Abstract
Patients with proteinuria are at high risk of cardiovascular and renal complications. Since this risk can be reduced by appropriate interventions, we hypothesized that remote dwellers, who are known to have lower access to health care, might have a higher risk of complications. Using a database of all adults with at least one measure of urine protein between May 2002 and March 2009, we examined the frequency of heavy proteinuria, quality of care delivery, and rates of adverse clinical outcomes across travel distance categories to the nearest nephrologist. Heavy proteinuria was defined by an albumin:creatinine ratio ⩾60 mg/mmol, protein:creatinine ratio ⩾100 mg/mmol, or protein ⩾2+ on dipstick urinalysis. Of 1,359,330 subjects in the study, 262,209 were remote dwellers. The overall prevalence of proteinuria was 2.3%, 2.9%, and 2.5% in those who live >200, 100.1–200, and 50.1–100 km, respectively, as compared to 1.5% in those who live within 50 km of the nearest nephrologist (P<0.001). Similarly, the prevalence of heavy proteinuria was increased among remote dwellers compared to urban dwellers (P=0.001 for trend). There were no differences in markers of good-quality care or the rate of adverse outcomes (all-cause mortality, heart failure, and renal outcomes) across distance categories. However, the rates of hospitalizations and stroke were significantly higher with increased distance from the nearest nephrologist (P<0.001and 0.02, respectively). In conclusion, heavy proteinuria was common in Alberta residents, especially in remote dwellers. Care seemed similar across distance categories of travel, but with higher risk of hospitalizations and stroke among remote dwellers. Further work is needed to understand the basis for the increased risk of hospitalizations and stroke.
Keywords: adverse clinical outcomes, population, proteinuria, quality of care, remote dwellers
INTRODUCTION
Increased urinary protein excretion is a risk factor for progression of chronic kidney disease (CKD) and is strongly associated with adverse cardiovascular outcomes.1, 2, 3, 4 The presence of proteinuria markedly increases cardiovascular and renal risk at any level of estimated glomerular filtration rate (eGFR) and identifies additional people who are at high risk despite normal or nearly normal eGFR.5 Proteinuria measurements have thus been used to identify patients who are most likely to benefit from treatment using current renoprotective strategies.1, 6, 7 These data highlight the importance of proteinuria as a prognostic marker in patients with CKD and also as a potential tool for guiding treatment.
Studies in patients with chronic diseases (including CKD) have suggested that remote-dwelling patients are at particularly high risk for suboptimal care and adverse outcomes—due in part to documented gaps between recommended practice and real-world clinical performance.7, 8, 9 This issue is especially germane for large countries such as Canada, where rural/remote residence is common and nephrologists often practice only in larger centers.
We sought to determine the prevalence of heavy proteinuria among remote dwellers in Alberta, investigate the association between remoteness and markers of good-quality care, and assess the association between such markers and clinical outcomes.
RESULTS
Of 3,897,684 eligible patients, 2,441,306 were excluded due to the absence of a measure of proteinuria (n=1,885,976) or eGFR (n=480,671), being underaged (n=69,102), and death out of province or having stage 5 CKD prior to the study start date (n=5557). The baseline demographic and clinical characteristics of the study population are shown in Table 1. Across distance categories, one-fifth of the population resided in remote areas >50 km from the closest nephrologist (Table 1). Remote dwellers were slightly older, were more likely to be Aboriginal, and were more likely to have hypertension, diabetes, and more advanced CKD compared to urban dwellers.
