Abstract
Approximately two out of three adult Americans are overweight or obese. Despite widespread recognition of this disorder, there has been little progress in the past 20 years in finding effective noninvasive treatments for weight loss. The consequences of obesity are increasingly well recognized and include increases in blood pressure, plasma lipids, the onset of type 2 diabetes, sleep apnea, asthma, osteoarthritis and a variety of cancers. Obesity can increase the rate of pregnancy complications and fetal malformations in normoglycemic women. Current medical approaches to obesity, including intensive lifestyle interventions and drug therapies, have been successful in achieving modest weight loss of 4–7%, less than the 1998 NIH Guidelines target of 10%. Surgical approaches, including laparoscopic adjustable gastric banding, vertical banded gastroplasty and Roux-en-Y gastric bypass, are much more successful, achieving weight loss of 15–50%. A treatment gap therefore exists in the management of obese and overweight patients, because many patients desire and would receive great health benefits by achieving weight loss of 7–15%. This review will discuss the dilemma of the treatment gap and explore possible ways by which it may be filled in the future by the use of innovative approaches.
Keywords: overweight, treatment, unmet need
Introduction
One-third of adult Americans are overweight, defined as a body mass index (BMI) between 25 and 30 kg m−2, and an additional one-third are obese, defined as a BMI of 30 kg m−2 or greater.1 There are promising data from the Centers for Disease Control and Prevention to suggest that the prevalence of overweight and obesity among adult Americans has recently reached a plateau compared with a continuous rise in prevalence from 1985 to 2005.2 However, the sober reality is that the weight of two-thirds of adult Americans is above ideal. In the US, obesity is striking Americans of all colors, in all states and at all income levels, although not proportionately.3 The prevalence of overweight and obesity has taken on greater significance among minority Americans, such as African Americans and Mexican Americans, where the prevalence exceeds that of Caucasians.3 Obesity is also more prevalent among Americans from lower socioeconomic status groups.3, 4, 5 Appropriately, several publications have proclaimed that there is an ‘obesity epidemic' in the US and internationally.
The purpose of this review is to examine the consequences of obesity, the available therapeutic options and what strategies may become available in the future to treat obesity, which is among the most widespread and costly health-care problem in the US.
The consequences of obesity
The health consequences of overweight and obesity are manifold. Increased body mass can increase blood pressure, and weight reduction can be effective in reducing both systolic and diastolic blood pressure, usually producing mean reductions of 1–5 mm Hg in systolic and diastolic blood pressure, depending on the starting level of blood pressure and degree of weight loss.6 Obesity is associated with a modest increase in LDL cholesterol and large increases in fasting triglycerides levels.7 Obesity is also associated with a marked increase in the risk for type 2 diabetes mellitus: the prevalence of diabetes in subjects with a BMI of less than 25 kg m−2 is 4.3% of US adults; this prevalence rises to 28.1% of adults whose BMI is greater than 40 kg m−2, a more than sixfold increase.8 In addition to these adverse effects on important health parameters—blood pressure, lipids and plasma glucose levels—obesity may modulate inflammatory markers that increase the risk of vascular pathology, such as increases in C-reactive protein and decreases in adiponectin.9 Obesity is also associated with an increased risk of obstructive sleep apnea,10 asthma11 and osteoarthritis.12
Multiple studies have reported associations of obesity with increased cancer risk, particularly esophageal, pancreatic, colorectal, postmenopausal breast, endometrial and kidney cancer.13 A recent review suggested a stepwise increase in overall cancer risk for obese women, relative to women with a BMI of 25 kg m−2 or less, of 8% higher for a BMI of 25–29.9; 18% higher for a BMI of 30–34.9; 32% higher for a BMI of 35–39.9; and 62% higher for a BMI of greater than or equal to 40. For men, the risk is also significant with a BMI of 30–34.9 elevating risk by 9% 35–39.9 by 20% and greater than or equal to 40 by 52%.14
In normoglycemic women who are obese at the time they become pregnant, obesity is associated with an increased rate of miscarriage, macrosomy (large infant), shoulder dystocia and a 2.5-fold increase in fetal malformations.15
Because of the adverse effects of obesity on these varied parameters of health, it has been estimated from an analysis of NHANES data that patients with a BMI between 30 and 40 kg m−2 lose approximately 1–6 years of life, and those with BMI greater than 45 kg m−2 lose up to 13 years of life.16 The cost of obesity to the medical care system in the US, including the cost to private and public payers, was estimated to be approximately $147 billion in 2008.17 Obesity is an ‘upstream' problem, according to population-health terminology, that undoubtedly has ‘downstream' effects on multiple health parameters.
