Abstract
Reconfiguration of extracellular matrix proteins appears to be necessary for the synaptic plasticity that underlies memory consolidation. The primary candidates involved in controlling this process are a family of endopeptidases called matrix metalloproteinases (MMPs); however, the potential role of MMPs in nicotine addiction-related memories has not been adequately tested. Present results indicate transient changes in hippocampal MMP-2, -3, and -9 expression following context dependent learning of nicotine-induced conditioned place preference (CPP). Members of a CPP procedural control group also indicated similar MMP changes, suggesting that memory activation occurred in these animals as well. However, hippocampal MMP-9 expression was differentially elevated in members of the nicotine-induced CPP group on days 4 and 5 of training. Inhibition of MMPs using a broad spectrum MMP inhibitor (FN439) during nicotine-induced CPP training blocked the acquisition of CPP. Elevations in hippocampal and prefrontal cortex MMP-3 expression—but not MMP-2 and -9—accompanied reactivation of a previously learned drug related memory. Decreases in the actin regulatory cytoskeletal protein cortactin were measured in the HIP and PFC during the initial two days of acquisition of CPP; however, no changes were seen following re-exposure to the drug related environment. These results suggest that MMP-9 may be involved in facilitating the intracellular and extracellular events required for the synaptic plasticity underlying the acquisition of nicotine-induced CPP. Furthermore, MMP-3 appears to be important during re-exposure to the drug associated environment. However, rats introduced into the CPP apparatus and given injections of vehicle rather than nicotine during training also revealed a pattern of MMP expression similar to nicotine-induced CPP animals.
Keywords: nicotine, conditioned place preference, matrix metalloproteinases, MMP-3, cortactin, FN439, relapse, adolescent rats
Introduction
Clinical reports indicate that human females are more susceptible to nicotine addiction and have greater difficulty quitting the use of tobacco products than males.1–4 Adolescent females are particularly vulnerable to nicotine addiction,5,6 as are young female rats.7–11 Conditioned place preference (CPP) is a frequently utilized protocol for determining the dependence and reward potential of a compound in animal models;12,13 however, there have been conflicting results concerning nicotine-induced CPP, particularly as related to dose. A wide range of doses have been reported to produce CPP in rats,14–18 with CPP absent at doses lower than 0.1 and higher than 1 mg/kg.19– 26 We recently determined that adolescent female Sprague-Dawley rats showed CPP to a nicotine dose of 0.03 mg/kg,27 significantly lower than the optimal dose of 0.6 mg/kg seen with adolescent female Wistar rats, as reported by Torres and colleagues.18
The addictive process appears to depend upon underlying alterations in the structural remodeling of synaptic connections.28,29 Thus, drugs of abuse modify learning-dependent changes in synaptic structure and function such that even after prolonged abstinence drug related cues are sufficiently salient to trigger drug seeking behavior.30–32 Once memories are consolidated they continue to be subject to reconsolidation following reactivation.33–35 Recent studies have shown that the disruption of the biochemical processes required for reconsolidation leads to an attenuation of drug seeking behavior.25,36–38 Thus, targeting the molecules involved in memory consolidation and reconsolidation, (eg, matrix metalloproteinases [MMPs]), could represent a useful strategy for blunting persistent drug dependence. MMPs are known to modify the extracellular matrix (ECM) proteins involved in regulating cytoskeletal reorganization and transcriptional activity; they also modify activation of proteases such as plasmin.39–42 We reasoned that if changes in MMP expression are required for the consolidation/reconsolidation of drug-related memories, then the inhibition of MMP activity may disrupt the development of drug dependence and context dependent relapse.
The present investigation measured MMP-2, -3, and -9 expression, and the intracellular cytoskeletal protein cortactin,43,44 in the hippocampus (HIP) and prefrontal cortex (PFC) during acquisition and context dependent relapse of nicotine-induced CPP in adolescent female rats. We selected these MMPs based on previous work from our laboratory and other laboratories indicating their importance in learning and memory. Furthermore, these MMPs are the most abundant in the mammalian brain,45,46 and Cortactin has also been implicated in memory consolidation.47 Based on previous results, the intracerebroventricular (ICV) infusion of the broad spectrum MMP inhibitor, FN439, was predicted to disrupt acquisition of drug associated learning and memory.48,49
Materials and Methods
All experiments adhered to the Guidelines for the Care and Use of Laboratory Animals, as required by the National Institutes of Health (NIH Publication No. 80–23); the protocols were approved by the Washington State University Institutional Animal Care and Use Committee.
Subjects
Four-week-old female Sprague-Dawley rats (breeding stock derived from Taconic, Germantown, NY) were housed 4–5 per cage in a room controlled for temperature and humidity and adapted to a 12 hour light– dark cycle. The cycle was initiated at 0600 hours in an American Association of Laboratory Animal Care approved vivarium at a temperature of 21 °C ± 1 °C. All animals had ad libitum access to Purina laboratory rat chow and water, except the night before surgery when food was removed from the cage.
Reagents
Nicotine hydrogen tartrate salt and FN439 were purchased from Sigma-Aldrich (Catalog # N5260 and #A4336, respectively, St. Louis, MO).
Surgical protocol
Rats were anesthetized using ketamine hydrochloride plus xylazine (100 and 2 mg/kg respectively, i.m.) and a chronic ICV guide cannula was positioned as described by Pederson et al.50 The scalp was shaved and cleaned with Betadine and 70% alcohol. A 4 cm midline incision superior on the skull was made and the scalp retracted. A trephine hole was placed with flat skull coordinates of 1 mm posterior to bregma and 1.5 mm lateral to the midline, per the atlas of Paxinos and Watson.51 A guide cannula fashioned from PE-60 tubing (Clay Adams, Parsippany, NJ), prepared with a heat bulge 2.5 mm above its beveled tip, was stereotaxically inserted and held in place using skull screws (#1–72 × 3/16′, Small Parts, Miami Lakes, FL) and dental acrylic (Lang Dental Supply, Wheeling, IL). The animals were allowed to recover for 5 days. Prior to CPP training, cannula placement was confirmed by a drinking response elicited by the ICV administration of angiotensin II (Catalog # A9525, Sigma-Aldrich, 100 pmol in 2 μl sterile artificial cerebrospinal fluid vehicle [aCSF: 124 mm NaCl, 3 mm KCl, 1.24 mm Na2PO4, 1.3 mm MgSO4, 2.0 mm CaCl2, 26 mm NaHCO3, 10 mm d-glucose] with pH set at 7.4). This dose of angiotensin II generally elicits a very reliable drinking response within 5 minutes of infusion if guide cannula placement is correct. Non-responding animals were removed from the study.52 We did not have to remove any rats from this study based on this testing criterion. All ICV infusions were accomplished using a hand held 10 μl Hamilton syringe connected by a 30 cm length of PE-20 tubing to a stainless steel tubing injector (31 gauge, 5.8 cm in length). Once inserted into the guide cannula the injector extended 3 mm beyond its tip, thus penetrating the roof of the lateral ventricle. Cannulas, injectors, and tubing were sterilized by overnight placement in CidexPlus (Johnson & Johnson, Arlington, TX). The infusant was delivered over a duration of 20 seconds. The injector was left in place for an additional 30 seconds before removal to avoid back flow.
