Abstract
Salt is one of the most important determinants of high blood pressure and increased cardiovascular risk worldwide. However, a high salt intake has other adverse effects beyond those involving the cardiovascular system, so that there is renewed interest in the relationships between high salt intake and other diseases.
Keywords: cardiovascular, health effects, salt or sodium intake
SALT INTAKE AND BLOOD PRESSURE
Raised blood pressure is the dominant cause of death and disability in adults worldwide, responsible for approximately 50% of deaths from coronary heart disease and over 60% of those from stroke. The risk of cardiovascular disease increases with increasing blood pressure and a reduction in blood pressure causes a significant reduction in vascular events.
Evidence from a wide variety of studies shows a consistent direct relationship between salt intake and blood pressure. A 4.4 g reduction in daily dietary intake of salt decreases blood pressure by about 4.2/2.1 mm Hg.1 Randomized controlled clinical trials have consistently shown dose–response effects.2 The blood pressure-lowering effect of reducing salt intake is effective in men and women, in all ethnic groups, in all age groups, and all starting blood pressures.1, 2
Population-based interventions indicate that when salt intake is reduced, blood pressure in the community falls. A moderate reduction in salt intake is therefore recommended worldwide for the prevention and control of high blood pressure.
SALT INTAKE AND CARDIOVASCULAR DISEASE
No randomized controlled trials have studied the effect of reducing the salt intake of populations on cardiovascular disease—the ethical and methodological problems with such trials are similar to those with trials involving tobacco and obesity—but the link is strong and is now accepted.2 In cohort studies, a 5 g per day higher salt intake (2000 mg of sodium) is associated with a 17% greater risk of total cardiovascular disease, and crucially a 23% greater risk of stroke.3 Since the 1970s, Finland has aimed to reduce the population's salt intake4 by collaboration with the food industry to develop food products with reduced salt and by raising awareness among consumers. Over 30 years salt intake has reduced by a third (6 g per person per day). Systolic blood pressure fell by over 10 mm Hg, whereas mortality from stroke and coronary heart disease decreased 75–80%, with an increase of 5–6 years in life expectancy.4 Similarly, in Japan, a government campaign to reduce salt intake saw a fall from 13.5 to 12.1 g/day (from 5400 to 4840 mg sodium) in a decade. This was associated with a substantial reduction in stroke mortality, despite increases in the population's fat intake, cigarette smoking, alcohol consumption, and body mass index.
A reduction in salt intake is associated with substantial cost savings.4 For example, in the United States, a mean population salt reduction of 3 g per day would result in an estimated annual gain of 194,000–392,000 quality-adjusted life years, a measure of added healthy life, and savings of $10–24bn (£6–15bn, €7–17bn) in health-care costs. This represents a $6–12 return on investment for each dollar spent on the regulatory program.
The global goal set by WHO is to reduce salt intake by at least 30% to less than 5 g (2000 mg of sodium) per person per day by 2025, with some countries aiming for even lower levels in the longer term. In the United States, a reduction in salt intake of 3 g per day would reduce the annual number of new cases of cardiovascular disease by approximately 10% (around 60,000–120,000 fewer cases of coronary heart disease, 32,000–66,000 strokes, and 54,000–99,000 heart attacks) with a corresponding reduction in all-cause mortality.4
SALT INTAKE AND OTHER CARDIOVASCULAR RISK FACTORS
Left ventricular hypertrophy is an important risk factor for premature cardiovascular disease. Salt intake, independent of blood pressure levels, is a predictor of left ventricular mass and a reduction in salt intake causes a regression of left ventricular hypertrophy.5
A high intake of salt causes an increase in renal blood flow and glomerular filtration rate and a reduction in salt intake appears to normalize the hyperfiltration seen in salt-sensitive normotensive individuals.6 Hyperfiltration often precedes the development of high blood pressure and it is reversed by a reduction in salt intake, particularly in those more sensitive to the detrimental effects of dietary salt.6
Finally, the association between salt intake and blood pressure has been described to be greater at higher levels of heart rate than at lower levels.7 The implications of these findings are as yet to be clarified.
