Abstract
Amniotic fluid (AF) contains a variety of cell types derived from fetal tissues that can easily grow in culture. These cells can be obtained during amniocentesis for prenatal screening of fetal genetic diseases, usually performed during the second trimester of pregnancy. Of particular interest, some expanded sub-populations derived from AF cells are capable of extensive self-renewal and maintain prolonged undifferentiated proliferation, which are defining properties of stem cells. These human AF stem cells (hAFSCs) exhibit multilineage potential and can differentiate into the three germ layers. They have high proliferation rates and express mesenchymal and embryonic markers, but do not induce tumor formation. In this study, hAFSCs derived from amniocentesis performed at 16–20 weeks of pregnancy were isolated, grown in culture, and characterized by flow cytometry and by their potential ability to differentiate into osteogenic, adipogenic, and chondrogenic lineages. After 4–7 passages, 5 × 105 hAFSCs were inoculated under the kidney capsule of Wistar rats that were subjected to an experimental model of chronic renal disease, the 5/6 nephrectomy model (Nx). After 30 days, Nx rats treated with hAFSCs displayed significant reductions in blood pressure, proteinuria, macrophages, and α-smooth muscle actin expression compared with Nx animals. These preliminary results suggest that hAFSCs isolated and expanded from AF obtained by routine amniocentesis can promote renoprotection in the Nx model. Considering that the AF cells not used for fetal karyotyping are usually discarded, and that their use does not raise ethical issues, they may represent an alternative source of stem cells for cell therapy and regenerative medicine.
Keywords: amniotic fluid stem cells, chronic kidney disease, mesenchymal stem cells
Stem cells (SCs) represent a potential and attractive cellular therapy for renal diseases. A large amount of research in this area has been provided through the use of adult stem cells, particularly mesenchymal SCs (mSCs). The main sources of mSCs include bone marrow (BMmSCs), peripheral blood umbilical cord blood, and other mesenchymal tissues, such as adipose tissue. The use of adult mSCs has some drawbacks and limitations. Besides the low number of mSCs that are available in the adult BM, which declines with increasing age, mSCs expand slowly and have a restricted differentiation potential.1 Pluripotent embryonic SCs have the potential to differentiate into a wide range of cell types, but their use in renal therapies involves ethical and technical problems, particularly the high risk of immunological rejection, teratoma formation, and carcinoma development.
An alternative source of SCs that has potential clinical therapeutic applications is amniotic fluid (AF). It is well known that AF contains various cell types, derived from embryonic and extra-embryonic tissues.2, 3 Human AF cells can be easily obtained during amniocentesis for prenatal diagnosis of fetal chromosomal abnormalities, routinely used in the last seven decades, with low risk for the mother and the fetus. For the screening of fetal genetic diseases, amniocentesis is usually performed during the second trimester, at 16–20 weeks of pregnancy. Amniotic fluid cells grow easily in culture. Of particular interest, some expanded sub-populations derived from AF cells are capable of extensive self-renewal and maintain prolonged undifferentiated proliferation, which are defining properties of SCs,4 and are termed human amniotic fluid stem cells (hAFSCs). The fetal origin of these cells collected from AF and cultured in vitro has been confirmed by human leukocyte antigen typing and by the identification of Y chromosome in the AF obtained by amniocentesis of mothers who are pregnant with male children.
CHARACTERISTICS OF AMNIOTIC FLUID CELLS
Amniotic fluid contains a wide variety of different cell types and properties that are mainly derived from fetal tissues.3 From the first trimester of pregnancy until the middle of the second trimester, AF is mostly formed by the passive movement of water through the fetal skin and amniotic membrane, as a result of simple diffusion and active transport of sodium and chloride, and is considered to be an ultrafiltrate of maternal blood. In this period, amniotic cells are derived from the sloughing of the yolk sac, fetus,5 placenta,6, 7 and amnion.3, 4, 6, 7, 8
Between the 17th and 20th week of gestation, keratinization of the fetal skin occurs, and most of the AF is composed of fetal urine9 and, to a minor extent, is derived from the fetal respiratory10 and digestive tracts.11 As a result of fluid dynamics, the cells in the AF are mainly derived not only from the urinary tract but also from respiratory and gastrointestinal tracts.
