Abstract
Effector (TEM) and central memory (TCM) T cells have been recently described as the main memory T-cell subsets generated after primary immune response, with a potential role in graft rejection after rechallenge with alloantigen. Because of their effector function, they could be involved in driving the response against the allograft, leading to rejection. In this study, we sought to investigate the different memory T-cell subpopulations in peripheral blood from a cohort of 90 patients who underwent consecutive renal transplant, and their association with acute rejection (AR) episodes and induction therapy. Twenty-one of them were monitored in the short term during the first 2 months after transplantation. Three of them suffered an AR but no changes in the circulating levels of either CD4+ or CD8+ TEM were observed as compared with rejection-free renal transplant patients. In total, 69 patients out of 90 were monitored in the long term. Even 2 years after transplantation, maintained increased numbers of peripheral blood CD4+ TEM were observed in patients suffering with AR. Interestingly, induction therapy with thymoglobulin, but not with basiliximab, produced an increase in circulating CD4+ TEM cells at 6 months after transplantation. In conclusion, our data suggest that AR episodes favor the induction of TEM cells in the periphery of renal transplant patients in the long term. It remains to be determined whether such an effect has any impact on long-term renal transplantation.
Keywords: acute rejection, effector T cells, kidney transplantation, memory T cells
T cells are under continuous education to be able to discern between self- and foreign antigens. After leaving the thymus, naïve T cells can differentiate into many different subsets, depending on the environment milieu in which they are activated.1 Upon activation by professional antigen-presenting cells, naïve T cells can differentiate into activated T cells, driving primary responses against the antigen. After clearance of the antigen, most of the T cells undergo apoptosis, whereas the remaining T cells will be recruited into the memory T-cell pool. Once the antigen re-enters into the host, those memory immune T cells are able to mount a more efficient and faster response against it.
In the transplantation setting, the memory cells can be generated by several ways after previous encounters with human leukocyte antigen molecules in sensitized patients (after pregnancy, blood transfusions), and in non-sensitized patients by cross-reactivity (molecular mimicry and bystander proliferation),2, 3 and homeostatic proliferation after lymphopenia.4
In mice and humans, several populations of memory T cells have been described with different effector functions, tissue localizations, and phenotypic characteristics.5 The central memory T cells (TCM) are confined to lymphoid tissues, and express CD62L and CD45RO. They have a silent phenotype and therefore could fit in a tolerance profile; however, after reactivation, they could develop into effector memory T cells (TEM), losing partially CD62L expression with very strong effector properties and the capacity to drive an effector response even without the requirement for co-stimulatory molecules. There are different markers that help to define these subpopulations: CD27, CD28, CD95, CD127, and chemokine receptor-5 and chemokine receptor-7, reviewed elsewhere.5
One important caveat is that most of the transplant animal models that assess mechanisms of tolerance and alloimmune responses are under pathogen-free conditions in which the memory arm could be impaired or absent. In spite of this argument, there are a number of models in which the establishment of tolerance is not achieved in the presence of memory T cells,6 although treatments blocking co-stimulatory pathways were able to prolong graft survival.7
The involvement of memory T cells in alloimmune responses is widely accepted and could have a role in the development of a rejection episode and, more specifically, in human renal transplantation. Nevertheless, such an issue has been poorly investigated. The present study addressed the changes in the number of circulating TCM and TEM in renal transplant recipients undergoing an acute rejection (AR) episode, as well as the effect that different immunosuppressant drugs have on them.
RESULTS AND DISCUSSION
CD4+ TEM cells and their association with acute rejection
Within the increasing number of potential subsets involved in the development of allograft rejection, the role of memory T cells in AR pathogenesis was demonstrated as early as in the 70s in murine models.8 These cells are able to drive an AR episode earlier and faster than naïve T cells.9
After 20 years, two new subsets of memory T cells with different properties were described (TCM and TEM).10 Although both populations of CD8+ TEM and TCM were able to mount an effective alloimmune response against the graft in a skin graft model, the CD8+ TEM rejected allografts better than TCM in the absence of secondary lymphoid tissues,11 pointing to the TEM subset as a main candidate to drive secondary alloresponses.