Reversibility of obesity-associated comorbidities
Many of the complications of obesity are reversible with weight loss. One of the best illustrations of this reversibility was shown in the Swedish Obese Subjects study in which 2010 obese subjects (average BMI of 42.4 kg m−2) underwent bariatric surgery (vertical banded gastroplasty, nonadjustable or adjustable banding or gastric bypass) and were compared with 2037 control subjects (average BMI of 40.1 kg m−2). Although only a fraction of the enrolled subjects had reached 10 and 15 years of post-surgery follow-up by the time of the 2007 publication, the magnitude of weight reduction was impressive: approximately 16–18% weight loss for gastroplasty, 13–14% for banding and 25–27% for gastric bypass at 10–15 years. The study also suggested a mortality benefit (hazard ratio of 0.76, CI of 0.59–0.99, P=0.04) in the subjects treated with bariatric surgery compared with the control subjects.18 There are numerous studies showing reversibility of diabetes after weight loss in obese diabetic patients, and recent consensus statements have therefore advocated bariatric surgery in selected obese diabetic patients.19 Many of the other cardiometabolic abnormalities, such as hypertension, dyslipidemia and adverse alteration in proinflammatory markers, are also improved by weight loss.20
Effectiveness of nonsurgical approaches for the management of obesity
On the basis of the ability of modest weight loss to improve health parameters, the National Institutes of Health's Clinical Guidelines (1998) recommended an initial goal of 10% weight loss in patients with obesity.21 Do therapies exist that can reliably produce and sustain a 10% weight loss? Although many programs that combine diet and exercise have been developed and promoted, there is no current consensus as to the most effective and widely applicable program. Some of the most publicized programs will be reviewed to illustrate how elusive the goal of 10% weight loss has been.
In the Diabetes Prevention Program (DPP) Study, 3234 subjects with abnormal glucose tolerance were enrolled in a multiyear study to compare standard lifestyle intervention, standard lifestyle intervention plus metformin (850 mg twice daily) and intensive lifestyle intervention in their abilities to decrease progression to diabetes, based on standard biochemical testing.22 Subjects assigned to intensive lifestyle intervention were instructed to eat a healthy low-calorie, low-fat diet and to engage in physical activity of moderate intensity for at least 150 min per week. In addition, subjects were provided with a 16-lesson curriculum covering diet, exercise and behavior modification designed to help subjects achieve these goals. Case managers taught an individualized curriculum on a one-to-one basis during the first 24 weeks after enrollment. Subjects were then provided with individual sessions (usually monthly) and group sessions with the case managers to reinforce behavioral changes.22 The DPP ‘model,' using a platform of individualized education about diet and exercise followed by reinforcement of principles, has been emulated at many centers and is one of the most commonly used approaches in the US today. The initial results from the DPP were impressive. The mean BMI at baseline for all participants was 34 kg m−2, and at 1 year the group randomized to intensive lifestyle intervention had lost an average of ∼7% of their body weight, compared with 3% in the metformin group and 0.5% in the group randomized to the standard lifestyle intervention. At 2 years, subjects in the intensive lifestyle intervention group showed approximately a 5% average weight loss from baseline. At 4 years, the average weight loss was 4% from baseline in the intensive lifestyle group compared with 0–1% in the metformin and standard lifestyle groups. Despite the modest reduction in weight, the intensive lifestyle intervention group showed a 58% reduction in the progression to type 2 diabetes over 4 years compared with standard lifestyle intervention. Thus, the DPP study showed that in obese subjects with abnormal glucose tolerance, an intensive educational program stressing the importance of diet and exercise could produce weight loss of 4–7% over 4 years and markedly reduce progression to type 2 diabetes.
