline developers and by the WHO.28 Indeed, a group of experts appointed by the same organization recently concluded that ‘metabolic syndrome is a concept … (that) may be considered useful as an educational concept, but with limited practical utility as a diagnostic or management tool'.29 New syndromic entities not supported by sufficient conceptual grounds should not be considered as neutral to the course of clinical research because these may have unpredictable effects on research orientation. Again, the metabolic syndrome can be taken as a case in point. Genetic epidemiology of the metabolic syndrome has become a much pursued research theme. Even though several meaningful associations between the individual components of the syndrome and various genes have been documented, as yet, no genetic factors that encompass all traits of the syndrome have been identified.30 This might not only simply reflect the lack of power of analyses performed so far but may also imply that this syndrome has no unifying basis.
Scoping questions
The ‘omics' technology now provides unprecedented opportunities for a new system biology-based approach31 to the study of the cardio–renal link and for describing the dynamics of cardio–renal risk factors in the evolution of human diseases in an integrated view that can match the Guytonian paradigm. The dawn of new medicine is visible but time is needed to build up new clinical science based on system biology and for this science to reap dividends in terms of health benefits. As to the specifics of the cardio–renal link, the questions and the problems on the table are disparate and scoping these questions is important to identify research priorities. Although deciphering the interrelationship of endothelial dysfunction, sympathetic overactivity, and the renin–angiotensin system with CV and renal diseases, an issue in part in the first phase of translational (T1) research,32 will certainly take advantage of the application of new technologies, an understanding of the blood pressure–CV risk relationship in these diseases and the application of this knowledge into clinical practice are already on a track that may rapidly mature to inform clinical practice (T2–T3 phases). The multiple connections of the arterial system and the heart and the kidney with other systems, from energy and protein balance to the musculoskeletal system, clearly require special focus and proper framing. Lingering questions also remain in relation to the application of extracorporeal treatments in kidney and heart diseases. Nephrologists are yet to fully understand why the application of dialysis has had only limited success in halting the parallel burden of CV and non-CV death in end-stage renal disease patients,33 an observation pointing to an array of risk factors having broad effects on major biological systems rather than to effects restricted to the CV system. Finally, cardiologists, intensivists, and nephrologists alike should settle whether and when extracorporeal ultrafiltration benefits patients with decompensated heart failure.34, 35 These are sparse but interconnected themes spanning from the basic science-clinical transition (T1) phase to clinical science (T2–3), epidemiology, and medical technology. We believe that it is important that investigators in the CV and renal area join their efforts to frame a common clinical research agenda. Herein, EUropean Renal and CV medicine investigators pose a series of research questions on key issues of the CV–renal link that appear mature for entering the translational phase of research and/or whose answers may yield useful information to improve diagnosis, prognosis, and treatment of CV and renal diseases.
AC has received consulting fees from Abbott Laboratories and received lecture fees from F. Hoffmann-La Roche, Amgen, and Fresenius Medical Care Holdings. AM-C has received consulting fees from Abbott Laboratories, Roche Spain, and Abbott Spain. AO has received grant support from the Spanish Government. AW has received lecture fees from Amgen, F. Hoffmann-La Roche, and Janssen-Cileg. AW has also received grant support from Astellas Pharma. DF has received funding from the EU. DG has received consulting fees and lecture fees from Shire, Genzyme, Novartis AG, Sandoz, Pfizer, and Fresenius Medical Care Holdings. FWD has received funding from Amgen and Baxter. GL has received consulting fees from Amgen and Sandoz. GL has also received lecture fees from Amgen, Sandoz, Genzyme, and Shire. PJB has received consulting fees from Medtronic and has received grant support from Ardian and Novartis AG. RA has received consulting fees from Amgen, Abbott Laboratories, Merck, Affymax, Takeda Pharmaceutical Company, Daiichi Sankyo, Celgene, Watson Pharmaceuticals, and Rockwell Medical. RA has also received lecture fees from Abbott Laboratories, Merck, and Medscape. ZM has received lecture fees from Amgen, Shire, Genzyme, FMC, and Merck Sharp & Dohme. ZM has received grant support from Baxter, Amgen, FMC, Shire, and Genzyme. The remaining authors declared no competing interests.
Footnotes
TO CITE THIS ARTICLE: Zoccali C, Goldsmith D, Agarwal R et al. The complexity of the cardio–renal link: taxonomy, syndromes, and diseases. Kidney Int Sup 2011; 1: 2–5.
