Abstract
Current interventions for the treatment of acute kidney injury (AKI) are not satisfactory, and it is time to approach new strategies in order to definitely take a step forward. At its beginning, cell therapy was innovative and promising. We have shown that mesenchymal stem cells (MSCs), isolated from human and murine bone marrow (BM), behave as an efficacious tool for the treatment of cisplatin-induced AKI in mice in terms of amelioration of renal function and structure, and animal survival. Although the mechanism has not been completely elucidated, we have provided data showing that BM-MSC-mediated renal recovery involves the release at the site of injury of the growth factor, insulin-like growth factor-1. Several biological effects have been observed in renal tissues of mice treated with BM-MSCs, including increased cell proliferation, hemodynamic changes, and cell apoptosis reduction. In the same experimental model, we have tested the effect of MSCs isolated from cord blood (CB-MSCs), which, similar to BM-MSCs, not only ameliorated renal function but also protected animals from death to a remarkably higher extent. Animals receiving CB-MSCs showed reduction of oxidative stress and activation of AKT prosurvival pathway in tubular cells. These results hold great promise for future studies in patients with AKI.
Keywords: acute renal failure, kidney regeneration, mesenchymal stem cells
The increased interest toward mesenchymal stem cells (MSCs) is associated with the prominent role that these cells have recently provoked in tissue regeneration. Mainly, they represent an important component of the hematopoietic stem cell niche in the bone marrow (BM), where they contribute to hematopoietic stem cell maturation.1, 2 In kidney regeneration, in which the quest for a pharmacological therapy in acute kidney injury (AKI) has been largely unsuccessful, BM-MSCs might represent a valid therapeutic tool.
Acknowledged evidence has proved that BM supplies kidney with cells for physiological turnover or regeneration of tubular epithelial cells.3 Our group investigated the use of BM-MSCs as cell therapy for AKI and documented for the first time that murine BM-MSCs contributed to renal repair and recovery from AKI.4 By means of intravenous injection, murine BM-MSCs ameliorated renal function and tubular injury of mice with AKI induced by the nephrotoxic anticancer agent cisplatin (Table 1).4, 5 In the same experimental model, hematopoietic stem cells had no protective effect.4 Therefore, it is important to understand which BM-MSCs properties make these cells responsible for their decisive role in kidney repair. In kidneys of AKI-injured mice supplied with BM-MSCs, we observed, by Ki67 staining, a marked increase of tubular cell proliferation, a fundamental step by which kidney restores normal architecture after acute damage. Proliferating cells were identified as endogenous renal cells, as the vast majority of the cells lacked Y chromosome, the marker used for identifying BM-MSC male cells given to female mice, and were negative for PKH-26, the cell tracker used to stain BM-MSCs before their administration.4, 5
Table 1. Bone marrow-derived mesenchymal stem cells (BM-MSCs) protected cisplatin-treated mice from renal function deterioration and renal histology changes.
| Control | Cisplatin+saline (4d) | Cisplatin+BM- MSCs (4d) | |
|---|---|---|---|
| Renal function | |||
| BUN (mg/dl) | 17.02±1.00 | 82.54±5.12* | 33.43±3.06°° |
| Renal histology | |||
| Casts | 0 | 1.77±0.20 | 0.50±0.19°° |
| Tubular cell degeneration | 0 | 1.54±0.14 | 0.62±0.18° |
| Cell loss | 0 | 1.38±0.18 | 0.12±0.12°° |
Mice were subcutaneously administered with cisplatin (12.7 mg/kg) and 24 h later BM-MSCs (2 × 105 cells) were given intravenously in the tail vein. Animals were killed at day 4 (4d). Renal function was measured as blood urea nitrogen (BUN). Renal histology was evaluated as periodic acid Shiff-positive droplets, cell debris in tubular lumens, and tubular degeneration consisting of brush border loss, nuclear changes, and vacuolization. BUN and histology data are mean values±s.e.m. and mean score±s.e.m., respectively. *P<0.01 versus control; °P<0.05, °°P<0.01 versus cisplatin+saline.
However, the mechanism responsible for the repair was still unclear. Indeed, in the face of a remarkable renoprotective and regenerative effect, in the kidney, the number of BM-MSCs was quantitatively very low, with an engraftment of PKH-26 MSC in the renal tissue that averaged 1.1±0.7 BM-MSC/105 renal cells.5 Moreover, the occasional differentiation of these cells into tubular cells could not explain and sustain per se such a therapeutic effect.
