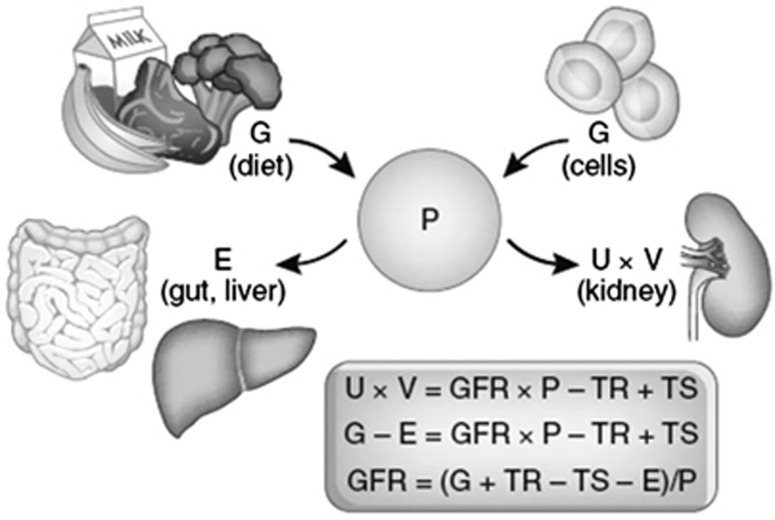
1.1: DEFINITION OF CKD
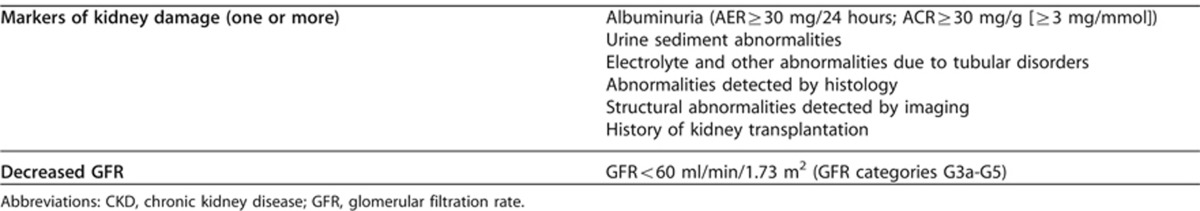
1.1.1: CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health (Table 2). (Not Graded)
Table 2. Criteria for CKD (either of the following present for 43 months).
RATIONALE
The definition of CKD remains intact, but we have clarified the classification and risk stratification as indicated below. The addition of ‘with implications for health' is intended to reflect the notion that a variety of abnormalities of kidney structure or function may exist, but not all have implications for health of individuals, and therefore need to be contextualized.
Kidney damage refers to a broad range of abnormalities observed during clinical assessment, which may be insensitive and non-specific for the cause of disease but may precede reduction in kidney function (Table 2). Excretory, endocrine and metabolic functions decline together in most chronic kidney diseases. GFR is generally accepted as the best overall index of kidney function. We refer to a GFR <60 ml/min/1.73 m2 as decreased GFR (Table 2) and a GFR <15 ml/min/1.73 m2 as kidney failure. AKI may occur in patients with CKD and hasten the progression to kidney failure.14
Complications include drug toxicity, metabolic and endocrine complications, increased risk for CVD, and a variety of other recently recognized complications, including infections, frailty, and cognitive impairment.15, 16, 17, 18 Complications may occur at any stage, often leading to death without progression to kidney failure. Complications may also arise from adverse effects of interventions to prevent or treat the disease and associated comorbidity.
Criteria for CKD
Defining terms: The following section aims to define specific terms and concepts so as to ensure clarity among all users. In addition, the rationale for including these terms is included.
Table 3 provides a justification for the criteria for CKD. The criteria for definition of CKD are objective and can be ascertained by means of simple laboratory tests without identification of the cause of disease, thereby enabling detection of CKD by non-nephrologist physicians and other health professionals.
Table 3. Criteria for definition of CKD19.

Duration >3 Months
Kidney diseases may be acute or chronic. We explicitly but arbitrarily define duration of >3 months (>90 days) as delineating “chronic” kidney disease. The rationale for defining chronicity is to differentiate CKD from acute kidney diseases (such as acute GN), including AKI, which may require different interventions, and have different etiologies and outcomes.7 We did not define acute kidney disease (AKD) because there does not appear be an evidence base for a precise definition.
The duration of kidney disease may be documented or inferred based on the clinical context. For example, a patient with decreased kidney function or kidney damage in the midst of an acute illness, without prior documentation of kidney disease, may be inferred to have AKI. Resolution over days to weeks would confirm the diagnosis of AKI. A patient with similar findings in the absence of an acute illness may be inferred to have CKD, and if followed over time would be confirmed to have CKD. In both cases, repeat ascertainment of kidney function and kidney damage is recommended for accurate diagnosis. The timing of the evaluation depends on clinical judgment, with earlier evaluation for the patients suspected of having AKI and later evaluation for the patient suspected of having CKD. For further details on the Evaluation of CKD, see Chapter 1.4.
Reversibility. Most kidney diseases do not have symptoms or findings until later in their course and are detected only when they are chronic. Most causes of CKD are irreversible with a life-long course, and treatment aimed at slowing progression to kidney failure. However, chronicity is not synonymous with irreversibility. In some cases, CKD is entirely reversible, either spontaneously or with treatment, and in other cases, treatment can cause partial regression of kidney damage and improvement in function (e.g., immunosuppressive therapies for GN). Even kidney failure may be reversed with transplantation. Because of the long course of most cases of CKD, patients often have one or more episodes of AKI, superimposed upon CKD.
Decreased GFR
The kidney has many functions, including excretory, endocrine and metabolic functions. The GFR is one component of excretory function, but is widely accepted as the best overall index of kidney function because it is generally reduced after widespread structural damage and most other kidney functions decline in parallel with GFR in CKD.
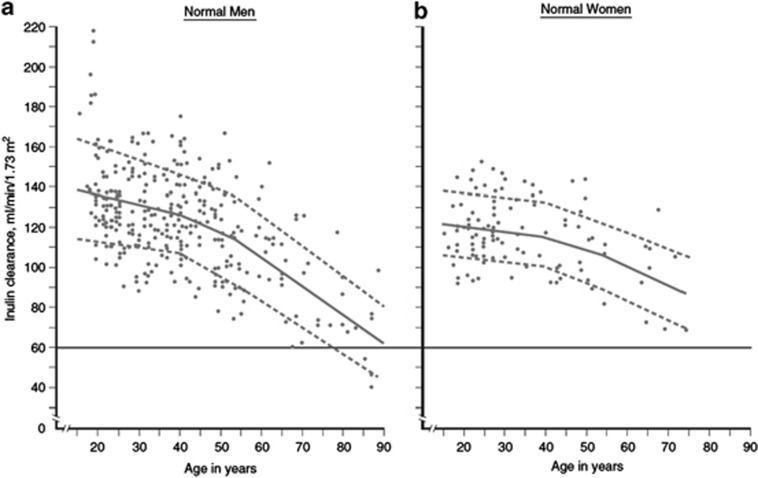
We chose a threshold of GFR <60 ml/min/1.73 m2 (GFR categories G3a-G5) for >3 months to indicate CKD. A GFR <60 ml/min/1.73 m2 is less than half of the normal value in young adult men and women of approximately 125 ml/min/1.73 m2. Figure 2 shows a compilation of GFR measurements in apparently healthy men and women in the US and Europe by age from more than 40 years ago.20 The age-associated GFR decline is observed in longitudinal as well as cross sectional studies, but varies substantially among individuals within the population.21 More recent data in kidney donors confirm these general trends.22, 23 Limited data are available for non-whites in the US and Europe or in other countries, although data suggest that the normal range for measured GFR and the age-associated decline is similar.24, 25, 26
Figure 2.
Normal values for GFR by age. GFR is shown for men (Panel a) and women (Panel b) of various ages, with the GFR measured as the urinary clearance of inulin. The horizontal line indicates a GFR value of 60 ml/min/1.73 m2, which is the threshold for the definition of CKD. Solid lines represent the mean value of GFR per decade of age, and dashed lines represent the value 1 SD from the mean value of GFR per decade of age. CKD, chronic kidney disease; GFR, glomerular filtration rate; SD, standard deviation. Adapted with permission from Wesson L.20 Physiology of the Human Kidney. Grune & Stratton: New York, 1969.
A GFR <60 ml/min/1.73 m2 can be detected by routine laboratory testing. Current estimating equations for GFR (eGFR) based on serum creatinine (SCr), but not SCr alone, are sensitive for detecting measured GFR<60 ml/min/1.73 m2.27 A decreased eGFR using SCr can be confirmed by GFR estimation using an alternative filtration marker (cystatin C) or GFR measurement, as necessary.
A GFR<60 ml/min/1.73 m2 is associated with a higher risk of complications of CKD than in subjects with CKD and conserved GFR. The causal mechanisms underlying these associations are not fully understood. We consider three main types of complications, which are of relevance to all patients with CKD and reduced GFR, irrespective of country, age or etiology:
Drug toxicity. Altered pharmacokinetics of drugs excreted by the kidney and an increased risk of drug-interactions are common and require adjustment in the dosage of many drugs (see Chapter 4.4).13 At lower GFR, altered pharmacokinetics and pharmacodynamics of drugs not excreted by the kidney may also be observed. Errors in drug dosing are common in patients with CKD and may be associated with toxicity to the kidney (resulting in AKI) or systemic toxicity, resulting in threats to patient safety.
Metabolic and endocrine complications. As GFR declines a variety of complications reflecting loss of endocrine or exocrine function of the kidneys develop including anemia, acidosis, malnutrition, bone and mineral disorders (described in Chapters 3 and 4).
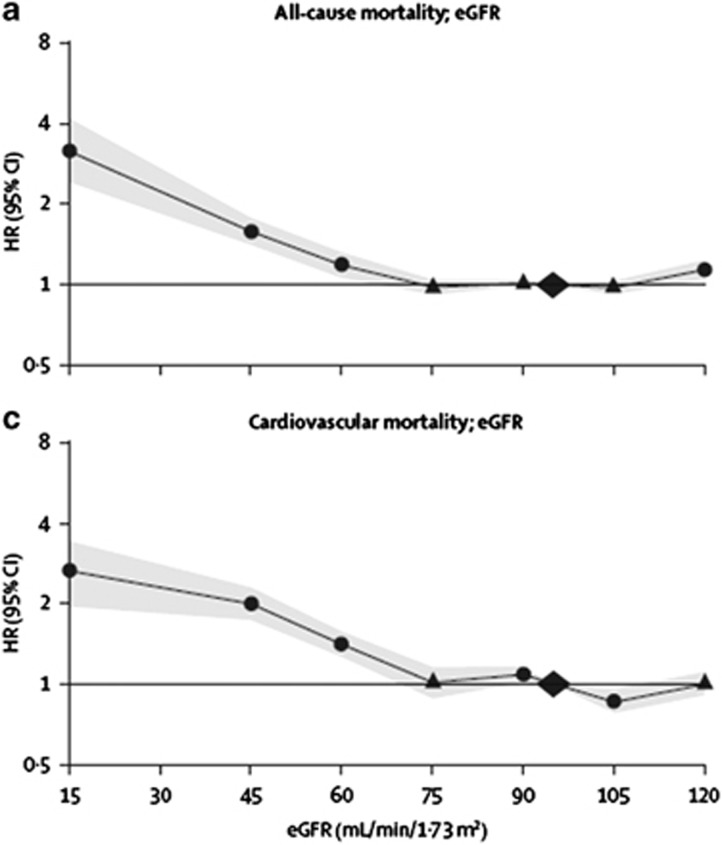
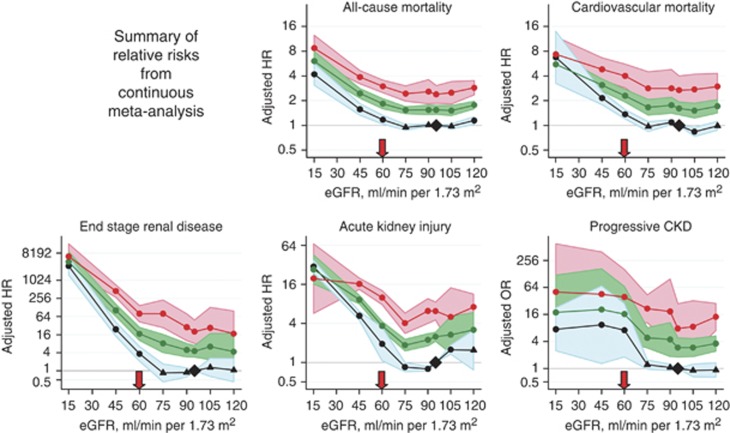
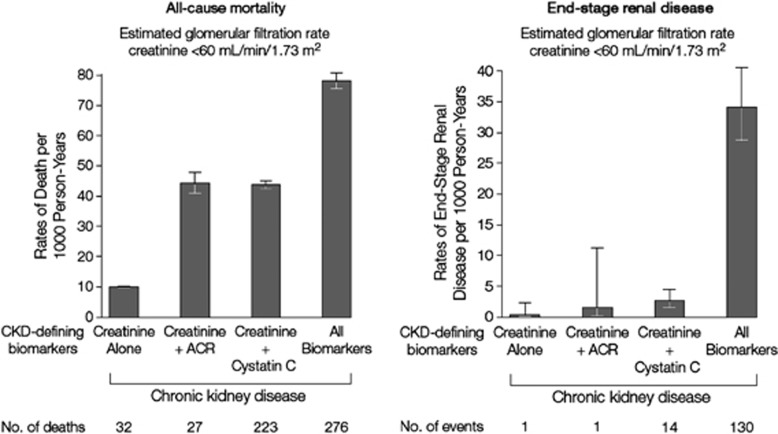
Risk of CVD and death. A meta-analysis by the CKD Prognosis Consortium demonstrated associations of eGFR <60 ml/min/1.73 m2 with subsequent risk of all-cause and cardiovascular mortality, kidney failure, AKI, and CKD progression in the general population and in populations with increased risk for CVD.3-5 Figure 3 shows the relationship for total and cardiovascular mortality in general population cohorts. The risk for all outcomes was relatively constant between eGFR of 75-105 ml/min/1.73 m2, with a suggestion of a U-shaped curve for total mortality. The increased relative risk (RR) for all outcomes was significant for eGFR of <60 ml/min/1.73 m2.
Figure 3.
Relationship of eGFR with mortality. HRs and 95% CIs for all-cause (a) and cardiovascular mortality (c) according to spline eGFR. HRs and 95% CIs (shaded areas) are adjusted for ACR, age, sex, ethnic origin, history of CVD, systolic BP, diabetes, smoking, and total cholesterol. The reference (diamond) was eGFR 95 ml/min/1.73 m2 and ACR 5 mg/g (0.6 mg/mmol), respectively. Circles represent statistically significant and triangles represent not significant. ACR, albumin-to-creatinine ratio; BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio. Reprinted from The Lancet, vol 375, Matshushita K, van de Velde M, Astor BC, et al.4 Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis, p. 2073-2081, 2010, with permission from Elsevier; accessed http://download.thelancet.com/pdfs/journals/lancet/PIIS0140673610606745.pdf
Kidney Damage
Damage to the kidney can be within the parenchyma, large blood vessels or collecting systems, and is most often inferred from markers rather than direct examination of kidney tissue. The markers of kidney damage often provide a clue to the likely site of damage within the kidney and in association with other clinical findings, the cause of kidney disease.
Proteinuria. Proteinuria is a general term for the presence of increased amounts of protein in the urine. Proteinuria may reflect abnormal loss of plasma proteins due to a) increased glomerular permeability to large molecular weight proteins (albuminuria or glomerular proteinuria), b) incomplete tubular reabsorption of normally filtered low-molecular-weight proteins (tubular proteinuria), or c) increased plasma concentration of low-molecular-weight proteins (overproduction proteinuria, such as immunoglobulin light chains). Proteinuria may also reflect abnormal loss of proteins derived from the kidney (renal tubular cell constituents due to tubular damage) and lower urinary tract. Albuminuria, tubular proteinuria and renal tubular cell constituents are pathognomonic of kidney damage. In addition, findings from experimental and clinical studies have suggested an important role for proteinuria in the pathogenesis of disease progression of CKD.28
Albuminuria. Albuminuria refers to abnormal loss of albumin in the urine. Albumin is one type of plasma protein found in the urine in normal subjects and in larger quantity in patients with kidney disease.
For a number of reasons, clinical terminology is changing to focus on albuminuria rather than proteinuria: a) albumin is the principal component of urinary protein in most kidney diseases; recent recommendations for measurement of urine proteins emphasize quantification of albuminuria rather than total protein; b) recent epidemiologic data from studies around the world demonstrate a strong graded relationship of the quantity of urine albumin with both kidney and CVD risk; and c) later recommendations in these guidelines classify kidney disease by level of albuminuria. In this guideline, we will refer to proteinuria when discussing general concepts and will refer either to total protein, albumin or other specific proteins when discussing measurements, patterns, and interpretation of proteinuria.
Albuminuria is a common but not uniform finding in CKD. It is the earliest marker of glomerular diseases, including diabetic glomerulosclerosis, where it generally appears before the reduction in GFR. It is a marker of hypertensive nephrosclerosis but may not appear until after the reduction in GFR. It is often associated with underlying hypertension, obesity, and vascular disease, where the underlying renal pathology is not known.
Normative values for albuminuria and proteinuria are generally expressed as the urinary loss rate. The urinary loss rate of albumin and protein has commonly been referred to as AER and protein excretion rate (PER), respectively, although in the strict physiological sense they are not excreted. The terms AER and PER will be retained herein.
We chose a threshold for urinary AER of ≥30 mg/24 hours sustained for >3 months to indicate CKD. This value is considered to be approximately equivalent to an ACR in a random untimed urine sample of ≥30 mg/g or ≥3 mg/mmol. The rationale for this threshold is as follows:
An AER of ≥30 mg/24 hours (ACR≥30 mg/g [≥3 mg/mmol]) is greater than 3 times the normal value in young adult men and women of approximately 10 mg/24 hours (ACR 10 mg/g or 1 mg/mmol).
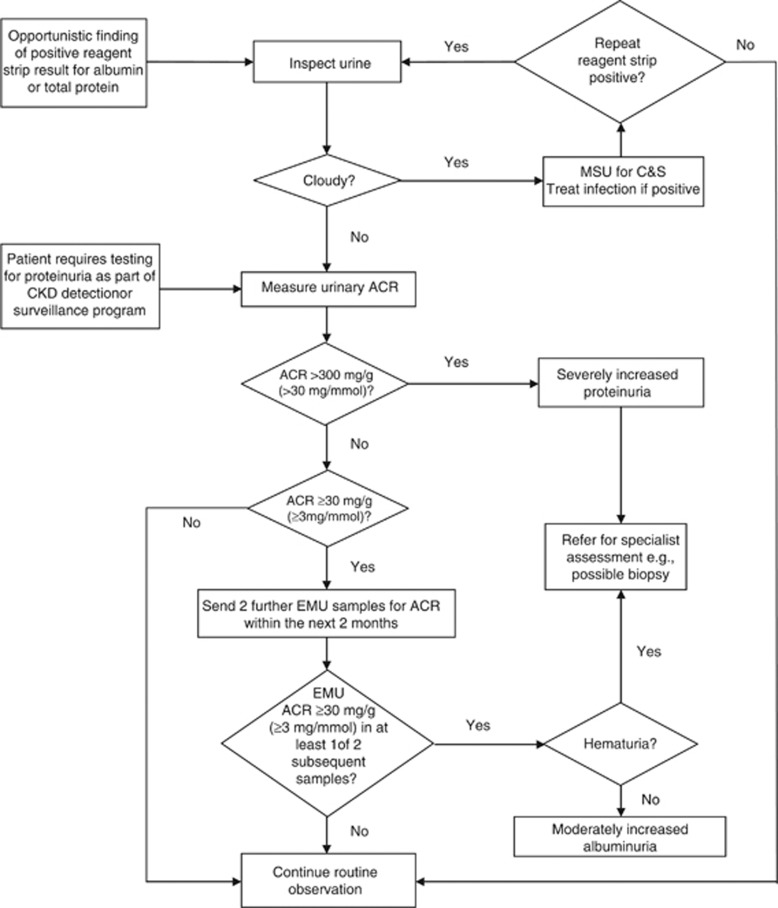
An AER of ≥30 mg/24 hours (ACR≥30 mg/g [≥3 mg/mmol]) may sometimes be detectable as ‘trace' using a urine reagent strip, depending on urine concentration, but this is not a consistent finding until AER exceeds approximately 300 mg/24 hours (ACR≥300 mg/g [≥30 mg/mmol]). As described later, trace or positive reagent strip values/readings can be confirmed by ACR, and an elevated ACR can be confirmed by urine AER in a timed urine collection, as necessary.
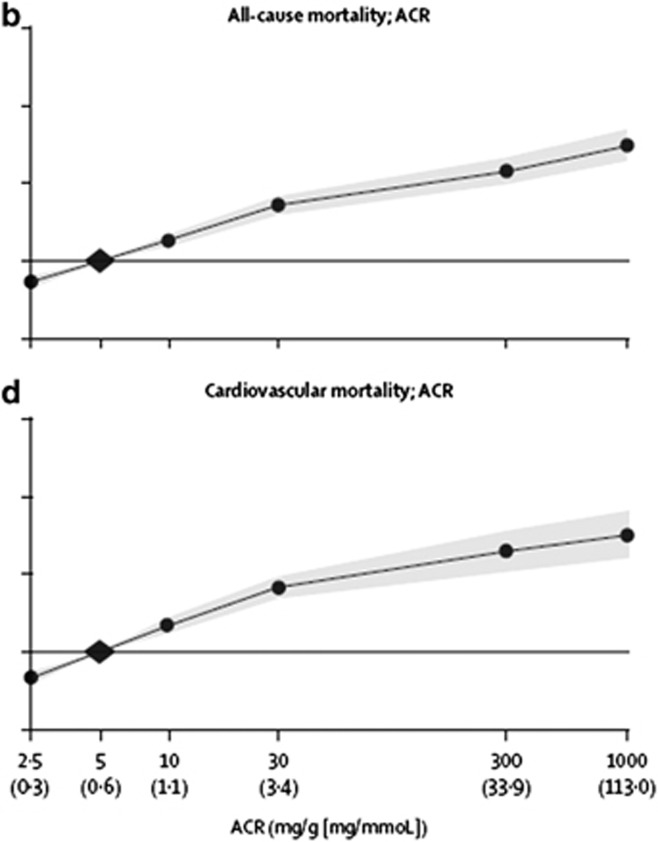
An AER≥30 mg/24 hours (ACR≥30 mg/g [≥3 mg/mmol]) is associated with an increased risk for complications of CKD. A meta-analysis by the CKD Prognosis Consortium demonstrated associations of an ACR≥30 mg/g (≥3 mg/mmol) or reagent strip 1+ protein with subsequent risk of all-cause and cardiovascular mortality, kidney failure, AKI, and CKD progression in the general population and in populations with increased risk for CVD3-5 (Figure 4).
Figure 4.
Relationship of albuminuria with mortality. HRs and 95% CIs for all-cause (b) and cardiovascular mortality (d) according to ACR. HRs and 95% CIs (shaded areas) are adjusted for age, sex, ethnic origin, history of CVD, systolic BP, diabetes, smoking, and total cholesterol and spline eGFR. The reference (diamond) was ACR 5 mg/g (0.6 mg/mmol) and eGFR 95 ml/min/1.73 m2, respectively. Circles represent statistically significant and triangles represent not significant. ACR plotted in mg/g. To convert ACR in mg/g to mg/mmol multiply by 0.113. Approximate conversions to mg/mmol are shown in parentheses. ACR, albumin-to-creatinine ratio; BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio. Reprinted from The Lancet, vol 375, Matshushita K, van de Velde M, Astor BC, et al.4 Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis, p. 2073-2081, 2010, with permission from Elsevier; accessed http://download.thelancet.com/pdfs/journals/lancet/PIIS0140673610606745.pdf
Urine sediment abnormalities. Formed elements, such as cells, casts, crystals, and microorganisms may appear in the urine sediment in a variety of disorders of the kidney and urinary tract, but renal tubular cells, red blood cell (RBC) casts, white blood cell (WBC) casts, coarse granular casts, wide casts, and large numbers of dysmorphic RBCs are pathognomonic of kidney damage.
Electrolyte and other abnormalities due to tubular disorders. Abnormalities of electrolytes and other solutes may result from disorders of renal tubular reabsorption and secretion. These syndromes are uncommon but pathognomonic of kidney disease. Often the diseases are genetic without underlying pathologic abnormalities. Other diseases are acquired, due to drugs or toxins, and are usually with prominent tubular pathologic lesions.
Pathologic abnormalities directly observed in kidney tissue obtained by biopsy. Evidence of abnormalities of renal parenchyma in kidney biopsies irrespective of eGFR or other markers of kidney damage must be acknowledged as an important parameter in defining kidney damage. The pathologic classification of diseases of the renal parenchyma reflects the localization of the disease to glomeruli, vessels, tubules and interstitium, or cysts. Renal biopsies are performed in the minority of CKD patients.
Imaging abnormalities. Imaging techniques allow the diagnosis of diseases of the renal structure, vessels and/or collecting systems. Thus, patients with significant structural abnormalities are considered to have CKD if the abnormality persists for greater than 3 months (note that this does not include simple cysts and clinical context is required for action).
History of kidney transplantation. Kidney transplant recipients are defined as having CKD, irrespective of the level of GFR or presence of markers of kidney damage. The rationale for this designation is that biopsies in kidney transplant recipients reveal pathologic abnormalities even in patients without decreased GFR or albuminuria. Kidney transplant recipients have an increased risk of mortality and kidney outcomes compared to the general population and they require specialized medical management.29
Implications for Health
CKD is associated with a wide range of complications leading to adverse health outcomes. For some complications, the causal pathway between kidney disease and adverse outcomes is well-known. For these complications, there are clinical practice guidelines for testing and treatment for modifiable factors to prevent adverse outcomes. Since 2002 a large number of epidemiologic studies have linked decreased GFR and albuminuria to the risk of adverse health outcomes not previously identified as CKD complications. The exploration of the mechanisms for the relationships of CKD with these complications is a rapidly growing topic for basic and clinical research. Because of the high prevalence, adverse outcomes, and high cost of CKD, especially kidney failure, some countries have developed public health programs for early identification and treatment of CKD and its complications. The effectiveness of these programs is being evaluated.