Table 1. Demographic and clinical characteristics of participants by distance to closest nephrologist.
| Urban | Rural | P | 0–50 km | 50.1–100 km | 100.1–200 km | >200 km | P for trend | |
|---|---|---|---|---|---|---|---|---|
| Na | 1,205,760 (88.8) | 151,689 (11.2) | — | 1,097,121 (80.7) | 106,326 (7.8) | 61,068 (4.5) | 94,815 (7.0) | — |
| eGFR, ml/min per 1.73 m2 | ||||||||
| ⩾60 | 1,115,496 (92.5) | 138,365 (91.2) | <0.001 | 1,018,217 (92.8) | 94,658 (89) | 54,604 (89.4) | 88,135 (93.0) | <0.001 |
| 45–59.9 | 61,208 (5.1) | 9054 (6.0) | <0.001 | 54,142 (4.9) | 7701 (7.2) | 4153 (6.8) | 4348 (4.6) | 0.56 |
| 30–44.9 | 22,182 (1.8) | 3238 (2.1) | <0.001 | 19,047 (1.7) | 2977 (2.8) | 1724 (2.8) | 1706 (1.8) | <0.001 |
| 15–29.9 | 6874 (0.6) | 1032 (0.7) | <0.001 | 5715 (0.5) | 990 (0.9) | 587 (1.0) | 626 (0.7) | <0.001 |
| Age, years | 47.7 (36, 59.8) | 51.4 (40.4, 62.8) | <0.001 | 47.6 (36, 59.5) | 53.0 (41.4, 65.4) | 52.2 (39.9, 65) | 47.0 (34.9, 58.2) | <0.001 |
| Male | 560,004 (46.4) | 72,118 (47.5) | <0.001 | 510,735 (46.6) | 49,317 (46.4) | 28,408 (46.5) | 44,527 (47) | 0.02 |
| Aboriginal | 16,296 (1.4) | 10,401 (6.9) | <0.001 | 13,434 (1.2) | 5707 (5.4) | 2070 (3.4) | 5570 (5.9) | <0.001 |
| Social assistance | 36,619 (3) | 3669 (2.4) | <0.001 | 32,882 (3.0) | 3198 (3.0) | 1922 (3.1) | 2325 (2.5) | <0.001 |
| Comorbidities | ||||||||
| Charlson scoreb | 0 (0, 1) | 0 (0, 1) | <0.001 | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Diabetes | 94,734 (7.9) | 15,650 (10.3) | <0.001 | 82,451 (7.5) | 12,089 (11.4) | 7087 (11.6) | 8928 (9.4) | <0.001 |
| Hypertension | 292,255 (24.2) | 44,683 (29.5) | <0.001 | 258,990 (23.6) | 34,818 (32.7) | 19,857 (32.5) | 23,736 (25.0) | <0.001 |
Abbreviations: AIDS, acquired immune deficiency syndrome; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula; HIV, human immunodeficiency virus; PVD, peripheral vascular disease.
1925 participants could not be classified according to urban or rural status.
Charlson score includes AIDS/HIV, metastatic cancers, non-metastatic cancers, cerebral vascular disease, chronic obstructive pulmonary disease, dementia, diabetes, heart failure, mild liver disease, moderate/severe liver disease, myocardial infarction, paraplegia, peptic ulcer, peripheral vascular disease, and rheumatological disease. N (%) or the median and inter-quartile range are presented.
Prevalence of heavy proteinuria
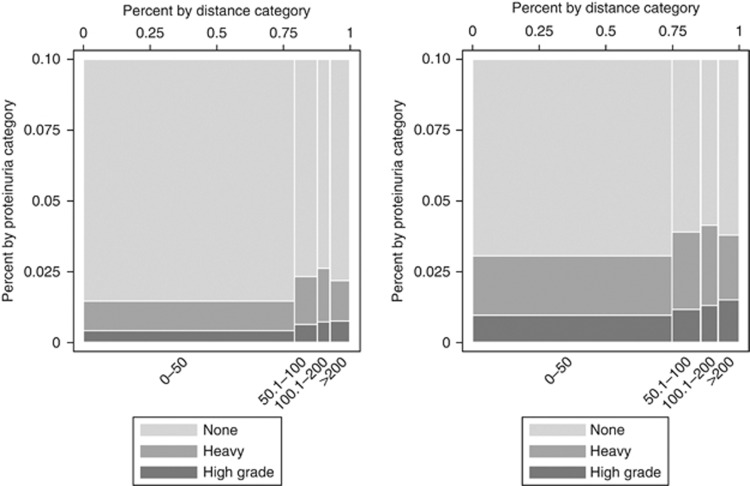
The overall prevalence of heavy proteinuria was 2.3%, 2.9%, and 2.5% in those who live >200, 100.1–200, and 50.1–100 km, respectively (P<0.001), as compared to 1.5% in those who live within 50 km of the closest nephrologist (Figure 1). The prevalence was 1.6% and 2.4% among urban and rural dwellers, respectively (P<0.001). The prevalence of proteinuria (using several definitions) was higher across all stages of CKD in the remote dwellers compared to the urban dwellers (Table 2).