Conditioned place preference protocol
The CPP apparatus consisted of a box 64 (L) × 20.5 (W) × 40 (H) cm constructed of wood and Plexiglas with two main compartments (28 × 20.5 cm) separated by a smaller compartment (8 × 20.5 cm). One of the main compartments was painted black and the other white. The black compartment had wire mesh flooring (1.2 cm squares) and the white compartment’s floor consisted of parallel metal rods (diameter = 4.8 mm) spaced 1 cm apart. The central compartment had a black wooden floor. A 15 W lamp was placed over the black compartment to compensate for high initial preference. A video camera was placed directly over the apparatus to record the activity of the rat. The camera was connected to a computer which recorded the activity; this activity was interpreted by video tracking software that provided quantifiable information on locomotive activity, time spent in each compartment, and number of entries into a compartment (San Diego Instruments software package). A biased paradigm was used in which the animal was assigned to the non-preferred compartment following nicotine administration. This protocol is thought to be more effective at producing CPP than the unbiased procedure in which the animal is randomly assigned to a chamber after nicotine injection.47–49
During preconditioning the rats were placed in the middle compartment and allowed free access to the entire box for 15 minutes. The time spent in each compartment was noted. The animals underwent preconditioning for 2 days and the mean of the two sessions for each animal was calculated for each compartment.
The conditioning phase began the day following preconditioning and at the same time of day for each animal. Three major groups of rats received two conditioning sessions per day, for 5 consecutive days. During one session members of Group 1 (FN439-Nic) were ICV infused with FN439 (14 μg in 2 μl of aCSF over 20 seconds, and then a delay of 30 seconds prior to removal of the injector), followed 15 minutes later by an injection of nicotine (0.03 mg/kg s.c. in 1 ml/kg sterile phosphate buffered saline [PBS], pH adjusted to 7.4). The animal was then placed in the non-preferred compartment for 15 minutes. During the other session each animal received an ICV injection of aCSF (2 μl over 20 seconds, followed by a delay of 30 seconds) with an injection of PBS (1 ml/kg s.c.) 15 minutes later. The animal was then placed in the preferred compartment for 15 minutes. These two sessions were counter-balanced and were separated by 3 hours. Members of Group 2 (aCSF-Nic) were treated equivalently to the rats of Group 1; however, each rat received an ICV injection of aCSF (2 μl over 20 seconds) during both sessions followed by nicotine. Members of Group 3 (aCSF-PBS) served as procedural controls and received ICV injections of aCSF (2 μl over 20 seconds) followed 15 minutes later by injections of PBS (1 ml/kg s.c.) during both sessions. Subgroups of 4–8 rats from each group were euthanized 3 hours following the second training session on each of the 5 conditioning days, in order to determine MMP-2, -3, -9 and cortactin levels in the HIP and PFC. The remaining animals from each group were tested for CPP on day 6. All animals in each subgroup were evaluated for MMP and cortactin levels. Naïve home cage control rats were included with each subgroup and their mean MMP and cortactin levels were utilized as the 100% normalized level.
During the post-conditioning phase (day 6) each rat was tested for CPP in a drug-free state. Each rat was placed in the middle compartment and allowed free access to the box for 15 minutes. Time spent in each compartment was measured.
To test for relapse the rats were maintained in their home cages for an additional 5 days without drug injection. On the following day (day 12) each animal was placed in the CPP apparatus in a drug-free state for 15 minutes. Times spent in the nicotine-paired and vehicle-paired compartments were measured. Three hours following behavioral testing these animals were also euthanized and MMP and cortactin levels were measured.
Western immunoblotting
Immediately following euthanization (by guillotine) the HIP and PFC from each hemisphere were extracted on ice. The hemispheres were separated by scalpel blade and each hippocampus was isolated using a tephlon-coated spatula and micro scissors (Roboz Instruments). The extreme anterior 1 cm portion of the prefrontal cortex was dissected (vertical cut) from each hemisphere. These tissues were immediately frozen in liquid nitrogen and stored at −80 °C until all samples were collected. Tissues were then weighed and homogenized on ice in homogenization buffer (50 mM Tris HCl pH 7.6, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij 35, 0.02% NaN3). Homogenates were centrifuged at 12000 RCF for 10 minutes at 4 °C and the supernatant was retained for immunoblotting. 20 μl of the supernatant were mixed 1:1 with 2 × Laemlli sample buffer plus β-mercaptoethanol. Samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently transferred onto a nitrocellulose membrane. Following transfer, membranes were pre-blocked in 5% milk/Tris-buffered saline before the addition of primary antibody. Membranes were incubated in primary antibody overnight at 4 °C (1:2000, MMP-9 [Abcam, Cambridge, MA, USA]; 1:2000, MMP-3 [RDI, Flanders, NJ, USA]; 1:1000, MMP-2 [Chemicon, Temecula, CA, USA]; 1:2000, cortactin [Upstate, Charlottesville, VA, USA]). After rinsing in alternating washes of Tris-buffered saline and TTBS (0.1% Tween 20 in Tris-buffered saline), blots were incubated for 2 hours with a 1:10,000 dilution of horseradish peroxidase-conjugated secondary antibody and rinsed again in Tris-buffered saline/TTBS. The membrane was incubated for 2 minutes in Super-Signal (Pierce, Rockford, IL, USA) and subsequently exposed to the phosphoroimager for visualization. Signal intensity per volume was measured using TotalLab Image Analysis software (AD Instruments Inc., Colorado Springs, CO, USA).
Statistical analysis
The comparison of ICV FN439 versus ICV aCSF-infused animals in the acquisition of nicotine-induced CPP on day 6 of training was accomplished by independent t-tests (n = 5/group). The comparison of HIP MMP levels on each day of training for the aCSF-Nic and aCSF-PBS rats (n = 4–8 rats/subgroup) versus naïve home cage controls were analyzed by 2 (groups) × 5 (days of training) ANOVAs, followed by Bonferroni post-hoc tests. Comparisons of aCSF-Nic treated and aCSF-PBS treated groups on each day of training were analyzed by independent t-tests. Comparisons of PFC MMP levels for aCSF-Nic and aCSF-PBS groups, each normalized against naïve home cage controls, were also analyzed by 2 (groups) × 5 (days of training) ANOVAs, followed by Bonferroni post-hoc tests. Changes in HIP cortactin levels during CPP training for aCSF-Nic treated and aCSF-PBS treated groups versus home cage control levels were analyzed by the same ANOVA and post-hoc tests. The PFC cortactin levels for these groups were similarly evaluated.
The comparison of percent time spent in the non-preferred chamber by the aCSF-Nic treated rats during initial preference trials versus day 6, and comparing day 6 preference with re-exposure on day 12, was accomplished using paired t-tests. Changes in HIP MMP levels during re-exposure compared to naïve home cage control levels were analyzed by independent t-tests. Changes in PFC MMP levels during re-exposure compared with naïve home cage control levels were also analyzed by independent t-tests. The alpha level for all tests was set at P < 0.01 or greater.