Implications for Clinical Practice and Public Policy
CKD was first defined in the 2002 KDOQI Guidelines and endorsed at subsequent KDIGO Controversies Conferences with minor modifications.30, 31 The definition of CKD proposed here is intended for use in clinical practice, research and public health, and has not changed. Thus, the updated version does not change any of the initiatives that have been commenced with respect to public policy. We recognize the variation around the world regarding measurement of urine albumin versus total protein in clinical practice, and we anticipate variation in implementation of the guideline until more widespread dissemination of the guideline has occurred. For additional discussion about methods for ascertainment of urine albumin versus total protein, see Recommendation 1.4.4 (Evaluation of albuminuria). The implications of highlighting the importance of albuminuria for general practitioners in evaluation and prognostication may help with identification and care planning. Nonetheless, a number of concerns about the definition remain, which are clarified below.30, 32, 33, 34, 35, 36
Areas of Controversy, Confusion, or Non-consensus and Clarification of Issues and Key Points
General concerns:
a) The use of single thresholds without consideration of patient specific factors
The use of single thresholds to define decreased GFR and increased AER, without consideration for cause of disease, age, sex, race-ethnicity and clinical context is consistent with the use of single thresholds for disease markers to define other chronic non-communicable diseases, such as hypertension, diabetes, and hypercholesterolemia, that primarily affect the elderly and are associated with an increased risk for cardiovascular mortality. Biologic variability and error in ascertainment of GFR and AER can lead to misclassification and false negative and false positive diagnosis. Furthermore, these single thresholds appear to differentiate groups of individuals and outcomes, irrespective of specific patient characteristics in a multitude of studies. However, they correspond to thresholds for RRs for complications, rather than predictions of absolute risk. Furthermore, as with any diagnostic tests, findings must be interpreted with considerations of likelihood of disease based on the clinical context but this should not negate the application of a standard definition for CKD.
Specific concerns:
b) Relationship of CKD criteria to aging
Epidemiologic studies show an increased prevalence of decreased eGFR and increased ACR in older subjects. There has been vigorous debate as to whether decreased GFR or increased ACR in older people represent a disease or “normal aging.” Numerous studies show pathologic abnormalities associated with aging, including glomerular sclerosis, tubular atrophy and vascular sclerosis. The cause for this association is not clear but has been hypothesized to reflect disparate processes, such as vascular disease or senescence.37, 38, 39 Irrespective of cause, there appears to be increased risk associated with decreased eGFR or increased ACR in older people, and for this reason, we consider all individuals with persistently decreased GFR or increased albuminuria to have CKD. Comparison of the magnitude of risk to younger individuals is complicated. As with other CVD risk factors, absolute risk appears to be higher in older than in younger individuals, but RR appears to be lower.3-5 Note is also made that healthy older individuals do not necessarily have decreased GFR, so that while one may expect some decline, levels below 60 ml/min/1.73 m2 in individuals without comorbidity is the exception.20
c) Isolated decreased GFR without markers of kidney damage
A variety of clinical circumstances are associated with GFR <60 ml/min/1.73 m2 for >3 months in the absence of known structural alterations. Below are examples of these conditions and the rationale for considering them as CKD:
Heart failure, cirrhosis of the liver, and hypothyroidism. Decreased GFR complicates the management of the primary disease and patients with these disorders with decreased GFR have a worse prognosis than those without decreased GFR. In addition, renal biopsy in these patients may reveal renal parenchymal lesions.
Kidney donors. The usual level of GFR in kidney donors after transplantation is approximately 70% of the pre-donation level, in the range of 60-90 ml/min/1.73 m2 in most donors. However, a minority of donors have GFR <60 ml/min/1.73 m2. The prognosis of these donors compared to those with higher GFR has not been carefully studied. However, as with decreased GFR due to recognized kidney diseases, donors with decreased GFR require closer follow-up for adjustment of drug doses.
Malnutrition. The level of GFR is affected by habitual protein intake.40 Healthy adults with lower protein intake may have lower mean GFR, but usually do not have GFR <60 ml/min/1.73 m2. Older studies of patients with protein-calorie malnutrition and more recent studies of subjects with anorexia nervosa have documented reduced measured GFR that can improve following restoration of nutritional status. However, renal biopsies may reveal structural abnormalities in these conditions and decreased GFR can complicate their management.
d) Isolated albuminuria without decreased GFR
As described later, transient ACR ≥30 mg/g (≥3 mg/mmol) can occur in disorders other than CKD. Remission of albuminuria within 3 months in association with recovery from these disorders is not defined as CKD. Patients with persistent albuminuria would be considered to have CKD. Below are examples of these conditions and the rationale for considering them as CKD:
Obesity and metabolic syndrome. Albuminuria can be associated with obesity and metabolic syndrome, and can remit during weight loss. The mechanism of albuminuria in these conditions is not known but renal biopsies may reveal prominent vascular lesions. Patients with obesity and metabolic syndrome are at increased risk for development of diabetes and hypertension. The risk of persistent albuminuria in this condition has not been carefully studied.
Orthostatic (postural) proteinuria.41 Albuminuria may rarely be observed in the upright but not recumbent posture in patients with the syndrome of postural proteinuria. This condition is not associated with an increased risk of long-term adverse outcomes but a thorough evaluation is required to exclude other causes of CKD. Exclusion is generally possible by studying a first pass early morning urine (EMU) after overnight recumbency: total protein loss of >1000 mg/24 hours is unlikely to be explained by orthostatic proteinuria.
e) Remission of decreased GFR or markers of kidney damage
If decreased GFR and markers of kidney damage resolve while on treatment, the patient would be considered to have treated CKD, consistent with nomenclature for treated hypertension, treated diabetes, or treated hypercholesterolemia if blood pressure, blood glucose and blood cholesterol are within normal range while on medications. If resolution of decreased GFR and markers of kidney damage is sustained after withdrawal of treatment, the patient would be considered to have a history of CKD.
f) Kidney disease in the absence of decreased GFR and markers of kidney damage
A GFR ≥60 ml/min/1.73 m2 may reflect a decline from a higher value, and an AER of <30 mg/24 hours (ACR <30 mg/g or <3 mg/mmol) may reflect a rise from a lower value. Both findings may be associated with a pathologic process, even in the absence of other markers of kidney damage. Although such patients do not fulfill the criteria for CKD, a clinician's high index of suspicion may warrant additional diagnostic testing or close follow-up to detect the onset of CKD.
Pediatric Considerations
In general the definition of CKD in adults applies to children (birth-18 years) with the following exceptions or allowances:
the criteria for duration >3 months does not apply to newborns or infants ≤3 months of age.
the criteria of a GFR <60 ml/min/1.73 m2 does not apply to children <2 years of age in whom an age appropriate value should be applied.
a urinary total protein or albumin excretion rate above the normal value for age may be substituted for albuminuria ≥30 mg/24 hours.
all electrolyte abnormalities are to be defined in light of age normative values.
Developmental renal abnormalities account for as many as 30-50% of the children with CKD or ESRD.42 As such many infants while born with normal SCr for age will in fact meet the definition of CKD based on structural abnormalities despite the appearance of a normal GFR and may be classified as such within the first few days of life.
Normal GFR in newborns is less than 60 ml/min/1.73 m2, and it is not until approximately 2 years of age that one expects to see body surface area (BSA) adjusted GFR values comparable to those seen in the adult.43, 44 The expected increases in GFR that occur in the first months of life are due to increases in mean arterial pressure (MAP), decrease in renal vascular resistance, and redistribution of intrarenal blood flows to the superficial cortical nephrons in the newborn and increases in glomerular size and capillary permeability in the infant.45, 46, 47, 48 As such direct application of the GFR threshold values in the current CKD definition would not be appropriate in children less than 2 years of age as their normative maximal values would be below those of the adult or older child; hence most neonates and infants would be classified a priori at a decreased GFR based not on a reduction in GFR from a higher value, but rather failure of maturity of the kidney.
Numerous references exist for fetal,49 neonatal term,44, 48 pre-term,46, 50, 51 infant, child and adolescent GFR values43, 44 and the reader is strongly encouraged to use such references when comparison to a normative range is required for approximating the reduction in renal clearance of the individual child. It should be noted that across these ages the method of GFR measurement has often varied with the majority of such measurements in the neonate (term or preterm) or infant being derived from urinary collections and creatinine clearance (CrCl) measurements, whereas the older children and adolescents are often investigated with exogenous markers including inulin, radionuclides, and other markers such as iohexol or iothalamate.
The most comprehensive list of GFR based on the gold standard of inulin clearance and stratified by age for both term and preterm babies and children up to the age of young adults can be found in Schwartz and Furth's review on GFR measurements and estimation in pediatric CKD.52
Similarly, age relevant normative values should be utilized when interpreting urinary protein (albumin) excretions as well as other important urinary and serum laboratory values. Such values may be found in a number of pediatric nephrology texts. For neonates and infants this includes Waters53 and for post-neonate to young adults, more comprehensive values can be found in Langlois.54
1.2 STAGING OF CKD
1.2.1: We recommend that CKD is classified based on cause, GFR category, and albuminuria category (CGA). (1B)
RATIONALE
This statement is worded in this way because a classification encompassing cause and severity, as expressed by the level of GFR and the level of albuminuria, links to risks of adverse outcomes including mortality and kidney outcomes. These factors will therefore guide management of CKD and this recommended classification is consistent with other classification systems of disease which are based on the general domains of cause, duration and severity which provide a guide to prognosis. We included only kidney measures as factors in the classification of kidney disease, although we acknowledge that factors other than kidney measures, such as level of BP, also affect prognosis in CKD.
This recommended staging with inclusion of two additional domains represents a revision of the previous CKD guidelines, which included staging only by level of GFR. Cause of disease is included because of its fundamental importance in predicting the outcome of CKD and choice of cause-specific treatments. With inclusion of cause of kidney disease in the classification, we considered that it was no longer necessary to retain the use of the letter “T” to refer to kidney transplant recipients. Albuminuria is included as an additional expression of severity of disease not only because it is a marker of the severity of injury but also because albuminuria itself strongly associates with progression of kidney disease. Numerous studies have identified the adverse prognostic implication of albuminuria irrespective of level of kidney function.
We propose that this classification of CKD by Cause, GFR and Albuminuria, respectively be referred to as CGA staging. It can be used to inform the need for specialist referral, general medical management, and indications for investigation and therapeutic interventions. It will also be a tool for the study of the epidemiology, natural history, and prognosis of CKD.
Pediatric Considerations
The principles inherent in this guideline are fully applicable to children.
While large scale trials in children relating cause, GFR and albuminuria or proteinuria are rare, the principles of a multimodal classification in these three spheres should apply to children.
To date the only large scale trial utilizing a validated exogenously measured GFR (iohexol) and urinary protein excretion in a well-described cohort of children with renal disease is the Chronic Kidney Disease in Children (CKiD) trial.55 They have enrolled over 600 children aged 1-16 years and have described GFR and urinary proteinuria related outcomes in the areas of neurodevelopment, cognition, behavior, cardiovascular health and risk, and somatic growth. They have also collected samples for ongoing and future genetic study. While these data are sparse in relation to overall adult numbers, this represents one of the largest pediatric nephrology trials. The use of true measured GFR, the quality and completeness of the data, and the long term longitudinal follow-up will form the basis for the best evidence-based outcomes in children with CKD for the foreseeable future. A recent review article by Copelovitch et al.56 summarizes the major findings of the trial up to the present time.
1.2.2: Assign cause of CKD based on presence or absence of systemic disease and the location within the kidney of observed or presumed pathologic-anatomic findings. (Not Graded)
RATIONALE
This statement has been included so as to ensure that clinicians are alerted to the fact that CKD is not a diagnosis in and of itself, and that the assignment of cause is important for prognostication and treatment.
The cause of CKD has been traditionally assigned based on presence or absence of underlying systemic diseases and location of known or presumed pathologic-anatomic abnormalities. The distinction between systemic diseases affecting the kidney and primary kidney diseases is based on the origin and locus of the disease process. In primary kidney disease the process arises and is confined to the kidney whereas in systemic diseases the kidney is only one victim of a specific process, for example diabetes mellitus. Certain genetic diseases cross this boundary by affecting different tissues, e.g., adult polycystic kidney disease. The location of pathologic-anatomic findings is based on the magnitude of proteinuria, findings from the urine sediment examination, imaging, and renal pathology. Table 4 represents an example of a classification of causes of kidney diseases based on these two domains.
Table 4. Classification* of CKD based on presence or absence of systemic disease and location within the kidney of pathologicanatomic findings.
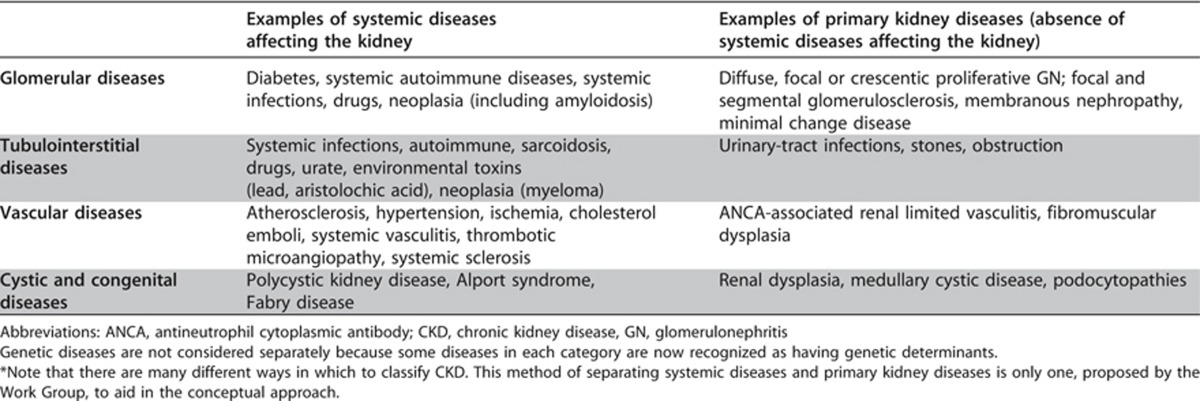
There is wide geographic variation in the cause of kidney disease. In developed countries, hypertension and diabetes are the most frequent causes of CKD, especially in the elderly. In populations with a high prevalence of diabetes and hypertension, it can be difficult to distinguish CKD due to hypertension and diabetes from CKD due to other disorders. In other countries, other causes of CKD may be as frequent as hypertension and diabetes (e.g., glomerular disease in East Asia) or coexist with them. Specialized diagnostic testing, such as kidney biopsy or invasive imaging studies are performed only when it is essential to confirm some diagnoses and the benefits justify the risks and cost. It is anticipated that cause of disease will not be known with certainty for many patients with CKD but can be either inferred or not known.
Pediatric Considerations
The principles inherent in this guideline are fully applicable to children.
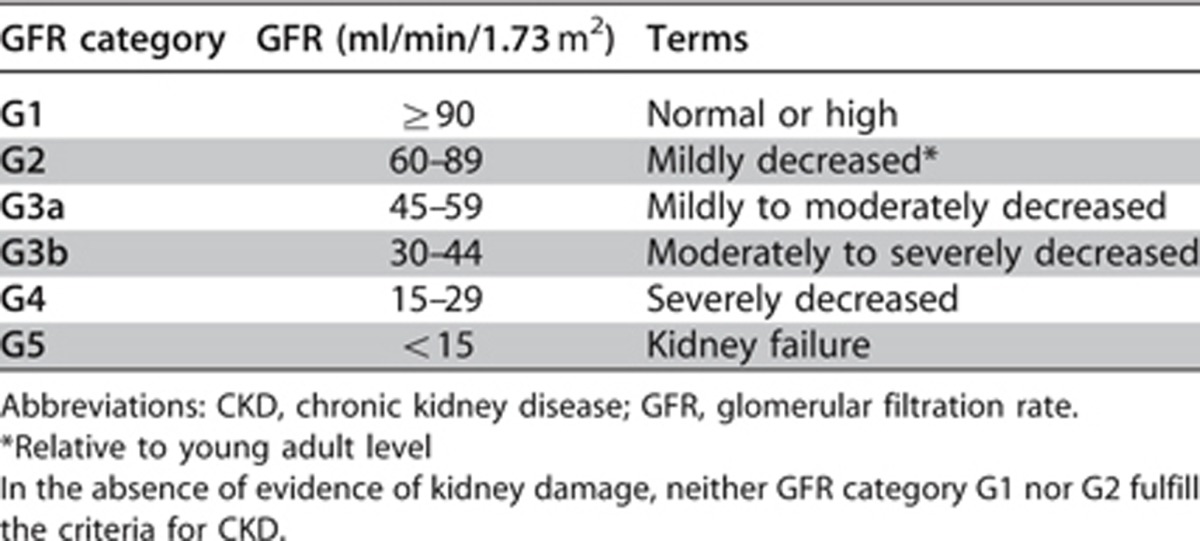
1.2.3: Assign GFR categories as follows [Table 5] (Not Graded):
Table 5. GFR categories in CKD.
RATIONALE
The purpose of this statement is to ensure clarity in communication. The terms associated with each of the GFR categories are descriptors which need to be taken in the context of the individual and are all references to normal young adults. Note that mildly decreased kidney function (G2) in the absence of other markers, does not constitute CKD.
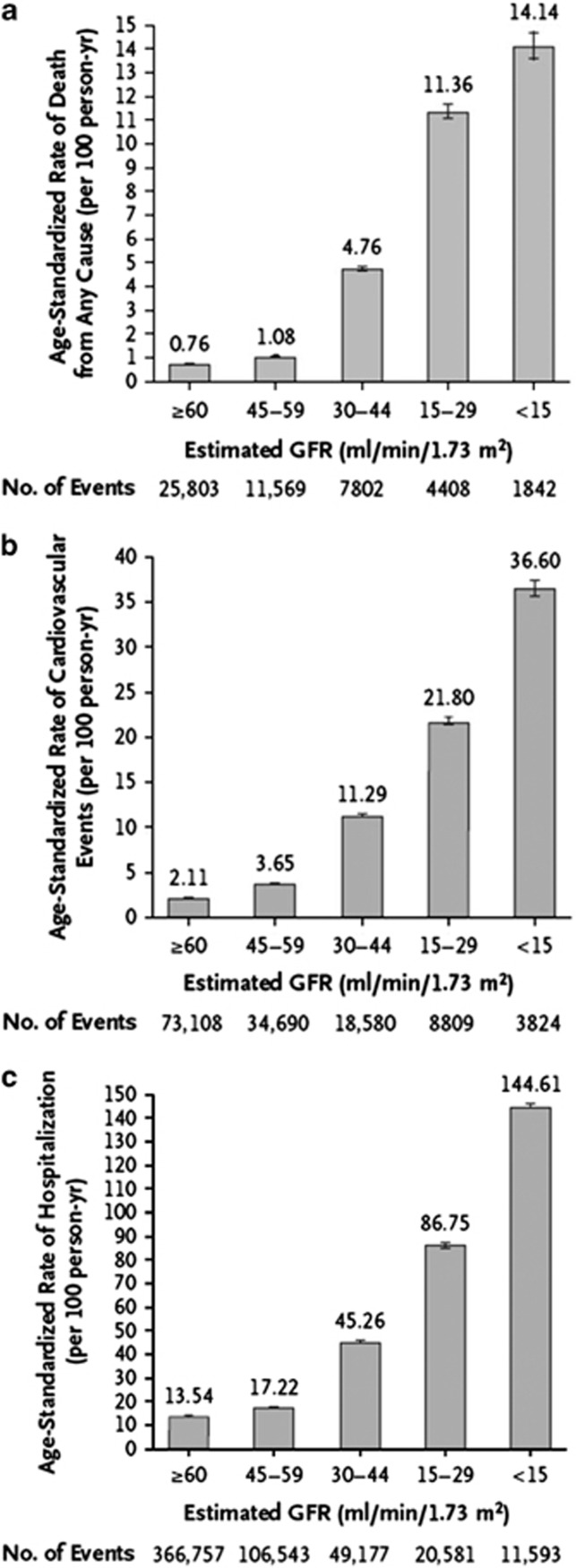
The associations of lower categories of GFR and risks of metabolic and endocrine complications formed the basis of the previous stratification into 5 stages. This current classification further acknowledges the importance of dividing Stage 3 based on data supporting different outcomes and risk profiles into categories G3a and G3b (Figure 5). A number of other concurrent complications are associated with decreased categories of GFR including infection, impaired cognitive and physical function, and threats to patient safety.57
Figure 5.
Age-standardized rates of death from any cause (panel a), cardiovascular events (panel b), and hospitalization (panel c), according to the eGFR among 1,120,295 ambulatory adults. eGFR, estimated glomerular filtration rate. From N Engl J Med, Go AS, Chertow GM, Fan D, et al.58 Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization, 351: 1296-1305. Copyright © (2004) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society; accessed http://www.nejm.org/doi/pdf/10.1056/NEJMoa041031
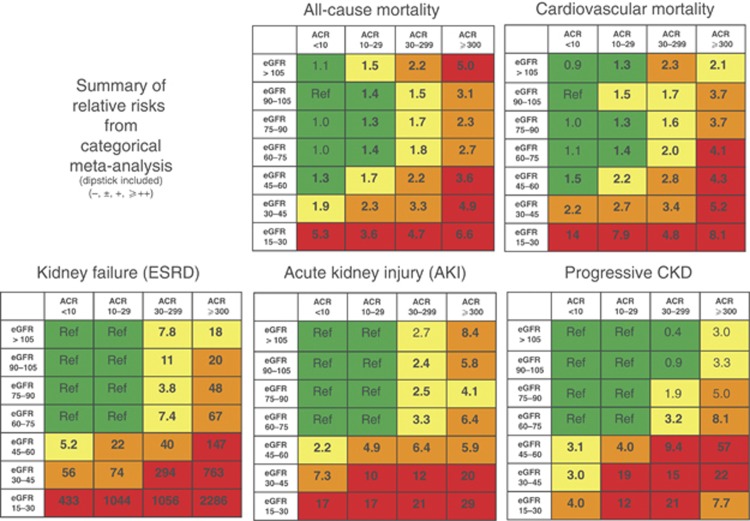
Figures 6 and 7 detail the RRs of decreased eGFR and increasing ACR with future complications, including mortality and kidney outcomes.30 Even for the group with the lowest value of albuminuria, the increased RR for all outcomes is significant for eGFRs below 60 ml/min/1.73 m2 in the continuous analysis and in the range of 45–59 ml/min/1.73 m2 for the categorical analysis.
Figure 6.
Summary of continuous meta-analysis (adjusted RRs) for general population cohorts with ACR. Mortality is reported for general population cohorts assessing albuminuria as urine ACR. Kidney outcomes are reported for general population cohorts assessing albuminuria as either urine ACR or reagent strip. eGFR is expressed as a continuous variable. The three lines represent urine ACR of <30, 30-299 and ≥300 mg/g (<3, 3-29, and ≥30 mg/mmol, respectively) or reagent strip negative and trace, 1+ positive, ≥2+ positive. All results are adjusted for covariates and compared to reference point of eGFR of 95 ml/min/1.73 m2 and ACR of <30 mg/g (<3 mg/mmol) or reagent strip negative (diamond). Each point represents the pooled RR from a meta-analysis. Solid circles indicate statistical significance compared to the reference point (P <0.05); triangles indicate non-significance. Red arrows indicate eGFR of 60 ml/min/1.73 m2, threshold value of eGFR for the current definition of CKD. ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; OR, odds ratio, RR, relative risk. Reprinted with permission from Macmillan Publishers Ltd: Kidney International. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int 2011; 80: 17-2830; accessed http://www.nature.com/ki/journal/v80/n1/full/ki2010483a.html
Figure 7.
Summary of categorical meta-analysis (adjusted RRs) for general population cohorts with ACR. Mortality is reported for general population cohorts assessing albuminuria as urine ACR. Kidney outcomes are reported for general population cohorts assessing albuminuria as either urine ACR or reagent strip. eGFR and albuminuria are expressed as categorical variables. All results are adjusted for covariates and compared to the reference cell (Ref). Each cell represents a pooled RR from a meta-analysis; bold numbers indicate statistical significance at P <0.05. Incidence rates per 1000 person-years for the reference cells are 7.0 for all-cause mortality, 4.5 for CVD mortality, 0.04 for kidney failure, 0.98 for AKI, and 2.02 for CKD progression. Colors reflect the ranking of adjusted RR. The point estimates for each cell were ranked from 1 to 28 (the lowest RR having rank number 1, and the highest number 28). The categories with a rank number 1-8 are green, rank numbers 9-14 are yellow, the rank numbers 15-21 are orange and the rank numbers 22-28 are colored red. (For the outcome of CKD progression, two cells with RR <1.0 are also green, leaving fewer cells as yellow, orange and red). ACR, albumin-to-creatinine ratio; AKI, acute kidney injury; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; RR, relative risk. Reprinted with permission from Macmillan Publishers Ltd: Kidney International. Levey AS, de Jong PE, Coresh J, et al.30 The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int 2011; 80: 17-28; accessed http://www.nature.com/ki/journal/v80/n1/full/ki2010483a.html
Pediatric Considerations
In children <2 years of age with CKD, the GFR categories as per the adult in Table 5 do not apply; these children should be categorized as having normal, moderately reduced, or severely reduced age-adjusted GFR.
No currently agreed upon set of international normative values or categories exist for GFR in children under the age of 1-2 years. However, the international pediatric nephrology community has embraced the adult CKD staging system as per the 2002 KDOQI guidelines in children over the age of 2 years, as suggested by Hogg et al.43
As indicated in Pediatric Considerations for Guideline 1.1, the normative GFR values for children less than 2 years vary quite widely by both age and method of measurement. More importantly these values are expected to increase in a non-linear fashion over the first 2 years of life with significant changes seen in the first few months post-birth and no current evidence of presence of comorbid conditions at any given level of measured or estimated GFR in this population. As such, specific categorization of G1-5 as suggested in this Recommendation would seem not be of value, and might be misleading if applied to a child less than 2 years of age.
With this in mind, it is suggested that based on the chosen method of GFR measurement or comparison for the individual (i.e., CrCl, radioactive or cold exogenous serum markers, or estimating formula), that one should attempt to classify the child under the age of 2 years as having normal, moderate or severe reductions in GFR based on the normative range and standard deviations (SDs) for the method. No evidence exists for this recommendation but recognition that values of GFR more than 1 SD below the mean would seem likely to raise concern of the clinician and foster the need for closer monitoring. For drug dosing adjustments it is suggested that those children with GFRs below the mean by >1 but <2 SD be classified as having a moderate reduction in GFR whereas those more than 2 SD below the mean for the method be classified as having a severe reduction in GFR.
