Abstract
Overweight (body mass index (BMI) 25 kg m−2) or obesity (BMI 30>kg m−2) affects more than two-thirds of Americans. Overweight and obesity are commonly associated with multiple coexisting conditions, such as hypertension, diabetes, dyslipidemia, cardiovascular disease, obstructive sleep apnea and cancer. Lifestyle modification can induce a modest weight loss, which is associated with the prevention or improvement of many of these comorbidities. A combination of diet, exercise and behavioral therapy is considered the cornerstone of treatment for all overweight and obese individuals. As the etiology and therapy of obesity is complex, what is needed for these patients is a multidisciplinary clinic where specialists from different disciplines share their knowledge and participate in the treatment of the obese patient.
Keywords: challenges, medical treatment, clinic organization
Introduction
The prevalence of overweight and obesity has increased markedly in the past decade and has become a major health problem. Currently, more than two-thirds of Americans are either overweight or obese.1 A major concern is that overweight and obesity have associated comorbidities, such as diabetes mellitus, cardiovascular disease and cancer among others, leading to increases in early mortality and decreases in life expectancy.2 Even a modest weight loss achieved through lifestyle modification is associated with the prevention or improvement of many of these comorbidities.3 Despite the negative effects of obesity on human health, interventions to mitigate obesity remain challenging. Potential obstacles to successful management of obesity include physician attitudes, paucity of effective therapies and inadequate time for counseling.
Challenges in treating obese patients
Patients with obesity are often stigmatized in the health-care setting owing to suboptimal facilities and the biases of health-care professionals. Many health-care facilities remain poorly equipped to treat obese patients. Common obstacles in standard medical clinics include inadequate sizes of waiting room chairs, hospital beds or even hospital gowns. Another potential problem relates to weight limitations of most imaging modalities, including CT and MRI. Remedying these obstacles requires concerted planning to design appropriate facilities and ensure the availability of imaging scanners with adequate weight and size capacity. It has been shown in several studies that obesity may adversely influence the judgment and practices of medical professionals.4 Many medical professionals do not feel comfortable treating obese patients partly because of the limitations associated with the physical examination in obese patients. Overweight and obese patients in turn may feel less comfortable pursuing appropriate medical care. These attitudes can manifest in reduced rates of preventive care. For example, populations of patients with high body mass index (BMI) have lower rates of age-appropriate cancer screening than patients with low BMIs.5
Compounding this problem further, many physicians are reluctant to address weight issues with their obese patients. Overweight is often considered a sensitive and personal subject, and as a result physicians fear offending their patients through direct discussion of these topics. Furthermore, physicians are often inadequately informed regarding appropriate therapeutic options for obesity or are frustrated by the paucity of successful interventions for the treatment of obesity. As a result, recommendations for behavior modification, medications or referrals of eligible patients for bariatric surgery are frequently underutilized. A lack of time to counsel obese patients on their weight is also often cited as a major factor for not addressing excess weight during the office visit.6, 7 The US Preventive Services Task Force recommended in 2003 that ‘clinicians should screen all adult patients for obesity and offer intensive counseling and behavioral interventions to promote sustained weight loss for obese adults.'8 Intensive counseling was defined as at least two visits per month for the first 3 months. A review of the treatment of obesity in primary care practice in the United States9 showed that high-intensity treatment may be effective, but low- and moderate-intensity counseling (less than two visits per month) was unlikely to result in clinically significant weight loss. With the already pressing demands of office practice and the absence of adequate reimbursement for counseling, the time and effort required to provide high-intensity weight-loss treatment prevents most practitioners from treating obesity.
It may be unrealistic to expect primary care physicians to provide effective weight management for all of their patients who require it, unless greater resources are provided in their practices, including appropriate reimbursement. Thus, many factors conspire to result in reduced care in obese patients compared with their non-obese counterparts. Obese patients require special care and a concerted approach in order to reverse these limitations and deliver appropriate therapeutic options for the management of their weight problem.