Figure 1.
Prevalence of heavy proteinuria by distance to the closest nephrologist. The x-axis represents the travel distance categories (km) with the width of each bar representing the proportion of participants (%) in each distance category. The y-axis represents the distribution of the various categories of proteinuria (%) (none, heavy, high grade). The height of each colored segment within a bar represents the proportion of participants in that category of proteinuria. None=no proteinuria; heavy=heavy proteinuria (ACR⩾60 mg/mmol, PCR⩾100 mg/mmol, or protein ⩾2+ on dipstick urinalysis); high grade=high-grade proteinuria (ACR⩾180 mg/mmol, PCR⩾300 mg/mmol, or protein ⩾3+ dipstick on urinalysis). The left panel shows all participants (N=1,359,330). The right panel shows participants at high risk for proteinuric CKD (N=394,354).
Table 2. Prevalence of clinically relevant proteinuria by distance to the closest nephrologist.
| Events (%) | Urban | Rural | P | 0–50 km | 50.1–100 km | 100.1–200 km | >200 km | P for trend |
|---|---|---|---|---|---|---|---|---|
| Heavy proteinuria | 19,399 (1.6) | 3673 (2.4) | <0.001 | 16,278 (1.5) | 2755 (2.5) | 1829 (2.9) | 2244 (2.3) | <0.001 |
| Proteinuria by eGFR | ||||||||
| ⩾60 | 13,331 (1.2) | 2522 (1.8) | <0.001 | 11,175 (1.1) | 1839 (1.9) | 1241 (2.2) | 1618 (1.8) | <0.001 |
| 45–59.9 | 2560 (4.1) | 521 (5.6) | <0.001 | 2132 (3.8) | 402 (5.1) | 276 (6.4) | 278 (6.2) | <0.001 |
| 30–44.9 | 2024 (8.8) | 363 (10.8) | <0.001 | 1731 (8.8) | 271 (8.9) | 189 (10.6) | 200 (11.1) | <0.001 |
| 15–29.9 | 1484 (20.9) | 267 (24.7) | 0.004 | 1240 (21) | 243 (23.5) | 123 (20.1) | 148 (22.9) | 0.28 |
| Persistent proteinuria | 9077 (0.7) | 1646 (1.1) | <0.001 | 7713 (.7) | 1341 (1.2) | 786 (1.3) | 901 (0.9) | <0.001 |
| Proteinuria as defined by ACR or PCR only | 4088 (0.3) | 773 (0.5) | <0.001 | 3534 (0.3) | 717 (0.7) | 326 (0.5) | 291 (0.3) | 0.13 |
| High-grade proteinuria | 5624 (0.5) | 1087 (0.7) | <0.001 | 4672 (0.4) | 754 (0.7) | 510 (0.8) | 789 (0.8) | <0.001 |
| Proteinuria in high-riska groups | 11,045 (3.2) | 2183 (4.1) | <0.001 | 9233 (3) | 1698 (4.1) | 1109 (4.7) | 1208 (4.1) | <0.001 |
| Proteinuria as defined by ACR or PCR only in high-risk groups | 3621 (1) | 711 (1.3) | <0.001 | 3127 (1) | 658 (1.6) | 296 (1.2) | 257 (0.9) | 0.42 |
| Incident proteinuria in high-risk groups | 8371 (2.4) | 1708 (3.2) | <0.001 | 6866 (2.2) | 1384 (3.3) | 956 (4) | 891 (3.1) | <0.001 |
Abbreviations: ACR, albumin:creatinine ratio; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula; PCR, protein:creatinine ratio.