References
- Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccali C, Kramer A, Jager KJ. Epidemiology of CKD in Europe: an uncertain scenario. Nephrol Dial Transplant. 2010;25:1731–1733. doi: 10.1093/ndt/gfq250. [DOI] [PubMed] [Google Scholar]
- Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Q. 2005;83:731–757. doi: 10.1111/j.1468-0009.2005.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Amann K, Wiest G, Zimmer G, et al. Reduced capillary density in the myocardium of uremic rats—a stereological study. Kidney Int. 1992;42:1079–1085. doi: 10.1038/ki.1992.390. [DOI] [PubMed] [Google Scholar]
- Bro S, Bentzon JF, Falk E, et al. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol. 2003;14:2466–2474. doi: 10.1097/01.asn.0000088024.72216.2e. [DOI] [PubMed] [Google Scholar]
- Massy ZA, Ivanovski O, Nguyen-Khoa T, et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- van Dokkum RP, Eijkelkamp WB, Kluppel AC, et al. Myocardial infarction enhances progressive renal damage in an experimental model for cardio-renal interaction. J Am Soc Nephrol. 2004;15:3103–3110. doi: 10.1097/01.ASN.0000145895.62896.98. [DOI] [PubMed] [Google Scholar]
- Hillege HL, van Gilst WH, Van Veldhuisen DJ, et al. Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ACE inhibition: the CATS randomized trial. Eur Heart J. 2003;24:412–420. doi: 10.1016/s0195-668x(02)00526-2. [DOI] [PubMed] [Google Scholar]
- Magri P, Rao MA, Cangianiello S, et al. Early impairment of renal hemodynamic reserve in patients with asymptomatic heart failure is restored by angiotensin II antagonism. Circulation. 1998;98:2849–2854. doi: 10.1161/01.cir.98.25.2849. [DOI] [PubMed] [Google Scholar]
- Cody RJ, Ljungman S, Covit AB, et al. Regulation of glomerular filtration rate in chronic congestive heart failure patients. Kidney Int. 1988;34:361–367. doi: 10.1038/ki.1988.189. [DOI] [PubMed] [Google Scholar]
- Damman K, van DV, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575–1581. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- Lewis T. Paroxysmal dyspnoea in cardiorenal patients: with particular reference to ‘cardiac' and ‘uremic' asthma. Br Med J. 1913;29:1417–1420. doi: 10.1136/bmj.2.2761.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung and Blood Institute (NHLBI) working group Cardiorenal connections in heart failure and cardiovascular disease. 20-8-2004 . http://www.nhlbi.nih.gov/meetings/workshops/cardiorenal-hf-hd.htm . Accessed 20 December2010
- Testani JM, Chen J, McCauley BD, et al. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besarab A, Horl WH, Silverberg D. Iron metabolism, iron deficiency, thrombocytosis, and the cardiorenal anemia syndrome. Oncologist. 2009;14 (Suppl 1:22–33. doi: 10.1634/theoncologist.2009-S1-22. [DOI] [PubMed] [Google Scholar]
- Keane WF. Metabolic pathogenesis of cardiorenal disease. Am J Kidney Dis. 2001;38:1372–1375. doi: 10.1053/ajkd.2001.29260. [DOI] [PubMed] [Google Scholar]
- de Mutsert R, Grootendorst DC, Axelsson J, et al. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23:2957–2964. doi: 10.1093/ndt/gfn167. [DOI] [PubMed] [Google Scholar]
- Levy WC, Mozaffarian D, Linker DT, et al. The seattle heart failure model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Simmons RK, Alberti KG, Gale EA, et al. The metabolic syndrome: useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia. 2010;53:600–605. doi: 10.1007/s00125-009-1620-4. [DOI] [PubMed] [Google Scholar]
- Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9:819–830. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock CE, Wheelock AM, Kawashima S, et al. Systems biology approaches and pathway tools for investigating cardiovascular disease. Mol Biosyst. 2009;5:588–602. doi: 10.1039/b902356a. [DOI] [PubMed] [Google Scholar]
- Lauer MS, Skarlatos S. Translational research for cardiovascular diseases at the National Heart, Lung, and Blood Institute: moving from bench to bedside and from bedside to community. Circulation. 2010;121:929–933. doi: 10.1161/CIRCULATIONAHA.109.917948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- Bart BA. Treatment of congestion in congestive heart failure: ultrafiltration is the only rational initial treatment of volume overload in decompensated heart failure. Circ Heart Fail. 2009;2:499–504. doi: 10.1161/CIRCHEARTFAILURE.109.863381. [DOI] [PubMed] [Google Scholar]
- Shin JT, Dec GW. Ultrafiltration should not replace diuretics for the initial treatment of acute decompensated heart failure. Circ Heart Fail. 2009;2:505–511. doi: 10.1161/CIRCHEARTFAILURE.109.862474. [DOI] [PMC free article] [PubMed] [Google Scholar]