The DPP study was published in 2002, and despite a decade of research to explore newer methodologies to enhance weight loss using diet and exercise approaches, recent clinical trials have been unable to improve on this efficacy.
In 2011, two important and innovative studies reported their results. Appel et al.23 studied computer-literate obese adults followed up in primary care practices with an average starting BMI of 37 kg m−2. Subjects were assigned to a control group or to one of two intervention groups. The control group met with a weight-loss coach at the time of randomization and, if desired, after the final data-collection visit at 24 months. They also received brochures and a list of recommended Internet sites promoting weight loss. Subjects in the two treatment groups received Internet-based education and online feedback regarding adherence to a diet and exercise program. Weight-loss coaches were assigned to each participant and had weekly contact (either one-on-one or in small group) for the first 3 months. Between months 3 and 6, subjects were randomized to in-person support 3 times per month versus monthly remote support. Beyond 6 months, subjects in the in-person support group were offered two monthly in-person contacts, whereas those in the remote support group received one monthly phone contact. At the end of 24 months, the mean change in weight from baseline was 0.8% in the control group, 4.9% in the in-person support group and 4.4% in the remote-support group.23
Wadden et al.24 reported results from a multicenter study in primary care practices. Obese subjects with average baseline BMIs of ∼38 kg m−2 were assigned to ‘usual' care, consisting of quarterly brief counseling sessions of 5–7 min duration with a primary care physician (PCP) to discuss weight change and review contents of weight management handouts; to ‘brief lifestyle counseling' that included the same quarterly visits with their PCP plus monthly meetings with a lifestyle coach who provided lessons from the DPP; or to ‘enhanced brief lifestyle counseling' in which participants received the same PCP and counseling visits as those assigned to brief lifestyle counseling, but in addition chose to take sibutramine, orlistat or meal replacements to increase weight loss, beginning 1 month after treatment commenced. At the end of 2 years, weight loss from baseline was 1.6, 2.9 and 4.7% in the usual care, brief lifestyle counseling, and enhanced brief lifestyle counseling groups, respectively.24
Thus, in the landmark DPP trial (2002) and two newer trials published in 2011, including the study by Wadden et al. that permitted the use of drug therapy to enhance weight loss, it is apparent that the average weight loss achieved in 2 years has been in the range of 4–5%. The goal of 10% sustained weight loss, as recommended by the NIH Clinical Guidelines, has not been achieved for most patients using diet and exercise approaches, creating a disappointing treatment gap in the management of obesity.
Effectiveness of pharmacologic therapies for the management of obesity
In the US in 2011, there are principally two pharmacological therapies that have received FDA approval and are currently in use: orlistat, an inhibitor of gastric and pancreatic lipases that induces a state of fat malabsorption, and phentermine hydrochloride, a sympathomimetic amine that decreases appetite centrally by releasing hypothalamic norepinephrine. There are several less-commonly prescribed sympathomimetic drugs that are FDA approved for short-term use for weight management, including diethylpropion, phendimetrazine and methamphetamine. These drugs are believed to be more habituating compared with phentermine, and are not commonly used for obesity management. The clinical results with orlistat show average weight loss of about 2.9 kg (∼2.9%) at 4 years among patients adhering with therapy.25 Orlistat is difficult to administer because it causes gastrointestinal side effects (flatulence, nausea, diarrhea and bloating) in most users. There have also been rare reports of severe liver injury in patients using orlistat. Phentermine is approved for short-term use (up to 3 months) in patients with obesity. There are scant data regarding its short-term efficacy at FDA-approved doses (maximum of 37.5 mg per day of phentermine hydrochloride). A recent report from one center using phentermine longer than approved by the FDA and in combination with a very low-carbohydrate (ketogenic) diet has shown impressive weight loss at 6 months and 2 years.26 Further follow-up will be needed to understand the generalizability of these results and the safety of chronic phentermine use. Common side effects of phentermine include insomnia, dry mouth, tremor and constipation. In general, currently approved pharmacotherapies, at doses and durations of treatment recommended by the FDA, do not appear to be more efficacious than intensive lifestyle programs that use diet, exercise and behavior modification. Perhaps, most importantly, the amount of average weight loss that is achieved with current drug treatment, as reported in controlled clinical trials of these therapies, falls short of the elusive 10% level recommended by the NIH.