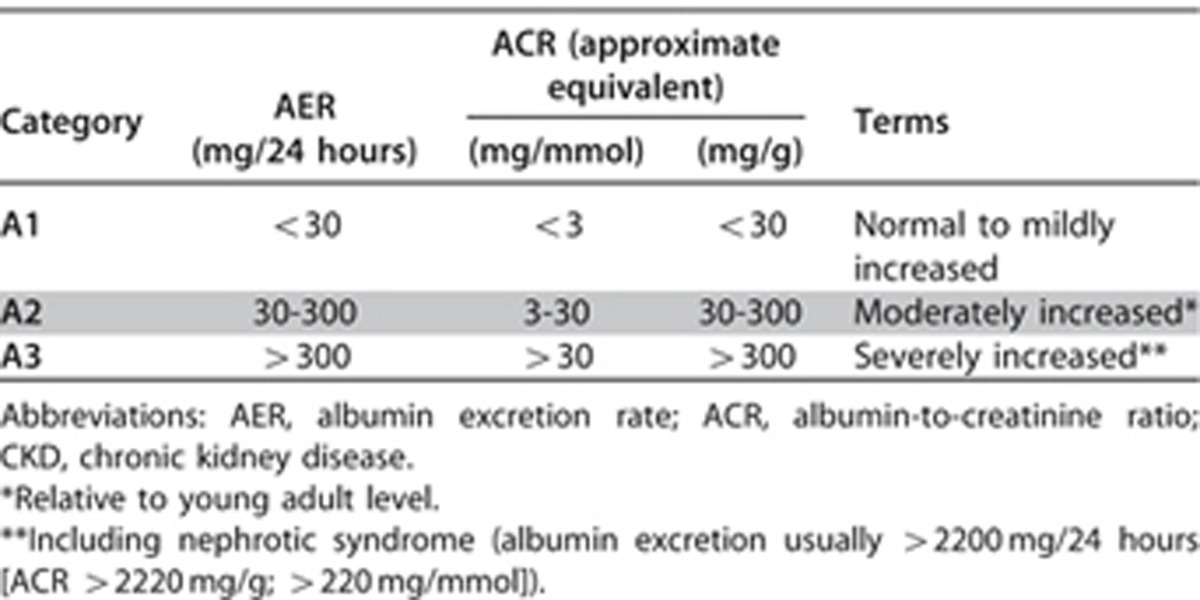
1.2.4: Assign albuminuria* categories as follows [Table 6] (Not Graded):
Table 6. Albuminuria categories in CKD.
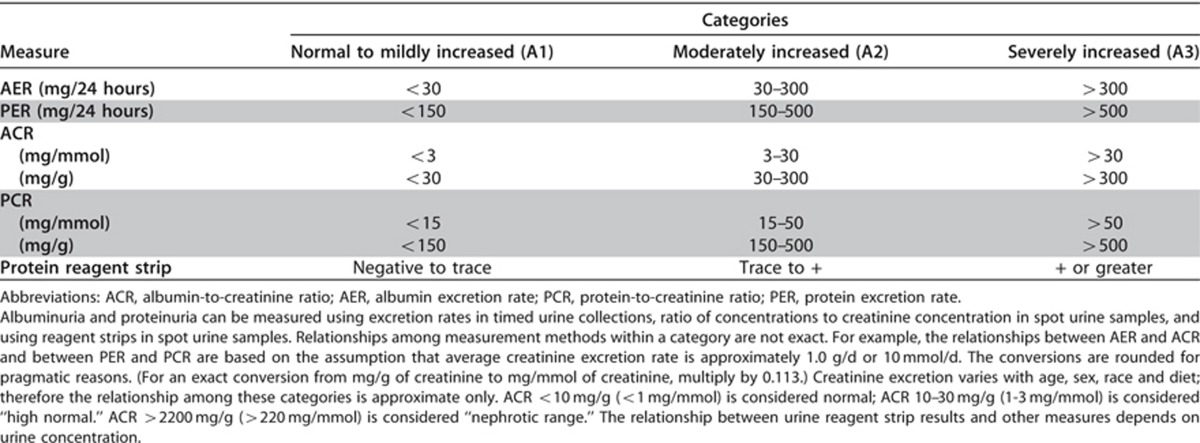
*note that where albuminuria measurement is not available, urine reagent strip results can be substituted (Table 7)
Table 7. Relationship among categories for albuminuria and proteinuria.
RATIONALE
The purpose of this statement is to ensure communication and to reflect that albuminuria category is an important predictor of outcomes. The association of high levels of proteinuria with signs and symptoms of nephrotic syndrome is well known. The detection and evaluation of lesser quantities of proteinuria have gained additional significance as multiple studies have demonstrated its diagnostic, pathogenic, and prognostic importance. There is a continuous risk associated with albuminuria but the use of a simple categorical approach was selected to simplify the concept for clinical practice. Several groups had suggested subdividing one or more GFR categories based on albuminuria category.
For the detection of diabetic nephropathy some guidelines recommend the use of different ACR thresholds for males and females (>25 mg/g [>2.5 mg/mmol] and >35 mg/g [>3.5 mg/mmol], respectively) to take into account variations in creatinine excretion. A single threshold is used in North America (30 mg/g or 3.4 mg/mmol). Earlier KDIGO guidance was reluctant to adopt gender-specific thresholds due to greater complexity, uncertainty about assay precision, and effects of race, ethnicity, diet and measures of body size on creatinine and this stance is maintained here. For simplicity, and to reflect the fact that it is an approximation, 3.4 mg/mmol as the current guideline threshold has been rounded to 3.0 mg/mmol.
There is a graded increase in risk for higher albuminuria categories, at all GFR categories, without any clear threshold value. Even for subjects with GFR >60 ml/min/1.73 m2, the increased RR is statistically significant for urine ACR ≥30 mg/g (≥3 mg/mmol) for mortality and kidney outcomes (Figures 6 and 7). The predictive ability of albuminuria at all categories of GFR supports the suggestion to add albuminuria categories to all GFR categories. Since the relationship with albuminuria is continuous, the selection of the number of categories and the cutoff values appears arbitrary. The Work Group has recommended the classification of albuminuria into only 3 categories, based on practical considerations, but recognized that further subdivisions within the category of <30 mg/24 hours (ACR<30 mg/g or <3 mg/mmol) may be useful for risk stratification, and that subdivisions within the category of >300 mg/24 hours (ACR>300 mg/g or >30 mg/mmol) may be useful for diagnosis and management. Specifically there is a recognition that nephrotic range proteinuria (AER>2200 mg/24 hours [ACR>2200 mg/g;>220 mg/mmol] PER>3000 mg/24 hours [>3000 mg/g;>300 mg/mmol]) confers unique additional risks and is usually associated with specific conditions (such as GN). As these are relatively rare in general practices, the simplicity of the AER categorization was preferred. Table 7 shows the approximate relationships of categories of AER to other measures of albuminuria and proteinuria.
Implications for Clinical Practice and Public Policy
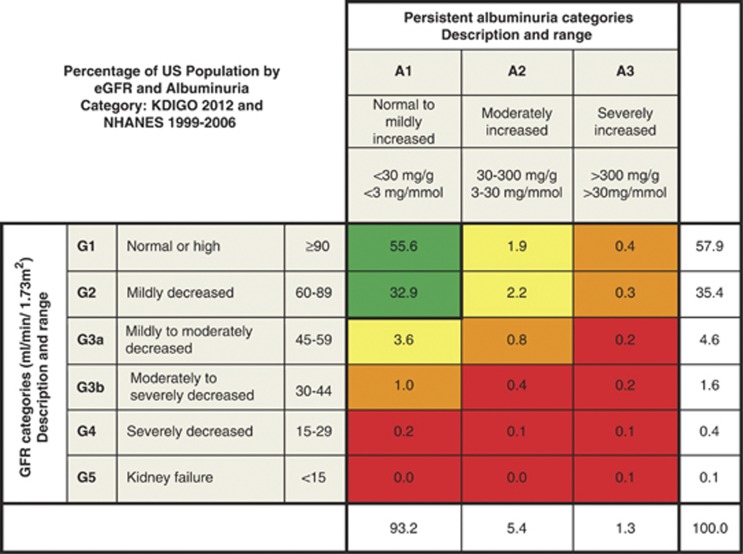
Data from around the world suggest that CKD prevalence is between 10-16% but information concerning population prevalence by category of GFR and ACR is scant. Figure 8 shows the proportion of adults in the US by categories of GFR and albuminuria.19 While CKD is common, few individuals have severely reduced GFR or kidney failure or severely increased albuminuria.
Figure 8.
Prevalence of CKD in the USA by GFR and albuminuria. Cells show the proportion of adult population in the USA. Data from the NHANES 1999-2006, N=18,026. GFR is estimated with the CKD-EPI equation and standardized serum creatinine.19 Albuminuria is determined by one measurement of ACR and persistence is estimated as described elsewhere.59 Values in cells do not total to values in margins because of rounding. Category of very high albuminuria includes nephrotic range. Green, low risk (if no other markers of kidney disease, no CKD); Yellow, moderately increased risk; Orange, high risk; Red, very high risk. ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; CKD-EPI, CKD Epidemiology Collaboration; GFR, glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey. Modified with permission from Macmillan Publishers Ltd: Kidney International. Levey AS, de Jong PE, Coresh J, et al.30 The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int 2011; 80: 17-28; accessed http://www.nature.com/ki/journal/v80/n1/full/ki2010483a.html
The classification of kidney disease by cause, category of GFR and category of albuminuria does not conform to the International Classification of Diseases (ICD) maintained by the World Health Organization (WHO). Currently the WHO is developing an update (ICD 11). It will be important to communicate and coordinate efforts with the kidney disease subgroup for ICD 11. However, the proposed current classification does address the need in clinical practice to acknowledge the multiple dimensions and variables by which individual patients are assessed. Table 8 gives examples of the use of CGA nomenclature.
Table 8. CGA staging of CKD: examples of nomenclature and comments.
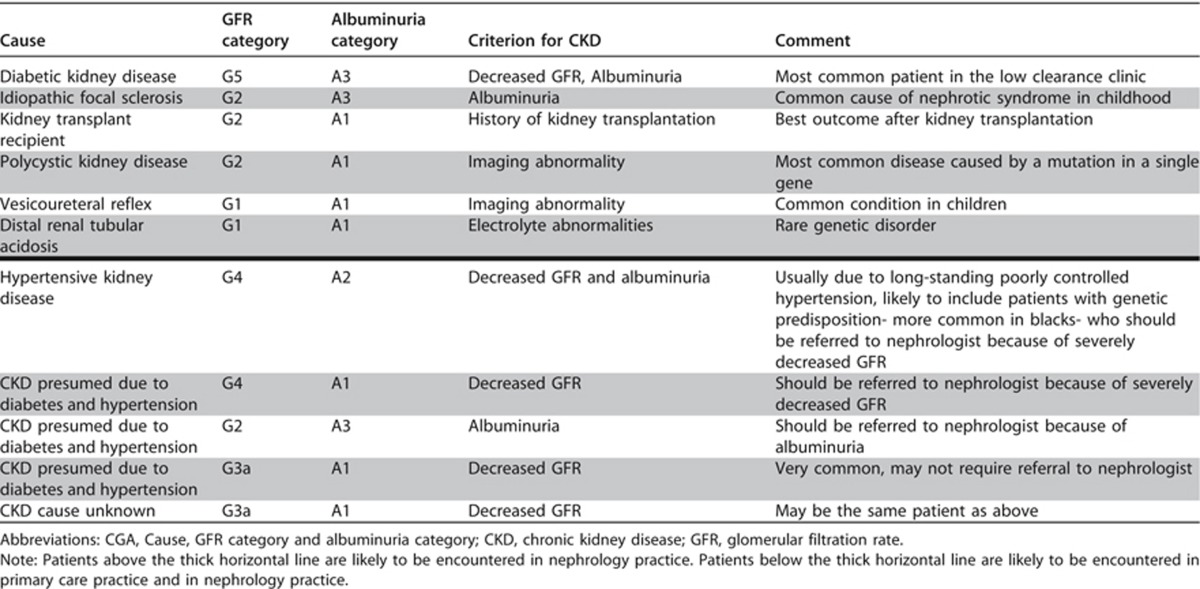
Definition of GFR categories have been deliberately based upon the concept of “true” GFR, whereas clinical practice and research has predominantly used creatinine-based estimates of GFR. The belief of the Work Group is that the non-GFR determinants of creatinine and the imprecision of creatinine-based GFR estimates have resulted in the absence of strong dose-dependent association of eGFR with clinical outcomes in the GFR range of >60 ml/min/1.73 m2. The Work Group felt confident that GFR levels of ≥90 ml/min/1.73 m2 portend better prognosis than GFR levels 60-89 ml/min/1.73 m2, if they could be estimated accurately. Therefore, the GFR categories include separate G1 (≥90 ml/min/1.73 m2) and G2 (60-89 ml/min/1.73 m2) designations despite limited data from creatinine-based estimates that prognosis differs between these two categories. It is also an acknowledgement that the degree of precision of some of our measurements may not be able to differentiate between these 2 categories reliably. As described later, studies that have used cystatin C have found gradients in prognosis at eGFR levels above 60 ml/min/1.73 m2, which supports the belief of the committee that separating these 2 GFR categories is appropriate for CKD classification.
Albuminuria categories are “wide” with respect to risk, with significant gradients within each category. The decision to propose only 3 categories is based on the perceived need for simplification in clinical practice. In specialized clinical nephrology centers, A3 (>300 mg/g or >30 mg/mmol) is often more precisely assessed and divided into additional categories. For example, nephrotic range proteinuria is defined as PER>3500 mg/24 hours or PCR (protein-to-creatinine ratio) >3500 mg/g [>350 mg/mmol] which is approximately equivalent to AER>2200 mg/24 hours or ACR>2200 mg/g [220 mg/mmol]. It is clearly recognized that these very high levels of proteinuria carry a different risk than lower values within the same category. Further differentiation after quantification and evaluation would inform treatment decisions for an individual patient. These categories serve as an initial assessment and prognostication tool; further classification is appropriate for specific circumstances and is not limited by the initial classification into only 3 categories.
Note that the term ‘microalbuminuria' is not used and is discouraged in this classification system. This will require a formal education program and review of existing guidelines in other disciplines so that consistency of terminology and understanding of the changes are universal (see Recommendation 1.4.4.2.1).
Pediatric Considerations
This statement would need to be altered for application in pediatric practice in the following way. In children with CKD any expression of abnormal urinary protein excretion, irrespective of the marker:
must account for variation in that measurement as seen across age, sex, puberty and or body size (height, weight, body mass index [BMI]).
should account for the possibility of tubular versus glomerular proteinuria dominance dependent on the underlying disease.
may utilize proteinuria in place of albuminuria.
There is no set standard encompassing all children with respect to the normal range of urinary protein (or albumin) excretion. Values vary across age, sex, race, pubertal status, the presence of obesity (high BMI) and may be modified by exercise, fever, and posture.60, 61, 62, 63
In general, neonates and young infants/ children are both expected and allowed to have higher urinary losses of both glomerular and tubular proteinuria due to lack of maturation in the proximal tubular reabsorption of proteins. The rough equivalences for ACR and PCR quoted in the pediatric literature are similar, but not identical to those quoted in the adult literature. Normal ranges vary but at least one reference suggests as much as 6-8 mg/m2/hr or >240 mg/m2/day of proteinuria as being acceptable at <6 months of age;64 normal ranges for urinary albumin losses are not known at this age.
The normal range of protein excretion for children 6-24 months of age in a 24-hour urine collection is quoted as being <4 mg/m2/hr (<150 mg/m2/day), whereas the first morning spot urine protein sample is said to be normal at levels of <500 mg/g creatinine (<50 mg/mmol). In children older than 24 months these values are <4 mg/m2/hr (<150 mg/m2/day) for the 24-hour collection and PCR <200 mg/g creatinine (<20 mg/mmol) in the first morning urine sample, or a first morning urine ACR <30 mg/g (<3 mg/mmol).43, 65
At all ages, total urinary protein excretion >40 mg/m2/hr (>3 grams/1.73 m2/day) is considered to represent ‘nephrotic range' loss of protein, with intermediate values, i.e., 4-40 mg/m2/hr or its equivalent representing abnormal but ‘non-nephrotic' losses.43, 65
Children older than 24 months of age are expected to achieve normal (‘adult') urinary protein values with the caveat of an exaggerated postural loss of glomerular proteins (albumin) as can commonly be seen in the 2-5% of the adolescent population (i.e., orthostatic proteinuria).62
Based on National Health and Nutrition Examination Survey III (NHANES III) data from just under 6000 healthy 6-19 year old children using either immunonephelometry or radioimmunoassay, the definition of urinary albumin excretion was determined to be 30-300 mg/24 h collection; 20-200 μg/min in an overnight collection and 30-300 mg/g creatinine (3-30 mg/mmol) in a first morning urine sample.66
Of note, to date the majority of studies that have examined the effects of urinary protein losses or therapeutic interventions have concentrated on so-called total protein excretion or random or first morning PCRs. The utility of measuring the albumin only fraction, and in particular quantitating this at the lower level of detection, i.e., <30 mg/g (<3 mg/mmol) creatinine, is only now being investigated in more detail in large pediatric studies. As such it should be recognized that in children the quantification of total protein, as compared to the albumin only fraction, may be the preferred method for assigning risk as it relates to the presence of urinary protein loss.
In summary, for children older than 2 years of age the assignment of ‘proteinuria' categories can be used as per the adult guidelines with the understanding that modification to the upper limit of expected values may be necessary in consideration of the factors outlined above. Although there is a preference for reporting albumin values, currently many clinicians still categorize these children based on total protein and in the child <2 years of age or the adolescent with demonstrable orthostatic proteinuria, the current albuminuria categories are unlikely to apply.
1.3: PREDICTING PROGNOSIS OF CKD
1.3.1: In predicting risk for outcome of CKD, identify the following variables: 1) cause of CKD; 2) GFR category; 3) albuminuria category; 4) other risk factors and comorbid conditions. (Not Graded)
1.3.2: In people with CKD, use estimated risk of concurrent complications and future outcomes to guide decisions for testing and treatment for CKD complications (Figure 9). (Not Graded)
1.3.3: In populations with CKD, group GFR and albuminuria categories with similar relative risk for CKD outcomes into risk categories (Figure 9). (Not Graded)
RATIONALE
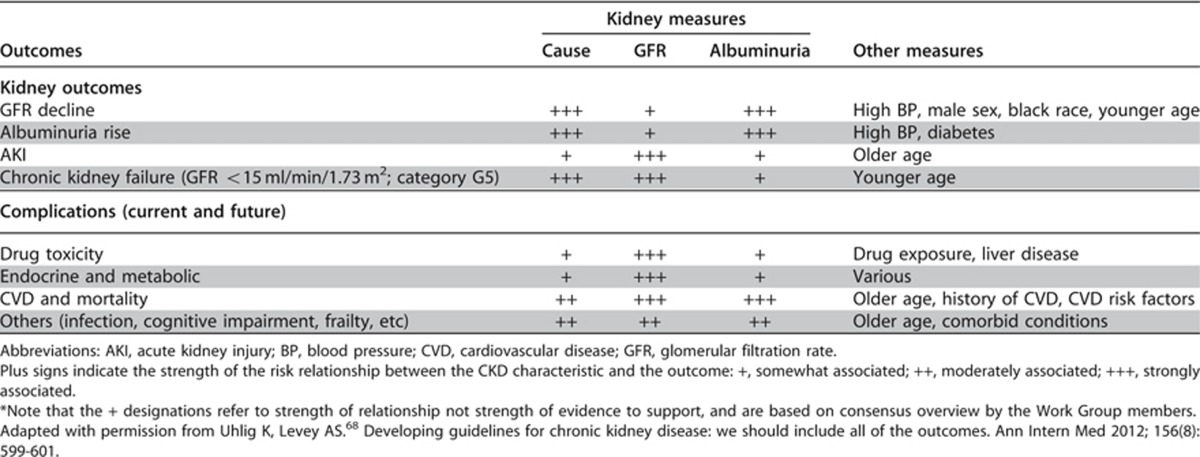
These statements are worded in this way because for all CKD complications, prognosis will vary depending on: 1) cause; 2) GFR; 3) degree of albuminuria; and 4) other comorbid conditions. The relative strength of each of these factors will vary for each complication or outcome of interest. Risk for kidney disease end points, such as kidney failure and AKI, is predominately driven by an individual patient's clinical diagnosis, GFR, and the degree of albuminuria or other markers of kidney damage and injury. For CVD, risk will be determined by history of CVD and traditional and non-traditional CVD risk factors. For other conditions, the risk will be determined by risk factors specific for those conditions. For all conditions, the cause of CKD, GFR category, and albuminuria category will still have important influence as “risk multipliers,” but will have smaller overall influence on disease prediction than risk factors specific for the condition. All these conditions have an impact on life expectancy and quality of life (QOL) and contribute substantially to predicting the prognosis of CKD. CKD is associated with numerous complications directly or indirectly related to the cause of CKD, decreased GFR, or albuminuria (Table 9).
Table 9. Prognosis of CKD: Relationship of outcomes and strength of relationship to Cause (C), GFR (G), Albuminuria (A) and other measures*67,68.
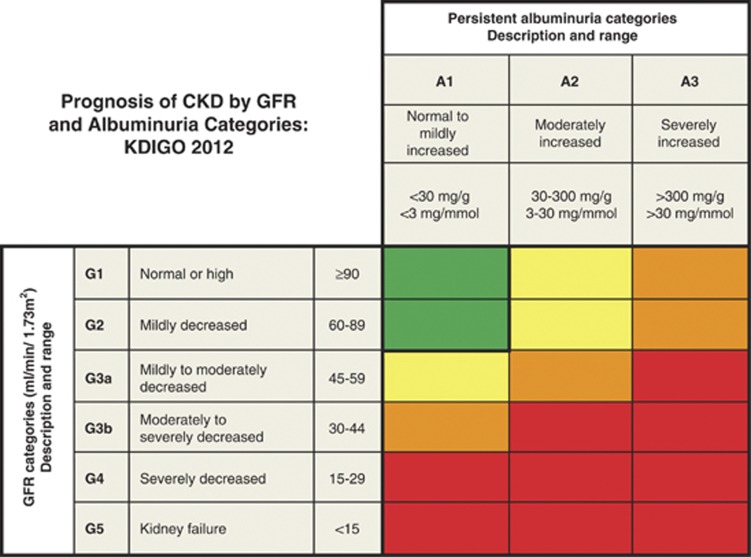
The risk associations of GFR and albuminuria categories appear to be largely independent of one another. Therefore, neither the category of GFR nor the category of albuminuria alone can fully capture prognosis for a patient with CKD. The magnitude and gradients of risk across categories of GFR and albuminuria will likely differ for each specific adverse event. This heterogeneity across the GFR and ACR grids in RRs for different outcomes makes it impractical to have a simple hierarchical staging of prognosis across all cells. Thus, the staging using CGA should be descriptive, but encompassing the ordered categories of GFR and ACR (Figure 9).
Figure 9.
Prognosis of CKD by GFR and albuminuria category. Green, low risk (if no other markers of kidney disease, no CKD); Yellow, moderately increased risk; Orange, high risk; Red, very high risk. CKD, chronic kidney disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes. Modified with permission from Macmillan Publishers Ltd: Kidney International. Levey AS, de Jong PE, Coresh J, et al.30 The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int 2011; 80: 17-28; accessed http://www.nature.com/ki/journal/v80/n1/full/ki2010483a.html
The CGA staging system proposed in this guideline provides a framework for future recommendations on CKD clinical management. At present, much of the evidence on clinical decision making in CKD is based solely on GFR. This recommendation serves to highlight the multidimensional aspect of CKD so as to ensure appropriate consideration of the complexity of the condition.
Evidence Base
The evidence base from which these statements are derived includes large observational cohort studies from diverse populations. For some outcomes, including mortality, CVD, and kidney disease progression, meta-analyses have summarized the risk associations. For outcomes that occur predominately in older adults (e.g., dementia, fracture), the evidence is largely limited to cohorts of older people.
Extensive work by the CKD Prognosis Consortium has defined the RRs across GFR and albuminuria categories for several important outcomes, including all-cause mortality, CVD, and kidney failure (Figures 6 and 7). Risk increases incrementally in both directions - down the GFR categories and across the albuminuria categories. Levels of risk can be identified and grouped into categories, but they may differ somewhat for each outcome. Additional research is needed to map these GFR and albuminuria categories and cause of kidney disease to other important outcomes of CKD (Table 9).
International Relevance
The above statements appear to be robust when applied in North America, Europe and Asia.30 Thus, it appears for all methods used to determine GFR and to detect albuminuria, the use of the 3 parameters (cause, category of GFR and category of albuminuria) influences prognosis irrespective of ethnicity or country of origin.
Implications for Clinical Practice and Public Policy
Providers must incorporate cause of kidney disease, GFR category and albuminuria category in order to better develop an accurate assessment of an individual's prognosis related to CKD. Many providers who are not nephrologists will need guidance in the local methods for requesting and interpreting a urine albumin assessment and an eGFR. Use of risk scores which are being developed and refined is advised.
Public policy and estimates of total burden of illness in a community need to take into account the incidence and prevalence of specific conditions (such as diabetes and congestive heart failure). In addition, knowledge of distribution of levels of eGFR and ACR may be valuable for resource planning. Community or health-system based interventions to reduce the incidence of kidney failure in populations should be targeted and prioritized based on these 3 criteria.
The primary impact on clinical practice will relate to kidney-specific complications of CKD and referral patterns to help prevent and manage them. Decisions related to screening and monitoring CKD disorders will be informed and guided by the CGA system. At present, this evidence for issues such as management of anemia, CKD bone and mineral disorders, and acid-base disorders has not been organized and presented in this way.
Decisions on screening and referral strategies have major impact on the costs and quality of health-care. The value of this revised system of classification is that it will allow the evaluation of different referral patterns and the impact of treatment strategies in those with diverse CGA assignment. In this way, we will develop additional evidence which will inform practice patterns. These will necessarily be developed locally and reflect the values and economic realities of each health-care system.
Areas of Controversy, Confusion, or Non-consensus and Clarification of Issues and Key points
Current clinical practice has not overtly incorporated these 3 variables into all decision making activities. The utility of the system will need to be vetted by those referring and those to whom patients are referred. The overt description of the 3 dimensions of diagnosis and staging of kidney disease which include the cause, the category of GFR and the category of albuminuria, should help to inform referral and treatment patterns of large groups of individuals. Risk calculators for specific events are under development.