In human heart and renal transplantation, the degree of AR was correlated with the degree of memory CD8+ T-cell infiltration.12, 13 However, in liver transplantation, an increased infiltration of naïve T cells was found.14 Further evidence of the role of memory T-cell activation in AR was demonstrated by the correlation of interferon-γ production measured by enzyme-linked immunosorbent spot with AR and poor graft outcome.15, 16
During the last decades, several groups have investigated AR biomarkers, although because of good short-term results with the use of new therapeutic strategies, the interest in those biomarkers decreased. Despite this, 5–10% of kidney transplant recipients still suffer from an AR episode. Within the candidate biomarkers, memory T subsets have gained interest within the transplant research community. In liver transplantation, a recent study showed a shift from naïve CD8+ cells to the different memory subsets before AR episodes.17 In human heart transplantation, an increase in naïve and a decrease in TCM CD4+ cells before heart transplantation in patients developing AR has been demonstrated.18 Our group has studied the different subsets of memory T cells in 21 renal transplant patients during the first 2 months after transplantation. Three out of the 21 patients developed an AR episode and showed a decrease in the percentage of CD4+ TEM and CD8+ TEM during the first 2 months after transplantation (Figure 1a), although not significantly, probably because of the less number of patients. We were able to measure the number of circulating T-cell subsets in an independent group of eight patients with deterioration of renal function at the time of biopsy because of suspicion of AR. In such a moment, the frequencies of peripheral blood CD4+ TEM cells were increased, with a trend toward decrease, or remain at the same level at 1 month after biopsy (Figure 1b). Circulating CD8+ TEM cells did not change at biopsy and at 1 month later (not shown). These findings point to a possible role of CD4+ TEM immediately after transplantation as inducers of the immune response responsible for the AR and as AR biomarkers.
Figure 1.
Changes in the frequencies of circulating TEM cells in early post-transplantation. (a) Frequencies of CD4+ TEM (left) and CD8+ TEM (right) cells in peripheral blood of renal transplant patients during the first 2 months after transplantation. Patients were grouped according to the development of acute rejection (A) or not (N). Each dot represents one patient and horizontal bars represent the mean value in each group. No significant differences were observed. (b) Changes in the frequencies of peripheral blood CD4+ TEM cells in a group of eight patients at the time of diagnosis of acute rejection by biopsy and 1 month after biopsy.
Induction therapy with thymoglobulin, but not with basiliximab, induces increased levels of circulating CD4+ TEM cells
One of the major limitations to the long-term success of transplantation is the need for immunosuppression during lifetime, and its deleterious effect on the graft and the recipient. Understanding the impact of different immunosuppressive regimens on memory T cells would be a very valuable tool in order to direct the therapy against the harmful subsets involved in transplantation. In an interesting in vitro model, after sorting CD8+ TEM cells cocultured with different immunosuppressant medications currently used in clinic, only the calcineurin inhibitor treatment was able to suppress their function, as compared with mammalian target of rapamycin inhibitors, steroids, or mycophenolic acid.19 This in vitro finding may have important consequences regarding immunosuppressant therapy in early post-transplant stages, as the TEM subset may have a role in driving an AR episode.
In transplant models, several studies have recently been published using pre-sensitized animals in which the inhibition of the memory arm after co-stimulation blockade induction with anti-CD134L (OX-40L), anti-CD122 (beta-chain of interleukin-2 receptor), anti-CD154, and anti-LFA-1 was effective to prolong heart graft survival but unable to achieve tolerance.7
With regard to induction therapy regimens, a fast recovery of blood TEM cells was observed after thymoglobulin treatment, whereas TCM levels were restored only after 3 months post-treatment.20 In renal transplant patients, Campath-1H induction evokes a severe lymphopenia, in which TCM cells are more resistant to depletion, and progressive restoration of TEM is found with time. Even one rejection episode was associated with a higher proportion of circulating CD4+ TEM cells.21 In our cohort population, we observed an increased proportion of CD4+ TEM cells at 6 months after transplantation in those patients who received thymoglobulin induction, whereas no differences were observed in patients without induction or in those on basiliximab treatment (Figure 2). No significant differences in CD8+ TEM cell frequencies were observed at 6 months after transplantation in patients undergoing thymoglobulin (30.7±15.0) or basiliximab induction (29.7±14), as compared with those patients who did not received induction therapy (33.5±12.0). Such a finding could be related to the phenomenon of homeostatic proliferation after the lymphopenia induced by thymoglobulin.4 However, basiliximab did not induce lymphopenia after induction treatment.