All analyses were performed using GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Inhibition of MMPs during acquisition of CPP
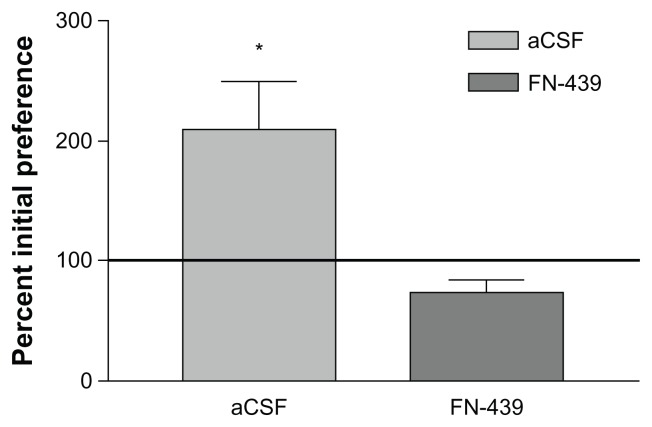
The results comparing the FN439-Nic and aCSF-Nic groups during each of the 5 CPP training days are presented in Figure 1. Members of the aCSF-Nic group acquired CPP as determined on test day 6; members of the FN439-Nic group failed to develop CPP and were behaviorally not different from the aCSF-PBS groups of rats. The FN439-Nic group indicated a mean of 392 seconds in the non-preferred compartment prior to CPP training and 306 seconds following training. The aCSF-Nic treated group revealed a mean of 365 seconds in the non-preferred compartment prior to CPP training and 767 seconds following training. Please refer to Table 1 for the t-value.
Figure 1.
Mean (SEM) percent shift in preference for the nicotine paired compartment on test day 6 for the icv-Nic and FN439-Nic groups.
Notes: The horizontal line represents the initial preference for the compartment in the absence of drug. n = 5 rats in each group. *P < 0.01.
Table 1.
Summary of statistical results.
| Data set | Statistic used | df | F or t value | Sig. level | Figure |
|---|---|---|---|---|---|
| CPP | 1 | ||||
| FN439-Nic vs. aCSF-Nic | Indep t-test | 9 | 4.16 | P < 0.01 | |
| HIP MMP-2 | 2A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,38 | 18.44 | P < 0.001 | ||
| Days | 4,38 | 169.68 | P < 0.001 | ||
| Grps × days | 4,38 | 9.45 | P < 0.005 | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,39 | 20.92 | P < 0.001 | ||
| Days | 4,39 | 129.02 | P < 0.001 | ||
| Grps × days | 4,39 | 8.17 | P < 0.005 | ||
| HIP MMP-3 | 2A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,38 | – | Not sig. | ||
| Days | 4,38 | 96.96 | P < 0.001 | ||
| Grps × days | 4,38 | 9.07 | P < 0.005 | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,41 | – | Not sig. | ||
| Days | 4,41 | 72.31 | P < 0.001 | ||
| Grps × days | 4,41 | 12.74 | P < 0.001 | ||
| HIP MMP-9 | 2A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,40 | 21.65 | P < 0.001 | ||
| Days | 4,40 | 79.70 | P < 0.001 | ||
| Grps × days | 4,40 | 8.84 | P < 0.005 | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,45 | 22.47 | P < 0.001 | ||
| Days | 4,45 | 12.04 | P < 0.001 | ||
| Grps × days | 4,45 | 6.85 | P < 0.005 | ||
| PFC MMP-2 | 3A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,26 | 14.26 | P < 0.001 | ||
| Days | 4,26 | 170.67 | P < 0.001 | ||
| Grps × days | 4,26 | 12.50 | P < 0.001 | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,26 | 18.93 | P < 0.001 | ||
| Days | 4,26 | 122.24 | P < 0.001 | ||
| Grps × days | 4,26 | 9.40 | P < 0.005 | ||
| PFC MMP-3 | 3A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,35 | 11.92 | P < 0.001 | ||
| Days | 4,35 | 248.69 | P < 0.001 | ||
| Grps × days | 4,35 | 4.35 | P < 0.01 | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,38 | 20.65 | P < 0.001 | ||
| Days | 4,38 | 198.07 | P < 0.001 | ||
| Grps × days | 4,38 | 4.48 | P < 0.005 | ||
| PFC MMP-9 | 3A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,38 | – | Not sig. | ||
| Days | 4,38 | 61.55 | P < 0.001 | ||
| Grps × days | 4,38 | – | Not sig. | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,40 | – | Not sig. | ||
| Days | 4,40 | 232/48 | P < 0.001 | ||
| Grps × days | 4,40 | 8.36 | P < 0.001 | ||
| HIP cortactin | 4A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,40 | – | Not sig. | ||
| Days | 4,40 | 8.60 | P < 0.001 | ||
| Grps × days | 4,40 | 11.43 | P < 0.001 | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,38 | – | Not sig. | ||
| Days | 4,38 | 9.59 | P < 0.001 | ||
| Grps × days | 4,38 | 3.81 | P < 0.02 | ||
| PFC cortactin | 5A | ||||
| aCSF-PBS vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,35 | – | Not sig. | ||
| Days | 4,35 | – | Not sig. | ||
| Grps × days | 4,35 | 17.91 | P < 0.001 | ||
| aCSF-Nic vs. Naïve HCC | 2 × 5 ANOVA | ||||
| Groups | 1,38 | – | Not sig. | ||
| Days | 4,38 | – | Not sig. | ||
| Grps × days | 4,38 | 25.28 | P < 0.001 | ||
| CPP: aCSF-Nic | 6 | ||||
| Init. pref. vs. Post-cond. | Paired t-test | 4 | 5.75 | P < 0.01 | |
| Init. pref. vs. Re-exp | Paired t-test | 4 | 8.65 | P < 0.001 | |
| Post-cond. vs. Re-exp | Paired t-test | 4 | 5.32 | P < 0.01 | |
| HIP Re-Exp. MMP-3 | 7A | ||||
| aCSF-PBS vs. naïve HCC | Indep t-test | 10 | 7.75 | P < 0.001 | |
| aCSF-Nic vs. naïve HCC | Indep t-test | 10 | 7.21 | P < 0.001 | |
| PFC Re-Exp. MMP-3 | 8B | ||||
| aCSF-PBS vs. naïve HCC | Indep t-test | 10 | 12.22 | P < 0.001 | |
| aCSF-Nic vs. naïve HCC | Indep t-test | 10 | 11.89 | P < 0.001 |
Abbreviations: aCSF, artificial cerebrospinal fluid; ANOVA, analysis of variance; cond, conditioning; CPP, conditioned place preference; df, degrees of freedom; exp, exposure; grps, groups; HCC, home cage control; HIP, hippocampus; indep, independent; init, initial; MMP, matrix metalloproteinase; Nic, nicotine; P, probability; PBS, phosphate buffered saline; PFC, prefrontal cortex; sig, significance (or significant); vs., versus.