The CGA classification system will be useful for quantifying risk for specific outcomes of CKD but its utility has not been fully assessed in clinical practice and research studies.
Additional evidence is required before decisions on screening, monitoring, and referral patterns can be fully informed.
Pediatric Considerations
For Recommendation 1.3.1 the rationale and principles behind this statement would apply to pediatrics though the data are not available.
Unlike in adults, the knowledge of risk of progression or outcomes of CKD is less robust in children, with the majority of such information gleaned from either registry datasets or longitudinal trials. In a 2008 report of a select group of patients enrolled by various North American pediatric nephrology centers in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry, 46% of nearly 7100 cases had reached a final ‘end point' with 86% progressing to ESRD over their time in the registry.69 Data from the prospective registry and population-based Italian Pediatric Registry of Chronic Renal Failure (ItalKid) study demonstrated a risk of progression to ESRD of ∼68% by age 20 years.70
Cause of CKD. Specific information related to rate of progression for all pediatric causes of CKD is not easily available. However data from the prospective longitudinal CKiD trial has demonstrated a more rapid decline in renal function in children whose underlying cause of CKD is classified as glomerular with an annualized rate of change in iohexol GFR of −10.5% as compared to those with a non-glomerular cause in whom the annualized rate of change is only −3.9%.71 In terms of absolute rates of change in measured iohexol GFR this translated, in a separate analysis from the same dataset, into a median change of GFR of −4.3 ml/min/1.73 m2 versus −1.5 ml/min/1.73 m2 in the glomerular versus non-glomerular groups, respectively.72 This paper also provides the only current individual disease-specific estimate of annual decline in a pediatric population. Table 10 illustrates that the median values for annualized change in GFR for various diagnosis categories.
Table 10. Annual percentage change in GFR across diagnosis categories.
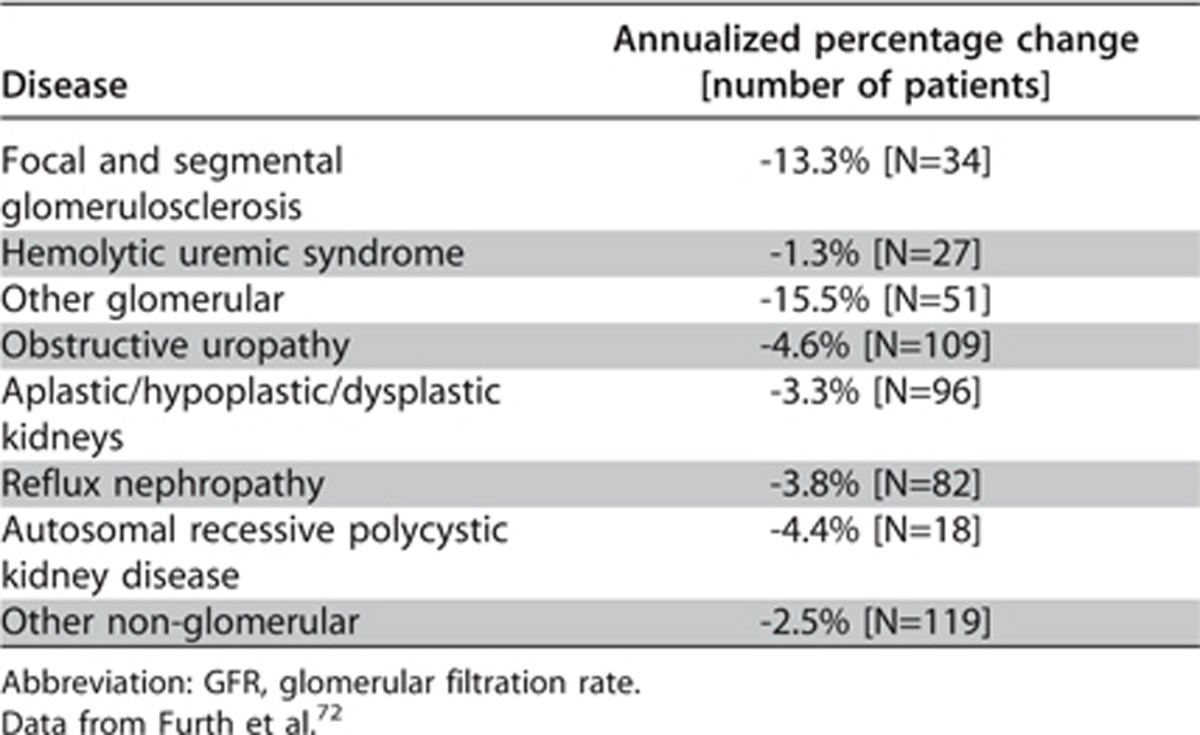
Similarly, a randomized controlled trial (RCT) from Europe73 examining the effects of diet on rate of progression demonstrated a statistically significant difference in CrCl between their glomerular and non-glomerular cohorts at 2 years of follow-up; with the mean decline [SD] in the glomerular group being −10.7 [11.3] versus −8.4 [13.5] ml/min/1.73 m2 in the non-glomerular patients (P = 0.048).
GFR category. It is also well recognized that there is an inverse relationship between the rates of progression of kidney disease to the level of kidney function present at that presentation with more rapid decline seen in patients with lower initial levels of GFR. Staples et al.74 in their retrospective review of the NAPRTCS CKD database involving nearly 4200 children registered with GFR categories G2-G4 (GFR 15-89 ml/min/1.73m2) demonstrated significantly higher rates of progression, defined by progression to GFR category G5 (GFR <15 ml/min/1.73m2) or initiation of dialysis or transplant, for children in GFR categories G3a-G4 (GFR 15–59 ml/min/1.73m2) as compared to those with CKD and GFR category G2 (GFR 60–89 ml/min/1.73m2) at time of enrollment: hazard ratio (HR) of GFR categories 3a and 3b (GFR 30–59 ml/min/1.73m2) (GFR category 2 (GFR 60–89 ml/min/1.73m2) = 1.00 as referent): 2.00; 95% confidence interval (CI) 1.64-2.42; P<0.0001 and HR of GFR category 4 (GFR 15–29 ml/min/1.73m2): 6.68; 95% CI 5.46-8.18; P<0.0001.
Albuminuria (proteinuria). Several studies have also demonstrated the effect of proteinuria on rate of progression of CKD in children. Using registry data, and in non-glomerular conditions the ItalKids trial75 demonstrated a significantly slower decline in CrCl in patients with baseline PCRs of <200 mg/g (20 mg/mmol) and 200–900 mg/g (20–90 mg/mmol) when compared to those patients with a PCR of >900 mg/g (>90 mg/mmol); slope +0.16±3.64 and −0.54±3.67 versus −3.61 ±5.47 (P <0.0001). This translated to higher rates of kidney survival over 5 years in the lower proteinuria groups, 96.7% and 94.1% versus 44.9%, (P <0.01). Multivariate analysis confirmed that the baseline PCR correlated with a more rapid decline in CrCl for any given level of baseline function.
In a prospective multicenter randomized trial of protein intake on rates of progression in children aged 2-18 years of age, Wingen et al. employed the Schwartz equation to estimate CrCl and demonstrated that baseline proteinuria in multivariate analysis was the most important independent predictor of change in CrCl. The authors reported a partial R2 of 0.259 at 2 years follow-up and similar results were found after the study was extended for a third year.73 Life-table analysis in this study also suggested a cutoff value of 50 mg/kg/day of proteinuria as a strong predictor of time to a decline in CrCl>10 ml/min/1.73 m2 and found a risk ratio of 4.01 (95% CI 2.23–7.25; P<0.001).
Finally Wong et al.76 used cross sectional data from the prospective longitudinal CKiD trial to demonstrate that even after controlling for age, race, BMI, cause of CKD and use of RAAS antagonists they could expect an average decline in measured GFR of 10% for every increase in urinary PCR of 14% (95% CI 10-18%).
Other risk factors and comorbid conditions. Many other risk factors and comorbid conditions have also been associated with greater risk of progression of CKD in adults but only a few of these have been convincingly proven in children due to lack of pediatric prospective trials.
Hypertension is by far the best studied of these risk factors in children, with clear evidence from multiple sources to document the value of aggressive BP control on slowing the rate of progression of CKD. Wingen et al.73 demonstrated the importance of systolic BP in rate of progression in both univariate and multivariate models. In this study Cox proportional hazards analysis demonstrated a systolic BP >120 mm Hg was an independent risk for decline in CrCl by >10 ml/min/1.73 m2; risk ratio was 3.1 (95% CI 1.74-5.53; P<0.001).
The most important prospective pediatric BP trial to date, the Effect of Strict Blood Pressure Control and ACE-Inhibition on Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) study, used ambulatory BP monitoring (ABPM) and a fixed dose of ramipril plus additional antihypertensive agents that do not target the RAAS to assess (as primary outcomes) the time to decline of 50% in GFR or development of ESRD. Their results demonstrated a 35% reduction in the risk of achieving the primary end point in the more intensely treated BP: HR 0.65; 95% CI 0.44-0.94; P=0.02. Further sub-analysis as reported in the KDIGO BP Guideline10 demonstrated that kidney survival was 66.1% at 5 year follow-up in patients with systolic BP<90th percentile for age whereas it was 41% in the patients who did not achieve this level of reduction (P=0.0002); similar numbers were seen if diastolic BP was the metric considered.
The issue of puberty and its effect on rate of progression has recently been addressed by the ItalKids investigators.77 While the methodology of their analysis is less than ideal as they did not determine actual Tanner stages in the majority of their cohort and used estimated rather than measured GFR, they do appear to demonstrate a decrease in kidney survival probability beginning around 10.9 years in girls and 11.6 years in boys with CKD. Of note, the rate of decline in kidney survival, using these age points as ‘inflection' or break points, is dramatically increased in both sexes based on their evidence provided in graphical form, although more precise analyses are not possible from the data provided.
As in adults, other factors for consideration and value in monitoring in children with respect to risk of progression include obesity, metabolic acidosis, anemia, calcium-phosphate metabolism, chronic inflammation, diabetes, hyperuricemia, dyslipidemia, and smoking.
The most comprehensive review of many of these factors in children comes from a retrospective study of the NAPRTCS CKD database. Staples et al.74 demonstrated that in a multivariate analysis of nearly 4200 children registered with CKD and GFR categories G2-G4 (GFR 15–89 ml/min/1.73m2), the following factors were significantly associated with the risk of CKD progression (defined by progression to GFR category G5 (GFR<15 ml/min/1.73m2) or initiation of dialysis or transplant): age; primary disease; GFR category; registration year; hypertension; corrected calcium, phosphorus, albumin, and hematocrit; and as proxies, the use of medications for anemia and short stature. The ability of this paper to prove causation or value in treating any of these conditions in hopes of delaying CKD progression is limited by its retrospective nature, and the fact that data were accrued from a voluntary registry.
There is optimism that prospective data from current large pediatric trials such as CKiD55 and the European Cardiovascular Comorbidity in Children with CKD (4C) trial78 will lead to a better understanding of how risk factors may be influencing the rate of progression of CKD in children.
For Recommendation 1.3.2 the rationale and principles behind this statement would apply to pediatrics, though the data are not available. Insufficient evidence currently exists with respect to the predictive value of prevalent risk factors to guide future decisions for testing or treatment for CKD complications in an individual child.
It is hoped that well powered, prospective trials with adequate follow-up, such as the CKiD55 and European 4C78 trials, will gather sufficient numbers of patients, comorbidities, and outcomes to allow for predictive models to be built in pediatric CKD that incorporate traditional and non-traditional cardiac risk factors including dyslipidemia and hypertension, proteinuria (albuminuria), specific disease-related issues (e.g., diabetes, tubulopathy), prematurity, and birth weight.
For Recommendation 1.3.3 the rationale and principles behind this statement would apply to pediatrics, though the data are not available. Current evidence and a paucity of numbers do not allow for the statistically relevant categorization of RR for CKD outcomes based solely on GFR and albuminuria or proteinuria. Again both the CKiD55 and European 4C78 trials may be able to address these shortcomings.
1.4: EVALUATION OF CKD
1.4.1: Evaluation of chronicity
- 1.4.1.1: In people with GFR <60 ml/min/1.73 m2 (GFR categories G3a-G5) or markers of kidney damage, review past history and previous measurements to determine duration of kidney disease. (Not Graded)
- If duration is >3 months, CKD is confirmed. Follow recommendations for CKD.
- If duration is not >3 months or unclear, CKD is not confirmed. Patients may have CKD or acute kidney diseases (including AKI) or both and tests should be repeated accordingly.
RATIONALE
When evidence of CKD is first ascertained, proof of chronicity can be obtained or confirmed by:
review of past measurements of GFR;
review of past measurements of albuminuria or proteinuria and urine examinations;
imaging findings such as reduced kidney size and reduction in cortical thickness;
pathological findings such as fibrosis and atrophy;
medical history especially duration of disorders known to cause CKD;
repeat measurements within and beyond the 3 month point.
Chronicity should not be assumed as AKI can present with similar abnormalities.
Pediatric Considerations
See Pediatric Considerations for next section.
- 1.4.2: Evaluation of cause
- 1.4.2.1: Evaluate the clinical context, including personal and family history, social and environmental factors, medications, physical examination, laboratory measures, imaging, and pathologic diagnosis to determine the causes of kidney disease. (Not Graded)
RATIONALE
Once the presence of CKD is proven it is essential to establish a cause for this which will inform specific management and modify risk projections. The diagnosis will be reached by standard clinical method (i.e., history examination) and special investigation, based on knowledge of the common causes of CKD and their manifestations. Not all evaluations are required in all patients, and will be directed by clinical context, and resource availability. For most patients the following evaluations are indicated:
Reagent strip urinalysis to detect hematuria or pyuria. If positive, use urine microscopy to detect RBC casts or WBC casts.
Ultrasound to assess kidney structure (i.e., kidney shape, size, symmetry and evidence of obstruction) as clinically indicated.
Serum and urine electrolytes to assess renal tubular disorders, as clinically indicated.
Many individuals found to have CKD will not have a primary kidney disease but kidney damage caused by diabetes mellitus, vascular disease, and hypertension. The issue for the clinician will be to decide whether the presence of these is a sufficient explanation and if not, to investigate further. The prevalence of other conditions will vary depending on region, age, and other factors.
It is beyond the scope of this guideline to describe how specific diagnoses are reached but non-nephrologists in the first instance should review the family history, medications, symptoms and signs for manifestations of systemic diseases. Urinalysis should be performed, along with imaging of the kidneys if obstruction of the urinary tract or polycystic kidney disease is considered.
Pediatric Considerations
For Recommendations 1.4.1.1 and 1.4.2.1, the statements would need to be altered for application in pediatric practice in the following way.
In any child with GFR<60 ml/min/1.73 m2 (or more than 1 SD below expected for their age and sex) or with markers of kidney damage, a complete review of their past history and previous measurement or estimate of renal function and full consideration of the clinical context, including prenatal history, drug exposures of fetus or mother, genetic conditions, coincident organ abnormalities, physical examination, fetal and post-natal laboratory measures including amniotic fluid, pre- and post-natal imaging and pathologic diagnosis including those of the fetus and placenta should be used to determine the cause(s) of kidney disease.
As noted in Pediatric Considerations for Recommendation 1.1.1, developmental renal abnormalities account for as many as 30-50% of the children with CKD.42 A careful review of all fetal or maternal exposures, genetic risks factors, and any relevant information on the intrauterine environment during gestation are all relevant to the determination of the presence of CKD either prior to or present immediately at the time of delivery. An infant may be born with CKD, leading to immediate classification within the CGA framework – up to and including that of dialysis dependency.
1.4.3 Evaluation of GFR
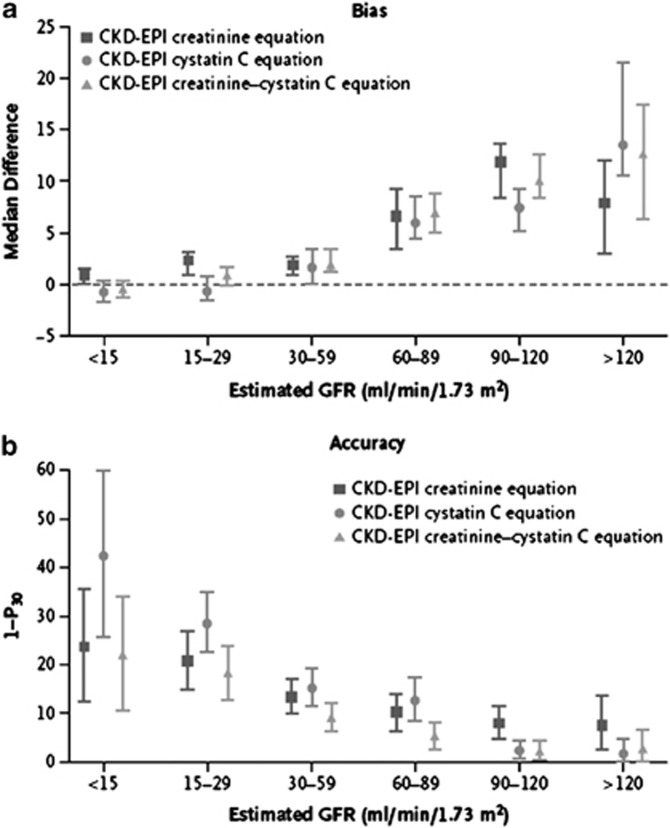
This section describes the various methods by which GFR can be estimated. We describe laboratory techniques that satisfy the requirements for robust result reporting and we compare the accuracy of available equations for the purpose of reporting eGFR using a single equation where applicable. We emphasize equations based on standardized measurements of SCr, but also consider newly developed equations based on standardized measurements of serum cystatin C (SCysC) because they are being introduced into clinical practice. We encourage practitioners to have a clear understanding of the value and limitations of both filtration markers, the importance of standardization of assays for both, and to understand that when an accurate assessment of kidney function is required, direct measurement should be undertaken.
- 1.4.3.1: We recommend using serum creatinine and a GFR estimating equation for initial assessment. (1A)
- 1.4.3.2: We suggest using additional tests (such as cystatin C or a clearance measurement) for confirmatory testing in specific circumstances when eGFR based on serum creatinine is less accurate. (2B)
RATIONALE
These statements specifically address the need to ensure that estimating equations are put into routine clinical practice, and that clinicians understand the utility of further evaluation with additional methods if required.
GFR is measured by the clearance of an exogenous or endogenous filtration marker.27 All clearance methods are complex so in clinical practice, GFR is estimated from the serum concentration of the endogenous filtration marker creatinine. Cystatin C is an alternative endogenous filtration marker; other filtration markers are also under evaluation. The principles of GFR estimation are discussed in the rationale for recommendations regarding the use of creatinine as a filtration marker but the concepts apply to GFR estimation from all endogenous filtration markers. Specific comments about GFR estimation using cystatin C are presented separately.
For most clinical circumstances, estimating GFR from SCr is appropriate for diagnosis, staging, and tracking the progression of CKD. However, like all diagnostic tests, interpretation is influenced by varying test characteristics in selected clinical circumstances and the prior probability of disease. In particular, an isolated decreased eGFR in otherwise healthy individuals is more likely to be false positive than in individuals with risk factors for kidney disease or markers of kidney damage. Confirmation of decreased eGFR by measurement of an alternative endogenous filtration marker (cystatin C) or a clearance measurement is warranted in specific circumstances when GFR estimates based on SCr are thought to be inaccurate and when decisions depend on more accurate knowledge of GFR, such as confirming a diagnosis of CKD, determining eligibility for kidney donation, or adjusting dosage of toxic drugs that are excreted by the kidneys.79 The choice of confirmatory test depends on the clinical circumstance and the availability of methods where the patient is treated.
Pediatric Considerations
For Recommendation 1.4.3.1, the statements would need to be altered for application in pediatric practice in the following way. The use of SCr and a recently derived pediatric specific GFR estimating equation, which incorporates a height term,80 is preferred over the use of SCr alone in the initial assessment of pediatric renal function.
For Recommendation 1.4.3.2, this guideline is fully applicable in pediatrics.
- 1.4.3.3: We recommend that clinicians (1B):
- use a GFR estimating equation to derive GFR from serum creatinine (eGFRcreat) rather than relying on the serum creatinine concentration alone.
- understand clinical settings in which eGFRcreat is less accurate.
RATIONALE
Estimating GFR from the SCr concentration alone requires implicit judgments that are difficult in routine clinical care, including reciprocal transformation, consideration of the non-GFR determinants, and conversion to the GFR scale. Using GFR estimating equations provides a more direct assessment of GFR than SCr alone. The SCr concentration is influenced by GFR and other physiological processes, collectively termed “non-GFR determinants,” including creatinine generation by muscle and dietary intake, tubular creatinine secretion by organic anion transporters, and extrarenal creatinine elimination by the gastrointestinal tract (Figure 10).
Figure 10.
Determinants of the serum level of endogenous filtration markers. The plasma level (P) of an endogenous filtration marker is determined by its generation (G) from cells and diet, extrarenal elimination (E) by gut and liver, and urinary excretion (UV) by the kidney. Urinary excretion is the sum of filtered load (GFR X P), tubular secretion (TS), and reabsorption (TR). In the steady state, urinary excretion equals generation and extrarenal elimination. By substitution and rearrangement, GFR can be expressed as the ratio of the non-GFR determinants (G, TS, TR, and E) to the plasma level. GFR, glomerular filtration rate. Reprinted with permission of American Society of Nephrology, Measured GFR as a confirmatory test for estimated GFR, Stevens LA, Levey AS.79 J Am Soc Nephrol 20: 2305-2313, 2009; permission conveyed through Copyright Clearance Center, Inc.; accessed http://jasn.asnjournals.org/content/20/11/2305.full.pdf
GFR estimating equations are developed using regression to relate the measured GFR to steady state SCr concentration and a combination of demographic and clinical variables as surrogates of the non-GFR determinants of SCr. By definition, GFR estimates using SCr concentration are more accurate in estimating measured GFR than the SCr concentration alone in the study population in which they were developed. Sources of error in GFR estimation from SCr concentration include non-steady state conditions, non-GFR determinants of SCr, measurement error at higher GFR, and interferences with the creatinine assays (Table 11). GFR estimates are less precise at higher GFR levels than at lower levels.
Table 11. Sources of error in GFR estimating using creatinine.
The clinician should remain aware of caveats for any estimating equation which may influence the accuracy in a given individual patient.
Because of the physiologic and statistical considerations in developing GFR estimating equations, GFR estimates are less precise at higher GFR levels than at lower levels. In principle, equations based on multiple endogenous filtration markers can overcome some of the imprecision of GFR estimates at higher levels, due to cancellation of errors from non-correlated non-GFR determinants.
Pediatric Considerations
This guideline is fully applicable in pediatrics.
- 1.4.3.4: We recommend that clinical laboratories should (1B):
- measure serum creatinine using a specific assay with calibration traceable to the international standard reference materials and minimal bias compared to isotope-dilution mass spectrometry (IDMS) reference methodology.
- report eGFRcreat in addition to the serum creatinine concentration in adults and specify the equation used whenever reporting eGFRcreat.
- report eGFRcreat in adults using the 2009 CKD-EPI creatinine equation. An alternative creatinine-based GFR estimating equation is acceptable if it has been shown to improve accuracy of GFR estimates compared to the 2009 CKD-EPI creatinine equation.
When reporting serum creatinine:
We recommend that serum creatinine concentration be reported and rounded to the nearest whole number when expressed as standard international units (μmol/l) and rounded to the nearest 100th of a whole number when expressed as conventional units (mg/dl).
When reporting eGFRcreat:
We recommend that eGFRcreat should be reported and rounded to the nearest whole number and relative to a body surface area of 1.73 m2 in adults using the units ml/min/1.73 m2.
We recommend eGFRcreat levels less than 60 ml/min/1.73 m2 should be reported as “decreased.”
RATIONALE
The statement is worded this way to acknowledge that calibration of assays is essential to interpretation of kidney function measures. This recommendation is directed to laboratories with the intent to clarify the details of such calibration and the use of specific equations so as to facilitate international standardization.81
There are numerous assay methods for creatinine for use in clinical laboratories. Variation in assigned values for SCr concentration among methods is greater at low concentrations, corresponding to high levels of GFR. Variation in assays at low SCr concentrations contributes to imprecision of GFR estimates at high GFR levels.
Currently available assays fall into two broad categories, the alkaline picrate (Jaffe) assay and enzymatic assays. In general, enzymatic assays are less biased compared to a standardized reference material and less susceptible to interferences. All assays are available on a number of platforms.
We recommend that laboratories use assays that are traceable to pure creatinine standards via a valid calibration hierarchy and that are specific and minimally-biased compared with isotope-dilution mass spectrometry (IDMS) reference method results. Results should be traceable to reference materials and methods listed on the Joint Committee for Traceability in Laboratory Medicine (JCTLM) database. Ideally laboratories should move to enzymatic assays for creatinine measurement: as a minimum, the use of traditional kinetic or end point Jaffe assays should cease and be replaced with IDMS aligned Jaffe methods.
Clinical laboratory information systems generally have access to patient age and sex and thus can report eGFR based on SCr age and sex, thus providing the clinician with the test result in units which are recommended for interpretation. Estimated GFR is now reported together with SCr when creatinine is ordered in more than 75% of clinical laboratories in the US.82 In the UK, 93% of NHS laboratories report eGFR with SCr,83 as is the case in Australia, Canada, and many European countries.
Selection of a single equation for use, where applicable, would facilitate communication among providers, patients, researchers and public health officials. Criteria for selection should be based on accuracy compared to measured GFR and usefulness in clinical care and public health.
The interpretation of measured and eGFR is based on comparison to normative values, which are adjusted for BSA because of the physiologic matching of GFR to kidney size, which is in turn related to BSA. The value of 1.73 m2 reflects the average value of BSA of 25-year old men and women in the USA in 1927.84 While it is known that modern populations may have different normal values for BSA, the 1.73 m2 value will be maintained for normalization purposes.
Drug dosing should be based on GFR which is not adjusted for BSA. The effect of drug dosing based on GFR adjusted for BSA compared to GFR unadjusted for BSA has not been studied rigorously and more precise recommendations are not available.
Flagging decreased values for eGFR can alert clinicians to the possibility of AKD or CKD, and may indicate the need for additional investigations or treatments, including adjustment of doses of drugs that are excreted by the kidney. However, values for GFR between 60 and 89 ml/min/1.73 m2 are mildly decreased compared to the usual values in young healthy people. Thus it is important that clinicians appreciate that eGFR values that are not flagged because they are >60 ml/min/1.73 m2 are not necessarily normal.
