Abstract
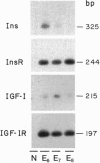
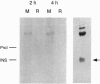
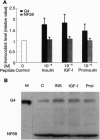
Early neurogenesis progresses by an initial massive proliferation of neuroepithelial cells followed by a sequential differentiation of the various mature neural cell types. The regulation of these processes by growth factors is poorly understood. We intend to understand, in a well-defined biological system, the embryonic chicken retina, the role of the insulin-related growth factors in neurogenesis. We demonstrate the local presence of signaling elements together with a biological response to the factors. Neuroretina at days 6-8 of embryonic development (E6-E8) expressed proinsulin/insulin and insulin-like growth factor I (IGF-I) mRNAs as well as insulin receptor and IGF type I receptor mRNAs. In parallel with this in vivo gene expression, E5 cultured neuroretinas synthesized and released to the medium a metabolically radiolabeled immunoprecipitable insulin-related peptide. Furthermore, insulin-related immunoreactive material with a HPLC mobility close to that of proinsulin was found in the E6-E8 vitreous humor. Exogenous chicken IGF-I, human insulin, and human proinsulin added to E6 cultured neuroretinas showed relatively close potencies stimulating proliferation, as determined by [methyl-3H]thymidine incorporation, with a plateau reached at 10(-8) M. These factors also stimulated neuronal differentiation, indicated by the expression of the neuron-specific antigen G4. Thus, insulin-related growth factors, interestingly including proinsulin, are present in the developing chicken retina and appear to play an autocrine/paracrine stimulatory role in the progression of neurogenesis.
Full text
PDF
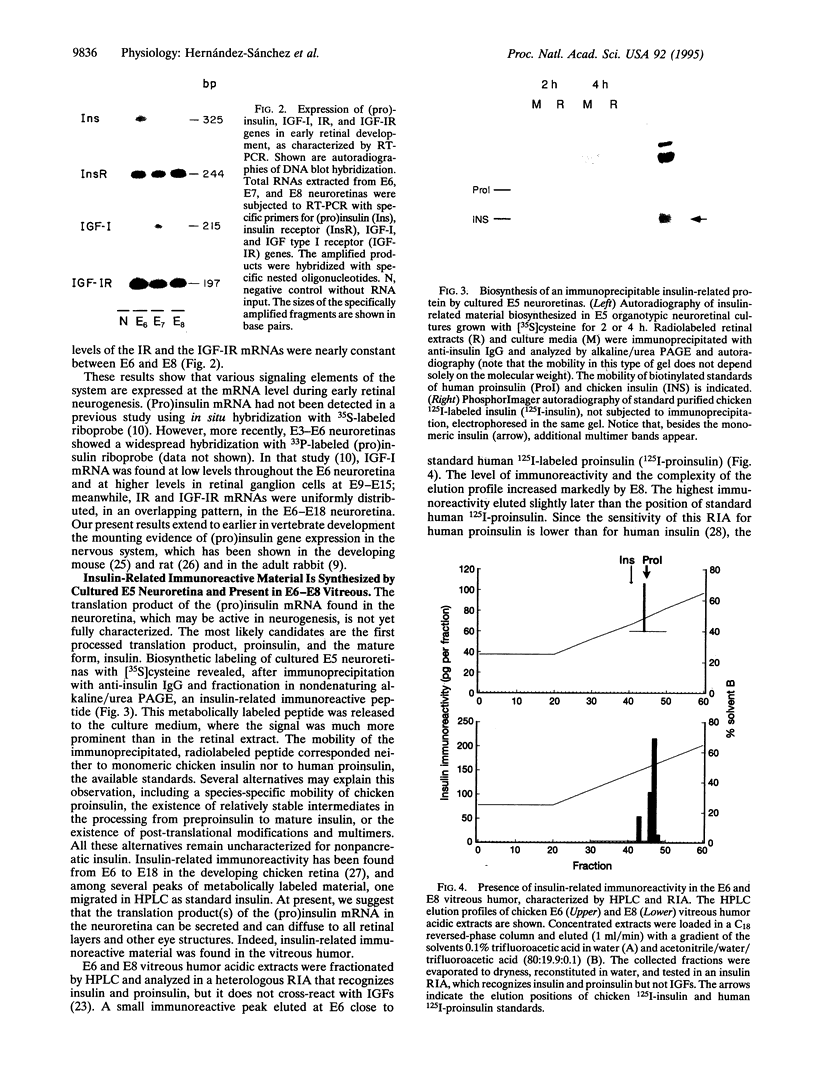
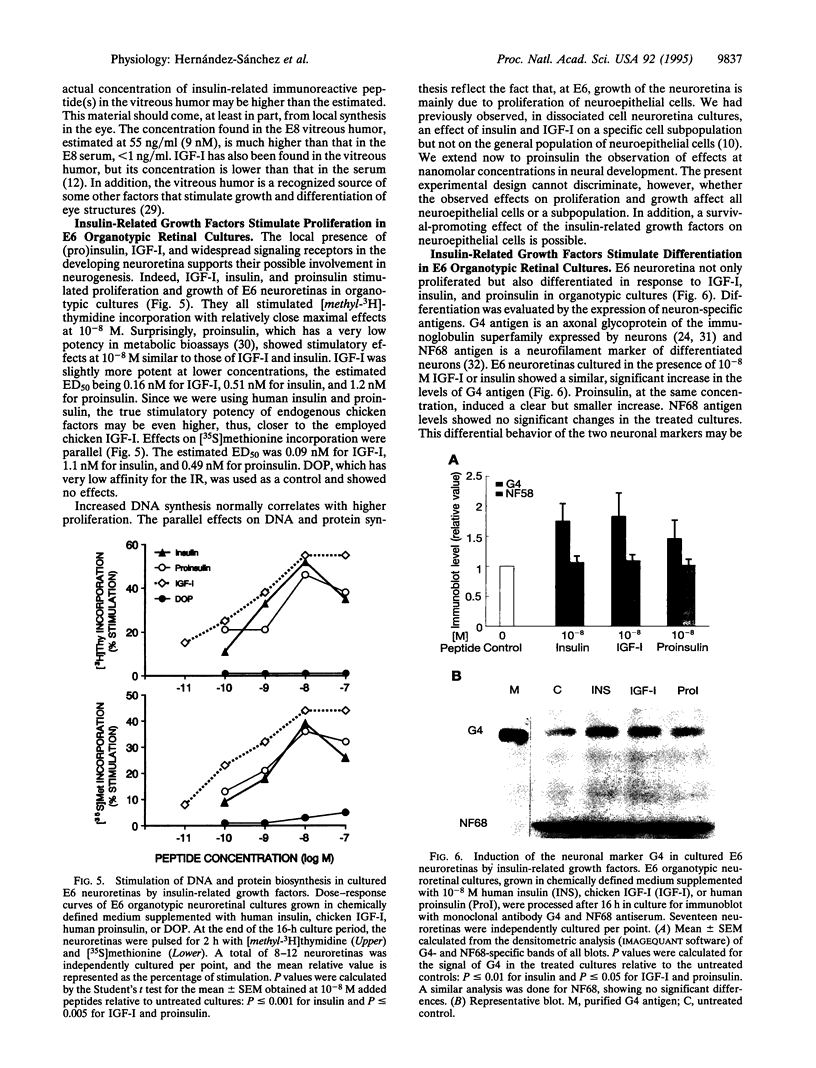

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón C., Lincoln B., Rhodes C. J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J Biol Chem. 1993 Feb 25;268(6):4276–4280. [PubMed] [Google Scholar]
- Bassas L., Girbau M., Lesniak M. A., Roth J., de Pablo F. Development of receptors for insulin and insulin-like growth factor-I in head and brain of chick embryos: autoradiographic localization. Endocrinology. 1989 Nov;125(5):2320–2327. doi: 10.1210/endo-125-5-2320. [DOI] [PubMed] [Google Scholar]
- Beck K. D., Powell-Braxton L., Widmer H. R., Valverde J., Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995 Apr;14(4):717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- De Pablo F., Roth J., Hernandez E., Pruss R. M. Insulin is present in chicken eggs and early chick embryos. Endocrinology. 1982 Dec;111(6):1909–1916. doi: 10.1210/endo-111-6-1909. [DOI] [PubMed] [Google Scholar]
- Deltour L., Leduque P., Blume N., Madsen O., Dubois P., Jami J., Bucchini D. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):527–531. doi: 10.1073/pnas.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaskar S. U., Giddings S. J., Rajakumar P. A., Carnaghi L. R., Menon R. K., Zahm D. S. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994 Mar 18;269(11):8445–8454. [PubMed] [Google Scholar]