Two considerations have been useful to advance a working hypothesis on the mechanism for regeneration of tubular epithelium: the first is that growth factors have the leading role in induction of a proliferative repair,6, 7 and the second is that BM-MSCs are responsible for the secretion of multiple bioactive factors.8, 9, 10, 11 Particularly, in the kidney, studies on rats with ischemia/reperfusion injury have indicated that BM-MSCs-mediated renal repair was associated with a high renal production of growth factors such as hepatocyte growth factor, vascular growth factor, and insulin-like growth factor-1 (IGF-1).8 Moreover, the effect of BM-MSCs was accompanied by the tissue downregulation of proinflammatory cytokines and upregulation of prosurvival mediators.8 These reasons prompted us to hypothesize that MSCs afford protection primarily through a paracrine pathway, and to investigate, among potential candidates, the contribution of IGF-1 to tissue protection. IGF-1 is constitutively expressed and secreted by BM-MSCs,8, 12 possesses mitogenic and antiapoptotic properties,13, 14 and is implicated as an important mediator in kidney regeneration in models of AKI.13, 15, 16 Our studies showed that in vitro, murine BM-MSCs induce proliferation of cisplatin-damaged tubular cells and protect tubular cells from cisplatin-induced apoptosis via IGF-1.5 Direct blocking of IGF-1, by specific IGF-1 small interfering RNAs or by specific antibody, confirmed that BM-MSC-derived IGF-1 mediated the proliferation of cisplatin-damaged tubular cells (Figure 1). Although partially, the inhibition of cisplatin-induced apoptosis on proximal tubular cells was also found to be IGF-1 mediated (Figure 1). In vivo, we have found confirmation of this evidence. Administration of IGF-1 gene-silenced BM-MSCs failed to protect renal function and tubular structure of mice injured with cisplatin. When BM-MSC localization within the kidney was explored, these cells were preferentially found in peritubular areas, further supporting the notion that their mitogenic and antiapoptotic action was exerted through a paracrine mechanism. These findings have opened up new extensive investigations on the effects of BM-MSCs principally in the surroundings of the tubuli. In the future perspective to use BM-MSCs in clinic, we used MSCs obtained from human BM aspirates to treat cisplatin-injured immunodeficient nonobese diabetes/severe combined immunodeficiency mice.17 Similar to the corresponding murine cells, human BM-MSCs were engaged to engraft the kidney and to preserve its tubular integrity and renal function, ultimately leading to a proliferation and reduced apoptosis of tubular cells. Moreover, we reported a clear effect on survival of mice receiving human BM-MSCs as compared with AKI mice that were administered saline.17 It is fully recognized that the main target of cisplatin-induced damage is tubular epithelial cell with DNA damage, mitochondrial dysfunction, and reactive oxygen species production, followed by apoptosis. However, cisplatin also induces intrarenal vasoactive mediators18 and proinflammatory factors, specifically tumor necrosis factor-α, which perturbs the peritubular endothelium19 leading to inflammatory cell migration and leukocyte-mediated changes of vascular tone and perfusion. The persistent vasoconstriction and reduction of blood flow, which develop later than the tubular epithelial damage, have been suggested to amplify the deleterious effects of tubular cell injury in AKI.20 These observations were confirmed in our AKI experimental model, where peritubular capillaries were significantly reduced in volume density and diameter. Notably, human BM-MSCs treatment not only protected against tubular cell damage but also almost completely normalized the endothelium and lumen density, as well as the capillary diameters (Figure 2).17 These findings add new intriguing information about the role of MSCs in kidney recovery from AKI. Amelioration of hemodynamic changes, likely consequence of a more pervious capillary, should yield advantageous effects increasing tissue oxygenation, reducing endothelial cell activation, and preserving microvascular integrity. Finally, an active role of BM-MSCs on endothelial cells cannot be excluded considering that these cells might induce prosurvival pathways and antioxidant mechanisms. However, several of these pathways still need to find experimental confirmation. At the moment, the overall findings can only suggest a hypothesis for the complex link of BM-MSCs-mediated regeneration. Following MSC recruitment to damaged tissues, the recovery process starts and passes through MSC-secreted growth factors, one of which is certainly IGF-1, which promotes prosurvival pathways and stimulates tubular cell proliferation (Figure 3).
Figure 1.