Evidence Base
Numerous equations have been developed to estimate GFR or CrCl in adults. In general, GFR estimating equations using creatinine include age, sex, race, and body size as surrogates for creatinine generation by muscle. For our review of GFR estimating equations, we only considered equations that were developing using assays that were traceable to reference methods and study populations in which SCr concentration was measured using traceable assays (Supplemental Table 1).85
Based on published data, only the Modification of Diet in Renal Disease (MDRD) Study equation, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and modifications of these equations were developed using creatinine assays traceable to the international reference material for creatinine (Table 12).86, 87 The Cockcroft and Gault formula and others were developed before standardization of creatinine assays but cannot be re-expressed for use with standardized creatinine assays (Supplemental Table 2).
Table 12. Equations based on serum creatinine assays in adults that are traceable to the standard reference material.
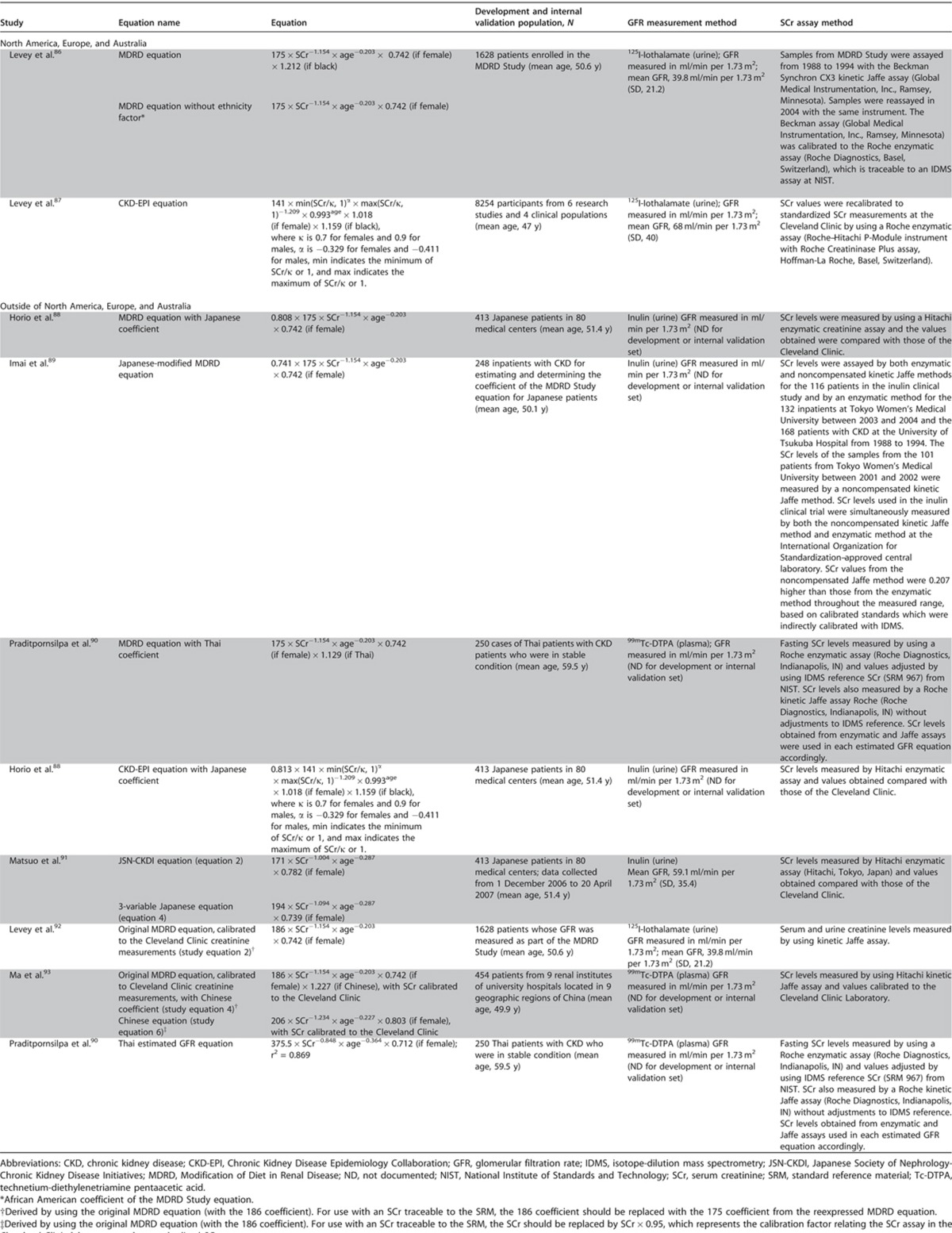
The MDRD Study equation was developed in 1999 and is currently recommended for eGFR reporting in adults by the National Kidney Disease Education Program (NKDEP) and by the Department of Health in the UK. It uses standardized SCr, age, sex, and race (black versus white and other) to estimate GFR adjusted for BSA (ml/min/1.73 m2).86, 94 Because of imprecision at higher GFR, NKDEP recommends that eGFR ≥60 ml/min/1.73 m2 computed using the MDRD Study equation not be reported as a numeric value. For a similar reason, the UK Department of Health recommends not reporting eGFR >90 ml/min/1.73 m2 using the MDRD Study equation as a numeric value.
The CKD-EPI equation was developed in 2009 and uses the same four variables as the MDRD Study equation.87 The CKD-EPI equation had less bias than the MDRD Study equation, especially at GFR≥60 ml/min/1.73 m2, a small improvement in precision, and greater accuracy (Figure 11). Most but not all studies from North America, Europe and Australia show that the CKD-EPI equation is more accurate than the MDRD Study equation, especially at higher GFR (Table 13),85 which enables reporting of numeric values across the range of GFR. At this time, large commercial clinical laboratories in the US have changed from using the MDRD Study equation to the CKD-EPI equation for eGFR reporting.
Figure 11.
Performance of the CKD-EPI and MDRD Study equations in estimating measured GFR in the external validation data set. Both panels show the difference between measured and estimated versus estimated GFR. A smoothed regression line is shown with the 95% CI (computed by using the lowest smoothing function in R), using quantile regression, excluding the lowest and highest 2.5% of estimated GFR. To convert GFR from ml/min per 1.73 m2 to ml/s per m2, multiply by 0.0167. CKI-EPD, Chronic Kidney Disease Epidemiology Collaboration; CI, confidence interval; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease. Reprinted with permission from Levey AS, Stevens LA, Schmid CH, et al.87 A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604-612.
Table 13. Performance comparison of creatinine-based GFR estimating equations in North America, Europe, and Australia.
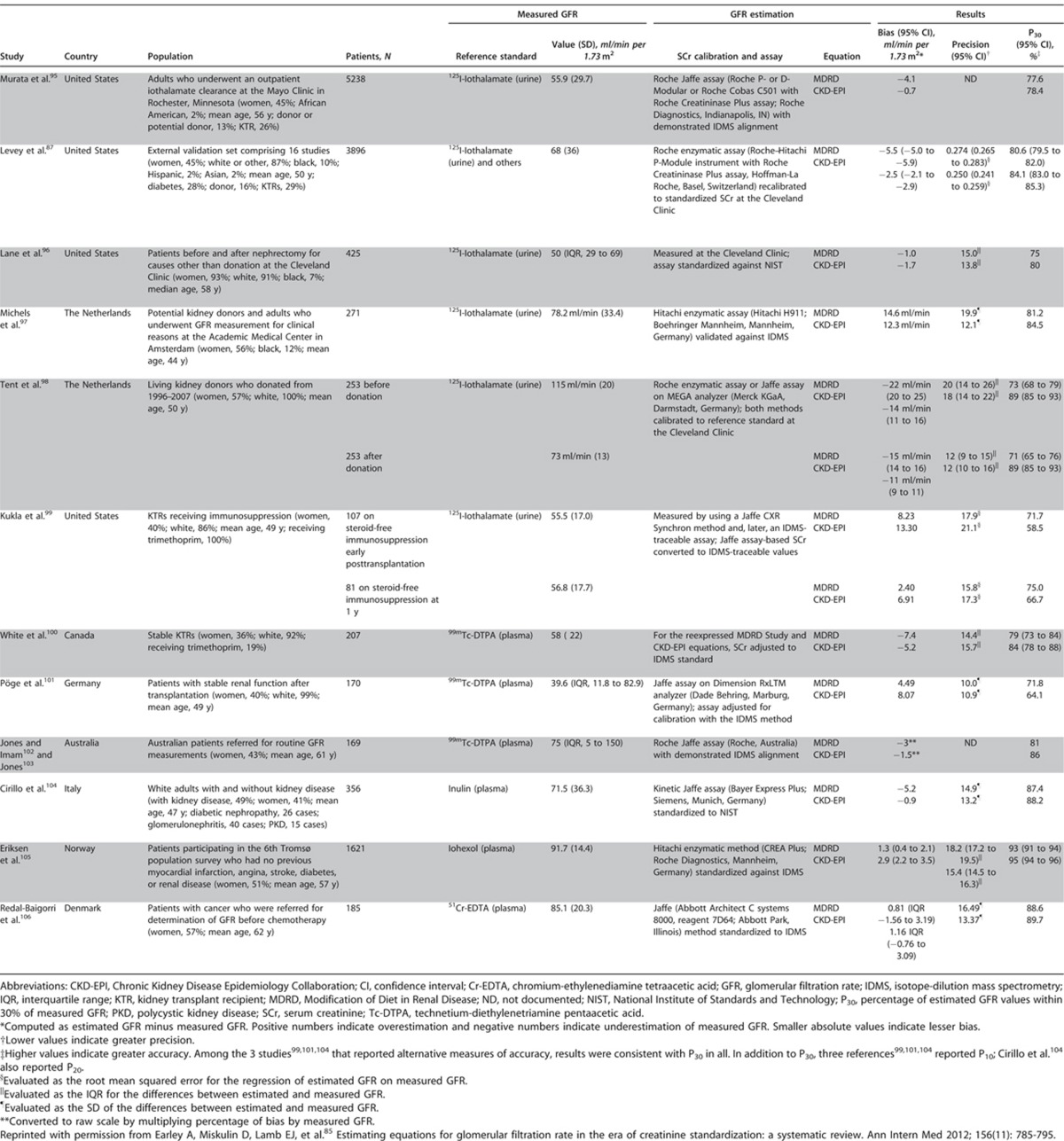
Lesser bias of the CKD-EPI equation compared to the MDRD Study equation reflects higher eGFR throughout most of the range for age and creatinine, especially in younger individuals, women and whites. Higher eGFR results in lower prevalence estimates for CKD in these groups (Figure 12), with more accurate risk relationships of lower eGFR and adverse outcomes (Figure 13).107
Figure 12.
Comparison of distribution of GFR and CKD prevalence by age (NHANES 1999-2004). GFR was categorized on the basis of the classification system established by the NKF-KDOQI. Top. Distribution of estimated GFR, by 4-ml/min per 1.73 m2 categories. Values are plotted at the midpoint. Bottom. Prevalence of CKD, by age. CKD, chronic kidney disease; GFR, glomerular filtration rate; NKF-KDOQI, National Kidney Foundation-Kidney Disease Outcomes Quality Initiative; NHANES, National Health and Nutrition Examination Survey. Reprinted with permission from Levey AS, Stevens LA, Schmid CH, et al.87 A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604-612.
Figure 13.
Meta-analysis of NRI for all-cause mortality, CVD mortality, and ESRD. NRI summarizes the risk of clinical outcomes among participants who are reclassified from one estimated GFR category using the MDRD Study equation to another estimated GFR category using the CKD-EPI equation compared with those who are not reclassified. NRI greater than zero favors the CKD-EPI equation. NRI less than zero favors the MDRD Study equation. The sizes of the data markers are proportional to the inverse of the variance of the NRIs. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, cardiovascular disease; ESRD, end-stage renal disease; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; NRI, net reclassification improvements. Reprinted with permission from Matsushita K, Mahmoodi BK, Woodward M, et al.107 Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration rate. JAMA 2012; 307(18): 1941-1951. Copyright © (2012) American Medical Association. All rights reserved.
To account for possible differences in muscle mass and diet according to race, ethnicity and geographic region, the MDRD Study and CKD-EPI equations have been modified for use in other racial and ethnic groups and in other countries. In some, but not all studies, these modifications are associated with increased accuracy (Table 14), and should be used in preference to unmodified equations. Where tested, the CKD-EPI equation and its modifications were generally more accurate than the MDRD Study and its modifications. In the absence of specific modifications for race, ethnicity, or regional difference, it is reasonable to use the CKD-EPI equation for GFR estimation. Reliance upon SCr alone is not an appropriate alternative since the uncertainty about the effect of non-GFR determinants affects interpretation of SCr as much as it affects interpretation of eGFR. More widespread testing of GFR estimating equations is necessary to resolve uncertainties about the need for racial, ethnic, and geographic modifications.108
Table 14. Performance comparison of creatinine-based GFR estimating equations outside of North America, Europe, and Australia.
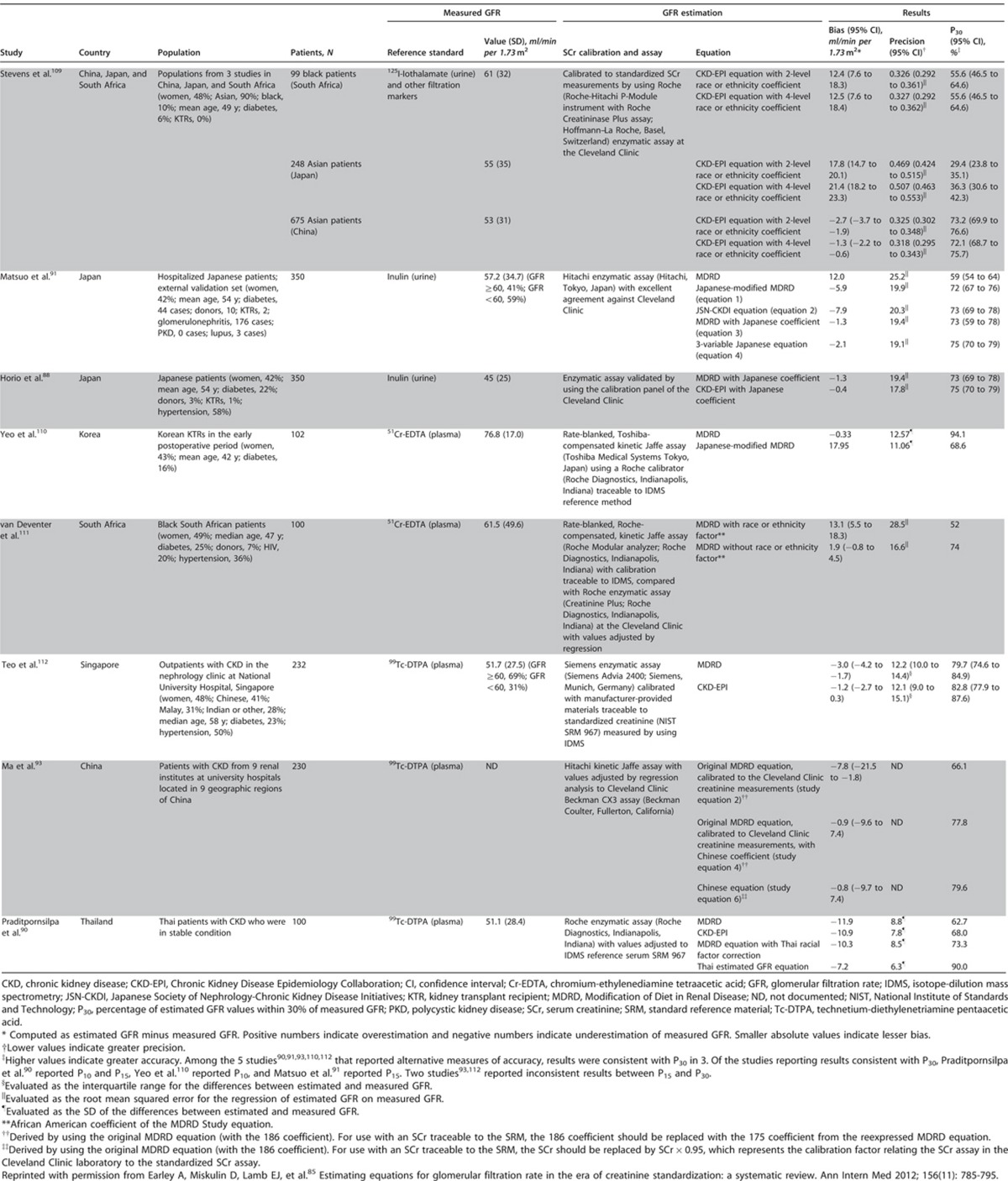
Pediatric Considerations
This recommendation would need to be altered for application in pediatric practice in the following way.
Creatinine measurements in all infants and children should be derived from methods that minimize confounders and are calibrated against an international standard.
eGFRcreat may only be reported when the height of the child is known by the laboratory.
If reporting eGFRcreat laboratories should utilize the most current and accurate pediatric derived equations based on the demographic and laboratory markers available.
In infants or small children the level of creatinine when measured is often below that of the normal ‘bottom range' of the adult assay. As such laboratories measuring creatinine in infants or small children must ensure their lower calibration samples include the lowest end of the expected range of values for the group of interest.
As the majority and the most accurate of the published pediatric eGFRcreat formulas require height, standard laboratory reporting of eGFRcreat is neither practical nor recommended in children. In a pediatric CKD population, and using the plasma disappearance of iohexol as the gold standard measure of GFR, Schwartz et al. derived a number of novel GFR prediction equations.80 Their analysis demonstrated the importance of the height/SCr variable in the population as it provided the best correlation with the iohexol GFR (R2=65%). The simplest of such formula, using only height and SCr and a constant of either 41.3 or 0.413 depending on whether height was expressed as meters or centimeters respectively, provided 79% of estimated GFRs within 30% of the iohexol values and 37% of estimated GFRs within 10% of the iohexol values.
Any eGFRcreat formula used in children will preferably be validated at the appropriate age and level of renal function, and the laboratory methods used locally will be calibrated or comparable to those used in the process of developing the formula being applied. Currently the most robust pediatric eGFR formulas, derived using iohexol disappearance and creatinine measurements which were measured centrally and calibrated and traceable to international standards come from the CKiDs study.80
The two most common creatinine-based formulas recommended for use in clinical practice include:
Updated “Bedside” Schwartz equation:
eGFR (ml/min/1.73m2)=41.3 × (height/SCr), where height is in meters and SCr is in mg/dl.
“1B” Equation (include blood urea nitrogen [BUN] not cystatin C):
eGFR (ml/min/1.73m2)=40.7 × (height/SCr)0.64 × (30/BUN)0.202, where height is in meters, SCr and BUN are in mg/dl.
The additional recommendation for laboratory reporting of SCr is fully applicable in pediatrics.
When the individual clinician has information regarding current and accurate height and applies the appropriate pediatric formula, the recommendation to report an individual child's eGFRcreat value of less than 60 ml/min/1.73 m2 as “decreased,” would be applicable in children over the age of 2 years.
- 1.4.3.5: We suggest measuring cystatin C in adults with eGFRcreat 45-59 ml/min/1.73m2 who do not have markers of kidney damage if confirmation of CKD is required. (2C)
- If eGFRcys/eGFRcreat-cys is also <60 ml/min/1.73 m2, the diagnosis of CKD is confirmed.
- If eGFRcys/eGFRcreat-cys is ≥60 ml/min/1.73 m2, the diagnosis of CKD is not confirmed.
RATIONALE
A major foundation of this guideline is that CKD classification and staging should be influenced primarily by clinical prognosis. As will be reviewed in the sections below, abundant evidence has shown that GFR estimates based on cystatin C are more powerful predictors of clinical outcomes than creatinine-based eGFR. These findings have been strongest for mortality and CVD events, and the prognostic advantage of cystatin C is most apparent among individuals with GFR >45 ml/min/1.73 m2. In addition, new findings show that using cystatin C in addition to SCr can lead to improved accuracy of GFR estimation, including CKD classification. In the opinion of the Work Group, these considerations warrant new recommendations for GFR estimation using cystatin C.
Evidence Base
Evidence supports the use of cystatin C-based eGFR within the population of persons diagnosed with CKD based on an eGFRcreat 45-59 ml/min/1.73 m2 (G3a) but without albuminuria (A1) or other manifestations of kidney damage. This group represents 3.6% of the US population and 41% of people in the US estimated to have CKD based on eGFRcreat and urine ACR alone (Figure 8), and there has been substantial controversy over whether or not these persons have CKD. Data described below indicate that use of cystatin C to estimate GFR in this population leads to more accurate estimation of GFR and prediction of risk for future adverse events.
In several studies, eGFRcys has been measured in populations with and without eGFRcreat <60 ml/min/1.73 m2, and participants were separated into those with and without eGFRcys <60 ml/min/1.73 m2 (Figure 14). Those with both eGFRcreat and eGFRcys<60 ml/min/1.73 m2, about two-thirds of those with eGFRcreat <60 ml/min/1.73 m2, had markedly elevated risks for death, CVD, and ESRD end points compared with persons with eGFRcreat >60 ml/min/1.73 m2. The Work Group therefore considers this group to have “confirmed CKD.” In contrast, about one-third of those with eGFRcreat<60 ml/min/1.73 m2 had eGFRcys>60 ml/min/1.73 m2 and this group were similar in risk for adverse outcomes as persons with eGFRcreat>60 ml/min/1.73 m2.
Figure 14.
Association of CKD definitions with all-cause mortality and ESRD. CKD, chronic kidney disease; ESRD, end-stage renal disease. Reprinted with permission from Peralta CA, Shlipak MG, Judd S, et al.114 Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA 2011; 305(15): 1545-1552. Copyright © (2011) American Medical Association. All rights reserved.
New data from CKD-EPI also showed improved accuracy in GFR estimation using both creatinine and cystatin C (eGFRcreat-cys) compared to either marker alone. In the subgroup with eGFRcreat 45-59 ml/min/1.73 m2, the combined equation correctly reclassified 16.8% of those with eGFR 45-59 ml/min/1.73 m2 to measured GFR ≥60 ml/min/1.73 m2.113
The consensus of the Work Group was therefore that the large group of persons with eGFRcreat 45-59 ml/min/1.73 m2 without markers of kidney damage, but with eGFRcys/eGFRcreat-cys ≥60 ml/min/1.73 m2 could be considered not to have CKD. The removal of the diagnosis and label of CKD may be reassuring to patients and may help clinicians to focus their efforts on higher risk CKD patients.
The guideline statement suggesting the use of eGFRcys/eGFRcreat-cys requires several important qualifiers. First, clinicians may not want or need to confirm the diagnosis of CKD in patients with eGFRcreat 45-59 ml/min/1.73 m2 without markers of kidney damage, either because the likelihood of CKD is high because of the presence of risk factors for CKD or presence of complications of CKD. Second, cystatin C is not universally available, so it may not be practical for a clinician to request a cystatin C blood test. Third, in certain clinical settings, the cost of measuring cystatin C (US $1–5) may be prohibitive. For all these reasons, the guideline statement 1.4.3.5 is stated as a suggestion.
In addition to the population described above, eGFRcys may be useful as a confirmatory test in situations where either the eGFRcreat may be inaccurate or biased, or when the clinical scenario warrants a secondary test (Recommendation 1.4.3.2). In these clinical situations, a clearance measurement using an exogenous filtration marker may be optimal when it is available. The measurement of eGFRcys/eGFRcreat-cys would be a relatively low-cost, feasible alternative when GFR measurement is not practical. The Work Group believed that measured urinary CrCl was an inferior confirmatory test relative to either GFR measurement or GFR estimation using both creatinine and cystatin C.
If cystatin C testing is desired, it is very important that clinicians understand principles of GFR estimation using cystatin C. As with creatinine, GFR should be estimated from cystatin C and an appropriate equation should be chosen for the specific clinical population (Recommendation 1.4.3.6), and an assay be chosen for measurement that is traceable to the international standard reference material (Recommendation 1.4.3.7).
Pediatric Considerations
The utility of this specific statement to pediatrics is unclear as the vast majority of children with significant reductions in GFR, e.g., below 60 ml/min/1.73 m2, have either structural abnormalities or findings of renal damage as evidenced by urinary or serum abnormalities. It is very unlikely that isolated reduction in GFR would occur as in older adults. As such, the confirmation of CKD will be made on criteria beyond that of GFR alone.
- 1.4.3.6: If cystatin C is measured, we suggest that health professionals (2C):
- use a GFR estimating equation to derive GFR from serum cystatin C rather than relying on the serum cystatin C concentration alone.
- understand clinical settings in which eGFRcys and eGFRcreat-cys are less accurate.
RATIONALE
Cystatin C is licensed for use in some countries in Europe and has been approved by the FDA as a measure of kidney function in the United States for nearly 10 years. In certain regions, notably Sweden and parts of China, eGFR is routinely estimated by both creatinine and cystatin C. As with creatinine, GFR estimates using cystatin C are more accurate in estimating measured GFR than the SCysC concentration alone. As with creatinine, sources of error in GFR estimation from SCysC concentration include non-steady state conditions, non-GFR determinants of SCysC, measurement error at higher GFR, and interferences with the cystatin C assays (Table 15).
Table 15. Sources of error in GFR estimating using cystatin C.
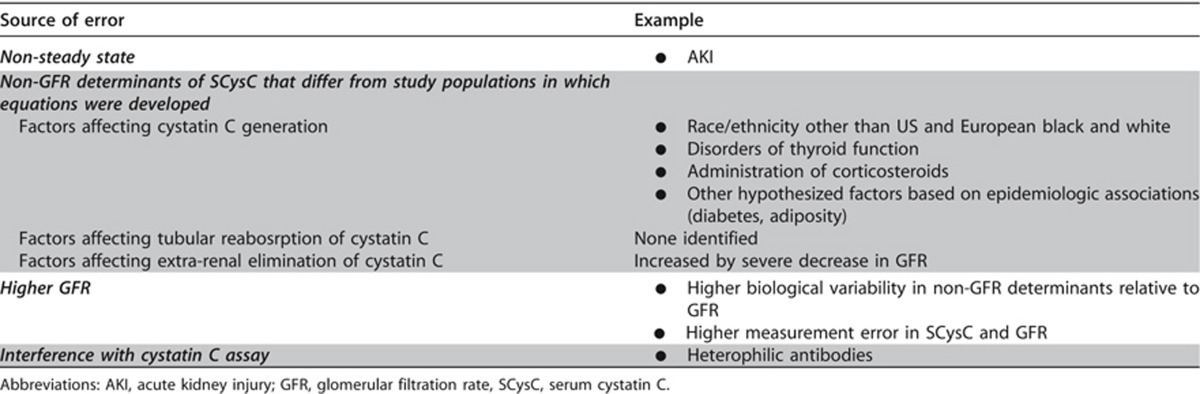
Pediatric Considerations
For Recommendation 1.4.3.6, this guideline is fully applicable in pediatrics. See Recommendation 1.4.3.7 for details.