There are three experimental pharmacotherapies that were evaluated but not approved by the FDA in 2010–2011. First, a controlled-release capsule containing low doses of phentermine and topiramate was effective in inducing weight loss of 8–10% in obese subjects at 1 year.20 FDA concerns about small increases in heart rate in phentermine/topiramate users and possible teratogenicity of topiramate in women of childbearing potential have been the key regulatory concerns. Second, lorcaserin, a selective 5HT2C receptor agonist, has been tested clinically as a potential weight-loss agent because of its ability to decrease appetite. In phase 3 clinical studies, patients randomized to lorcaserin lost an average of almost 6% body weight from baseline at 1 year.27 One of the key regulatory concerns for lorcaserin has been the development of mammary tumors in rats during preclinical toxicology testing; the significance of this finding to humans is being explored. Third, a combination of naltrexone and bupropion has been tested and shown to achieve 5–6% average weight reduction in obese subjects at 1 year.28 This combination was recommended for approval by the FDA Endocrine Advisory Committee, but was not approved because of FDA concerns that insufficient high-risk cardiac patients were included in the clinical trial program. New trials that will include such patients are anticipated in the future. Hopefully, new pharmacotherapies will become available for weight loss in obese individuals, in the near future to help achieve the target of 10% weight loss.
Effectiveness of bariatric surgery for the management of obesity
Bariatric surgery has emerged during the past two decades as the most effective therapy for patients with moderate to severe obesity (BMI of 35 kg m−2 or greater). Although the reported efficacy varies, the weight loss at 2 years for patients undergoing laparoscopic adjustable gastric banding (LAPG) ranges from 12 to 18% and for vertical banded gastroplasty (VBG) and Roux-en-Y gastric bypass (RYGB) from 25 to 50%.29 In a 2004 review, the mean short-term mortality rate was 0.05% for LAPG, 0.31% for VBG and 0.50% for RYGB.29 The median morbidity rates were 11.3, 25.7 and 23.6% for the three procedures, respectively.29 Long-term complications of fat malabsorption, hypoglycemia, vitamin and mineral depletion and weight regain continue to be challenges faced by patients who have undergone bariatric procedures.30 The complications from these procedures, although decreasing as surgical expertise grows, continue to be significant.