- Devaskar S. U., Singh B. S., Carnaghi L. R., Rajakumar P. A., Giddings S. J. Insulin II gene expression in rat central nervous system. Regul Pept. 1993 Oct 20;48(1-2):55–63. doi: 10.1016/0167-0115(93)90335-6. [DOI] [PubMed] [Google Scholar]
- Halfter W., Deiss S. Axonal pathfinding in organ-cultured embryonic avian retinae. Dev Biol. 1986 Apr;114(2):296–310. doi: 10.1016/0012-1606(86)90194-6. [DOI] [PubMed] [Google Scholar]
- Hernández-Sánchez C., Frade J. M., de la Rosa E. J. Heterogeneity among neuroepithelial cells in the chick retina revealed by immunostaining with monoclonal antibody PM1. Eur J Neurosci. 1994 Jan 1;6(1):105–114. doi: 10.1111/j.1460-9568.1994.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Kahn A. J. An autoradiographic analysis of the time of appearance of neurons in the developing chick neural retina. Dev Biol. 1974 May;38(1):30–40. doi: 10.1016/0012-1606(74)90256-5. [DOI] [PubMed] [Google Scholar]
- Kajimoto Y., Rotwein P. Structure and expression of a chicken insulin-like growth factor I precursor. Mol Endocrinol. 1989 Dec;3(12):1907–1913. doi: 10.1210/mend-3-12-1907. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R. Non-parallel evolution of metabolic and growth-promoting functions of insulin. Nature. 1981 Aug 13;292(5824):644–646. doi: 10.1038/292644a0. [DOI] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993 Jul;13(7):2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Nissley S. P., Rechler M. M., Moses A. C., Short P. A., Podskalny J. M. Proinsulin binds to a growth peptide receptor and stimulates DNA synthesis in chick embryo fibroblasts. Endocrinology. 1977 Sep;101(3):708–716. doi: 10.1210/endo-101-3-708. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Nawa H. Neuronal differentiation factors/cytokines and synaptic plasticity. Cell. 1993 Jan;72 (Suppl):123–137. doi: 10.1016/s0092-8674(05)80032-7. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Prada Carmen, Puga José, Pérez-Méndez Luisa, López Rosario, Ramírez Galo. Spatial and Temporal Patterns of Neurogenesis in the Chick Retina. Eur J Neurosci. 1991 Jun;3(6):559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Påhlman S., Meyerson G., Lindgren E., Schalling M., Johansson I. Insulin-like growth factor I shifts from promoting cell division to potentiating maturation during neuronal differentiation. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9994–9998. doi: 10.1073/pnas.88.22.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Villamil B., de la Rosa E. J., Morales A. V., de Pablo F. Developmentally regulated expression of the preproinsulin gene in the chicken embryo during gastrulation and neurulation. Endocrinology. 1994 Dec;135(6):2342–2350. doi: 10.1210/endo.135.6.7988416. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993 Oct 29;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Rathjen F. G., Wolff J. M., Frank R., Bonhoeffer F., Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987 Feb;104(2):343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. C., Tager H. S., Rubenstein A. H. Biologic and clinical importance of proinsulin. N Engl J Med. 1984 May 3;310(18):1165–1175. doi: 10.1056/NEJM198405033101807. [DOI] [PubMed] [Google Scholar]