In terms of clinical settings where eGFRcys might be less accurate, it should be noted that Schwartz et al. determined that the only variable that explained the outlier values of estimated GFR (in both univariate and multivariate formulas) was heavier weight; race, high blood pressure, albumin levels and use of steroids did not contribute.115
- 1.4.3.7: We recommend that clinical laboratories that measure cystatin C should (1B):
- measure serum cystatin C using an assay with calibration traceable to the international standard reference material.
- report eGFR from serum cystatin C in addition to the serum cystatin C concentration in adults and specify the equation used whenever reporting eGFRcys and eGFRcreat-cys.
- report eGFRcys and eGFRcreat-cys in adults using the 2012 CKD-EPI cystatin C and 2012 CKD-EPI creatinine-cystatin C equations, respectively, or an alternative cystatin C-based GFR estimating equations if they have been shown to improve accuracy of GFR estimates compared to the 2012 CKD-EPI cystatin C and 2012 CKD-EPI creatinine-cystatin C equations.
When reporting serum cystatin C:
We recommend reporting serum cystatin C concentration rounded to the nearest 100th of a whole number when expressed as conventional units (mg/l).
When reporting eGFRcys and eGFRcreat-cys:
We recommend that eGFRcys and eGFRcreat-cys be reported and rounded to the nearest whole number and relative to a body surface area of 1.73 m2 in adults using the units ml/min/1.73 m2.
We recommend eGFRcys and eGFRcreat-cys levels less than 60 ml/min/1.73m2 should be reported as “decreased.”
RATIONALE
As for SCr, reporting eGFR using cystatin C in addition to cystatin C will facilitate clinician's use of cystatin C for GFR estimation. It is important to acknowledge that calibration of assays is essential to interpretation of kidney function measures. Cystatin C is measured by a variety of immunoassays and, as for creatinine, there can be variation among methods in reported SCysC concentration but reported analytic variation appears less common than with creatinine. In June 2010 the Institute for Reference Materials and Measurements (IRMM) released a reference material (ERM-DA471/IFCC) for cystatin C measurement. Reagent manufacturers are in the process of recalibrating their assays against this standard which will enable standardized reporting of cystatin C and eGFR results. This recommendation is directed to laboratories with the intent to clarify the details of such calibration and the use of specific equations so as to facilitate international standardization.
Evidence Base
Numerous equations have been developed to estimate GFR. Some equations include cystatin C as the only variable, while others include, age, sex, or race, but the magnitude of coefficients for these variables are smaller than in creatinine-based equations, presumably reflecting less contribution of muscle to cystatin C generation than to creatinine generation. Equations without race are a potential advantage for cystatin C-based estimating equations in non-black, non-white populations.
For our review of GFR estimating equations, we only considered equations that were developed using assays that were traceable to the new reference methods and study populations in which SCysC concentration was measured using traceable assays. At this time, only the equations developed by CKD-EPI are expressed for use with standardized SCysC (Table 16), including equations developed in CKD populations in 2008116, 117 and re-expressed for use with standardized cystatin C in 2011, and equations developed in diverse populations in 2012.113 Equations using assays that are not traceable to the the reference standard are listed in Supplemental Table 3.
Table 16. Equations based on IDMS traceable creatinine and IFCC traceable cystatin C assays.
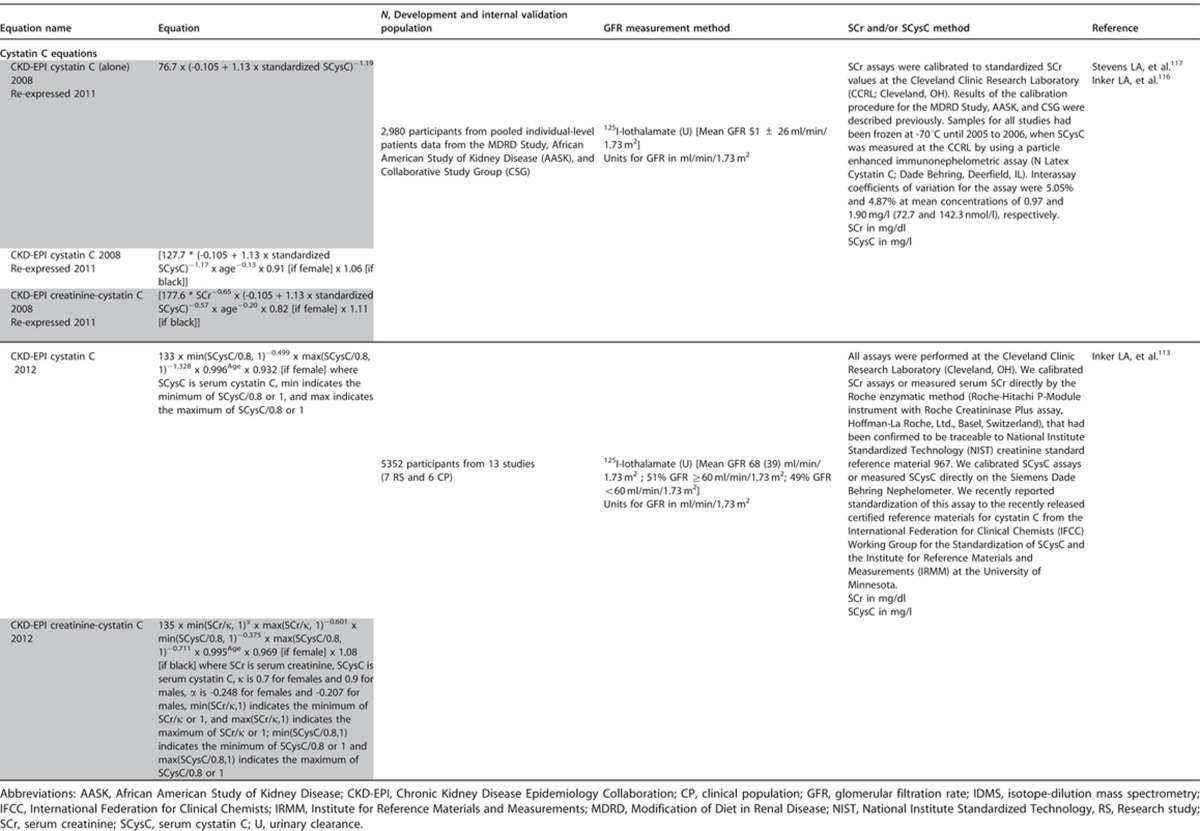
The 2012 creatinine-cystatin C equation is more accurate than equations using creatinine or cystatin C separately (Figure 15), and more accurate than the 2008 creatinine-cystatin C equation (Table 17). The average of the GFR computed by the equations using creatinine and cystatin C separately is similar to the GFR computing using the creatinine-cystatin C equations. The 2012 cystatin C equation has similar accuracy to the 2009 creatinine equation described above but does not require use of race, and may be more accurate in non-black, non-white populations or in clinical conditions with variation in non-GFR determinants of SCr. We anticipate the development of additional equations using cystatin C in the future and recommend that they be compared with the CKD-EPI 2012 cystatin C and creatinine-cystatin C equations as well as with the CKD-EPI 2009 creatinine equation.
Figure 15.
Performance of three equations for estimating GFR. Panel a shows the median difference between measured and estimated GFR. The bias is similar with the equation using creatinine alone, the equation using cystatin C alone, and the combined creatinine–cystatin C equation. Panel b shows the accuracy of the three equations with respect to the percentage of estimates that were greater than 30% of the measured GFR (1 – P30). Whiskers indicate 95% confidence intervals. GFR, glomerular filtration rate; P30, percentage of estimated GFR values within 30% of measured GFR. From N Engl J Med, Inker LA, Schmid CH, Tighiouart H, et al.113 Estimating glomerular filtration rate from serum creatinine and cystatin C. 367: 20-29. Copyright © 2012 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Table 17. Performance comparison of cystatin C-based estimating equations in North American and European populations.
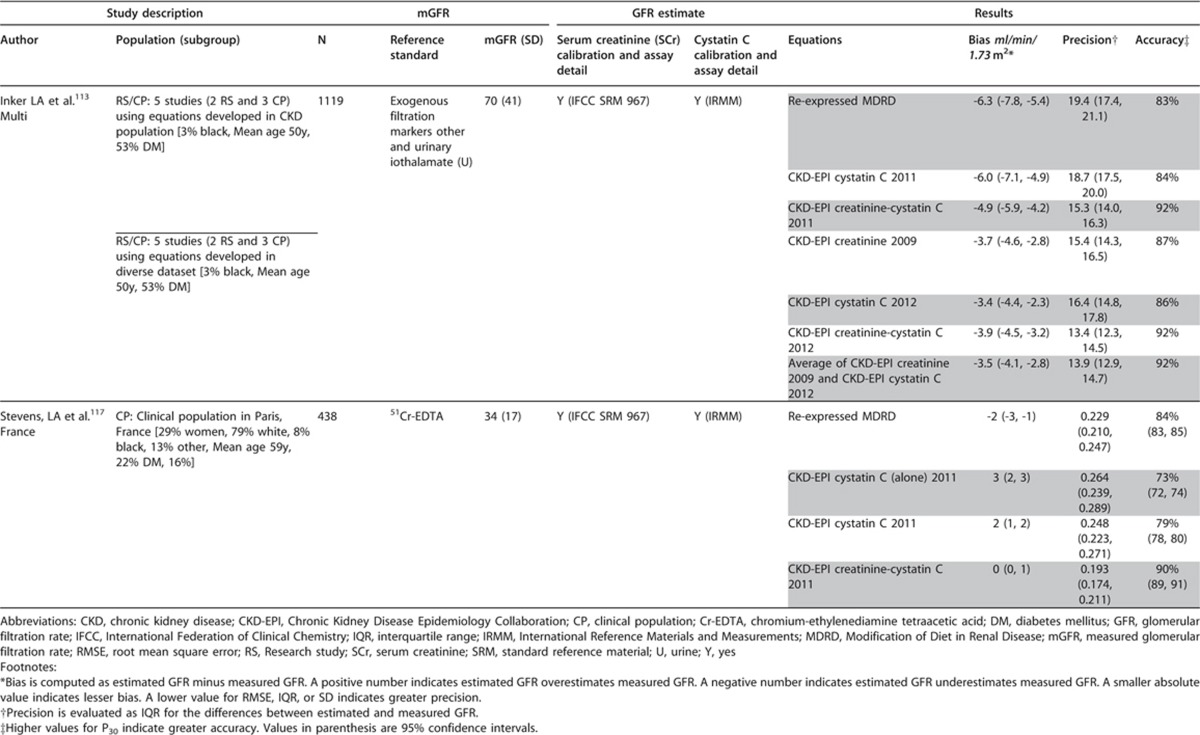
Pediatric Considerations
For Recommendation 1.4.3.7 this set of statements would need to be altered for application in pediatric practice in the following way:
Measure SCysC using an immunonephelometrically determined method in which the assay is calibrated and traceable to the international standard reference material.
Report eGFRcys in addition to the SCysC concentration in children.
Report eGFRcys in children specifying the specific equation used.
Based on their recent work comparing particle-enhanced nephelometric to turbidometric immunoassays for cystatin C in a pediatric population with significant reduction in GFR (median GFR ∼45 ml/min/1.73 m2), Schwartz et al. demonstrated less bias for the nepholometric value and that its reciprocal showed a substantially improved correlation to the iohexol GFR (0.87 versus 0.74) when compared to that of the turbidometric assay.115 This demonstrates the importance of using an assay calibrated and traceable to the international standard reference material.
Numerous pediatric specific and derived eGFRcys formulas have been published, the most current and recent by Schwartz115 who derives the newest available from a validation set from the CKiD study and compares those results to 3 other well-recognized formulas from the literature, namely Zapitelli et al.,118 Filler and Lepage,119 and Hoek et al.120
Their results demonstrate that the newest univariate cystatin C formula derived from the CKiD cohort has excellent accuracy with 82.6% of eGFRcys within 30% of the true measured iohexol GFR and 37.6% within 10% of the true measured iohexol GFR. Likewise the bias of 0.3% and correlation of 0.85 are the best of all formulas reported to date. The formula they use to obtain these values is: 70.69 × (cystatin c)-0.931.
Of note, their final multivariate equation, when applied to the validation set and using height/SCr, nepholemetric cystatin C, BUN, sex, and an adjusted height term demonstrated the best accuracy reported in pediatric studies to date, 91% and 45% within 30% and 10% of the true GFR, respectively; with a bias of only −0.2 and correlation of 0.92.
1.4.3.8: We suggest measuring GFR using an exogenous filtration marker under circumstances where more accurate ascertainment of GFR will impact on treatment decisions. (2B)
RATIONALE
In clinical practice, there may be a requirement to measure GFR when the need for a ‘truer' more precise value is identified (such as for organ donation or for dosing of toxic drugs). The intention of this statement is to recognize that specialty centers for kidney disease, usually tertiary referral centers, should have the capacity to measure GFR using exogenous filtration markers as a recognized specialist service. We recognize that this ability does not currently constitute the definition of specialty kidney referral centers and that it may be problematic, but resources to ensure accurate measurement ought to be made available. Given that these specific measurements require levels of rigor and reproducibility similar to those of laboratory calibration issues, specialist centers would be the right place to suggest that these facilities be made available.
Evidence Base
GFR is measured as the clearance of an exogenous filtration marker. The “gold standard” method is the urinary clearance of inulin during a continuous intravenous infusion. To simplify the procedure there are a number of alternative clearance methods and alternative filtration markers, with minor differences among them.79 For all measurement methods, measured GFR should be reported as described for eGFR.
Table 18 summarizes the strengths and limitations of clearance methods and filtration markers for clearance measurements. Thus measured GFR may also be associated with error, and in evaluation of GFR estimating equations, random error in GFR measurement is a source of some of the imprecision in GFR estimating equations.27, 121 In principle, the magnitude of random error in GFR measurements is likely to be smaller than errors in GFR estimation using creatinine and cystatin C due to conditions listed in Tables 11 and 15.
Table 18. Strengths and limitations of GFR measurement methods and markers.
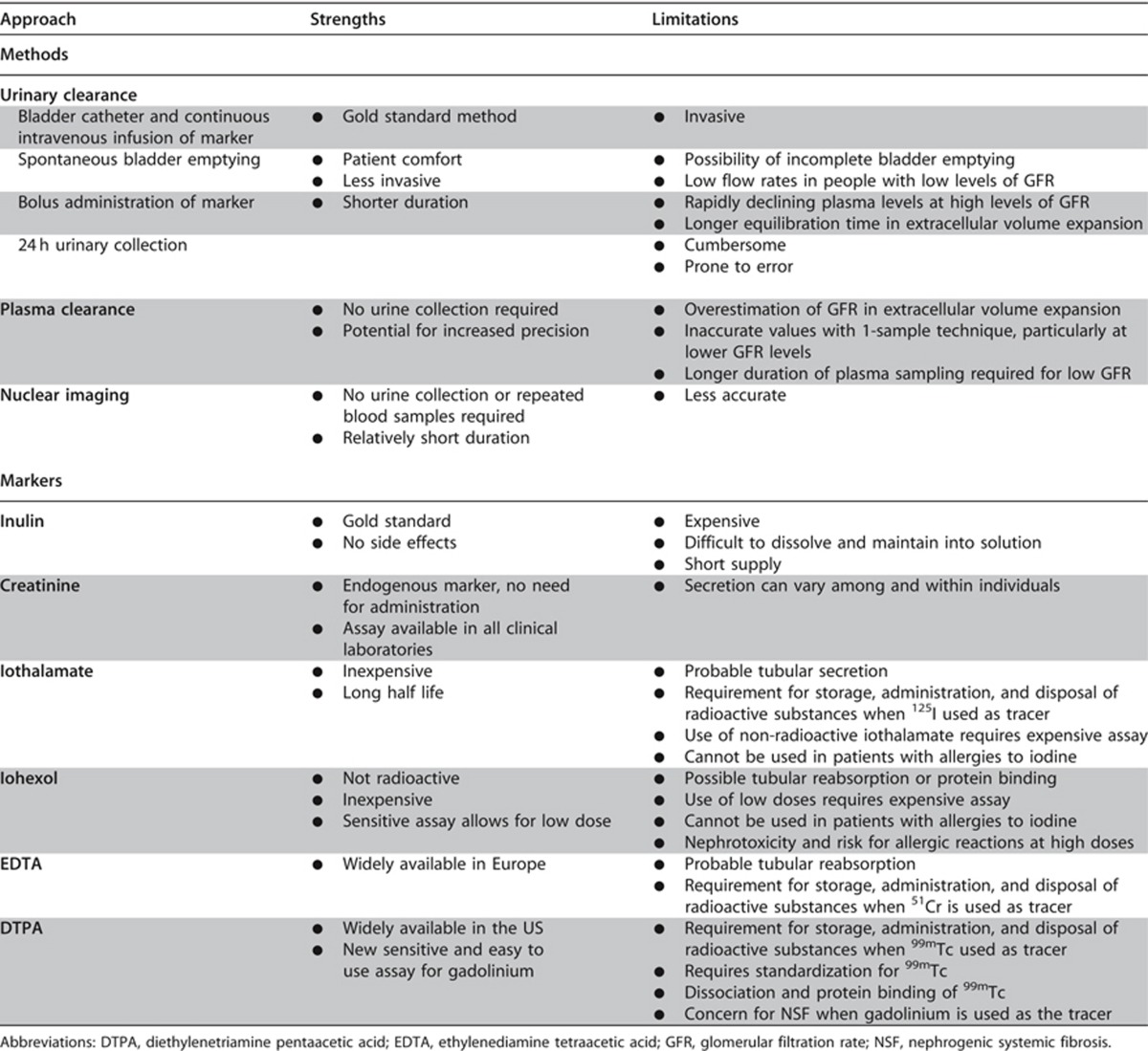
International Relevance
The calculation of eGFR using these equations usually requires computer programming and some processes for quality monitoring. Nonetheless the statements are here to serve as ‘best practice' recommendations so that these can be aspired to over time in those locations where these recommendations are currently not able to be implemented.
The Work Group appreciated that not all laboratories have capabilities to assay cystatin C. Different countries and regions will have different availabilities for measurement of GFR. The statement about GFR measurements mostly applies to countries with tertiary care services such as kidney transplantation and oncology.
Implications for Clinical Practice and Public Policy
It is important for clinicians to understand various methods for estimating and measuring kidney function and the situations in which specific methods may be superior in clinical decision making about treatment and referral.
Standardized assays and robust equations are important for epidemiological and planning purposes so that public policy can be informed by more accurate estimates of CKD, which may be possible with improved standardization of both assays and equations.
In different parts of the world, different assays are used and equations for estimating eGFR may differ. Thus, appreciating and understanding local standards is important for individual patients who may travel, and for comparative research across countries or regions.
In the event that a clinician requires measurement of GFR instead of an estimate, knowledge of these different tests and availability of them is important. Situations in which measurement would be required are likely quite infrequent but include donor evaluation in kidney transplantation and use of toxic drugs which have a narrow therapeutic range. We acknowledge that drug development and clinical observation programs may not define the various thresholds with sufficient granularity to require greater accuracy than is provided by eGFRcreat. Guidance is evolving regarding kidney function evaluation during drug development programs.13 There are no direct implications for public policy for the statement about GFR measurement.
Areas of Controversy, Confusion, or Non-consensus
The Work Group recognizes that no single creatinine-based estimating equation will perform optimally in all clinical circumstances and that there may be changes in the performance of estimating equations over time and in different regions. However, for the purpose of eGFR reporting, it is important to select a single equation within a region or country. At the writing of this guideline, in North America, Europe, and Australia, the advantages of the CKD-EPI equation at higher GFR make it more applicable than the MDRD Study equation for general practice and public health.
While cystatin C offers some advantages over SCr as the basis of estimating equations, the cost of the assay and potential lack of standardization across laboratories for this ‘newer' test limit our ability to recommend it as a preferred or even usual second test after creatinine. We recognize that these factors may lead to variations in implementation. The recommendation to consider confirmatory or additional testing if there is a need for more accurate determination of GFR is important. That there are other laboratory markers to estimate GFR (i.e., cystatin C) is stated here as there has been accumulating data to support its use in these situations. We have specifically mentioned cystatin C because of these data.
Clarification of Issues and Key Points
It is important for clinicians to appreciate the need for standardized assays and standardized equations for laboratory reporting of eGFR. Changes in laboratory assays or calculation methods should be reported to clinicians in order to avoid confusion when serially following individuals. This is because values in an individual might indicate a worsening or improvement in eGFR which may be attributable to different assays or calculation methods, rather than a reflection of true change.
When precise information about GFR is required, direct measurement using reliable methods should be pursued.
Pediatric Considerations
For Recommendation 1.4.3.8 this guideline is fully applicable in pediatrics.
- 1.4.4 Evaluation of albuminuria
- 1.4.4.1: We suggest using the following measurements for initial testing of proteinuria (in descending order of preference, in all cases an early morning urine sample is preferred) (2B):
- urine albumin-to-creatinine ratio (ACR);
- urine protein-to-creatinine ratio (PCR);
- reagent strip urinalysis for total protein with automated reading;
- reagent strip urinalysis for total protein with manual reading.
- 1.4.4.2: We recommend that clinical laboratories report ACR and PCR in untimed urine samples in addition to albumin concentration or proteinuria concentrations rather than the concentrations alone. (1B)
- 1.4.4.2.1: The term microalbuminuria should no longer be used by laboratories. (Not Graded)
- 1.4.4.3: Clinicians need to understand settings that may affect interpretation of measurements of albuminuria and order confirmatory tests as indicated (Not Graded):
- Confirm reagent strip positive albuminuria and proteinuria by quantitative laboratory measurement and express as a ratio to creatinine wherever possible.
- Confirm ACR ≥30 mg/g (≥3 mg/mmol) on a random untimed urine with a subsequent early morning urine sample.
- If a more accurate estimate of albuminuria or total proteinuria is required, measure albumin excretion rate or total protein excretion rate in a timed urine sample.
RATIONALE
We recommend measurement of urinary albumin because it is relatively standardized and because it is the single most important protein lost in the urine in most chronic kidney diseases. Use of urinary albumin measurement as the preferred test for proteinuria detection will improve the sensitivity, quality, and consistency of approach to the early detection and management of kidney disease.
By contrast, laboratory tests purporting to measure urinary total protein are commonly flawed, often being standardized against, and predominantly sensitive to, albumin. They have poor precision at low concentrations and demonstrate poor between-laboratory agreement while being insensitive, non-specific, and susceptible to a range of false-positive and false-negative problems. There may occasionally be clinical reasons for a specialist to use PCR instead of ACR to quantify and monitor significant levels of proteinuria (e.g., in patients with monoclonal gammopathies).
Commonly used reagent strip devices measuring total protein are insufficiently sensitive for the reliable detection of proteinuria, do not adjust for urinary concentration, and are only semi-quantitative. Furthermore, there is no standardization between manufacturers. The use of such strips should be discouraged in favor of quantitative laboratory measurements of albuminuria or proteinuria. When used, reagent strip results should be confirmed by laboratory testing (Figure 16).
Figure 16.
Suggested protocol for the further investigation of an individual demonstrating a positive reagent strip test for albuminuria/proteinuria or quantitative albuminuria/proteinuria test. Reagent strip device results should be confirmed using laboratory testing of the ACR on at least two further occasions. Patients with two or more positive (≥30 mg/g or ≥3 mg/mmol) tests on early morning samples 1-2 weeks apart should be diagnosed as having persistent albuminuria. The possibility of postural proteinuria should be excluded by the examination of an EMU. PCR measurement can be substituted for the ACR but is insensitive in the detection of moderately increased albuminuria/proteinuria. Approximate PCR equivalent to an ACR of 30 mg/mmol is 50 mg/mmol. ACR, albumin-to-creatinine ratio; C&S, culture and sensitivity; CKD, chronic kidney disease; EMU, early morning urine; MSU, mid-stream urine; PCR, protein-to-creatinine ratio. aConsider other causes of increased ACR (e.g., menstrual contamination, uncontrolled hypertension, symptomatic urinary tract infection, heart failure, other transitory illnesses, and strenuous exercise), especially in the case of type 1 diabetes present for less than 5 years. The presence of hematuria may indicate non-diabetic renal disease. This figure was published and adapted from Lamb EJ, Price CP.122 Kidney function tests, in Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, eds Burtis CA, Ashwood E, Bruns DE, 5th edition, pp 669-708, 2012. Copyright Elsevier.
The combination of reagent strips with automated reader devices can improve inter-operator variability. More recently launched reagent strip devices capable of producing albumin or total protein results as a ratio to urinary creatinine require further evaluation to provide evidence that they have equivalent sensitivity and specificity to laboratory tests and are economically advantageous.
Although the reference point remains the accurately timed 24-hour specimen, it is widely accepted that this is a difficult procedure to control effectively and that inaccuracies in urinary collection may contribute to errors in estimation of protein losses. In practice, untimed urine samples are a reasonable first test for ascertainment of albuminuria. An EMU (‘first pass') sample is preferred since it correlates well with 24-hour protein excretion, has relatively low intra-individual variability, and is required to exclude the diagnosis of orthostatic (postural) proteinuria. However, a random urine sample is acceptable if no EMU sample is available.
The concentration of protein or albumin in a urine sample will be affected by hydration (i.e., how diluted or concentrated a urine sample is). Creatinine excretion is considered to be fairly constant throughout the day and it has become customary to correct for urinary concentration by expressing either the protein or albumin concentrations as a ratio to the creatinine concentration in the same sample.
Timed urine collections may be used for confirmatory purposes but are not required except in circumstances in which untimed urine ACR is less accurate. It is worthwhile noting that albumin and protein excretion display considerable biological variability and may be increased by a variety of pathological and non-pathological factors. Consequently, confirmation of increased excretion rates is recommended.
Evidence Base
Why is albumin measurement being recommended instead of total protein?