Treatment options for obesity
At this time, there are no evidence-based guidelines or defined practice standards to treat obesity, leaving many academic and nonacademic weight management centers to develop their own approaches. A consensus on the best approach to treat obesity is lacking. Lifestyle modifications are considered the basis of treatment for all overweight and obese individuals. Lifestyle modifications include three main components: diet, physical activity and behavioral therapy. Many excellent studies have shown that a comprehensive lifestyle modification program can induce a weight loss of approximately 10% of initial weight in 16–26 weeks of therapy.10 Unfortunately, many patients regain the weight they lost.11, 12 Thus, weight loss maintenance represents a very challenging problem for patients and experts in the treatment of obesity. If patients have a BMI of 30 kg m−2 or greater (or a BMI of ⩾27 kg m−2 in the presence of significant comorbidities), pharmacotherapy can be added to lifestyle interventions. Many physicians remain unfamiliar with the use of medications for weight loss, and thus pharmacologic therapy is not widely used for the treatment of obesity. In addition, there are few FDA-approved drugs specifically indicated for weight management. Weight-loss surgery can be considered for patients with a BMI ⩾40 kg m−2 (or 35 kg m−2 with significant comorbidities) who fulfill eligibility criteria. However, weight-loss surgery is performed on fewer than 2% of the eligible patients per year.13 In summary, there is a large treatment gap between obese and overweight patients needing treatment for their condition and available therapeutic options and medical professionals comfortable with treating obesity. To address this treatment gap, major medical centers should strive to establish weight management centers.
Organization of the multidisciplinary weight center
My colleagues from our weight management team and I propose a multidisciplinary approach to treat the obese or overweight patient, which has been shown to be successful in several academic centers and many clinical studies.14, 15 A multidisciplinary approach combines the skills of several experts to aid the patient in the treatment of the obesity. The multidisciplinary team should include a physician with expertise in the treatment of obesity, a dietitian, a behavioral therapist, an exercise specialist and nursing staff.
As the prevention of obesity is almost as important as its treatment, the clinic population is not restricted to those already obese, but also incorporates preventive strategies aimed toward patients with a BMI of ⩾27 or ⩾25 kg m−2 with comorbidities. The goals of this proposed 1-year program are a 10% sustainable weight loss, the reduction of comorbidities and an improvement in physical fitness. Patients will also be expected to learn tools to aid them in weight maintenance. The 10% weight-loss goal might appear quite modest, and patients will often be disappointed when they are told that it is not realistic to expect a larger weight loss after the first year. However, even just a 10% weight loss can significantly improve obesity-related comorbidities.16, 17 In addition, patients must realize that it often took years to gain the excess weight, and thus it is reasonable and realistic to expect that it is likely to require years and significant investment to lose the weight.
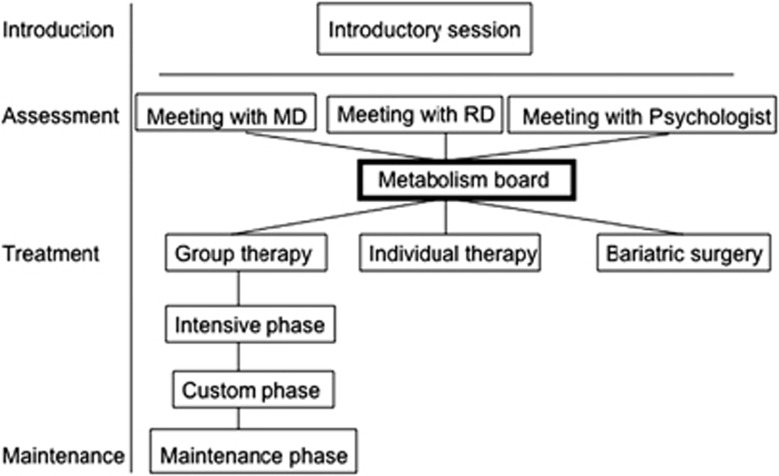
To take full advantage of the multidisciplinary team approach, my colleagues and I propose the following structure to our weight management center. After an introductory presentation concerning the structure and goals of the program, interested patients will have individual meetings with the physician, the registered dietitian, the behavioral therapist and the exercise specialist. After these meetings, the providers meet to discuss the best therapeutic approach for each patient. We refer to these provider meetings, involving representatives from different disciplines, as the ‘metabolism board', analogous to tumor boards that convene for patients with cancer. There are several different treatment options available for the patients. To appropriately pair patients and treatments, several factors are considered including the severity of obesity and comorbidities, the patient's preferences, his/her knowledge about nutrition and previous attempts at weight loss. Options include group therapy (which will be discussed in more detail below), individual therapy and direct referral to bariatric surgery (Figure 1).
Figure 1.
Organization of the Stanford weight management center.
A direct referral to bariatric surgery would be recommended, for example, for patients with obesity-related comorbidities who have unsuccessfully attempted lifestyle modification and pharmacotherapy and are interested in a surgical approach. Another patient group that would be referred comprise obese patients without significant comorbidities who have tried alternative approaches without success and would like to undergo bariatric surgery.
Patients who have undergone bariatric surgery will follow-up with the medical providers on a regular basis to adjust medications (if necessary) and evaluate for nutrient deficiencies.