HIP and PFC MMP levels following each day of CPP training
As indicated above, ICV injection of FN439 effectively blocked CPP in members of the FN439-Nic group. These rats also failed to show changes in MMPs different from naïve home cage control levels over the 5 days of training. This confirms the ability of this broad spectrum MMP inhibitor to interfere with CPP contextual-induced MMP expression. Thus, subsequent analyses focused on results from animals assigned to the aCSF-Nic and aCSF-PBS treated groups.
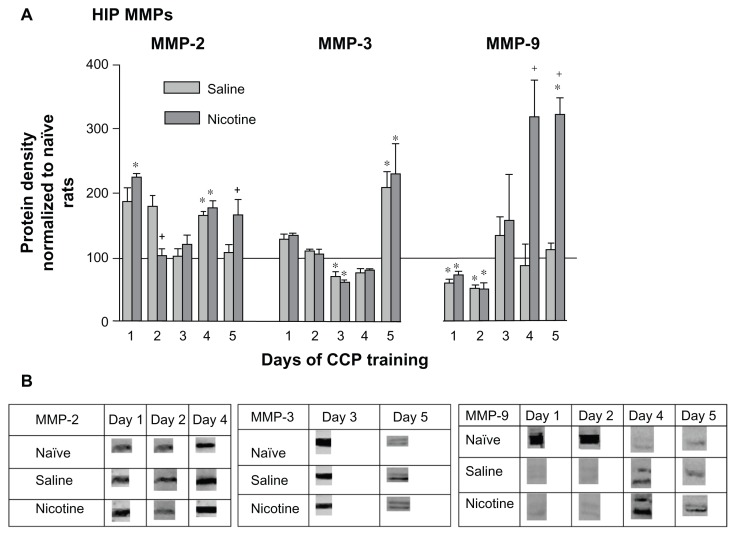
When compared with naïve home cage control levels, there was a significant increase in HIP pro-MMP-2 (72 kDa) in both PBS and nicotine treated groups on days 1 and 4 of CPP training (Fig. 2A). These groups showed significantly different levels of MMP-2 from one another on days 2 and 5. On day 2 the pro-MMP-2 levels of nicotine treated rats were lower than the saline treated rats but were significantly higher than the saline group on day 5. The middle panel indicates that on day 3 of training there was a significant decrease in pro-MMP-3 (57/59 kDa doublet) by both nicotine and saline treated animals. However, on day 5 of conditioning both nicotine and saline treated animals exhibited a similar increase in pro-MMP-3 protein. The right panel indicates that pro-MMP-9 levels (92/89 kDa doublet) in the nicotine and saline groups were initially below the naïve home cage control level on days 1 and 2, but were significantly elevated in members of the nicotine treated group on days 4 and 5. Please refer to Table 1 for F values and Figure 2B for representative bands for each pro-MMP.
Figure 2.
Changes in HIP MMPs 3 hours following each of 5 days of CPP training. (A) HIP MMP proteins were assayed by Western blotting. These data were normalized to naïve protein levels and are represented as mean ± SEM percentage MMP protein levels of naïve home cage control rats (horizontal line). Saline subgroups: n = 4–8; Nicotine subgroups: n = 5–8; Naïve home cage control groups: n = 4–5. *P < 0.01, Comparing each group with naïve home cage control levels. *P < 0.01, Comparing the aCSF-Saline and aCSF-Nic groups. (B) Protein densities of representative bands from Western blots of HIP tissues.
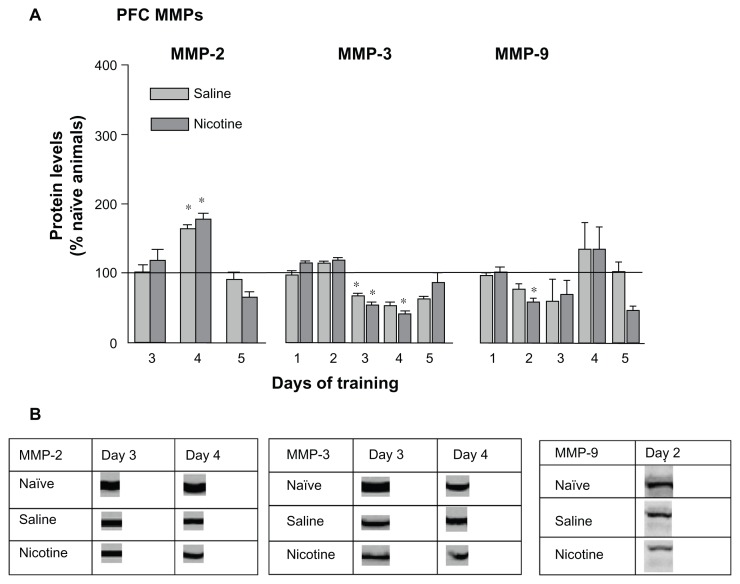
Both the saline and nicotine treated groups exhibited significant increases in PFC pro-MMP-2 levels on day 4 (Fig. 3A, left panel). Similar to the HIP results pro-MMP-3 levels significantly decreased on days 3 and 4 for members of both groups; however, unlike the HIP no late surge in pro-MMP expression was measured on day 5. The levels of pro-MMP-9 in the PFC exhibited little change with a modest decrease by the nicotine treated group on day 2. Please refer to Table 1 for F values and Figure 3B for representative bands for each pro-MMP.
Figure 3.
(A) Changes in prefrontal cortex MMP levels 3 hours following each of 5 days of CPP training. Terminology is the same as Figure 2. Saline subgroups: n = 4–8; Nicotine subgroups: n = 5–8; Naïve home cage control groups: n = 4–5. *P < 0.01. (B) Protein densities of representative bands from Western blots of PFC tissues.