Urine albumin measurement provides a more specific and sensitive measure of changes in glomerular permeability than urinary total protein.123, 124, 125 There is substantial evidence linking increased albuminuria to outcomes of CKD4, 30 (e.g., CKD Prognosis Consortium2, 3, 4, 5, Nord-Trøndelag Health Study [HUNT 2]125a, Prevention of Renal and Vascular Endstage Disease [PREVEND]125b). There is also evidence that urinary albumin is a more sensitive test to enable detection of glomerular pathology associated with some other systemic diseases including diabetes, hypertension and systemic sclerosis.126, 127, 128, 129
In health, relatively small amounts of albumin (<30 mg/24 hours) are lost in the urine. Because of this, and additionally because total protein assays are imprecise and insensitive at low concentrations, relatively large increases in urine albumin excretion can occur without causing a significant measurable increase in urinary total protein.125
Total protein measurement is problematic in urine due to: large sample-to-sample variation in the amount and composition of proteins; high and variable concentrations of non-protein interfering substances relative to the protein concentration; and high inorganic ion content. All these factors affect the precision and accuracy of the various methods. Most laboratories currently use either turbidimetry or colorimetry130 to measure total protein and as with urine reagent strip analysis, these methods do not give equal analytical specificity and sensitivity for all proteins which can contribute to diverse estimates of proteinuria prevalence.131, 132 Most methods tend to react more strongly with albumin than with globulin and other non-albumin proteins.34, 133, 134, 135 There are significant interferences causing falsely high results.136, 137, 138. There is no reference measurement procedure and no standardized reference material for urinary total protein listed by the JCTLM. The variety of methods and calibrants in use means that there is inevitably significant between-laboratory variation.139, 140, 141 Since a variable mixture of proteins is measured, it is difficult to define a standardized reference material.
How should albumin be measured and reported?
Albumin should be measured using immunological assays capable of specifically and precisely quantifying albumin at low concentrations and of producing quantitative results over the clinically relevant range. Currently urinary albumin is predominantly measured by diagnostic laboratories using turbidimetric assays.130 At present there is no reference measurement procedure or standardized reference material for urine albumin listed by the JCTLM, although the NKDEP and the International Federation of Clinical Chemistry and Laboratory Medicine have recently established a joint committee to address these issues.142, 143 At present, most assays are standardized against a serum-based calibrant (CRM 470) distributed by the IRMM of the European Commission, as has previously been recommended by KDIGO.31
Albumin concentration should be reported as a ratio to urinary creatinine concentration (mg/mmol or mg/g). ACR results should be expressed to one decimal place (mg/mmol) or whole numbers (mg/g). Both enzymatic and Jaffe assays are suitable for the measurement of creatinine in urine. We suggest that the term ‘microalbuminuria' no longer be used because it can be misleading in suggesting that the albumin may be small or different in some way. The proposed albuminuria categories A1-3 are a more clinically meaningful way to express information about categories within the continuum of albumin excretion.
Reagent strip point-of-care testing devices capable of measuring low concentrations of albumin are also available producing both semi-quantitative and fully quantitative ACR results. Reasonable analytical144, 145, 146, 147 and diagnostic performance has been demonstrated.148, 149, 150 While studies of these devices have been somewhat limited in size, they demonstrate their potential to play a significant role in the care pathway of patients suspected of having CKD.
Why are reagent strip devices for protein measurement considered less accurate than laboratory measurement?
Reagent strip devices for proteinuria detection have been in use for more than 50 years. As discussed earlier, a positive reagent strip result is also associated with outcomes of CKD. Such devices have been used to support screening programs in some countries,151, 152, 153 although there appears to be no evidence supporting such screening of unselected populations.154
Although purporting to measure total protein, the reagent pad is most sensitive to albumin.155, 156, 157 There is evidence that strips from different manufacturers perform differently at the cutoff (‘+') concentration of 300 mg/l and degrees of ‘plus-ness' between different manufacturers don't always correspond to the same nominal concentration of protein in urine.124 Concentrated urines may give a color change in the positive range of a reagent strip device even though protein loss remains normal and vice versa. False-positive results may occur if the urine is alkalinized (e.g., due to urinary tract infection) or in the presence of quaternary ammonium compounds that alter the pH of the urine. The performance of reagent strips is operator-dependent158 and affected by the presence of colored compounds such as bilirubin and certain drugs (e.g., ciprofloxacin, quinine, and chloroquine).159 Reagent strips cannot reliably distinguish between proteinuria categories124, 157 and show relatively poor diagnostic accuracy for proteinuria detection.160, 161 In the Australian Diabetes, Obesity and Lifestyle (AusDiab) study, a reagent strip reading of + or greater had 58% and 99% sensitivity for detecting ACR ≥30 mg/g (≥ 3 mg/mmol) and ≥300 mg/g (≥30 mg/mmol), respectively. 47% of individuals who tested + or greater had an ACR ≥30 mg/g (≥ 3 mg/mmol) on laboratory testing.162
Automated devices capable of reading the color changes of reagent strips using reflectance spectrometry are available. These reduce inter-operator variability and improve diagnostic accuracy.150, 158, 163 A creatinine test pad has been added to some reagent strip systems to enable a PCR to be reported and so reduce the intra-individual variation seen with random urine collections. Such devices have been shown to be suitable for ruling out significant proteinuria (>300 mg/24 hours) in an outpatient setting.149
Correcting for urinary dilution. Since creatinine excretion in the urine is fairly constant throughout the 24-hour period, measurement of ACR (or PCR) allows correction for variations in urinary concentration.164, 165 ACR is a suitable alternative to timed measurement of urine albumin loss.143, 166, 167, 168, 169, 170 PCR on random or early morning untimed samples shows good diagnostic performance and correlation with 24-hour collection.160, 163, 171, 172, 173, 174, 175, 176, 177
Expressing albumin as a ratio to creatinine reduces intra-individual variability: lowest variability for the ACR has been reported in EMU samples as opposed to other untimed samples or timed collections.142, 178 In one study albumin variability was reduced from 80% to 52% when expressed as an ACR rather than an albumin concentration.179 The within-subject biological variation for urinary ACR in an EMU has been reported to be 31%, compared to 36% for urinary albumin concentration.180 The same study reported variability for ACR of 103% and 85% in random and timed 24-hour collections, respectively.180 Intra-individual variation for protein loss is also significantly reduced when reported as a PCR compared to protein concentration in random urine samples collected throughout the day (a mean reduction from 97% to 39%).179
Why and how should a finding of albuminuria be confirmed?
Given the high biological variation and other pathological and physiological causes of albuminuria (Table 19),143 repeat testing to confirm albuminuria, ideally using an EMU and laboratory testing, is recommended (Figure 16).
Table 19. Factors affecting urinary ACR.
There has been extensive discussion in the literature about the appropriate urine sample to use for the investigation of protein loss. It is generally recognized that a 24-hour sample is the definitive means of demonstrating the presence of proteinuria. However, overnight, first void in the morning (i.e., EMU), second void in the morning, or random sample collections can also be used. In a systematic review random urine PCR was shown to have better performance as a test for ruling out significant proteinuria than as a “rule-in” test; the authors suggested that positive PCR results may still require confirmation with a 24-hour collection.185 If an EMU is unavailable, subsequent samples can give a reliable indication of the 24-hour urine protein loss.174
International Relevance
The recommendation to replace urinary total protein with albumin as the test of choice in testing for proteinuria is consistent with most,1,31, 130, 186, 187 but not all,188, 189 current national and international guidance. It is accepted that cost pressures may affect implementation of this recommendation and may differ across the world.
Most international guidelines have also discouraged the use of reagent strip analysis for proteinuria detection.186, 189, 190, 191 Nevertheless, in the present guideline we acknowledge that these devices may have a role, particularly in settings where access to laboratory services may be limited.
ACRs in North America tend to be reported in mg/g whereas in other parts of the world usage of mg/mmol predominates. This difference appears unlikely to be resolved in the foreseeable future. When publishing data authors should ensure either that both units are cited or that a conversion factor is provided.
There is increasing adoption of the term ‘albuminuria' instead of microalbuminuria by international and national laboratory and some clinical organizations.
Implications for Clinical Practice and Public Policy
Direct reagent costs of total protein measurement are generally lower than those of albumin measurement, which requires antibody-based reagents. It is often considered that reagent strip analyses are a cheaper option. Therefore some health-care systems may struggle to justify the recommendations in this guideline.
Costs of diagnostic tests vary depending on local financial agreements between hospitals and suppliers. In England, the National Institute for Health and Clinical Excellence (NICE) sampled a small random number of laboratories and estimated the average cost of an ACR to be £2.16 whereas a PCR cost £1.42.186 It is acknowledged that increased use of ACR testing may reduce the unit cost on the basis of economies of scale. In Canada, laboratory analysis costs (Canadian dollars) of $2.81 for reagent strip, $11.67 for PCR, and $29.23 for ACR have been cited.192 In relation to albumin-specific reagent strips, a cost of approximately $4 for a Micral test II (Roche Diagnostics) compared to $2 for a laboratory ACR has been reported.193
The cost- and clinical-effectiveness of an approach utilizing reagent strip testing followed by laboratory measurement compared to an approach in which samples are submitted directly to the laboratory (for either albumin or protein measurement) has recently been evaluated in a health economics model.186 The model favored abandoning the use of reagent strips for identification of proteinuria.
Areas of Controversy, Confusion, or Non-consensus
Some data suggest that ACR is a poorer predictor of 24-hour total protein loss than PCR194 and has no advantage over PCR as a predictor of renal outcomes and mortality in patients with CKD.195, 196 In the prediction of future transplant rejection, PCR has been reported to have equal utility to ACR,192 although in a separate study ACR was found to be a better predictor.197
In the setting of preeclampsia, proteinuria is generally defined as ≥300 mg/24 hours or a PCR ≥300 mg/g (≥30 mg/mmol).175 Currently, there is insufficient evidence to substitute urine albumin measurement for total protein in this setting.172
Creatinine excretion is affected by a variety of non-renal influences (Table 19) and it therefore follows that different cutoffs for ACR (and PCR) may be required in different individuals.194, 198 While age-related cutoffs have not generally been applied in clinical practice, clinicians should bear this in mind when interpreting urine ACR data in older individuals or those with very low body mass, as these will impact the urine creatinine excretion.
While most guidelines agree that an ACR greater than approximately 3 mg/mmol (30 mg/g) is pathological in the setting of diabetes, in the non-diabetic population a higher threshold has commonly been used to define proteinuria. In the NICE guideline in England and Wales, proteinuria in non-diabetic individuals was defined as ≥30 mg/mmol (≥300 mg/g), with higher level proteinuria being >70 mg/mmol (>700 mg/g).186 Confirmation of results lying between 30 and 70 mg/mmol (300-700 mg/g) was recommended.186 The present guideline proposes a lower threshold definition for albuminuria for use in both diabetic and non-diabetic individuals.
A study from Italy in type 2 diabetes has reported that, although intra-individual biological variation of albuminuria is large, a single sample (either ACR or timed collection) can accurately classify patients into albuminuria categories, negating the need for multiple collections.178
Some data suggest that a significant proportion of albumin present in urine may be non-immunoreactive,199, 200, 201, 202 although this finding has been questioned.203, 204
There is a substantial existing literature using the term microalbuminuria and many existing guidelines use this term especially in the context of diabetes and cardiovascular risk, as its presence confers risk. Nonetheless, the Work Group believes that it is important for this international guideline to foster ‘best practices' and clarity of communication, and since the risk of adverse events is continuous throughout the spectrum of albuminuria, we encourage adoption of the term ‘albuminuria' with subsequent quantification of the level or amount.
Pediatric Considerations
For Recommendation 1.4.4.1, this set of statements would need to be altered for application in the pediatric practice as follows:
We suggest using the following measurements for initial testing of proteinuria in children (in descending order of preference):
urine PCR, EMU sample preferred;
urine ACR, EMU sample preferred;
reagent strip urinalysis for total protein with automated reading;
reagent strip urinalysis for total protein with manual reading.
For Recommendations 1.4.4.2 and 1.4.4.3, this set of statements would need to be altered for application in the pediatric practice as follows:
Currently the urinary PCR should be favored over the urine ACR in children. Unlike in adults where powerful evidence exists in support of the use of measures of albumin rather than total protein to predict adverse outcomes, this level of evidence is currently lacking in children.205 However, current longitudinal trials such as CKiD55 and European 4C78 may eventually shed light on this issue.
In children the underlying conditions associated with the diagnosis of CKD are also important considerations as to which form of testing is most valuable. Unlike adults where the majority of patients with CKD are attributed to an underlying glomerular disease or hypertensive damage, the vast majority of children have underlying developmental abnormalities often referred to as CAKUT (congenital anomalies of the kidney and urinary tract).70 This relative paucity of glomerular conditions makes the use of albumin excretion a less sensitive test for diagnostic purposes as many children will have underlying tubular conditions and hence tend to excrete more Tamm-Horsfall protein and other low-molecular-weight proteins that will not be captured by the albumin-to-creatinine (or formal albumin excretion) assay.
For Recommendations 1.4.4.2 and 1.4.4.2.1 this guideline is fully applicable in pediatrics. The recommendation that clinical laboratories report ACR and PCR in untimed urine samples in addition to albumin concentration or proteinuria concentrations rather than the concentrations alone is valid and useful in the pediatric population. As per Recommendation 1.2.4, however, note should be made that age-related normal values for urinary protein losses must be considered when laboratories choose to report either ACR or PCR.
Albuminuria in children, whether measured as an absolute value per day, an excretion rate, or as an albumin to creatinine ratio is fraught with more uncertainity than in adults as they are known to vary across categories of age, sex, height, weight, and Tanner staging.206
In two recent reviews by Rademacher206 and Tsioufis et al.,205 both groups examined the results of all relevant studies on normative values of AER or ACR. Rademacher's paper in particular provides detailed information on the mean AER values (with SD) across a variety of studies, ages, sex, and race, and provides a normative estimate for overnight AER of between 2-6 μg/minute or a 95th percentile value from 4.5–28 μg/minute. Similarly, they summarize results for ACR in normal children and suggest that the mean for children older than 6 years would seem to fall between 8-10 mg/g (0.8-1.0 mg/mmol).
For Recommendation 1.4.4.3, this guideline is fully applicable in pediatrics.
1.4.4.4: If significant non-albumin proteinuria is suspected, use assays for specific urine proteins (e.g., α1-microglobulin, monoclonal heavy or light chains, [known in some countries as “Bence Jones”proteins]). (Not Graded)
RATIONALE
Testing for tubular proteinuria using a total protein approach almost certainly has very poor sensitivity for detecting tubular disease. When an isolated tubular lesion is suspected (Table 3), this is probably best investigated by measuring a specific tubular protein (e.g., α1-microglobulin) using an immunoassay approach.
Evidence Base
There have been concerns that replacing urinary total protein measurement with albumin measurement may cause non-albuminuric (effectively tubular and overproduction) proteinuria to be missed. Low-molecular-weight proteinuria is a defining feature in some uncommon kidney diseases (e.g., Dent's disease).207 However, for some of the reasons already discussed, total protein assays will also be poor at detecting tubular proteinuria. When investigating patients for tubular proteinuria, it is advisable to use assays targeted at specific tubular proteins.
In the AusDiab study, of those with proteinuria (2.4% of the general population, defined as a PCR >23 mg/mmol [230 mg/g]) 92% had albuminuria (defined as an ACR >3.4 mg/mmol [34 mg/g]); 8% had an ACR within the reference range.208 These individuals were less likely to have diabetes than those with both proteinuria and albuminuria, but no further information is available as to the nature of the proteinuria in these individuals or its likely significance. The authors speculate that these individuals could have had light chain proteinuria or interstitial nephropathies. Using albuminuria testing to identify proteinuria had a specificity of 95%. The negative predictive value was 99.8% and the positive predictive value was 32.4%. The authors concluded that testing for albuminuria rather than proteinuria was supported.
As discussed above, quite significant increases in urinary albumin loss have to occur before such an increase is detectable on the background of a total protein assay. The situation is even more extreme for tubular proteins which, in health, are present in urine at lower concentrations than albumin (e.g., normal daily losses of retinol binding protein, α1-microglobulin and β2-microglobulin are 0.08, 3.6, and 0.1 mg/d, respectively).209 This problem will be exacerbated by the fact that the recognition of tubular proteins by some total protein assays is poor.210
In disease states concentrations of tubular proteins, at least collectively, can reach levels detectable by total protein assays. For example, among patients with tubulointerstitial disease but without renal insufficiency, median concentrations of α1-microglobulin were 37 mg/l, with concentrations up to 100 mg/l being observed; higher concentrations were seen in patients with decreased GFR.211 Among a group of patients with acute tubular necrosis requiring dialysis treatment, median α1-microglobulin concentration was 35 mg/mmol of creatinine.212 However, although tubular proteinuria is characterized by a relative increase in low-molecular-weight protein concentrations, generally albumin still remains a significant component of the total protein concentration. Indeed, it is thought that tubular disease results in an increase in albumin loss as a result of decreased tubular reabsorption of filtered albumin. For example, it has been estimated that when tubular absorption fails completely, β2-microglobulin loss increases to 180 mg/24 hours (approximately 1800-fold normal) but there will also be an increase in urinary albumin loss to about 360 mg/24 hours (approximately 20-fold normal).209 In a series of patients with Dent's disease, a classical tubular disorder, 21 of the 23 patients demonstrating increased urinary α1-microglobulin and β2-microglobulin loss also had increased urinary albumin loss: those who did not had borderline increases in tubular protein losses that would not have been detectable using a total protein measurement approach.207 The authors comment that in those patients in whom proteinuria was marked (>1 g/d), urinary albumin loss was also markedly increased. In some situations, however, tubular proteinuria in the absence of albuminuria has been reported (e.g., in some children with type 1 diabetes213 and in kidney scarring in reflux nephropathy214).
International Relevance
There is no reason to believe that there are significant differences around the world with respect to incidence or prevalence of conditions in which measurement of non-albumin proteins would be required. The availability of reliable tests for these alternative proteins, however, may be different in different regions.
Implications for Clinical Practice and Public Policy
The incidence and prevalence of tubular disorders will vary geographically with the clinical setting (e.g., adult or pediatric practice) and factors such as occupational exposure. Clinicians should agree with their local laboratories a suitable approach to the detection of tubular proteinuria and laboratories should be able to advise on suitable sample handling procedures. It is acknowledged that many laboratories do not currently offer assays of tubular proteins.
In patients with suspected myeloma, monoclonal heavy or light chains (known in some countries as Bence Jones) protein should be sought in concentrated urine using electrophoresis with immunofixation of any identified protein bands in accordance with current myeloma guidelines.215 Simultaneous albumin measurement is needed when the possibility of immunoglobulin light chain (AL) amyloid or light chain deposition disease is suspected.
Non-albumin proteinuria may also be suspected in patients with disorders of tubular function (see Table 3).
Areas of Controversy, Confusion, or Non-consensus
Testing for proteinuria using a urine albumin rather than total protein first-line approach may occasionally miss cases of tubular proteinuria but the significance of this problem is probably overestimated and should be the subject of further research.
Earlier guidance from KDOQI1 suggested that proteinuria in children should be detected with total protein rather than albumin assays due to the higher prevalence of non-glomerular diseases in this group of patients. For the reasons outlined above, we do not think total protein assays are suitable for this purpose and would ideally recommend testing for albumin and for specific tubular proteins when non-glomerular disease is suspected.
Pediatric Considerations
For Recommendation 1.4.4.4, this statement is fully applicable in pediatrics. In children the likelihood of any form of overflow proteinuria such as seen in conditions of heavy or light chain production is extremely low; however a significant number of underlying genetic tubular disorders do exist and protein electrophoresis can assist the practitioner in determining the presence of such a condition or the concurrent finding of severe tubular injury in addition to a glomerular condition.
DISCLAIMER
While every effort is made by the publishers, editorial board, and ISN to see that no inaccurate or misleading data, opinion or statement appears in this Journal, they wish to make it clear that the data and opinions appearing in the articles and advertisements herein are the responsibility of the contributor, copyright holder, or advertiser concerned. Accordingly, the publishers and the ISN, the editorial board and their respective employers, office and agents accept no liability whatsoever for the consequences of any such inaccurate or misleading data, opinion or statement. While every effort is made to ensure that drug doses and other quantities are presented accurately, readers are advised that new methods and techniques involving drug usage, and described within this Journal, should only be followed in conjunction with the drug manufacturer's own published literature.
Footnotes
SUPPLEMENTARY MATERIAL
Supplemental Table 1: Search strategy.
Supplemental Table 2: Equations based on serum creatinine assays in adults that are not traceable to the standard reference material.
Supplemental Table 3: Equations based on serum cystatin C assays in adults that are not traceable to standard reference material.
Supplementary material is linked to the online version of the paper at http://www.kdigo.org/clinical_practice_guidelines/ckd.php
References
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Ordonez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailpern SM, Melamed ML, Cohen HW, et al. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2007;18:2205–2213. doi: 10.1681/ASN.2006101165. [DOI] [PubMed] [Google Scholar]
- James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376:2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- James MT, Quan H, Tonelli M, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis. 2009;54:24–32. doi: 10.1053/j.ajkd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Wilhelm-Leen ER, Hall YN, M KT, et al. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey Am J Med 2009122664–671.e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- Wesson L. Physiology of the human kidney. Grune & Stratton: New York; 1969. [Google Scholar]
- Rowe JW, Andres R, Tobin JD. Letter: Age-adjusted standards for creatinine clearance. Ann Intern Med. 1976;84:567–569. doi: 10.7326/0003-4819-84-5-567. [DOI] [PubMed] [Google Scholar]
- Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75:1079–1087. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barai S, Gambhir S, Prasad N, et al. Levels of GFR and protein-induced hyperfiltration in kidney donors: a single-center experience in India. Am J Kidney Dis. 2008;51:407–414. doi: 10.1053/j.ajkd.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Eastwood JB, Kerry SM, Plange-Rhule J, et al. Assessment of GFR by four methods in adults in Ashanti, Ghana: the need for an eGFR equation for lean African populations. Nephrol Dial Transplant. 2010;25:2178–2187. doi: 10.1093/ndt/gfp765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar TH, Islam M, Jessani S, et al. Level and determinants of kidney function in a South Asian population in Pakistan. Am J Kidney Dis. 2011;58:764–772. doi: 10.1053/j.ajkd.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Greene T, et al. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KDIGO Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- Eckardt KU, Berns JS, Rocco MV, et al. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Eknoyan G. Chronic kidney disease definition and classification: no need for a rush to judgment. Kidney Int. 2009;75:1015–1018. doi: 10.1038/ki.2009.53. [DOI] [PubMed] [Google Scholar]
- El Nahas M. Cardio-Kidney-Damage: a unifying concept. Kidney Int. 2010;78:14–18. doi: 10.1038/ki.2010.123. [DOI] [PubMed] [Google Scholar]
- Levey AS, Astor BC, Stevens LA, et al. Chronic kidney disease, diabetes, and hypertension: what's in a name. Kidney Int. 2010;78:19–22. doi: 10.1038/ki.2010.115. [DOI] [PubMed] [Google Scholar]
- Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75:1009–1014. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- Silva FG. The aging kidney: a review -- part I. Int Urol Nephrol. 2005;37:185–205. doi: 10.1007/s11255-004-0873-6. [DOI] [PubMed] [Google Scholar]
- Silva FG. The aging kidney: a review--part II. Int Urol Nephrol. 2005;37:419–432. doi: 10.1007/s11255-004-0874-5. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17:302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Levey AS. Dietary protein and renal function. J Am Soc Nephrol. 1993;3:1723–1737. doi: 10.1681/ASN.V3111723. [DOI] [PubMed] [Google Scholar]
- Vehaskari VM. Orthostatic proteinuria. Arch Dis Child. 1982;57:729–730. doi: 10.1136/adc.57.10.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seikaly MG, Ho PL, Emmett L, et al. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatr Nephrol. 2003;18:796–804. doi: 10.1007/s00467-003-1158-5. [DOI] [PubMed] [Google Scholar]
- Hogg RJ, Furth S, Lemley KV, et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111:1416–1421. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- Aperia A, Broberger O, Elinder G, et al. Postnatal development of renal function in pre-term and full-term infants. Acta Paediatr Scand. 1981;70:183–187. doi: 10.1111/j.1651-2227.1981.tb05539.x. [DOI] [PubMed] [Google Scholar]
- Bueva A, Guignard JP. Renal function in preterm neonates. Pediatr Res. 1994;36:572–577. doi: 10.1203/00006450-199411000-00005. [DOI] [PubMed] [Google Scholar]
- Fetterman GH, Shuplock NA, Philipp FJ, et al. The Growth and Maturation of Human Glomeruli and Proximal Convolutions from Term to Adulthood: Studies by Microdissection. Pediatrics. 1965;35:601–619. [PubMed] [Google Scholar]
- Guignard JP, Torrado A, Da Cunha O, et al. Glomerular filtration rate in the first three weeks of life. J Pediatr. 1975;87:268–272. doi: 10.1016/s0022-3476(75)80600-7. [DOI] [PubMed] [Google Scholar]
- Haycock GB. Development of glomerular filtration and tubular sodium reabsorption in the human fetus and newborn. Br J Urol. 1998;81 Suppl 2:33–38. doi: 10.1046/j.1464-410x.1998.0810s2033.x. [DOI] [PubMed] [Google Scholar]
- Gallini F, Maggio L, Romagnoli C, et al. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol. 2000;15:119–124. doi: 10.1007/s004670000356. [DOI] [PubMed] [Google Scholar]
- Vieux R, Hascoet JM, Merdariu D, et al. Glomerular filtration rate reference values in very preterm infants. Pediatrics. 2010;125:e1186–e1192. doi: 10.1542/peds.2009-1426. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–1848. doi: 10.1007/s00467-006-0358-1. [DOI] [PubMed] [Google Scholar]
- Waters AM.Chapter 6, Part 2: Functional development of the nephronIn: Geary DF, Schaefer F (eds)Comprehensive Pediatric Nephrology Mosby Elsevier: Philadelphia, PA; 2008111–129. [Google Scholar]
- Langlois V.Laboratory evaluation at different agesIn: Geary DF, Schaefer F (eds)Comprehensive Pediatric Nephrology Mosby Elsevier: Philadelphia, PA; 200839–54. [Google Scholar]
- Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelovitch L, Warady BA, Furth SL. Insights from the Chronic Kidney Disease in Children (CKiD) study. Clin J Am Soc Nephrol. 2011;6:2047–2053. doi: 10.2215/CJN.10751210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seliger SL, Zhan M, Hsu VD, et al. Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol. 2008;19:2414–2419. doi: 10.1681/ASN.2008010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- Burgert TS, Dziura J, Yeckel C, et al. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond) 2006;30:273–280. doi: 10.1038/sj.ijo.0803136. [DOI] [PubMed] [Google Scholar]
- Csernus K, Lanyi E, Erhardt E, et al. Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur J Pediatr. 2005;164:44–49. doi: 10.1007/s00431-004-1546-2. [DOI] [PubMed] [Google Scholar]
- Houser MT, Jahn MF, Kobayashi A, et al. Assessment of urinary protein excretion in the adolescent: effect of body position and exercise. J Pediatr. 1986;109:556–561. doi: 10.1016/s0022-3476(86)80143-3. [DOI] [PubMed] [Google Scholar]
- Trachtenberg F, Barregard L. The effect of age, sex, and race on urinary markers of kidney damage in children. Am J Kidney Dis. 2007;50:938–945. doi: 10.1053/j.ajkd.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Brem AS. Neonatal hematuria and proteinuria. Clin Perinatol. 1981;8:321–332. [PubMed] [Google Scholar]
- Hogg RJ, Portman RJ, Milliner D, et al. Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE) Pediatrics. 2000;105:1242–1249. doi: 10.1542/peds.105.6.1242. [DOI] [PubMed] [Google Scholar]
- Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J. Should the K/DOQI definition of chronic kidney disease be changed. Am J Kidney Dis. 2003;42:626–630. doi: 10.1016/s0272-6386(03)00827-8. [DOI] [PubMed] [Google Scholar]
- Uhlig K, Levey AS. Developing guidelines for chronic kidney disease: we should include all of the outcomes. Ann Intern Med. 2012;156:599–601. doi: 10.7326/0003-4819-156-8-201204170-00009. [DOI] [PubMed] [Google Scholar]
- NAPRTCS 2008 Annual Report. ( https://web.emmes.com/study/ped/annlrept/Annual%20Report%20-2008.pdf ). Accessed September 7, 2012.