Those with diabetes, hypertension, coronary disease, peripheral vascular disease, and/or eGFR<60 ml/min per 1.73 m2.
Heavy proteinuria=presence of ACR ⩾60 mg/mmol, PCR ⩾100 mg/mmol or protein ⩾2+ on dipstick urinalysis.
High-grade proteinuria=presence of ACR ⩾180 mg/mmol, PCR ⩾300 mg/mmol or protein ⩾3+ dipstick on urinalysis.
Persistent proteinuria defined as two or more measurements demonstrating proteinuria within 6 months of the index date.
Guideline-recommended care in patients with heavy proteinuria
There was no significant negative association between the presence of heavy proteinuria and markers of quality care and remoteness or rural residence (Table 3).
Table 3. Care and clinical outcomes by distance to the closest nephrologist in subjects with heavy proteinuria.
| Events/N | Rural | 0-50 km | 50.1–100 km | 100.1–200 km | >200 km | P for trend | |
|---|---|---|---|---|---|---|---|
| ACEi/ARB use in ⩾65 years | 4128/7760 | 0.85 (0.74, 0.96) | 1.0 | 0.88 (0.76, 1.01) | 0.84 (0.71, 0.99) | 1.04 (0.87, 1.24) | 0.97 |
| Statin use in ⩾65 years | 2468/7760 | 0.99 (0.87, 1.14) | 1.0 | 1.00 (0.86, 1.16) | 0.98 (0.83, 1.17) | 1.00 (0.83, 1.20) | 0.98 |
| Timely referral | 4602/22,599 | 0.72 (0.63, 0.83) | 1.0 | 0.82 (0.71, 0.94) | 0.52 (0.43, 0.63) | 0.46 (0.38, 0.56) | <0.001 |
| HR (95% CI) | |||||||
| All-cause mortality | 4307/22,599 | 0.99 (0.91, 1.08) | 1.0 | 1.15 (1.05, 1.27) | 1.10 (0.98, 1.23) | 1.04 (0.93, 1.17) | 0.32 |
| Myocardial infarction | 675/22,599 | 0.78 (0.62, 0.99) | 1.0 | 1.00 (0.78, 1.27) | 0.62 (0.42, 0.90) | 1.12 (0.86, 1.46) | 0.68 |
| Stroke | 600/22,599 | 1.13 (0.90, 1.41) | 1.0 | 1.19 (0.93, 1.53) | 1.37 (1.03, 1.83) | 1.35 (1.03, 1.78) | 0.02 |
| Heart failure | 1120/22,599 | 1.07 (0.91, 1.26) | 1.0 | 1.23 (1.03, 1.47) | 0.95 (0.75, 1.20) | 0.89 (0.70, 1.12) | 0.31 |
| Doubling of SCr | 1350/22,599 | 1.00 (0.85, 1.16) | 1.0 | 1.17 (0.98, 1.38) | 1.11 (0.89, 1.39) | 1.06 (0.88, 1.29) | 0.47 |
| ESRDa | 1927/22,599 | 0.91 (0.79, 1.03) | 1.0 | 1.13 (0.98, 1.30) | 0.83 (0.68, 1.02) | 1.03 (0.87, 1.21) | 0.93 |
| Relative rate (95% CI) | |||||||
| Hospitalizations | 34,481/22,599 | 1.33 (1.29, 1.38) | 1.0 | 1.54 (1.49, 1.59) | 1.58 (1.52, 1.65) | 1.57 (1.51, 1.64) | <0.001 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin:creatinine ratio; ARB, angiotensin receptor blocker; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula; ESRD, end-stage renal disease; HR, hazard ratio; OR, odds ratio; PCR, protein:creatinine ratio; SCr, serum creatinine ratio.