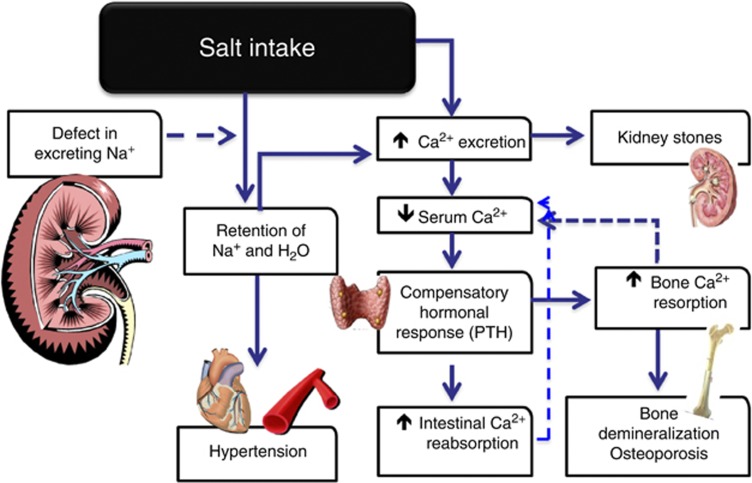
SALT INTAKE, HYPERCALCIURIA, KIDNEY STONES, AND BONE METABOLISM
Evidence from animal, clinical, and epidemiological studies suggests that high blood pressure is associated with abnormalities of calcium metabolism, leading to increased calcium loss, secondary activation of the parathyroid gland, increased movement of calcium from bone, and increased risk of urinary tract stones.8 Some of these abnormalities are detectable in children and young people and continue throughout adult life.9 The cluster of abnormalities may be due either to a primary renal tubular defect (‘renal calcium leak' hypothesis) or to the effect of central volume expansion seen in hypertension (‘central blood volume' hypothesis).8 A high salt intake is known to aggravate these abnormalities and their consequences. Salt (sodium) intake is one of the major determinants of urinary calcium excretion, the other being calcium intake. Within a population, calcium excretion is directly related to sodium excretion, that is, salt intake. A dietary increase of 100 mmol of sodium produces an increase in urinary calcium of 1 mmol.10 A high salt intake, by causing high urinary calcium losses, causes increased levels of parathyroid hormone, 1,25-dihydroxy-vitamin D, and serum osteocalcin (marker of bone formation), as well as urinary cyclic AMP and urinary hydroxyproline (marker of bone resorption).8 If substantial calcium loss related to high blood pressure is sustained over many decades, increased excretion of calcium in the urine may result in an increased risk of urinary tract stones, and the increased movement of calcium from bone may result in higher rates of bone mineral loss, thereby increasing the risk of osteoporosis (Figure 1). Hypercalciuria is the most common risk factor for urolithiasis in adults, being present in up to 80% of patients with kidney stones. In the general population, high salt intake is associated with a greater incidence of kidney stones.11 A reduction in salt intake is an effective strategy to reduce hypercalciuria in hypertensive patients12 as well as in kidney stone formers.13 Sustained hypercalciuria in people with high blood pressure may lead to an increased risk of bone mineral loss. In postmenopausal women, high blood pressure is associated with faster bone mineral density loss.14 In turn, bone mineral density is inversely associated with stroke incidence and cardiovascular mortality.8 A high salt intake in humans causes (a) reduction in the activity of biomarkers of bone formation, (b) increase in biomarkers of bone resorption, (c) reduction in peak bone mass in young girls, and (d) higher rate of bone mineral loss in postmenopausal women.8
Figure 1.
Schematic representation of the effects of salt intake on calcium metabolism and its implications for kidney stone formation and bone health.