The treatment gap in the management of overweight and obese patients
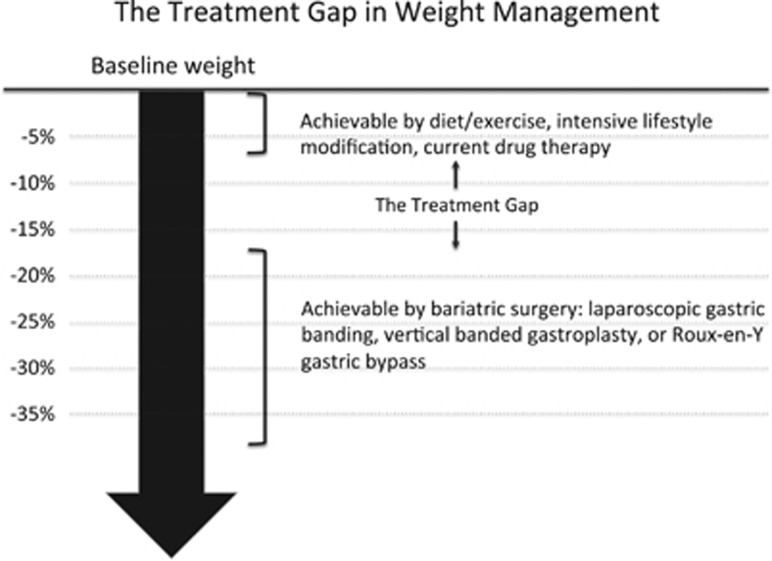
On the basis of the therapies that have been described, medical practitioners today face a therapeutic dilemma when trying to manage patients who are overweight or obese. Clinical data support the recommendation that such patients, particularly if they have one or more comorbidities, should attempt to achieve 10% weight loss as their initial goal, because this amount of weight loss may be sufficient to reverse many of the weight-related complications. Although achieving 10% weight loss may appear modest, achieving this goal has been highly challenging on a population level. At present, nonsurgical measures that do not use drug therapy, such as intensive lifestyle modifications that emphasize diet and exercise, appear only able to achieve a 4–7% reduction in BMI. Approved drug therapies, such as orlistat and phentermine, appear to have efficacy similar to intensive lifestyle intervention. Surgical alternatives, although efficacious, in some ways overshoot the target for patients who only require a 5–15% reduction in BMI to improve their metabolic parameters. Thus, there is a significant treatment gap between what is currently achievable through medical/behavioral and surgical approaches. This gap in the availability of efficacious and implementable treatments has also created a second gap, namely, that the majority of overweight and obese patients do not seek therapy.31 Why seek therapy when the efficacy of nonsurgical approaches is so small? Figure 1 illustrates the efficacy treatment gap that has been described herein.
Figure 1.
The treatment gap in weight management. This figure illustrates the magnitude of weight loss that can be achieved by current interventions. Nonsurgical approaches, shown in the top part of the figure, achieve weight loss of up to 7% of baseline weight. Bariatric surgical techniques are more successful, achieving weight loss of 15% or greater. Many Americans, however, would benefit from weight loss between 7 and 15%, leaving a gap in current treatment options.
Future approaches to filling the treatment gap
The ideal weight-loss approach for overweight and obese patients would be as low risk as diet and exercise programs but result in a 10% or greater weight loss. New and relatively noninvasive endoluminal methods, such as the intragastric balloon, are being explored that may produce a sense of satiety—much as is achieved by laparascopic gastric banding—without requiring surgery.32 Another possibility that has not been fully explored is the combination of intensive lifestyle intervention and drug therapy to suppress appetite. As an example, phentermine could be used with intensive lifestyle intervention, perhaps as has been reported by Hendricks et al.,26 with the goal of achieving additivity of the two treatment effects. Newer oral agents, such as the three therapies that were reviewed by the FDA in 2010, may obtain sufficient safety and efficacy data to justify approval. However, it is unlikely that a single pharmacologic agent will be able to suppress appetite in a sustained manner in all patients because of redundancy in feeding/satiety mechanisms. In fact, a recent study that examined hormonal factors showed compelling post-weight-loss increases in hormones that stimulate appetite (such as Ghrelin) and decreases in those that mediate satiety (such as leptin).33 This hormonal ‘conspiracy' explains the profound drive to eat and regain weight after dieting. Clearly, a multipronged approach to combating overweight and obesity—using lifestyle modification, drugs, less invasive devices and behavioral therapy—will be needed to fill the treatment gap that exists in our approach to this vexing public health problem. Further development of what this might involve in terms of an adult weight management clinic is described in this supplement by Maja Artandi.
Acknowledgments
I would like to acknowledge Dr Christopher Gardner for his generous help and suggestions during the process of review and revision of this manuscript and to Dr Maja Artandi for her critical review and suggestions. I received no financial or grant support related to preparing this manuscript. Publication of this supplement was partially supported by Nutrilite Health Institute with an unrestricted educational contribution to Stanford Prevention Research Center.