Behavioral modification component of the program
We modeled the group therapy approach closely after the Diabetes Prevention Program (DPP) and the Look AHEAD study. Both the DPP and the Look AHEAD study used lifestyle modification techniques that many weightloss centers have incorporated into their own programs. The DPP was a 27-center randomized clinical trial to determine whether lifestyle intervention or pharmacologic therapy with metformin would prevent or delay the onset of diabetes in individuals with impaired glucose tolerance who are at high risk for the disease.18 The Look AHEAD study is a multicenter, randomized controlled trial designed to determine whether intentional weight loss reduces cardiovascular morbidity and mortality in overweight individuals with type 2 diabetes.19 Both studies are excellent examples of using behavioral treatment for the benefits they confer.20, 21
The goal of each trial was to induce a mean weight loss of ⩾7% and to increase the participant's physical activity, although different teaching methods were used. The DPP provided each participant with 16 individual treatment sessions, consisting of one-on-one meetings between the patient and a lifestyle coach, during the first 6 months. However, we propose combining group therapy and individual sessions to teach lifestyle interventions as was done in the Look AHEAD study. Patients participate in groups consisting of 10–20 people with whom they attend group therapy sessions for the whole year.22 It has been shown that group therapy is superior to individual therapy in inducing weight loss, regardless of the individual's preferred method of treatment.23 Group therapy is more cost-effective, provides social support and can create a potentially healthy dose of competition. Adding individual therapy gives participants the chance to discuss progress and review specific questions or problems. It also helps to address individual needs including those related to cultural or ethnic differences. Individual therapy might also help the participants form a stronger bond with the program. Our group therapy approach is divided into a 26-week intervention phase and a 26-week maintenance phase. The intervention phase is further divided into an intensive phase (week 1–8) and a custom phase (week 9–26). Restriction of caloric intake is the primary method of achieving weight loss. Patients are provided with a very specific meal plan and a shopping list to stay within their caloric goals. To aim for a weight loss of 10% of initial weight, the goals are 1200–1500 kcal per day for individuals weighing 250 pounds (114 kg) or less at baseline and 1600–2000 kcal per day for individuals who weigh more than 250 pounds. These calorie levels should promote a weight loss of approximately one to two pounds per week, as it takes a caloric deficit of approximately 3500 kcal to lose one pound of body weight.
Despite the success of many weight-loss interventions with meal replacements,24 we have decided not to use these routinely in our multidisciplinary clinic but rather to provide the patients with detailed menu plans and accompanying shopping lists for the first 8 weeks. It has been shown that this approach also facilitates significant weight loss.25 Our rationale for this approach is to expose the patients from the beginning to ‘real-world foods' to facilitate continuing weight loss and weight maintenance later on. Stanford will provide three similar meal plans from which a patient can choose his/her preferences. The plans contain 1200, 1600 and 2000 calories per day consisting of 40–50% carbohydrates, 20–30% protein and 20–30% polyunsaturated and monounsaturated fats with less than 7% of saturated fats. After 8 weeks on these detailed menu plans, the patient can choose when to initiate the custom phase. In this phase, the patient maintains a daily caloric limit but can choose the composition of each meal within that limit. If the weight loss is not satisfactory at any time after the first 4 weeks, the patient will be given toolbox options. These toolbox options include meal replacements, more individualized care and the use of weight-loss medications.
Exercise is also an integral part of weight management. It has been shown that regular exercise is an important predictor of weight maintenance after weight loss.26, 27 Our initial goal for exercise is 150 min per week, which can be increased to 300 min per week in the weight maintenance phase. These recommendations follow the guidelines for physical activity goals determined by the American College of Sports Medicine. The patients have frequent group meetings interspersed with individual meetings with different specialists, including a physician, a registered dietician and a psychologist. In the first 12 weeks, the group meetings are on a weekly basis, whereas group meetings are held twice a month during weeks 13–26. Once the patients enter the maintenance phase after week 26, the group meetings are conducted once a month with a bimonthly telephone or e-mail contact. The focus of the weight management shifts after week 26 from losing weight to maintaining the lost weight. Patients are encouraged to stabilize their weight instead of losing more weight. If more weight loss is desired, participants will be encouraged to restart losing weight again after 6 months of maintenance.
Pharmacotherapy as a component of the program
Pharmacotherapy is an important adjunct option in those patients who are not able to lose sufficient weight with lifestyle modification alone.
A crucial part of pharmacotherapy is to carefully review the patient's list of chronic medications and try to substitute known adipogenic drugs with drugs that are either weight neutral or promote weight loss (for example, switch from insulin to exenatide).
In patients with a BMI above 30 or 27 kg m−2 with comorbidities, weight loss medications can be prescribed.
Drugs given for weight loss can be divided into two categories—lipase inhibitors and appetite suppressants—on the basis of their putative mechanism. Unfortunately, there are few FDA-approved weight-loss drugs available, and most of these weight-loss medications are only approved for short-term use (3 months).