Includes eGFR <15 ml/min per 1.73 m2.
Values are shown as OR (95% CI) unless otherwise indicated.
Results were adjusted for eGFR (⩾60, 45–59.9,30–44.9,15–29.9), age (18–49.9, 50–69.9, ⩾70), gender, aboriginal, social assistance, and comorbidities (Charlson score, hypertension).
Clinical outcomes among those with heavy proteinuria
The clinical outcomes of all-cause mortality, myocardial infarction, stroke, heart failure, doubling of serum creatinine (Scr), and end-stage renal disease (ESRD) occurred overall in 4307 (19.1), 675 (3.0), 600 (2.7), 1120 (5.0), 1350 (6.0), and 1927 (8.5) patients, respectively. There were no significant differences in the likelihood of all-cause mortality, myocardial infarction, heart failure, doubling of Scr, and development of ESRD across the travel distance categories (Table 3); however, there was a higher incidence of stroke in those travelling a greater distance to the nephrologist (hazards ratio (HR): 1.37 (95% confidence interval 1.03–1.83)) in the 100.1–200 km distance category; and HR: 1.35 (1.03–1.78) in the >200 km distance category; P for trend 0.02. The all-cause hospitalization rate was significantly greater in remote dwellers as compared with urban dwellers (relative rate: 1.33 (1.29–1.38); P<0.001).
Sensitivity analysis
Sensitivity analyses on the subgroup of subjects with diabetes and heavy proteinuria showed similar results (data not shown).
DISCUSSION
This study examined the burden of heavy proteinuria—focusing on the link between quality of care and clinical outcomes in people with this condition who live in remote Alberta communities. We aimed to identify opportunities to improve clinical outcomes in remote dwellers with heavy proteinuria. In this study of over 1.3 million people, we found that the prevalence of heavy proteinuria is especially common in people living in rural and remote areas of Alberta. Although markers of high-quality care (i.e. use of angiotensin-converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs) and statins) were equally common in remote dwellers and urban dwellers, we noted an increased risk of stroke and all-cause hospitalizations in remote dwellers.
In previous studies, we have demonstrated that markers of good-quality care are less prevalent among rural and remote dwellers with non-dialysis-dependent CKD, and among remote dwellers with ESRD,9, 10 and that remote dwellers also have worse outcomes as compared to otherwise similar clinical outcomes. What this study adds to the existing literature is the finding that heavy proteinuria is common in the community and even more common in remote dwellers. However, unlike the general CKD population, gaps in care are equally pronounced in both remote and urban dwellers. This information has significant implications for policy-makers in planning clinical care for patients with proteinuria and CKD living in remote locations of Alberta and elsewhere. Specifically, since remote dwellers have a higher burden of heavy proteinuria, strategies aimed at improving care in this population will have to take into account the additional barriers to care faced by rural dwellers.8 The large numbers of affected people suggest that nephrologists will be unable to address the problem alone. For example, decision makers might consider co-management of patients by community practitioners (including primary-care physicians, community health workers, nurse practitioners) using pre-specified management guidelines and/or protocols.
Why did remote dwellers have a higher frequency of proteinuria? The major established risk factors for proteinuria include diabetes, hypertension, obesity, cardiovascular disease, smoking, age, and race. In our study, the remote dwellers were indeed older and more likely to have diabetes, hypertension, and to be Aboriginal than the urban dwellers. Of note, our sensitivity analyses stratified based on presence/absence of diabetes did not show any significant differences on quality-of-care delivery and clinical outcomes in the study population.