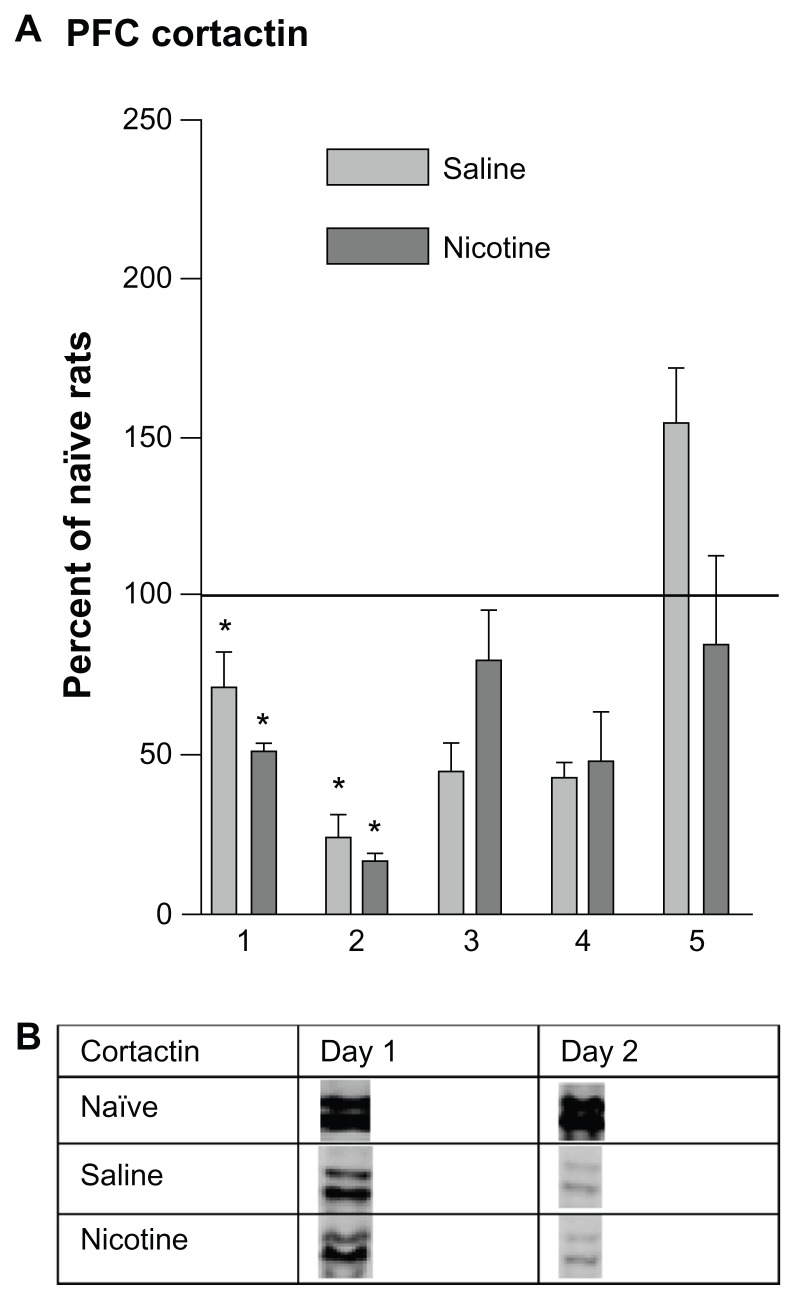
HIP and PFC Cortactin levels following CPP training
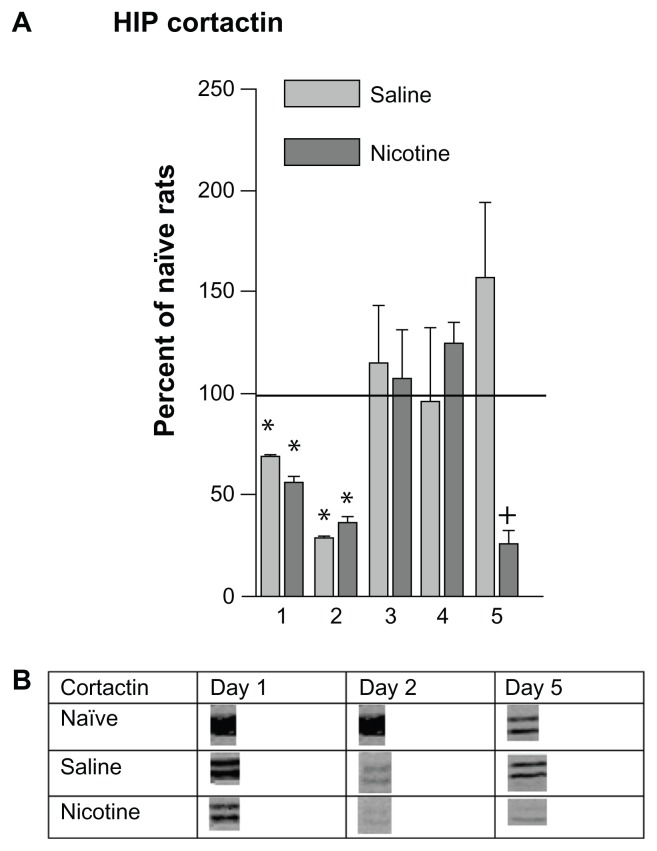
HIP cortactin levels decreased significantly in members of both the saline and nicotine treated groups during the first two days of conditioning; they then returned to naïve home cage control levels on days 3 and 4 (Fig. 4A). Cortactin levels in the nicotine treated rats decreased on day 5 while those of the saline group remained at control level. Please refer to Table 1 for F values and Figure 4B for representative bands.
Figure 4.
Changes in HIP cortactin levels 3 hours following each of 5 days of CPP training. (A) These data were normalized to naïve home cage control protein levels and are represented as mean (± SEM) percentage MMP protein levels (horizontal line). Saline subgroups: n = 4–8; Nicotine subgroups: n = 5–8; Naïve home cage control groups: n = 4. *P < 0.01, Comparing each group with naïve home cage control levels. *P < 0.01, Comparing the aCSF-Saline and aCSF-Nic groups. (B) Protein densities of representative bands from Western blots HIP tissues.
The pattern of PFC cortactin changes (Fig. 5A) were similar to those seen in the HIP (Fig. 4A), indicating significant decreases below baseline on days 1 and 2 in both the nicotine and saline treated groups, followed by a return to baseline by day 5. Unlike the HIP, no decline was measured in members of the nicotine group on day 5. Please refer to Table 1 for F values and Figure 5B for representative bands.
Figure 5.
(A) Prefrontal cortex cortactin levels 3 hours following each of 5 days of CPP training. The horizontal line represents cortactin levels of naïve home cage control animals on each day. Saline subgroups: n = 4–8; Nicotine subgroups: n = 5–7; Naïve home cage control groups: n = 4–7. *P < 0.01. (B) Protein densities of representative bands from Western blots PFC tissues.
Conditioned place preference in nicotine treated rats
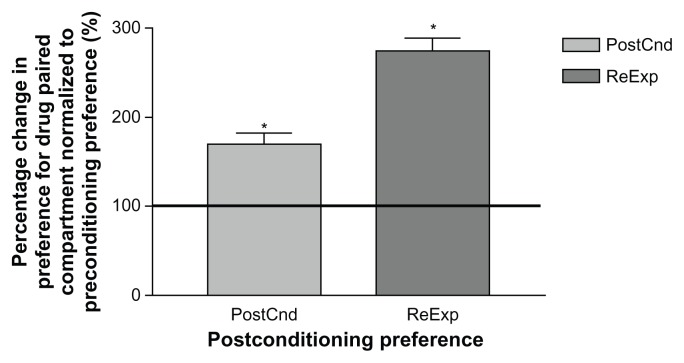
There was a significant increase in preference for the nicotine paired compartment by members of the aCSF-Nic treated group on test day 6 following CPP training (170%) as compared with their precondition preference (100%, Fig. 6). This preference for the nicotine paired compartment was more robust when the animals were re-exposed to the CPP chambers after 5 days of rest on test day 12 (274%). Please refer to Table 1 for t values.
Figure 6.
Mean (SEM) CPP results for nicotine treated rats following reexposure to the chamber.
Notes: Data were normalized to preconditioning preference and are represented as mean (±SEM) percentage change in preference for the drug paired side. The horizontal line indicates the initial preference for the drug paired compartment. PostCnd = preference for drug paired compartment on day 6 following 5 days of nicotine conditioning. ReExp = preference for drug paired compartment on day 12 following 5 days of rest. PostCnd group: n = 5; ReExp group: n = 5; *P < 0.01.