Bone marrow-mesenchymal stem cells (BM-MSCs) stimulated proximal tubular cell (PTEC) proliferation and inhibited cisplatin-induced apoptosis via insulin-like growth factor-1 (IGF-1). (a) Proliferation of PTECs treated with cisplatin (2.5 μM, 6 h), alone or in coculture for 4 days with BM-MSCs transfected with irrelevant (irrel) or IGF-1 small interfering (si) RNAs. Blocking of IGF-1 by RNA silencing (si-IGF-1) led to a strong reduction in the proliferation of cisplatin-treated PTECs as compared with BM-MSCs transfected with si-irrel. °P<0.01 versus PTECs; *P<0.01 versus PTECs+cisplatin; #P<0.05 versus si-irrel BM-MSCs. Data are means±s.e.m. (b) Cisplatin-induced apoptosis on PTECs is reduced by BM-MSCs treatment via IGF-1. Untreated PTECs and cisplatin-treated PTECs, alone or in coculture for 4 days with si-irrel BM-MSCs or si-IGF-1 BM-MSCs, were analyzed by fluorescence-activated cell sorter to determine late apoptosis (expression of caspase 3 and 7, propidium iodide). °P<0.01 versus PTECs; *P<0.01 versus PTECs+cisplatin; #P<0.05 versus si-irrel BM-MSCs and PTECs+cisplatin. Data are means±s.e.m.
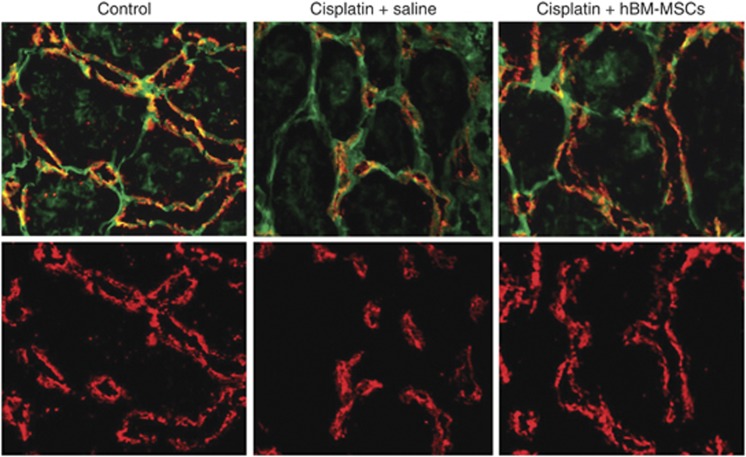
Figure 2.
Effect of human bone marrow-mesenchymal stem cells (hBM-MSCs) on peritubular capillaries in immunodeficient mice with cisplatin-induced acute kidney injury. Representative micrografts of kidney tissues of control mouse and cisplatin-treated mice that were administered saline or hBM-MSCs at 4 days after cisplatin. The peritubular capillary endothelium was labeled with MECA-32 (red), whereas renal structures were stained with fluorescein isothiocyanate-labeled lectin wheat germ agglutinin (green). Volume density of endothelial cells and capillary lumen was markedly reduced in cisplatin-treated mice that were administered saline as compared with control mice, as well as their capillary diameter. Treatment with hBM-MSCs normalized all these parameters. Original magnification, × 630.
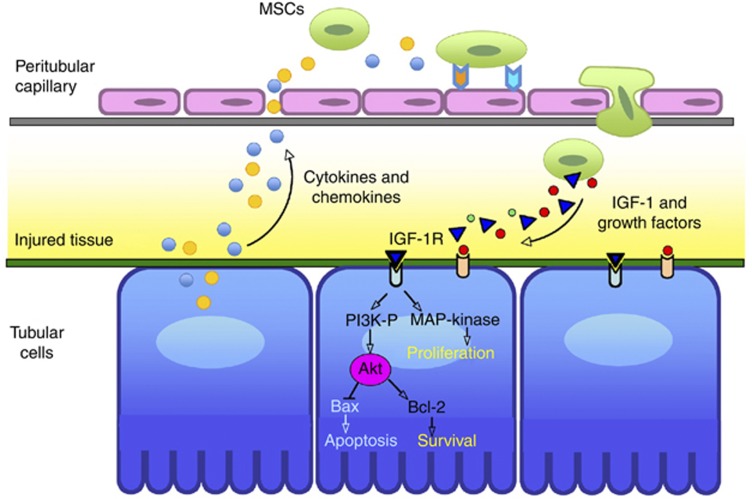
Figure 3.