Figure 2.
Frequencies of CD4+ TEM cells in peripheral blood of long-term follow-up renal transplant recipients at 6 months after transplantation according to the induction therapy received. Significant increased frequencies were observed in the thymoglobulin (TG)-treated patients as compared with basiliximab (anti-CD25)-treated patients or those not receiving induction therapy. Each dot represents one patient and horizontal bars represent the mean value in each group. Empty dots represent patients who did not receive induction therapy. Filled dots represent patients who received induction therapy with anti-CD25. Triangles represent patients who received induction therapy with TG.
Kinetics of peripheral blood memory T cells in the long-term follow-up
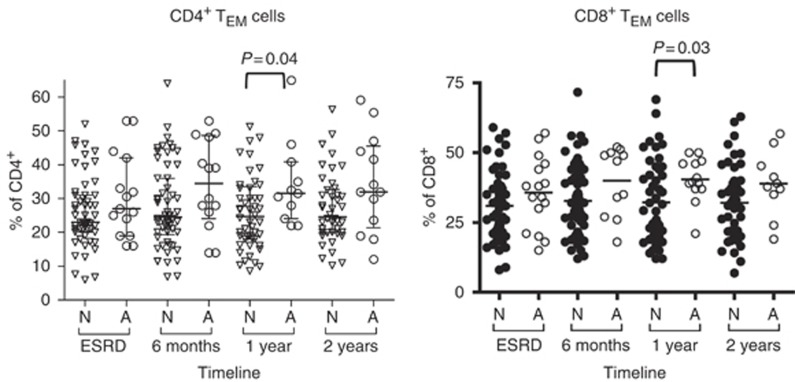
Although memory T cells may be mainly involved in AR, with new induction therapies and depleting treatments, these populations could have an important role in long-term graft loss. There is evidence showing infiltration of memory populations in biopsies of renal transplant patients diagnosed with chronic allograft nephropathy.22 This is indirectly in agreement with previous data from our group showing decreased number of TCM CD3+ cells in patients with long-term failed grafts and reintroduced in the waiting list.23 In the present study, we observed that even several months after diagnosis, those patients suffering with an AR episode maintained increased percentages of CD4+ TEM and CD8+ TEM cells (Figure 3), although it did not affect graft survival. This is in contrast with the apparent decrease of both TEM subsets in the short-term follow-up of those patients suffering AR (Figure 1a and b). Nevertheless, the possible effect of memory T cells on long-term prognosis and chronic rejection in renal transplantation still awaits investigation.
Figure 3.
Changes in the frequencies of circulating TEM cells in late post-transplantation. Frequencies of CD4+ TEM (left) and CD8+ TEM (right) cells in peripheral blood of renal transplant patients during the first 2 years after transplantation. Patients were grouped according to the development of acute rejection (A) or not (N). Each dot represents one patient and horizontal bars represent the mean value in each group. Data were collected at 6 months, 1 year, and 2 years after transplantation. P-value is indicated when statistically significant difference was found. Triangles in the left figure represent those patients who did not suffer acute rejection (N) whereas empty dots represent patients who developed acute rejection (A). In the right figure, filled dots represent patients who did not develop acute rejection (N) whereas empty dots represent patients who developed acute rejection (A). ESRD, end-stage renal disease.
CONCLUDING REMARKS
Despite the well-accepted role of memory T cells as initiators of the potent secondary immune response, they have not been particularly addressed as clinical biomarkers in the setting of renal transplantation. In the present study, we evaluated the quantitative changes in the two main memory T-cell subsets, TCM and TEM, in the peripheral blood of renal transplant recipients. Our data point to a possible role of CD4+ TEM immediately after transplantation as inducers of the immune response that would finally activate the effector CD8+ TEM responsible for AR. There is practically no data regarding the association between memory T cells and chronic rejection in human renal transplantation. The different immunosuppresive regimens seem to have no quantitative effect on memory T cells. However, induction therapy with thymoglobulin, but not with basiliximab, induced increased numbers of circulating CD4+ TEM cells.