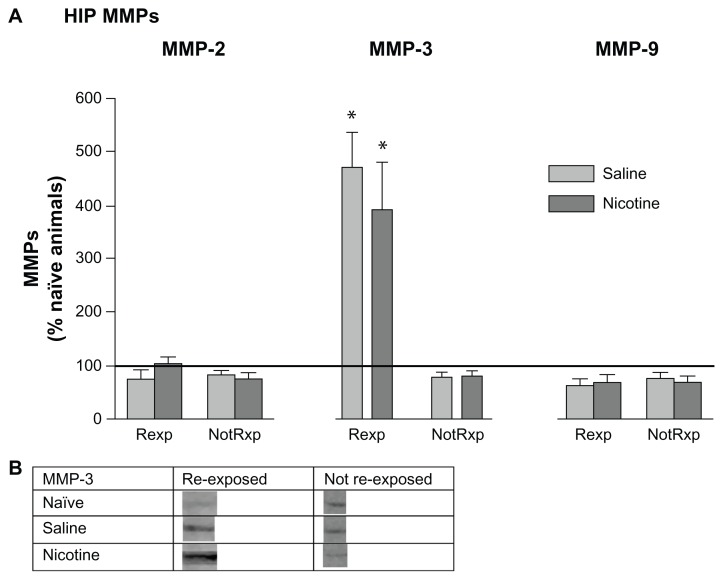
MMPs in the HIP and PFC following re-exposure
Following re-exposure testing on day 12, HIP pro-MMP-2 and -9 levels in re-exposed and not reexposed groups were not different from naïve home cage control levels, suggesting that these proteins may not be involved in reconsolidation of CPP memory (Fig. 7A). However, members of both the saline and nicotine treated groups re-exposed to the CPP chamber showed a selective and marked increase in pro-MMP-3 levels. Please refer to Table 1 for t values and Figure 7B for representative bands for pro-MMP-3.
Figure 7.
Mean (±SEM) changes in HIP MMP levels following re-exposure to the CPP chamber after 5 days of rest. (A) Data were normalized to naïve home cage control protein levels (horizontal line). Rexp = re-exposed to the CPP chamber. NotRxp = not re-exposed to the CPP chamber. Naïve home cage control group: n = 4; aCSF-PBS group: n = 7; aCSF-Nic group: n = 7. *P < 0.01. (B) Protein densities of representative bands from Western blots of HIP tissues.
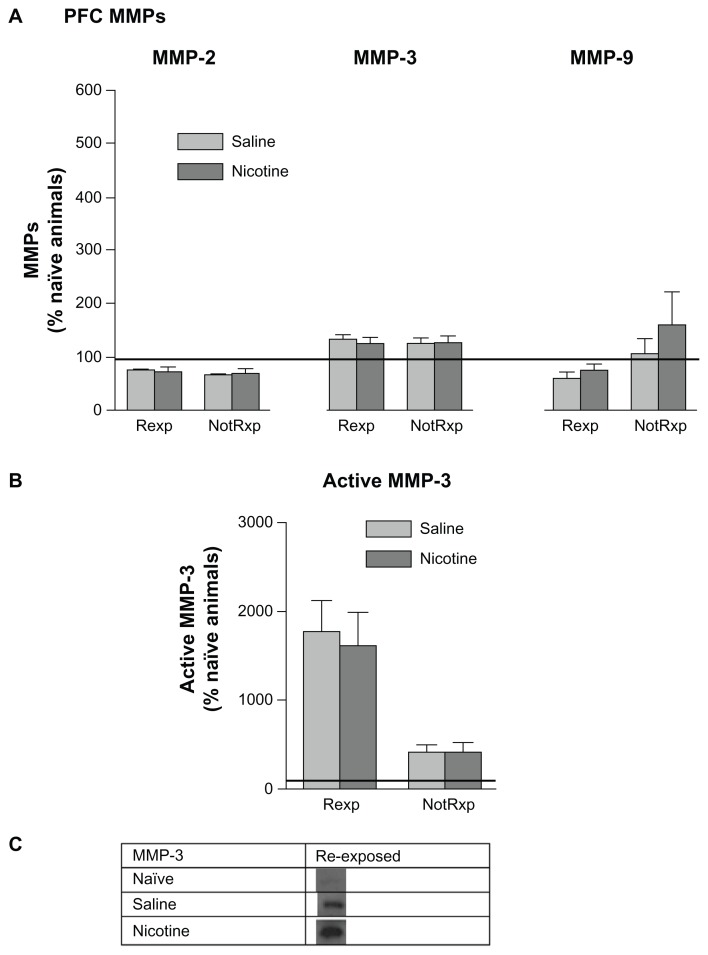
No significant changes in pro-MMPs were noted in the PFC comparing saline and nicotine treated groups, or between groups re-exposed to the CPP chamber and those that were not re-exposed (Fig. 8A). Representative bands for active MMP-3 (45 kDa) are shown in Figure 8C. Both nicotine and saline treated groups showed a significant increase in active MMP-3 levels following re-exposure to the CPP chamber; while no changes were evident in the non-re-exposed groups. This elevation in MMP-3 in the PFC following re-exposure suggests the involvement of MMP-3 in the reconsolidation process. Please refer to Table 1 for t values.
Figure 8.
Mean (±SEM) changes in PFC MMP levels following re-exposure to the CPP chamber on day 12 following 5 days of rest. Refer to Figure 7 for an explanation of terminology. (A) There were no significant differences among the levels of pro-MMPs among groups. (B) An elevation in the 45 kDa active MMP-3 form was measured in the re-exposed group. *P < 0.01. (C) Representative protein bands from Western blots of 45 kDa active MMP-3 measured in PFC tissues.
Cortactin levels in the HIP and PFC following re-exposure
No differences were noted in the levels of HIP or PFC cortactin in saline and nicotine treated groups reexposed or not re-exposed (n = 4–7/subgroup). Data not shown.
Discussion
Matrix metalloproteinases are known to alter intracellular signaling and influence cytoskeletal protein restructuring.53–55 The actin regulatory protein cortactin is concentrated in dendritic spines of HIP neurons; it is involved in regulating activity dependent changes in dendritic morphology. There appears to be an inverse relationship between MMP activity and cortactin expression, ie. increases in MMP activity often correlate with temporary decreases in cortactin expression.56 This correlation appears to be necessary in order for synaptic plasticity to occur, followed by increases in the levels of tissue inhibitors of MMPs (TIMPs) designed to terminate MMP enzymatic activity. Reports from our laboratories47,57–62 and other laboratories55,63 have suggested the involvement of MMPs and TIMPs in neural plasticity, memory consolidation, and memory reconsolidation. However, the temporal pattern of MMP expression during learning and drug related memory is not clearly understood.