- Scavo L. M., Serrano J., Roth J., de Pablo F. Genes for the insulin receptor and the insulin-like growth factor I receptor are expressed in the chicken embryo blastoderm and throughout organogenesis. Biochem Biophys Res Commun. 1991 May 15;176(3):1393–1401. doi: 10.1016/0006-291x(91)90441-9. [DOI] [PubMed] [Google Scholar]
- Soos M. A., Field C. E., Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J. 1993 Mar 1;290(Pt 2):419–426. doi: 10.1042/bj2900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S. G., Robson J. A. An autoradiographic analysis of neurogenesis in the chick retina in vitro and in vivo. Neuroscience. 1989;32(3):801–812. doi: 10.1016/0306-4522(89)90300-x. [DOI] [PubMed] [Google Scholar]
- Tesoriere G., Calvaruso G., Vento R., Giuliano M., Lauricella M., Carabillò M. Insulin synthesis in chick embryo retinas during development. Neurochem Res. 1994 Jul;19(7):821–825. doi: 10.1007/BF00967450. [DOI] [PubMed] [Google Scholar]
- Travis J. Innovative techniques on display at Boston meeting. Science. 1993 Aug 27;261(5125):1112–1113. doi: 10.1126/science.8356444. [DOI] [PubMed] [Google Scholar]
- Tripathi B. J., Tripathi R. C., Livingston A. M., Borisuth N. S. The role of growth factors in the embryogenesis and differentiation of the eye. Am J Anat. 1991 Dec;192(4):442–471. doi: 10.1002/aja.1001920411. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Obrocka M. A., Lee V. M. Distribution of neurofilament subunits in neurons and neuronal processes: immunohistochemical studies of bovine cerebellum with subunit-specific monoclonal antibodies. J Histochem Cytochem. 1985 Jun;33(6):557–563. doi: 10.1177/33.6.3889140. [DOI] [PubMed] [Google Scholar]
- Yang Y. W., Brown D. R., Robcis H. L., Rechler M. M., de Pablo F. Developmental regulation of insulin-like growth factor binding protein-2 in chick embryo serum and vitreous humor. Regul Pept. 1993 Oct 20;48(1-2):145–155. doi: 10.1016/0167-0115(93)90343-7. [DOI] [PubMed] [Google Scholar]
- de Pablo F., Dashner R., Shuldiner A. R., Roth J. Xenopus laevis oocytes, eggs and tadpoles contain immunoactive insulin. J Endocrinol. 1994 Apr;141(1):123–129. doi: 10.1677/joe.0.1410123. [DOI] [PubMed] [Google Scholar]
- de Pablo F., de la Rosa E. J. The developing CNS: a scenario for the action of proinsulin, insulin and insulin-like growth factors. Trends Neurosci. 1995 Mar;18(3):143–150. doi: 10.1016/0166-2236(95)93892-2. [DOI] [PubMed] [Google Scholar]
- de la Rosa E. J., Bondy C. A., Hernández-Sánchez C., Wu X., Zhou J., López-Carranza A., Scavo L. M., de Pablo F. Insulin and insulin-like growth factor system components gene expression in the chicken retina from early neurogenesis until late development and their effect on neuroepithelial cells. Eur J Neurosci. 1994 Dec 1;6(12):1801–1810. doi: 10.1111/j.1460-9568.1994.tb00573.x. [DOI] [PubMed] [Google Scholar]