Orlistat, a lipase inhibitor, is the only weight-loss medication approved for longer-term (2 years) use in obese patients. However, the additional weight loss with orlistat after 4 years averages just 2.9%.28 The most commonly prescribed appetite suppressant is phentermine. Phentermine and other appetite suppressants are approved for short-term use by the FDA. It is a common practice, however, to prescribe these drugs for longer periods of time (off-label use). Other medications that can help with weight loss are naltrexon, bupropion, topiramate, zonisamide and metformin.29 These drugs are not FDA approved for weight loss, but are often used ‘off-label' to treat obesity.
When using medications to help the patient lose weight, it is important to realize that not all patients respond equally to the same medication. The success of a drug during the first month of therapy usually predicts the ultimate weight loss.30 It is also important to discuss with the patient that medications are often used ‘off-label' so that the patient can make an informed decision to start these drugs.
Dr Neil Gesundheit describes the effectiveness of pharmacologic therapies for the management of obesity in more detail in an earlier article in this supplement.
Summary
We advocate a holistic approach to weight loss that emphasizes diet management, improved exercise, and behavioral and social support to maintain adherence to a new lifestyle. By using a multidisciplinary approach that includes pharmacological, behavioral and surgical methods, we believe we can enhance patient self-efficacy and encourage long-term adherence to new diet and exercise programs.
Acknowledgments
I thank Dr Neil Gesundheit for his support and help with the manuscript. Publication of this supplement was partially supported by Nutrilite Health Institute with an unrestricted educational contribution to Stanford Prevention Research Center.
The author declares no conflict of interest.
References
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241. [DOI] [PubMed] [Google Scholar]
- Lee IM, Paffenbarger Jr RS. Change in body weight and longevity. JAMA 1992; 268: 2045–2049. [PubMed] [Google Scholar]
- Seagle HM, Strain GW, Makris A, Reeves RS. Position of the American dietetic association: weight management. J Am Diet Assoc 2009; 109: 330–346. [DOI] [PubMed] [Google Scholar]
- Puhl R, Brownell KD. Bias, discrimination, and obesity. Obes Res 2001; 9: 788–805. [DOI] [PubMed] [Google Scholar]
- Cohen SS, Palmieri RT, Nyante SJ, Koralek DO, Kim S, Bradshaw P et al. Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer 2008; 112: 1892–1904. [DOI] [PubMed] [Google Scholar]
- Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med 1995; 24: 546–552. [DOI] [PubMed] [Google Scholar]
- Kolasa KM, Rickett K. Barriers to providing nutrition counseling cited by physicians: a survey of primary care practitioners. Nutr Clin Pract 2010; 25: 502–509. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med 2003; 139: 930–932. [DOI] [PubMed] [Google Scholar]
- Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med 2009; 24 (9): 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology 2007; 132: 2226–2238. [DOI] [PubMed] [Google Scholar]
- Perri MG, Sears Jr SF, Clark JE. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. Diabetes Care 1993; 16: 200–209. [DOI] [PubMed] [Google Scholar]
- Vogels N, Westerterp-Plantenga MS. Successful long-term weight maintenance: a 2-year follow-up. Obesity 2007; 15: 1258–1266. [DOI] [PubMed] [Google Scholar]
- Kohn GP, Galanko JA, Overby DW, Farrell TM. Recent trends in bariatric surgery case volume in the United States. Surgery 2009; 146: 375–380. [DOI] [PubMed] [Google Scholar]
- Golay A, Fossati M, Deletraz M, De Luzy F, Howles MN, Ybarra J. Multidisciplinary approach to obesity treatment. Diabetes Obes Metab 2003; 5: 274–279. [DOI] [PubMed] [Google Scholar]
- Vansant G, Hulens M, van der Borght W, Demyttenaere K, Lysens R, Muls E. A multidisciplinary approach to the treatment of obesity. Int J Obes Relat Metab Disord 1999; 23 (Suppl 1): 65–68. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obesity Res 1998; 6 (Suppl 2): 51S–209S. [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999; 22: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003; 24: 610–628. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity 2011; 19: 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006; 14: 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol 2001; 69: 717–721. [PubMed] [Google Scholar]
- Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 2003; 27: 537–549. [DOI] [PubMed] [Google Scholar]
- Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord 1996; 20: 56–62. [PubMed] [Google Scholar]
- Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005; 82 (1 Suppl): 222S–225S. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American college of sports medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exercise 2009; 41: 459–471. [DOI] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 2007; 335: 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 2005; 142: 532–546. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ, Yanovski JA. Obesity. N Engl J Med 2002; 346: 591–602. [DOI] [PubMed] [Google Scholar]