The present results indicate that transient changes in MMP-2, -3, and -9 occurred in the HIP and PFC during the active phase (rising phase of the acquisition curve when performance is improving) of learning and memory consolidation of nicotine-induced CPP in rats of the aCSF-Nic and aCSF-PBS groups, as compared with naïve home cage control levels (horizontal line, Fig. 2). Specifically, members of these two groups showed significant elevations in MMP-2 on day 4 of training, while only the nicotine treated group revealed significant elevations on days 1 and 5 of training. Both groups evidenced significant elevations in MMP-3 on day 5; only the nicotine treated group revealed significant elevations in MMP-9 on days 4 and 5. It also appears that MMP-3, but not MMP-2 or -9, may be involved in reactivation of consolidated memory (Fig. 7). Members of both the nicotine and saline treated groups evidenced significant elevations in MMP-3 during re-exposure. Changes in PFC MMP levels were less dramatic (Fig. 3). Members of both groups indicated significant changes in MMP-2 on day 4 of training, and significant decreases in MMP-3 on day 3. The nicotine treated group showed significant decreases in MMP-3 on day 4 and MMP-9 on day 2 of training. PFC cortactin levels were depressed in members of both groups on days 1 and 2 (Fig. 5). Finally, the inhibition of MMP expression by FN439 in rats assigned to the FN439-Nic group resulted in a failure to develop nicotine-induced CPP (Fig. 1).
MMP expression in the HIP following each day of conditioning suggests the presence of two types of learning. One type appears to be “context dependent” and the other “drug dependent”. The nicotine and saline treated rats showed significantly different MMP-2 patterns of expression on days 1 and 5, and in particular MMP-9 on days 4 and 5. These nicotine-induced changes in MMP-9 expression suggest that this protein may be involved in mediating drug related learning. No such differential protein expression patterns were measured with MMP-3 when comparing nicotine and saline treated groups. At the time that animals were presumably finalizing memory consolidation of the CPP task (day 5), HIP MMP-3 levels were significantly elevated in members of both the nicotine and saline treated groups (Fig. 2A). The nicotine treated group indicated significant elevations in MMP-9 on both days 4 and 5 of training, whereas the saline group showed levels at home cage control values. When the animals were re-exposed to the drug associated context following a 5 day rest period, MMP-3 levels increased significantly in the HIP (Fig. 7) and PFC (Fig. 8B), suggesting its involvement in memory reconsolidation. It appears that elevations in MMP-3 may be accompanying the learning that occurs due to handling and the novelty of exposure to the CPP apparatus; this is in contrast to MMP-9 changes, which are more specific to drug related learning, especially late in CPP training. Inhibition of MMP activity by the application of FN439 resulted in the disruption of CPP, thus supporting the hypothesis that MMP activation is required for CPP learning. These results further support the contention that MMPs are involved in the development of CPP for nicotine. However, it is clear that changes in MMP expression also occur due to the experience of being placed in the apparatus. Related to this finding, Brown and colleagues (2008) have reported increases in PFC MMP-9 levels following cocaine primed reinstatement of CPP. The animals used in the present investigation did not receive a drug priming injection during re-exposure to the CPP environment, which may explain the absence of PFC MMP-9 changes.
Decreases in cortactin expression were measured in the HIP and PFC during the first two days of conditioning (Figs. 4 and 5) in both saline and nicotine treated rats; this suggests that the dendritic cytoskeleton underwent restructuring on those days irrespective of drug treatment. It would thus appear that learning, independent of the drug treatment, triggered significant decreases in cortactin cytoskeleton early in training. There were no changes in cortactin when compared with home cage control values during days 3 and 4 of conditioning; however, nicotine treated animals showed a significant decrease on day 5. Thus, by the completion of 5 days of conditioning, synaptic changes may have been complete. There is also the possibility that cytoskeletal protein modifications at the later stages of learning were programmed to occur at a different time point than those selected for tissue collection in this study. It is further possible that cortactin changes may be highly localized in the HIP or PFC, making detection difficult using current gross dissections techniques.
This study focused on the involvement of MMP-2, -3 and -9 in a contextual learning paradigm but other brain MMPs could be involved. A particularly attractive candidate is MMP-7, known to regulate HIP dendritic spine structure.39 MMP-7 has also been implicated in affecting the structure and function of neurons by modulating synaptic proteins at the active zone where synaptic vesicle recycling is thought to occur.64
Our MMP inhibition studies employed FN439, a broad spectrum MMP inhibitor, in order to block the expressions of MMP-2, -3, and -9, and thus their impact on the remodeling process. It should be acknowledged that FN439 is particularly specific to MMP-2 and -9, although it has been used to inhibit MMP-3 as well.65 Since a limited number of brain MMPs are probably involved in CPP learning, the use of more specific inhibitors to interfere with nicotine-induced CPP may provide more detailed information concerning the respective roles of each MMP. Given the widespread role of MMPs in multiple physiological processes, such an approach is appropriate if MMP inhibition is to be considered as a therapeutic option for the treatment of addictive behaviors.
Conclusion
Studies in humans indicate that adolescent girls are particularly vulnerable to nicotine addiction.5,6,66 Smoking appears to be accompanied by the formation of associations among taste, olfaction, motor, and visual cues; these associations facilitate craving for nicotine. In agreement with findings from humans, the results of this study indicate that nicotine induces CPP in adolescent female rats at a very low dose, much lower than those efficacious in adolescent male rats.27 Brain MMP expression appears to be important to the synaptic reorganization that accompanies memory consolidation. In order to break these learned associations that maintain nicotine dependence, it may be possible to employ an MMP inhibitor or siRNA that prevents the expression of MMPs, and thus memory reconsolidation that mediates nicotine craving. Taken together the present results support the hypothesis that MMPs are involved in learning of a nicotine-associated contextual memory and that the inhibition of MMP expression may be an efficacious intervention technique to weaken memory reconsolidation. However, it is also clear that animals exposed to such training without nicotine treatment also evidenced similar increases in MMP expression. It is therefore clear that much experimental work remains to be completed in order to gain a full understanding of the relationship among nicotine addiction, brain MMP and cortactin expressions, and memory consolidation/reconsolidation.
Footnotes
Author Contributions
Conceived and designed the experiments: RN, JWH, JWW. Analyzed the data: RN, JWW. Wrote the first draft of the manuscript: RN. Contributed to the writing of the manuscript: JWH, JWW. Agree with manuscript results and conclusions: RN, JWH, JWW. Jointly developed the structure and arguments for the paper: RN, JWH, JWW. Made critical revisions and approved final version: RN, JWH, JWW.
Competing Interests
The authors disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
This research was supported by funds provided by the Departments of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, and Psychology, Washington State University, and the Edward E. and Lucille I. Lainge Endowment for Alzheimer’s disease research.