SALT INTAKE AND STOMACH CANCER
More than 30 years ago, an association between stroke mortality and incidence of stomach cancer was reported. Soon after it was suggested that high salt intake could be the underlying common factor. Stomach cancer is still a common neoplasia and an important, at least partly, preventable public health problem. Given that high salt intake has been associated with Helicobacter pylori infection, as promoter of damage of gastric mucosa, hypergastrinemia, and cell proliferation, numerous case–control studies in the past have investigated the relationship between salt consumption and risk of gastric cancer and have reported a positive statistical association. Since then prospective studies clearly indicate an association between salt consumption and stomach cancer. In a recent meta-analysis of prospective population studies, dietary salt intake was directly associated with risk of gastric cancer, with progressively increasing risk across consumption levels.15 In the pooled analysis, ‘high' and ‘moderately high' versus ‘low' salt intake were both associated with increased risk of gastric cancer (relative risk=1.68 (95% confidence interval: 1.17–2.41), P=0.005 and 1.41 (1.03–1.93), P=0.032, respectively). The association was stronger in the Japanese population, and higher consumption of selected salt-rich foods was also associated with greater risk.
A number of experimental studies provide possible mechanistic explanations for a causal role of an elevated salt intake on the risk of gastric cancer. High intragastric sodium concentrations cause mucosal damage and inflammation, which in turn increase cell proliferation and endogenous mutations. High sodium intake appears to change the viscosity of the protective mucous barrier and to increase the colonization by H. pylori, a recognized risk factor for gastric cancer.
SALT INTAKE AND FLUID RETENTION
Salt is one of the major substrates contributing to total body fluid balance. High salt intake induces thirst and increased fluid intake that is then retained in the intravascular compartment causing an increase in total blood volume. Both the heart and the kidney are the main organs to compensate for these changes through hormonal responses (renin–angiotensin–aldosterone axis and atrial natriuretic peptide), increased systolic ejection, increased blood flow, and renal clearance. Dysregulations of these systems may lead to fluid retention that can be aggravated by high salt intake and ameliorated by a reduction in salt intake. Congestive heart failure may be triggered by a high salt intake and ‘rebound' sodium, and fluid retention following diuretic withdrawal can be minimized or avoided by low salt intake before withdrawal.16
SALT INTAKE AND CATARACT
Some observational studies in Italy and Australia have described a significant association between high salt intake (measured by food frequency questionnaire) and the prevalence of cataract.9 Higher levels of extracellular sodium might make it more difficult for sodium pumps to maintain the low levels of intracellular sodium necessary for lens transparency. However, no intervention trials to date are available to support causality.
SALT INTAKE AND ASTHMA
Early clinical and epidemiological observations suggested a potential link between excessive salt intake and both the etiology and physiology of asthma.9 However, more recently, a Cochrane review identified nine small studies in relation to sodium manipulation and asthma, of which five were in people with asthma, and four in people with exercise-induced asthma. There were no significant benefits of salt restriction on the control of asthma. There was some evidence from the exercise-induced asthma studies that a low sodium diet may improve lung function after exercise and possibly baseline lung function, but this is based on findings from a very small numbers of participants.17
SALT INTAKE AND MENIERE'S DISEASE
Meniere's disease is a chronic illness that affects many patients every year worldwide. The disease is characterized by intermittent episodes of vertigo, fluctuating sensorineural hearing loss, tinnitus and aural pressure.18 Currently there is no cure. However, it has been suggested that a low-salt diet might relieve symptoms by reducing endolymphatic pressure in endolymphatic hydrops. International guidelines do mention a reduced salt diet as a lifestyle change recommended for the management of Meniere's syndrome. However, so far the hypothesis does not seem to have been tested in randomized clinical trials.19
CONCLUSIONS
The case for a reduction in salt intake for the prevention of cardiovascular disease is overwhelming. The present short review highlights many additional health benefits, some with more direct evidence and some still providing circumstantial support (Table 1). The latter provide clear indication for research gaps to be followed to extend our knowledge of the benefit of a moderate reduction in salt intake.