Suggested mechanism for mesenchymal stem cell (MSC)-mediated tubular repair after acute injury. Administered MSCs are attracted to the site of injury following cytokine and chemokine release from damaged tubular cells. Recruited MSCs release growth factors such as insulin-like growth factor-1 (IGF-1), which may affect tubular functional and structural repair by induction of cell proliferation and inhibition of apoptosis. MAP-kinase, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase.
Studies conducted by our group on MSCs obtained from a different source, the human cord blood (CB-MSCs), confirmed and extended some of the findings highlighted for BM-MSCs.21 Specifically, data in murine model of cisplatin-induced AKI showed the great potential of CB-MSC in terms of protection from renal function impairment and remarkable prolongation of animal survival. CB-MSC treatment led to reduction of apoptosis and tubular cell proliferation. Moreover, the favorable effect on renal tissues was associated with inhibition of tubular oxidative damage, in terms of nitrotyrosine expression and induction of the phosphorylation of the prosurvival factor Akt.21 Similar to the MSCs derived from BM, CB-MSCs almost exclusively localized in the peritubular areas where they are likely to promote regeneration through paracrine action. In support to this statement are in vitro experiments showing that the proregenerative growth factors fibroblast growth factor, heparin binding-epidermal growth factor-like growth factor, vascular endothelial growth factor, and hepatocyte growth factor are increased in the supernatant of cisplatin-treated proximal tubular cells cocultured with CB-MSCs. The release of inflammatory cytokines, such as interleukin-1α and transforming growth factor-β, was found to be decreased.21
Studies that aimed to further clarify the mechanism responsible for renoprotection by MSCs obtained from different sources are now essential to support an educated answer to the question of which cell type will definitely represent the best therapeutic strategy for kidney regeneration.
Acknowledgments
We are really indebted to Professor Giuseppe Remuzzi for valuable comments and suggestions.
All the authors declared no competing interests.
Footnotes
TO CITE THIS ARTICLE: Imberti B, Morigi M, Benigni A. Potential of mesenchymal stem cells in the repair of tubular injury. Kidney inter., Suppl. 2011; 1: 90–93.
References
- Mitsiadis TA, Barrandon O, Rochat A, et al. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Forbes SJ, Hodivala-Dilke K, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- Imberti B, Morigi M, Tomasoni S, et al. Insulin-like growth factor-1 sustains stem cell-mediated renal recovery following an acute injury. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- Gobe G, Zhang XJ, Willgoss DA, et al. Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol. 2000;11:454–467. doi: 10.1681/ASN.V113454. [DOI] [PubMed] [Google Scholar]
- Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Abboud SL, Bethel CR, Aron DC. Secretion of insulinlike growth factor I and insulinlike growth factor-binding proteins by murine bone marrow stromal cells. J Clin Invest. 1991;88:470–475. doi: 10.1172/JCI115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kopple JD, Cohen A, et al. Recombinant human insulin-like growth factor-I accelerates recovery and reduces catabolism in rats with ischemic acute renal failure. J Clin Invest. 1993;91:2281–2287. doi: 10.1172/JCI116456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R, Ding H. Mechanisms of insulin-like growth factor-I-induced accelerated recovery in experimental ischemic acute renal failure. Miner Electrolyte Metab. 1998;24:211–219. doi: 10.1159/000057373. [DOI] [PubMed] [Google Scholar]
- Miller SB, Martin DR, Kissane J, et al. Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Natl Acad Sci USA. 1992;89:11876–11880. doi: 10.1073/pnas.89.24.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlaender M, Popovtzer MM, Weiss O, et al. Insulin-like growth factor-1 (IGF-1) enhances recovery from HgCl2-induced acute renal failure: the effects on renal IGF-1, IGF-1 receptor, and IGF-binding protein-1 mRNA. J Am Soc Nephrol. 1995;5:1782–1791. doi: 10.1681/ASN.V5101782. [DOI] [PubMed] [Google Scholar]
- Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- Huang Q, Dunn RT, II, Jayadev S, et al. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol Sci. 2001;63:196–207. doi: 10.1093/toxsci/63.2.196. [DOI] [PubMed] [Google Scholar]
- Zhang B, Ramesh G, Norbury CC, et al. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007;72:37–44. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- Morigi M, Rota C, Montemurro T, et al. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513–522. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]