Our study has several potential strengths. First, it was a population-based study of a single Canadian province, involving a relatively homogenous population. Second, it included more than 1.3 million subjects from which individuals with heavy proteinuria were identified. However, our study also has some limitations, including the known inaccuracies of urine dipstick analysis, and the fact that most subjects in the study had only a single measurement of proteinuria and eGFR. However, results were similar in the subset of participants with multiple measures and/or persistent proteinuria.
In conclusion, heavy proteinuria is common in Alberta residents, especially in remote dwellers. Given the higher risk of adverse outcomes in those with proteinuria, strategies aimed at improving care in this high-risk population will have to take into account the additional barriers to care faced by the remote dwellers. Care and outcomes seems similar across categories of travel distance, but with higher risk of hospitalizations and stroke among remote dwellers. This has policy and practice implications for CKD care in remote communities, and further work is needed to understand the basis of increased risk of hospitalizations and stroke, which may be partly related to a higher burden of proteinuria and comorbidities among the remote dwellers.
METHODS
Population and data sources
We studied all adults, 18 years and older, residing in Alberta with at least one measure of urine protein (albumin:creatinine ratio (ACR), protein:creatinine ratio (PCR), or protein dipstick urinalysis) and a measure of Scr concentration between May 2002 and March 2009. Participants with ESRD (eGFR<15 ml/min per 1.73 m2; chronic dialysis; prior kidney transplant) at baseline were excluded. Data were drawn from Alberta Health and Wellness, Alberta Blue Cross, the Northern and Southern Alberta Renal Programs (NARP and SARP), and the provincial laboratories of Alberta.12
Definitions and classifications
Heavy proteinuria was defined by the presence of ACR ⩾60 mg/mmol, PCR ⩾100 mg/mmol,13 or protein ⩾2+ on dipstick urinalysis. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and categorized as ⩾60, 45–59.9, 30–44.9, and 15–29.9 ml/min per 1.73 m2. In a sensitivity analysis, we evaluated persistent proteinuria, defined as two or more measurements demonstrating proteinuria within 6 months of the index date, and high-grade proteinuria, defined as the presence of ACR ⩾180 mg/mmol, PCR ⩾300 mg/mmol, or protein ⩾3+ on dipstick urinalysis.
Demographic variables included age (categorized as 18–49.9, 50–69.9, and ⩾70), gender, Aboriginal (registered First Nations or recognized Inuit), and social assistance. We used validated algorithms to define the Charlson comorbidities and hypertension14 using the AHW physician claims and hospitalization databases. The Charlson score was based on the Deyo classification15 of the following comorbidities: cerebrovascular disease, peripheral vascular disease, congestive heart failure, cancer, COPD, dementia, diabetes with and without complications, AIDS/HIV, metastatic solid tumor, myocardial infarction, mild liver disease, moderate/severe liver disease, paralysis, peptic ulcer disease, and rheumatic disease.
Evaluation of residence location
We calculated the geographic coordinates for each patient's residence using the Canadian Postal Code conversion file (PCCF),16 and determined the practice location of the closest nephrologist and closest internal medicine specialist. The geographic coordinates for each 6-digit postal code were determined using the Statistics Canada PCCF (www.statcan.ca). These coordinates were entered into ESRI ArcInfo 9.3 software (www.esri.com) to determine the shortest distance by road (in km) between the residence of each patient and the practice location of the closest specialist. As in our previous work, we categorized driving distance to the closest specialist into the following a priori categories: 0–50, 50.1–100, 100.1–200, and >200 km.10 Rural or urban residence was defined at the postal code level using the Statistics Canada definition as recorded in the PCCF.
Markers of good quality of care (process-based outcomes) among patients with proteinuric CKD
Markers of good quality of care were: referral to any nephrologist within 18 months of the index date, ACEi or ARB, and statin usage. Prescription use was evaluated in the subset of participants aged 65 years and above, all of whom had government-sponsored drug insurance. Medication usage was defined as at least one prescription within 6 months of the index date.