References
- 1.Pauly JR. Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci. 2008;13:505–16. doi: 10.2741/2696. [DOI] [PubMed] [Google Scholar]
- 2.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 3.Pogun S, Yararbas G. Sex-differences in nicotine action. Handb Exp Pharmacol. 2009;192:261–91. doi: 10.1007/978-3-540-69248-5_10. [DOI] [PubMed] [Google Scholar]
- 4.Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Women’s Health. 2007;16:1211–8. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Millar MJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- 6.Cropsey KL, Linker JA, Waite DE. An analysis of racial and sex differences for smoking among adolescents in a juvenile correctional center. Drug Alcohol Depend. 2008;92:156–63. doi: 10.1016/j.drugalcdep.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–9. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 8.Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Shram MJ, Lê AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2010;206:240–4. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Trauth JA, Seidler FJ, Slotkin TA. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res. 2000;880:167–72. doi: 10.1016/s0006-8993(00)02823-7. [DOI] [PubMed] [Google Scholar]
- 11.Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58:374–82. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Kitanaka N, Kitanaka J, Hall FS, et al. Attenuation of methamphetamine-induced conditioned place preference in mice after a drug-free period and facilitation of this effect by exposure to a running wheel. J Exp Neurosci. 2012;6:11–9. [Google Scholar]
- 13.O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharm Biochem Behav. 2009;91:481–8. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology. 2004;175:134–42. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- 15.Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci. 2002;22:8653–60. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry. 2003a;8:50–9. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- 17.Laviolette SR, van der Kooy D. The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the alpha7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology. 2003b;166:306–13. doi: 10.1007/s00213-002-1317-6. [DOI] [PubMed] [Google Scholar]
- 18.Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–12. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashby CR, Jr, Paul M, Gardner EL, et al. Systemic administration of 1R,4S-4-amino-cyclopent-2-ene-carboxylic acid, a reversible inhibitor of GABA transaminase, blocks expression of conditioned place preference to cocaine and nicotine in rats. Synapse. 2002;44:61–3. doi: 10.1002/syn.10052. [DOI] [PubMed] [Google Scholar]
- 20.Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR., Jr A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav. 1986;25:1041–9. doi: 10.1016/0091-3057(86)90083-3. [DOI] [PubMed] [Google Scholar]
- 22.Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–41. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- 23.Horan B, Smith M, Gardner EL, Leipore M, Ashby CR., Jr Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–4. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Jorenby DE, Steinpreis RE, Sherman JE, Baker TB. Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacology. 1990;101:533–38. doi: 10.1007/BF02244233. [DOI] [PubMed] [Google Scholar]
- 25.Papp M, Gruca P, Willner P. Selective blockade of drug-induced place preference conditioning by ACPC, a functional NDMA-receptor antagonist. Neuropsychopharmacology. 2002;27:727–43. doi: 10.1016/S0893-133X(02)00349-4. [DOI] [PubMed] [Google Scholar]
- 26.Rogers DT, Barron S, Littleton JM. Neonatal ethanol exposure produces a hyperalgesia that extends into adolescence, and is associated with increased analgesic and rewarding properties of nicotine in rats. Psychopharmacology. 2004;171:204–11. doi: 10.1007/s00213-003-1574-z. [DOI] [PubMed] [Google Scholar]
- 27.Natarajan R, Wright JW, Harding JW. Nicotine-induced conditioned place preference in adolescent rats. Pharmacol Biochem Behav. 2011;99:519–23. doi: 10.1016/j.pbb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–57. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 29.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–5. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MJ, Malenka RC. Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond B Biol Sci. 2003;358:815–9. doi: 10.1098/rstb.2002.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artinian J, De Jaeger X, Fellini L, de Saint Blanquat P, Roullet P. Reactivation with a simple exposure to the experimental environment is sufficient to induce reconsolidation requiring protein synthesis in the hippocampal CA3 region in mice. Hippocampus. 2007;17:181–91. doi: 10.1002/hipo.20256. [DOI] [PubMed] [Google Scholar]
- 34.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–38. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 35.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–5. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 36.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–7. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–84. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2007;40:1362–78. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright JW, Harding JW. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Prog Neurobiol. 2004;72:263–93. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Helwani FM, Kovacs EM, Paterson AD, et al. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–62. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 45.Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J Neurosci Res. 2007;85:2813–23. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- 46.Huntley GW. Synaptic circuit remodeling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–57. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102:2085–96. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- 48.Brown TE, Wilson AR, Cocking DL, Sorg BA. Inhibition of matrix metalloproteinase activity disrupts reconsolidation but not consolidation of a fear memory. Neurobiol Learn Mem. 2009;91:66–72. doi: 10.1016/j.nlm.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright JW, Brown TE, Harding JW. Inhibition of hippocampal matix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plast. 2007;2007:73813. doi: 10.1155/2007/73813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pederson ES, Harding JW, Wright JW. Attenuation of scopolamine-induced spatial learning impairments by an angiotensin IV analog. Regul Pept. 1998;74:97–103. doi: 10.1016/s0167-0115(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. Burlington, MA: Elsevier Academic Press; 2005. [Google Scholar]
- 52.Wright JW, Roberts KA, Harding JW. Drinking to intracerebroventricularly infused angiotensins II, III, and IV in the SHR. Peptides. 1988;9:979–84. doi: 10.1016/0196-9781(88)90076-9. [DOI] [PubMed] [Google Scholar]
- 53.Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23:11759–69. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 2009;29:6007–12. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagy V, Bozdagi O, Matynia A, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–34. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meighan SE, Meighan PC, Choudhury P, et al. Effects of extracellular matrix-degrading poteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96:1227–41. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- 57.Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- 59.Olson ML, Meighan PC, Brown TE, et al. Hippocampal MMP-3 elevation is associated with passive avoidance conditioning. Regul Pept. 2008;146:19–25. doi: 10.1016/j.regpep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Smith AS, Nealey KA, Wright JW, Walker BM. Plasticity associated with negative reinforcement learning during ethanol dependence requires intact matrix metalloproteinase systems. Neurobiol Learn Mem. 2011;96:199–206. doi: 10.1016/j.nlm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiediger RV, Wright JW. Influence of dorsal hippocampal lesions and MMP inhibitors on spontaneous recovery following a habituation/classical conditioning head-shake task. Neurobiol Learn Mem. 2009;92:504–11. doi: 10.1016/j.nlm.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Wright JW, Wilson WL, Wakeling V, et al. The hepatocyte growth factor/c-Met antagonist, divalinal-angiotensin IV, blocks the acquisition of methamphetamine dependent conditioned place preference in rats. Brain Sci. 2012;2:298–318. doi: 10.3390/brainsci2030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizoguchi H, Hamada K, Nabeshima T. Neuropsychotoxicity of abused drugs: Involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J Pharmacol Sci. 2008;106:91–14. doi: 10.1254/jphs.fm0070139. [DOI] [PubMed] [Google Scholar]
- 64.Szklarczyk A, Conant K, Owens DF, Ravin R, McKay RD, Gerfen C. Matrix metalloproteinase-7 modulates synaptic vesicle recycling and induces atrophy of neuronal synapses. Neuroscience. 2007;149:87–98. doi: 10.1016/j.neuroscience.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Falo MC, Fillmore HL, Reeves TM, Phillips LL. Matrix metalloproteinase-3 expression profile differentiates adaptive and maladaptive synaptic plasticity induced by traumatic brain injury. J Neurosci Res. 2006;84:768–81. doi: 10.1002/jnr.20986. [DOI] [PubMed] [Google Scholar]
- 66.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–15. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]