Table 1. Effects of salt intake on health conditions.
| Condition | Level of evidencea |
|---|---|
| Blood pressure | ++++ |
| Stroke | +++ |
| Cardiovascular disease | +++ |
| Left ventricular hypertrophy | +++ |
| Glomerular filtration | +++ |
| Calcium excretion | ++++ |
| Kidney stones (Ca oxalate) | +++ |
| Bone demineralization | ++ |
| Bone fractures | + |
| Stomach cancer | ++ |
| Fluid retention | ++++ |
| Cataract | + |
| Asthma | + |
| Meniere's disease | + |
Based on an arbitrary scale.
FPC has received consulting fees from Boehringer Ingelheim and Daiichi Sankyo. Publication costs for this article were supported by the Turkish Society of Hypertension and Renal Diseases, a nonprofit national organization in Turkey.
References
- He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzullo P, D'Elia L, Kandala N-B, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Capewell S, Lincoln P, et al. Policy options to reduce population salt intake. BMJ. 2011;343:d4995. doi: 10.1136/bmj.d4995. [DOI] [PubMed] [Google Scholar]
- Schmieder RE. Dietary salt intake and left ventricular hypertrophy. Nephrol Dial Transplant. 1997;12:245–248. doi: 10.1093/ndt/12.2.245. [DOI] [PubMed] [Google Scholar]
- Barba G, Cappuccio FP, Russo L, et al. Renal function and blood pressure response to dietary salt restriction in normotensive men. Hypertension. 1996;27:1160–1164. doi: 10.1161/01.hyp.27.5.1160. [DOI] [PubMed] [Google Scholar]
- Rastenyte D, Tuomilehto J, Moltchanov V, et al. Association between salt intake, heart rate and blood pressure. J Hum Hypertens. 1997;11:57–62. doi: 10.1038/sj.jhh.1000385. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Kalaitzidis RG, Duneclift S, et al. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol. 2000;13:169–177. [PubMed] [Google Scholar]
- Cappuccio FP, MacGregor GA. Dietary salt restriction: benefits for cardiovascular disease and beyond. Curr Opinion Nephrol Hypertension. 1997;6:477–482. doi: 10.1097/00041552-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Blackwood AM, Sagnella GA, Cook DG, et al. Urinary calcium excretion, sodium intake and blood pressure in a multi-ethnic population: results of the Wandsworth Heart and Stroke Study. J Hum Hypert. 2001;15:229–237. doi: 10.1038/sj.jhh.1001171. [DOI] [PubMed] [Google Scholar]
- Cirillo M, Laurenzi M, Panarelli W, et al. Urinary sodium to potassium ratio and urinary stone disease. Kidney Int. 1994;46:1133–1139. doi: 10.1038/ki.1994.376. [DOI] [PubMed] [Google Scholar]
- Saggar-Malik AK, Markandu ND, MacGregor GA, et al. Moderate salt restriction for the management of hypertension and hypercalciuria. J Hum Hypertens. 1996;10:811–813. [PubMed] [Google Scholar]
- Timio F, Kerry SM, Anson KM, et al. Calcium urolithiasis, blood pressure and salt intake. Blood Pressure. 2003;12:122–127. doi: 10.1080/08037050310001084. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Meilahn E, Zmuda JM, et al. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Lancet. 1999;354:971–975. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- D'Elia L, Rossi G, Ippolito R, et al. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31:489–498. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Missouris CG, Cappuccio FP, Markandu ND, et al. Diuretics and oedema: how to avoid rebound sodium retention. Lancet. 1992;339:1546. doi: 10.1016/0140-6736(92)91317-2. [DOI] [PubMed] [Google Scholar]
- Pogson Z, McKeever T. Dietary sodium manipulation and asthma. Cochrane Database Syst Rev. 2011;3:CD000436. doi: 10.1002/14651858.CD000436.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi H, Paparella MM. Meniere's disease. Lancet. 2008;372:406–414. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- James A, Thorp M. Meniere's disease. BMJ Clin Evid. 2007;3:505. [PMC free article] [PubMed] [Google Scholar]