Clinical outcomes
Clinical outcomes included all-cause mortality; number of hospitalizations; cardiovascular events including heart failure, myocardial infarction, and stroke; ESRD; and sustained doubling of Scr concentration (a surrogate measure for progressive kidney disease) as previously defined.5
Statistical analyses
The analyses were done with Stata/MP 11.1 (www.stata.com). Baseline descriptive statistics were reported as counts and percentages, or medians and interquartile ranges, as appropriate. Prevalence of heavy proteinuria was calculated overall and for CKD-EPI eGFR subgroups, by distance to the closest nephrologist, and rural or urban residence. In sensitivity analyses, heavy proteinuria was defined by ACR or PCR measurements alone.
The associations between distance and quality-of-care outcomes were estimated using logistic, Cox, and Poisson regression models as appropriate. Follow-up was censored when a participant died, moved out of province, or was at the end of study (March 2009). Models were adjusted for all variables presented in Table 1. The threshold P for statistical significance was set at 0.05. We did sensitivity analyses on the subgroup of subjects with diabetes and proteinuria.
Acknowledgments
This work was supported by an interdisciplinary team grant from the Alberta Heritage Foundation for Medical Research (AHFMR), the Kidney Foundation of Canada, University Hospital Foundation (UHF), University of Alberta Hospital, Edmonton, AB. Drs Bello and Thompson were supported by the Division of Nephrology, Department of Medicine, University of Alberta. Dr Tonelli was supported by a Government of Canada Research Chair in the optimal care of people with chronic kidney disease. Drs Tonelli, Hemmelgarn and Klarenbach were supported by career salary awards from AHFMR. Drs Hemmelgarn, Klarenbach, Manns and Tonelli were all supported by a joint initiative between Alberta Health and Wellness and the Universities of Alberta and Calgary. Publication of this article was supported in part by the National Health and Medical Research Council of Australia through an Australia Fellowship Award (511081: theme Chronic Disease in High Risk Populations) to Dr Wendy Hoy, School of Medicine, the University of Queensland, and the National Institutes of Health—NIDDK DK079709, NCRR RR026138, and NIMHD MD000182.
DISCLOSURE
All the authors declared no competing interests.
References
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde M, Halbesma N, de Charro FT, et al. Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol. 2009;20:852–862. doi: 10.1681/ASN.2008060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Chen G, Campbell NR, et al. Regional variations in not treating diagnosed hypertension in Canada. Can J Cardiol. 2010;26:409–413. doi: 10.1016/s0828-282x(10)70434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean G, Guthrie B, Sutton M. Differences in the quality of primary medical care services by remoteness from urban settlements. Qual Saf Health Care. 2007;16:446–449. doi: 10.1136/qshc.2006.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli M, Hemmelgarn B, Culleton B, et al. Mortality of Canadians treated by peritoneal dialysis in remote locations. Kidney Int. 2007;72:1023–1028. doi: 10.1038/sj.ki.5002443. [DOI] [PubMed] [Google Scholar]
- Rucker D, Hemmelgarn BR, Lin M, et al. Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int. 2011;79:210–217. doi: 10.1038/ki.2010.376. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D. Albuminuria, not only a cardiovascular/renal risk marker, but also a target for treatment. Kidney Int Suppl. 2004;92:S2–S6. doi: 10.1111/j.1523-1755.2004.09201.x. [DOI] [PubMed] [Google Scholar]
- Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. doi: 10.1186/1471-2369-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–1162. doi: 10.1503/cmaj.080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423–1428. doi: 10.1161/HYPERTENSIONAHA.109.139279. [DOI] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Ng E, Wilkins R, Perras A. How far is it to the nearest hospital? Calculating distances using the Statistics Canada Postal Code Conversion File. Health Rep. 1993;5:179–188. [PubMed] [Google Scholar]