Dr Gesundheit is a consultant to and shareholder in VIVUS, Inc., Mountain View, CA, USA. VIVUS is developing pharmacological therapies for the treatment of obesity. Dr Gesundheit is also a consultant to Astellas Pharma in an area unrelated to obesity research or treatment.
References
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ, Yanovski JA. Obesity prevalence in the United States—up, down, or sideways? N Engl J Med 2011; 364: 987–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011; 34: 717–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MJ. Epidemic of obesity expands its spread to developing countries. JAMA 2002; 287: 1382–1386. [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378: 804–814. [DOI] [PubMed] [Google Scholar]
- Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D. Long-term weight loss from lifestyle intervention benefits blood pressure?: a systematic review. Hypertension 2009; 54: 756–762. [DOI] [PubMed] [Google Scholar]
- Karaouzene N, Merzouk H, Aribi M, Merzouk SA, Berrouiguet AY, Tessier C et al. Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: a comparison of older with young men. Nutr Metab Cardiovasc Dis 2011; 21: 792–799. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cheng YJ, Narayan KM, Thompson TJ, Williamson DF. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med 2007; 45: 348–352. [DOI] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004; 350: 2362–2374. [DOI] [PubMed] [Google Scholar]
- Acioglu E, Yigit O, Volkan Sunter A, Taskin U, Bercik Inal B, Sahin M. Obesity and obstructive sleep apnea syndrome. J Otolaryngol Head Neck Surg 2010; 39: 744–751. [PubMed] [Google Scholar]
- Kent BD, Lane SJ. Twin epidemics: asthma and obesity. Int Arch Allergy Immunol 2011; 157: 213–214. [DOI] [PubMed] [Google Scholar]
- Holliday KL, McWilliams DF, Maciewicz RA, Muir KR, Zhang W, Doherty M. Lifetime body mass index, other anthropometric measures of obesity and risk of knee or hip osteoarthritis in the GOAL case-control study. Osteoarthritis Cartilage 2011; 19: 37–43. [DOI] [PubMed] [Google Scholar]
- Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep 2011; 13: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–1638. [DOI] [PubMed] [Google Scholar]
- Owens LA, O'Sullivan EP, Kirwan B, Avalos G, Gaffney G, Dunne F. ATLANTIC DIP: the impact of obesity on pregnancy outcome in glucose-tolerant women. Diabetes Care 2010; 33: 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003; 289: 187–193. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs (Project Hope) 2009; 28: w822–w831. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357: 741–752. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med 2011; 28: 628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 1341–1352. [DOI] [PubMed] [Google Scholar]
- NIH. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, 1998. [PubMed]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011; 365: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011; 365: 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 2007; 335: 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring) 2011; 19: 2351–2360. [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010; 363: 245–256. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010; 376: 595–605. [DOI] [PubMed] [Google Scholar]
- Chapman AE, Kiroff G, Game P, Foster B, O'Brien P, Ham J et al. Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic literature review. Surgery 2004; 135: 326–351. [DOI] [PubMed] [Google Scholar]
- Fujioka K, DiBaise JK, Martindale RG. Nutrition and metabolic complications after bariatric surgery and their treatment. JPEN J Parenter Enteral Nutr 2011; 35: 52S–59S. [DOI] [PubMed] [Google Scholar]
- Mold F, Forbes A. Patients' and professionals' experiences and perspectives of obesity in health-care settings: a synthesis of current research. Health expectations: an international journal of public participation in health care and health policy 2011; Health Expect; e-pub ahead of print 7 June 2011. [DOI] [PMC free article] [PubMed]
- Familiari P, Boskoski I, Marchese M, Perri V, Costamagna G. Endoscopic treatment of obesity. Expert Rev Gastroenterol Hepatol 2011; 5: 689–701. [DOI] [PubMed] [Google Scholar]
- Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365: 1597–1604. [DOI] [PubMed] [Google Scholar]