PATIENTS AND METHODS
Patients
A cohort of 90 patients who underwent consecutive renal transplant in the Hospital Universitario Marqués de Valdecilla from September 2007 was recruited, and informed consent given for participation in this study. All the clinical data were recorded at the Nephrology Department. The patients were divided into two groups depending on the time of follow-up: the first group consisted of 69 patients with long-term follow-up (6 months, 1 and 2 years after transplantation), whereas the second one consisted of 21 patients with a short-term follow-up during 2 months. Within long-term follow-up group, 15 patients suffered an AR episode (21.7%), whereas within the short-term follow-up group 14.3% had an AR episode. Immunological, demographic, and clinical parameters are summarized in Table 1. No significant differences in these parameters between the non-AR and the AR group were observed in any of the cohorts studied.
Table 1. Demographic, immunological, and clinical data from short-term and long-term follow-up renal transplant patients.
| Short-term follow-up | Long-term follow-up | |
|---|---|---|
| N | 21 | 69 |
| Donor age (years, mean±s.d.) | 58±17.3 | 50.4±15.7 |
| Recipient age (years, mean±s.d.) | 63.3±7.2 | 52.8±12 |
| Time on dialysis (days, mean±s.d.) | 287±271 | 25.9±18.6 |
| Number of transplants (1/2/3) | (21/0/0) | (49/15/5) |
| Acute rejection (yes/no, %) | 3/18 (14.3) | 15/54 (21.7) |
| Banff classification (BL/Ia/Ib/IIa/IIb/H) | 2/0/0/1/0/0 | 3/5/1/3/1/2 |
| Kidney disease (% of GN) | 33 | 36 |
| Mismatches (A/B/DR, mean) | 1.1/1.0/1.0 | 0.91/1.27/1.0 |
| Induction therapy (no/thymoglobulin/basiliximab) | Not determined | 46/6/17 |
| Serum creatinine (mg/dl, mean±s.d.)a | 2.27±0.67 | 6 months: 1.52±0.44 |
| 1 year: 1.47±0.38 | ||
| 2 years: 1.51±0.46 | ||
| MDRD (ml/min per 1.73 m2)a | 37.1±25.6 | 6 months: 51±14.8 |
| 1 year: 52±12.5 | ||
| 2 years: 51.3±14.9 |
Abbreviations: BL, borderline; GN, glomerulonephritis; MDRD, modification of diet in renal disease; s.d., standard deviation.
Data in the short-term follow-up group are at 2 months after transplantation. In the long-term follow-up, data are indicated at (6 months, 1 year, 2 years).
Phenotype analysis
Four-color flow cytometry analyses were performed on peripheral whole blood collected in heparin anticoagulant tubes, within 2 h after collection. The blood cells were stained with the following monoclonal antibodies (BD Biosciences, San Jose, CA): anti-CD62L-fluorescein isothiocyanate, anti-CD45RO-phycoerythrin, anti-CD4-peridinin chlorophyll protein, anti-CD8-peridinin chlorophyll protein, and anti-CD3-allophycocyanine. TEM cells were defined by their phenotype CD45RO+CD62L+, whereas the TCM cells were defined by CD45RO+CD62 L−.
Cells were incubated for 30 min in the dark at room temperature. Subsequently, the erythrocytes were lysed after 10-min incubation with FACS lysing solution (BD Biosciences) and washed with phosphate-buffered saline. After centrifugation, the cells were resuspended in 0.2 ml of phosphate-buffered saline before acquisition of 50 000 events in lymphocyte gate by FACScalibur flow cytometer. All the data were analyzed using the CellQuest Pro software (BD Biosciences).
Statistical analysis
The data from both long- and short-term groups were nonparametrically distributed (Kolmogorov–Smirnov test). Differences in the percentage of CD4+CD45RO+CD62 L− TEM cells between the group suffering an AR episode and those who were rejection-free were analyzed by using the Mann–Whitney U test. The clinical and demographical parameters were analyzed using Student's t-test and Mann–Whitney U test as appropriate. P-values <0.05 were considered significant.
Acknowledgments
This work was partially supported by grants from the Fondo de Investigaciones Sanitarias-ISCIII (PI070683, PI080157, REDINREN 06/16 ISCIII) and from the Fundación Mutua-Madrileña (ACI 07/01). DSS is a recipient of a Lopez-Albo grant from the Fundación Marqués de Valdecilla-IFIMAV. IB and MG are technicians supported by grants from the Fundación Marqués de Valdecilla-IFIMAV.
All the authors declared no competing interests.