- Ardissino G, Dacco V, Testa S, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111:e382–e387. doi: 10.1542/peds.111.4.e382. [DOI] [PubMed] [Google Scholar]
- Pierce CB, Cox C, Saland JM, et al. Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol. 2011;174:604–612. doi: 10.1093/aje/kwr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth SL, Abraham AG, Jerry-Fluker J, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2132–2140. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingen AM, Fabian-Bach C, Schaefer F, et al. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet. 1997;349:1117–1123. doi: 10.1016/s0140-6736(96)09260-4. [DOI] [PubMed] [Google Scholar]
- Staples AO, Greenbaum LA, Smith JM, et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010;5:2172–2179. doi: 10.2215/CJN.07851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardissino G, Testa S, Dacco V, et al. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol. 2004;19:172–177. doi: 10.1007/s00467-003-1268-0. [DOI] [PubMed] [Google Scholar]
- Wong CS, Pierce CB, Cole SR, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–819. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardissino G, Testa S, Dacco V, et al. Puberty is associated with increased deterioration of renal function in patients with CKD: data from the ItalKid Project. Arch Dis Child. 2012. [DOI] [PubMed]
- Querfeld U, Anarat A, Bayazit AK, et al. The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol. 2010;5:1642–1648. doi: 10.2215/CJN.08791209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- Miller WG. Estimating glomerular filtration rate. Clin Chem Lab Med. 2009;47:1017–1019. doi: 10.1515/CCLM.2009.264. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Verrill H. A national audit of estimated glomerular filtration rate and proteinuria reporting in the UK. Ann Clin Biochem. 2011;48:558–561. doi: 10.1258/acb.2011.011083. [DOI] [PubMed] [Google Scholar]
- McIntosh JF, Moller E, Van Slyke DD. Studies of urea excretion. III: The influence of body size on urea output. J Clin Invest. 1928;6:467–483. doi: 10.1172/JCI100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley A, Miskulin D, Lamb EJ, et al. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156:785–795. doi: 10.7326/0003-4819-156-11-201203200-00391. [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio M, Imai E, Yasuda Y, et al. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41–50. doi: 10.1007/s10157-006-0453-4. [DOI] [PubMed] [Google Scholar]
- Praditpornsilpa K, Townamchai N, Chaiwatanarat T, et al. The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol Dial Transplant. 2011;26:2780–2785. doi: 10.1093/ndt/gfq815. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Levey AS, Greene T, Kusek J, et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Murata K, Baumann NA, Saenger AK, et al. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol. 2011;6:1963–1972. doi: 10.2215/CJN.02300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BR, Demirjian S, Weight CJ, et al. Performance of the chronic kidney disease-epidemiology study equations for estimating glomerular filtration rate before and after nephrectomy. J Urol. 2010;183:896–901. doi: 10.1016/j.juro.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Michels WM, Grootendorst DC, Verduijn M, et al. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tent H, Rook M, Stevens LA, et al. Renal function equations before and after living kidney donation: a within-individual comparison of performance at different levels of renal function. Clin J Am Soc Nephrol. 2010;5:1960–1968. doi: 10.2215/CJN.08761209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukla A, El-Shahawi Y, Leister E, et al. GFR-estimating models in kidney transplant recipients on a steroid-free regimen. Nephrol Dial Transplant. 2010;25:1653–1661. doi: 10.1093/ndt/gfp668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CA, Akbari A, Doucette S, et al. Estimating glomerular filtration rate in kidney transplantation: is the new chronic kidney disease epidemiology collaboration equation any better. Clin Chem. 2010;56:474–477. doi: 10.1373/clinchem.2009.135111. [DOI] [PubMed] [Google Scholar]
- Poge U, Gerhardt T, Stoffel-Wagner B, et al. Validation of the CKD-EPI formula in patients after renal transplantation. Nephrol Dial Transplant. 2011;26:4104–4108. doi: 10.1093/ndt/gfr183. [DOI] [PubMed] [Google Scholar]
- Jones GR, Imam SK. Validation of the revised MDRD formula and the original Cockcroft and Gault formula for estimation of the glomerular filtration rate using Australian data. Pathology. 2009;41:379–382. doi: 10.1080/00313020902884980. [DOI] [PubMed] [Google Scholar]
- Jones GR. Use of the CKD-EPI equation for estimation of GFR in an Australian cohort. Pathology. 2010;42:487–488. doi: 10.3109/00313025.2010.494291. [DOI] [PubMed] [Google Scholar]
- Cirillo M, Lombardi C, Luciano MG, et al. Estimation of GFR: a comparison of new and established equations. Am J Kidney Dis. 2010;56:802–804. doi: 10.1053/j.ajkd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Eriksen BO, Mathisen UD, Melsom T, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78:1305–1311. doi: 10.1038/ki.2010.321. [DOI] [PubMed] [Google Scholar]
- Redal-Baigorri B, Stokholm KH, Rasmussen K, et al. Estimation of kidney function in cancer patients. Dan Med Bull. 2011;58:A4236. [PubMed] [Google Scholar]
- Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule AD, Teo BW. GFR estimation in Japan and China: what accounts for the difference. Am J Kidney Dis. 2009;53:932–935. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo Y, Han DJ, Moon DH, et al. Suitability of the IDMS-traceable MDRD equation method to estimate GFR in early postoperative renal transplant recipients. Nephron Clin Pract. 2010;114:c108–c117. doi: 10.1159/000254383. [DOI] [PubMed] [Google Scholar]
- van Deventer HE, George JA, Paiker JE, et al. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin Chem. 2008;54:1197–1202. doi: 10.1373/clinchem.2007.099085. [DOI] [PubMed] [Google Scholar]
- Teo BW, Xu H, Wang D, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis. 2011;58:56–63. doi: 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012. [DOI] [PMC free article] [PubMed]
- Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappitelli M, Parvex P, Joseph L, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula. Pediatr Nephrol. 2003;18:981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56:39–49. doi: 10.1053/j.ajkd.2010.02.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb EJ, Price CP.Kidney function tests Tietz Textbook of Clinical Chemistry and Molecular Diagnosticseds Burtis CA, Ashwood E, Bruns DE.Elsevier; 5th edition2012669–708. [Google Scholar]
- Ballantyne FC, Gibbons J, O'Reilly DS. Urine albumin should replace total protein for the assessment of glomerular proteinuria. Ann Clin Biochem. 1993;30 ( Pt 1:101–103. doi: 10.1177/000456329303000119. [DOI] [PubMed] [Google Scholar]
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured. Ann Clin Biochem. 2009;46:205–217. doi: 10.1258/acb.2009.009007. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Thakkar H, Medcalf EA, et al. Use of urine albumin measurement as a replacement for total protein. Clin Nephrol. 1995;43:104–109. [PubMed] [Google Scholar]
- Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsma AH, Bakker SJ, Hillege HL, et al. Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008;23:3851–3858. doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]
- Dawnay A, Wilson AG, Lamb E, et al. Microalbuminuria in systemic sclerosis. Ann Rheum Dis. 1992;51:384–388. doi: 10.1136/ard.51.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabi ZK, Konen JC, O'Connor ML. Albuminuria vs urinary total protein for detecting chronic renal disorders. Clin Chem. 1991;37:621–624. [PubMed] [Google Scholar]
- Martin H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin Biochem Rev. 2011;32:97–102. [PMC free article] [PubMed] [Google Scholar]
- Waugh J, Bell SC, Kilby M, et al. Effect of concentration and biochemical assay on the accuracy of urine dipsticks in hypertensive pregnancies. Hypertens Pregnancy. 2001;20:205–217. doi: 10.1081/PRG-100106970. [DOI] [PubMed] [Google Scholar]
- Waugh J, Bell SC, Kilby MD, et al. Urine protein estimation in hypertensive pregnancy: which thresholds and laboratory assay best predict clinical outcome. Hypertens Pregnancy. 2005;24:291–302. doi: 10.1080/10641950500281019. [DOI] [PubMed] [Google Scholar]
- McElderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein compared. Clin Chem. 1982;28:356–360. [PubMed] [Google Scholar]
- Nishi HH, Elin RJ. Three turbidimetric methods for determining total protein compared. Clin Chem. 1985;31:1377–1380. [PubMed] [Google Scholar]
- Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977;79:544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- de Keijzer MH, Klasen IS, Branten AJ, et al. Infusion of plasma expanders may lead to unexpected results in urinary protein assays. Scand J Clin Lab Invest. 1999;59:133–137. doi: 10.1080/00365519950185869. [DOI] [PubMed] [Google Scholar]
- Marshall T, Williams KM. Extent of aminoglycoside interference in the pyrogallol red-molybdate protein assay depends on the concentration of sodium oxalate in the dye reagent. Clin Chem. 2004;50:934–935. doi: 10.1373/clinchem.2003.030478. [DOI] [PubMed] [Google Scholar]
- Yilmaz FM, Yucel D. Effect of addition of hemolysate on urine and cerebrospinal fluid assays for protein. Clin Chem. 2006;52:152–153. doi: 10.1373/clinchem.2005.057547. [DOI] [PubMed] [Google Scholar]
- Chambers RE, Bullock DG, Whicher JT. External quality assessment of total urinary protein estimation in the United Kingdom. Ann Clin Biochem. 1991;28 (Pt 5:467–473. doi: 10.1177/000456329102800508. [DOI] [PubMed] [Google Scholar]
- Heick HM, Begin-Heick N, Acharya C, et al. Automated determination of urine and cerebrospinal fluid proteins with Coomassie Brilliant Blue and the Abbott ABA-100. Clin Biochem. 1980;13:81–83. doi: 10.1016/s0009-9120(80)91243-6. [DOI] [PubMed] [Google Scholar]
- Marshall T, Williams KM. Total protein determination in urine: elimination of a differential response between the coomassie blue and pyrogallol red protein dye-binding assays. Clin Chem. 2000;46:392–398. [PubMed] [Google Scholar]
- Miller WG. Urine albumin: Recommendations for standardization. Scand J Clin Lab Invest Suppl. 2008;241:71–72. doi: 10.1080/00365510802150125. [DOI] [PubMed] [Google Scholar]
- Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare products Regulatory Agency. MHAR 04086 Point of care devices for the quantitation of microalbuminuria2004
- Medicines and Healthcare products Regulatory Agency. MHRA 04098. Point of care devices for the detection and semi-quantitation of microalbuminuria.2004
- Parsons M, Newman DJ, Pugia M, et al. Performance of a reagent strip device for quantitation of the urine albumin: creatinine ratio in a point of care setting. Clin Nephrol. 1999;51:220–227. [PubMed] [Google Scholar]
- Parsons MP, Newman DJ, Newall RG, et al. Validation of a point-of-care assay for the urinary albumin:creatinine ratio. Clin Chem. 1999;45:414–417. [PubMed] [Google Scholar]
- Graziani MS, Gambaro G, Mantovani L, et al. Diagnostic accuracy of a reagent strip for assessing urinary albumin excretion in the general population. Nephrol Dial Transplant. 2009;24:1490–1494. doi: 10.1093/ndt/gfn639. [DOI] [PubMed] [Google Scholar]
- Guy M, Newall R, Borzomato J, et al. Diagnostic accuracy of the urinary albumin: creatinine ratio determined by the CLINITEK Microalbumin and DCA 2000+ for the rule-out of albuminuria in chronic kidney disease. Clin Chim Acta. 2009;399:54–58. doi: 10.1016/j.cca.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Waugh JJ, Bell SC, Kilby MD, et al. Optimal bedside urinalysis for the detection of proteinuria in hypertensive pregnancy: a study of diagnostic accuracy. BJOG. 2005;112:412–417. doi: 10.1111/j.1471-0528.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- Iseki K, Iseki C, Ikemiya Y, et al. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49:800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100:919–921. doi: 10.1542/peds.100.6.919. [DOI] [PubMed] [Google Scholar]
- Kitagawa T. Lessons learned from the Japanese nephritis screening study. Pediatr Nephrol. 1988;2:256–263. doi: 10.1007/BF00862602. [DOI] [PubMed] [Google Scholar]
- Boulware LE, Jaar BG, Tarver-Carr ME, et al. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- Bowie L, Smith S, Gochman N. Characteristics of binding between reagent-strip indicators and urinary proteins. Clin Chem. 1977;23:128–130. [PubMed] [Google Scholar]
- Gyure WL. Comparison of several methods for semiquantitative determination of urinary protein. Clin Chem. 1977;23:876–879. [PubMed] [Google Scholar]
- James GP, Bee DE, Fuller JB. Proteinuria: accuracy and precision of laboratory diagnosis by dip-stick analysis. Clin Chem. 1978;24:1934–1939. [PubMed] [Google Scholar]
- Rumley A. Urine dipstick testing: comparison of results obtained by visual reading and with the Bayer CLINITEK 50. Ann Clin Biochem. 2000;37 (Pt 2:220–221. doi: 10.1258/0004563001899041. [DOI] [PubMed] [Google Scholar]
- Scotti da Silva-Colombeli A, Falkenberg M. Analytical interferences of drugs in the chemical examination of urinary protein. Clin Biochem. 2007;40:1074–1076. doi: 10.1016/j.clinbiochem.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Ralston SH, Caine N, Richards I, et al. Screening for proteinuria in a rheumatology clinic: comparison of dipstick testing, 24 h urine quantitative protein, and protein/creatinine ratio in random urine samples. Ann Rheum Dis. 1988;47:759–763. doi: 10.1136/ard.47.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh JJ, Clark TJ, Divakaran TG, et al. Accuracy of urinalysis dipstick techniques in predicting significant proteinuria in pregnancy. Obstet Gynecol. 2004;103:769–777. doi: 10.1097/01.AOG.0000118311.18958.63. [DOI] [PubMed] [Google Scholar]
- White SL, Yu R, Craig JC, et al. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis. 2011;58:19–28. doi: 10.1053/j.ajkd.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Saudan PJ, Brown MA, Farrell T, et al. Improved methods of assessing proteinuria in hypertensive pregnancy. Br J Obstet Gynaecol. 1997;104:1159–1164. doi: 10.1111/j.1471-0528.1997.tb10940.x. [DOI] [PubMed] [Google Scholar]
- Beetham R, Cattell WR. Proteinuria: pathophysiology, significance and recommendations for measurement in clinical practice. Ann Clin Biochem. 1993;30 (Pt 5:425–434. doi: 10.1177/000456329303000502. [DOI] [PubMed] [Google Scholar]
- Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- Claudi T, Cooper JG. Comparison of urinary albumin excretion rate in overnight urine and albumin creatinine ratio in spot urine in diabetic patients in general practice. Scand J Prim Health Care. 2001;19:247–248. doi: 10.1080/02813430152706774. [DOI] [PubMed] [Google Scholar]
- Gatling W, Knight C, Mullee MA, et al. Microalbuminuria in diabetes: a population study of the prevalence and an assessment of three screening tests. Diabet Med. 1988;5:343–347. doi: 10.1111/j.1464-5491.1988.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Hutchison AS, O'Reilly DS, MacCuish AC. Albumin excretion rate, albumin concentration, and albumin/creatinine ratio compared for screening diabetics for slight albuminuria. Clin Chem. 1988;34:2019–2021. [PubMed] [Google Scholar]
- Marshall SM. Screening for microalbuminuria: which measurement. Diabet Med. 1991;8:706–711. doi: 10.1111/j.1464-5491.1991.tb01688.x. [DOI] [PubMed] [Google Scholar]
- Marshall SM, Alberti KG. Screening for early diabetic nephropathy. Ann Clin Biochem. 1986;23 (Pt 2:195–197. doi: 10.1177/000456328602300209. [DOI] [PubMed] [Google Scholar]
- Chitalia VC, Kothari J, Wells EJ, et al. Cost-benefit analysis and prediction of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin Nephrol. 2001;55:436–447. [PubMed] [Google Scholar]
- Cote AM, Brown MA, Lam E, et al. Diagnostic accuracy of urinary spot protein:creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ. 2008;336:1003–1006. doi: 10.1136/bmj.39532.543947.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson EH, Will EJ, Davison AM, et al. Use of the urinary protein creatinine index to assess proteinuria in renal transplant patients. Nephrol Dial Transplant. 1992;7:450–452. [PubMed] [Google Scholar]
- Ginsberg JM, Chang BS, Matarese RA, et al. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- Leanos-Miranda A, Marquez-Acosta J, Romero-Arauz F, et al. Protein:creatinine ratio in random urine samples is a reliable marker of increased 24-hour protein excretion in hospitalized women with hypertensive disorders of pregnancy. Clin Chem. 2007;53:1623–1628. doi: 10.1373/clinchem.2007.089334. [DOI] [PubMed] [Google Scholar]
- Lemann J, Jr, Doumas BT. Proteinuria in health and disease assessed by measuring the urinary protein/creatinine ratio. Clin Chem. 1987;33:297–299. [PubMed] [Google Scholar]
- Ruggenenti P, Gaspari F, Perna A, et al. Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 h urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ. 1998;316:504–509. doi: 10.1136/bmj.316.7130.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese G, Solini A, Fondelli C, et al. Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency And Cardiovascular Events (RIACE) study. Nephrol Dial Transplant. 2011;26:3950–3954. doi: 10.1093/ndt/gfr140. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Pugia MJ, Lott JA, et al. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta. 2000;294:139–155. doi: 10.1016/s0009-8981(00)00181-9. [DOI] [PubMed] [Google Scholar]
- Howey JE, Browning MC, Fraser CG. Selecting the optimum specimen for assessing slight albuminuria, and a strategy for clinical investigation: novel uses of data on biological variation. Clin Chem. 1987;33:2034–2038. [PubMed] [Google Scholar]
- Carter JL, Tomson CR, Stevens PE, et al. Does urinary tract infection cause proteinuria or microalbuminuria? A systematic review. Nephrol Dial Transplant. 2006;21:3031–3037. doi: 10.1093/ndt/gfl373. [DOI] [PubMed] [Google Scholar]
- Heathcote KL, Wilson MP, Quest DW, et al. Prevalence and duration of exercise induced albuminuria in healthy people. Clin Invest Med. 2009;32:E261–E265. doi: 10.25011/cim.v32i4.6616. [DOI] [PubMed] [Google Scholar]
- Leung AK, Wong AH. Proteinuria in children. Am Fam Physician. 2010;82:645–651. [PubMed] [Google Scholar]
- Boger CA, Chen MH, Tin A, et al. CUBN is a gene locus for albuminuria. J Am Soc Nephrol. 2011;22:555–570. doi: 10.1681/ASN.2010060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CP, Newall RG, Boyd JC. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem. 2005;51:1577–1586. doi: 10.1373/clinchem.2005.049742. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. NICE clinical guideline 73. Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care2008
- Montanes Bermudez R, Gracia Garcia S, Perez Surribas D, et al. Consensus document. Recommendations on assessing proteinuria during the diagnosis and follow-up of chronic kidney disease. Nefrologia. 2011;31:331–345. doi: 10.3265/Nefrologia.pre2011.Jan.10807. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Jones GR, Mathew TH, et al. Chronic kidney disease and measurement of albuminuria or proteinuria: a position statement. Med J Aust. 2012;197:224–225. doi: 10.5694/mja11.11468. [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network. Guideline 103. Diagnosis and management of chronic kidney disease2008
- Caring for Australasians with Renal Impairment http://www.cari.org.au/guidelines.php .
- Clarke W, Frost SJ, Kraus E, et al. Renal function testingIn: Nichols JH (ed)Evidence-based Practice for Point-of-Care Testing National Academy of Clinical Biochemistry; 2006126–134. [Google Scholar]
- Panek R, Lawen T, Kiberd BA. Screening for proteinuria in kidney transplant recipients. Nephrol Dial Transplant. 2011;26:1385–1387. doi: 10.1093/ndt/gfq503. [DOI] [PubMed] [Google Scholar]
- Incerti J, Zelmanovitz T, Camargo JL, et al. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant. 2005;20:2402–2407. doi: 10.1093/ndt/gfi074. [DOI] [PubMed] [Google Scholar]
- Methven S, MacGregor MS, Traynor JP, et al. Assessing proteinuria in chronic kidney disease: protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant. 2010;25:2991–2996. doi: 10.1093/ndt/gfq140. [DOI] [PubMed] [Google Scholar]
- Methven S, MacGregor MS, Traynor JP, et al. Comparison of urinary albumin and urinary total protein as predictors of patient outcomes in CKD. Am J Kidney Dis. 2011;57:21–28. doi: 10.1053/j.ajkd.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Methven S, Traynor JP, Hair MD, et al. Stratifying risk in chronic kidney disease: an observational study of UK guidelines for measuring total proteinuria and albuminuria. QJM. 2011;104:663–670. doi: 10.1093/qjmed/hcr026. [DOI] [PubMed] [Google Scholar]
- Nauta FL, Bakker SJ, van Oeveren W, et al. Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;57:733–743. doi: 10.1053/j.ajkd.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Ellam TJ. Albumin:creatinine ratio--a flawed measure? The merits of estimated albuminuria reporting. Nephron Clin Pract. 2011;118:c324–c330. doi: 10.1159/000323670. [DOI] [PubMed] [Google Scholar]
- Comper WD, Osicka TM, Clark M, et al. Earlier detection of microalbuminuria in diabetic patients using a new urinary albumin assay. Kidney Int. 2004;65:1850–1855. doi: 10.1111/j.1523-1755.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- Comper WD, Osicka TM, Jerums G. High prevalence of immuno-unreactive intact albumin in urine of diabetic patients. Am J Kidney Dis. 2003;41:336–342. doi: 10.1053/ajkd.2003.50041. [DOI] [PubMed] [Google Scholar]
- Magliano DJ, Polkinghorne KR, Barr EL, et al. HPLC-detected albuminuria predicts mortality. J Am Soc Nephrol. 2007;18:3171–3176. doi: 10.1681/ASN.2007030359. [DOI] [PubMed] [Google Scholar]
- Osicka TM, Comper WD. Characterization of immunochemically nonreactive urinary albumin. Clin Chem. 2004;50:2286–2291. doi: 10.1373/clinchem.2004.039743. [DOI] [PubMed] [Google Scholar]
- Sviridov D, Drake SK, Hortin GL. Reactivity of urinary albumin (microalbumin) assays with fragmented or modified albumin. Clin Chem. 2008;54:61–68. doi: 10.1373/clinchem.2007.092825. [DOI] [PubMed] [Google Scholar]
- Sviridov D, Meilinger B, Drake SK, et al. Coelution of other proteins with albumin during size-exclusion HPLC: Implications for analysis of urinary albumin. Clin Chem. 2006;52:389–397. doi: 10.1373/clinchem.2005.057323. [DOI] [PubMed] [Google Scholar]
- Tsioufis C, Mazaraki A, Dimitriadis K, et al. Microalbuminuria in the paediatric age: current knowledge and emerging questions. Acta Paediatr. 2011;100:1180–1184. doi: 10.1111/j.1651-2227.2011.02291.x. [DOI] [PubMed] [Google Scholar]
- Rademacher ER, Sinaiko AR. Albuminuria in children. Curr Opin Nephrol Hypertens. 2009;18:246–251. doi: 10.1097/MNH.0b013e3283294b98. [DOI] [PubMed] [Google Scholar]
- Wrong OM, Norden AG, Feest TG. Dent's disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. QJM. 1994;87:473–493. [PubMed] [Google Scholar]
- Atkins RC, Briganti EM, Zimmet PZ, et al. Association between albuminuria and proteinuria in the general population: the AusDiab Study. Nephrol Dial Transplant. 2003;18:2170–2174. doi: 10.1093/ndt/gfg314. [DOI] [PubMed] [Google Scholar]
- Gosling P.In: Marshall WJ, Bangert SK (eds)Clinical Biochemistry: Metabolic and Clinical Aspects2nd Ed.Elsevier; 2008156–173. [Google Scholar]
- Goren MP, Li JT. The Coomassie Brilliant Blue method underestimates drug-induced tubular proteinuria. Clin Chem. 1986;32:386–388. [PubMed] [Google Scholar]
- Weber MH, Verwiebe R. Alpha 1-microglobulin (protein HC): features of a promising indicator of proximal tubular dysfunction. Eur J Clin Chem Clin Biochem. 1992;30:683–691. [PubMed] [Google Scholar]
- Herget-Rosenthal S, Poppen D, Husing J, et al. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004;50:552–558. doi: 10.1373/clinchem.2003.027763. [DOI] [PubMed] [Google Scholar]
- Ginevri F, Piccotti E, Alinovi R, et al. Reversible tubular proteinuria precedes microalbuminuria and correlates with the metabolic status in diabetic children. Pediatr Nephrol. 1993;7:23–26. doi: 10.1007/BF00861555. [DOI] [PubMed] [Google Scholar]
- Tomlinson PA, Smellie JM, Prescod N, et al. Differential excretion of urinary proteins in children with vesicoureteric reflux and reflux nephropathy. Pediatr Nephrol. 1994;8:21–25. doi: 10.1007/BF00868252. [DOI] [PubMed] [Google Scholar]
- Bird JM, Owen RG, D'Sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]