Footnotes
TO CITE THIS ARTICLE: Segundo DS, Fernández-Fresnedo G, Gago M et al. Changes in the number of circulating TCM and TEM subsets in renal transplantation: relationship with acute rejection and induction therapy. Kidney Int Sup 2011; 1: 31–35.
References
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Molecular mimicry and autoimmune disease. Cell. 1987;50:819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Jones ND. Memory T cells: how might they disrupt the induction of tolerance. Transplantation. 2009;87 (Suppl 9:S74–S77. doi: 10.1097/TP.0b013e3181a2b83b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Chen J, Wang F, et al. Monoclonal antibody treatment to prolong the secondary cardiac allograft survival in alloantigen-primed mice. Scand J Immunol. 2010;71:345–352. doi: 10.1111/j.1365-3083.2010.02387.x. [DOI] [PubMed] [Google Scholar]
- Hall BM, Dorsch S, Roser B. The cellular basis of allograft rejection in vivo. II. The nature of memory cells mediating second set heart graft rejection. J Exp Med. 1978;148:890–902. doi: 10.1084/jem.148.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BM, Dorsch S, Roser B. The cellular basis of allograft rejection in vivo. I. The cellular requirements for first-set rejection of heart grafts. J Exp Med. 1978;148:878–889. doi: 10.1084/jem.148.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Oberbarnscheidt MH, Ng YH, Chalasani G. The roles of CD8 central and effector memory T-cell subsets in allograft rejection. Am J Transplant. 2008;8:1809–1818. doi: 10.1111/j.1600-6143.2008.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S, Dawson DV, Van Trigt P, et al. Differential infiltration by CD45RO and CD45RA subsets of T cells associated with human heart allograft rejection. Am J Pathol. 1993;142:1794–1803. [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S, Dawson DV, Sanfilippo F. Predominant infiltration of rejecting human renal allografts with T cells expressing CD8 and CD45RO. Transplantation. 1995;59:724–728. doi: 10.1097/00007890-199503150-00015. [DOI] [PubMed] [Google Scholar]
- Dollinger MM, Howie SE, Plevris JN, et al. Intrahepatic proliferation of ‘naive' and ‘memory' T cells during liver allograft rejection: primary immune response within the allograft. FASEB J. 1998;12:939–947. doi: 10.1096/fasebj.12.11.939. [DOI] [PubMed] [Google Scholar]
- Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- Augustine JJ, Siu DS, Clemente MJ, et al. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5:1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yin S, Xie H, et al. Immunophenotypic shift of memory CD8 T cells identifies the changes of immune status in the patients after liver transplantation. Scand J Clin Lab Invest. 2009;69:789–796. doi: 10.3109/00365510903268818. [DOI] [PubMed] [Google Scholar]
- Lanio N, Sarmiento E, Gallego A, et al. The potential role of T-cell memory distribution as predisposing factor for rejection in heart transplant recipients. Transplant Proc. 2009;41:2480–2484. doi: 10.1016/j.transproceed.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Jones DL, Sacks SH, Wong W. Controlling the generation and function of human CD8+ memory T cells in vitro with immunosuppressants. Transplantation. 2006;82:1352–1361. doi: 10.1097/01.tp.0000241077.83511.be. [DOI] [PubMed] [Google Scholar]
- Louis S, Audrain M, Cantarovich D, et al. Long-term cell monitoring of kidney recipients after an antilymphocyte globulin induction with and without steroids. Transplantation. 2007;83:712–721. doi: 10.1097/01.tp.0000255683.66156.d3. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Zilvetti M, Friend P, et al. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006;82:1342–1351. doi: 10.1097/01.tp.0000239268.64408.84. [DOI] [PubMed] [Google Scholar]
- Yehia M, Matheson PJ, Merrilees MJ, et al. Predictors of chronic allograft nephropathy from protocol biopsies using histological and immunohistochemical techniques. Nephrology (Carlton) 2006;11:261–266. doi: 10.1111/j.1440-1797.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- Segundo DS, Fernández-Fresnedo G, Gago M, et al. Kidney transplant recipients show an increase in the ratio of T-cell effector memory/central memory as compared to nontransplant recipients on the waiting list. Transplant Proc. 2010;42:2877–2879. doi: 10.1016/j.transproceed.2010.07.072. [DOI] [PubMed] [Google Scholar]