Amniotic fluid cells obtained by amniocentesis grow rapidly in routine culture. The adherent AF cells can be classified into three groups based on their morphological, biochemical, and growth characteristics: epithelioid E-type cells, AF-specific AF-type cells, and fibroblastic F-type cells. The epithelioid E-type cells (likely derived from the fetal skin) and the AF-specific AF-type cells (which produce estrogen, chorionic gonadotropin, and progesterone, and are likely derived from placental trophoblast tissue) are found in the initial passages of cultivation. The fibroblastic F-type cells (derived from fetal mesenchymal tissue and dermal fibroblasts) are adherent cells and exhibit characteristics of rapid proliferation with phenotypes and multilineage differentiation similar to BMmSCs.3, 4, 5, 12, 13
The adherent human AF cells expanded in culture have been extensively characterized by flow cytometry. Similar to BMmSCs, they express CD29, CD44 (hyaluronan receptor), CD73, CD90, CD105 (endoglin), CD166, and human leukocyte antigen class I, but not human leukocyte antigen class II.4, 14, 15, 16 They are multipotent cells, capable of differentiating into a variety of cell types including, adipogenic, osteogenic, and chondrogenic lineages.4, 14, 15, 17, 18 Owing to these characteristics, these cells are usually termed human AF mSCs (hAF-mSCs).4, 14, 15, 17, 18
Aside from mesenchymal markers, hAFSCs simultaneously express markers associated with pluripotency, such as Oct-4 (an embryonic SC marker), Nanog protein (responsible for pluripotency), and stage-specific embryonic antigen-4, which are specific markers of human embryonic cells.15, 19 Indeed, hAFSCs express genes characteristic of endodermal, mesodermal, and ectodermal germ layers,20 and exhibit, under specific culture conditions, the potential to differentiate into neuronal, myogenic, and hepatogenic cell lineages.15, 18, 21
Similar to the embryonic SCs that exhibit telomerase activity, hAFSCs also have high levels of telomerase. Telomerase activity protects against the progressive shortening of chromosomal telomeres, protecting against senescence. hAF-mSCs have significantly greater telomere length compared with BMmSCs,17, 22 providing them a great proliferative capacity. In fact, hAFSCs have a high expansion rate in vitro, with a proliferative rate in culture greater than adult mSCs.
Although hAFSCs have a marked high proliferative and differentiation capacity, they do not undergo neoplastic transformation in vitro17 or induce teratoma transformation similar to embryonic SCs. Accordingly, hAFSCs are not tumorigenic in vivo. Injections of hAF-mSCs into immunodeficient animals does not induce tumor formation,17 which is an additional advantage for their eventual clinical use in regenerative medicine.
Atala et al.23 isolated a sub-population of c-kit (CD117)-positive SC from AF cells, which represents about 1% of the total AF cell population. CD117 (the receptor for SC factor) is a cell surface marker expressed on human embryonic SCs, pluripotent hematopoietic progenitor cells, mast cells, and other somatic SCs. c-Kit+ cells can be immunoselected from AF and expanded in culture as a clonal cell line. They express the transcription factor Oct-4 and have the potential to differentiate into the three germ layers24 and form embryoid bodies.25 They also express surface markers characteristic of mSCs, including CD29, CD44, CD73, CD90, and CD105, and differentiate into adipogenic, osteogenic, myogenic, endothelial, neurogenic, and hepatic lineages. This fetal pluripotent lineage has a high proliferation rate with a doubling time of ∼36 h. c-kit+ hAFSCs do not induce tumor formation in mice.24
hAFSCs AND THE KIDNEY
Perin et al.26 analyzed the effect of c-kit+ hAFSCs on kidney differentiation by injecting these cells into isolated murine embryonic kidneys. After 10 days, histological analysis and live imaging showed that hAFSCs transfected with green fluorescent protein and Lac-Z integrated into kidney structures, including the tubules and developing nephrons.26 A possible role for hAFSCs in renal differentiation was provided by the marked expression of human zona occludens-1, claudin, and glial-derived neurotrophic factor within the developing kidney.26 Further evidence for the contribution of c-kit+ hAFSCs in renal differentiation was recently provided by Siegel et al.27 by cultivating labeled hAFSCs mixed with murine embryonic kidneys that had been dissociated into single cells. An analysis of the chimeric aggregates showed hAFSCs within the structures expressing PAX-2, E-cadherin, calbindin, Wt1, and laminin, which are markers found in renal structures.27
The potential ability of hAFSCs to undergo mesenchymal-to-epithelial transition was also demonstrated by the sequential cultivation of hAFSCs with epidermal growth factor/platelet-derived growth factor, and fibroblast growth factor-4/hepatocyte growth factor, which induced the expression of the epithelial markers CD51 and human zona occludens-1, and the podocyte markers CD2AP and NPHS2.28 Recently, Da Sacco et al.20 found different expression levels of the renal markers nephrin, human zona occludens-1, PDGF-αreceptor, glial-derived neurotrophic factor, aquaporin-1, PAX-2, LIM-1, occludin, tyrosine kinase A, E-cadherin, CD24, and OB-cadherin in AF cells obtained by amniocentesis between 15 and 20 weeks of pregnancy and cultured for 4–5 passages. Given that the AF in this period of gestation mostly consists of fetal urine, the cells with this renal marker expression profile in the AF are likely derived from the fetal kidney structures. Moreover, in this study, a specific AF cell population of nephron precursors characterized by the co-expression of CD24 and OB-cadherin (markers that are also expressed in the metanephric mesenchyma) was expanded in culture. On the basis of the expression of nephrin and podocalyxin (markers of podocytes), PDGF-αreceptor (mesangial cell marker), E-cadherin (MET marker), and tyrosine kinase A (stromogenic cortical mesenchymal cell marker), five sub-populations of renal progenitor cells could be identified in the AF.
The effect of hAFSCs administration in an experimental model of acute kidney injury has already been analyzed. Perin et al.29 showed that the intra-renal injection of c-kit+ hAFSCs into the glycerol-induced rhabdomyolysis model of acute tubular necrosis ameliorated kidney injury, as demonstrated by decreased blood urea nitrogen and creatinine levels, and provided protection against tubular structural damage cast formation. The protective effect of c-kit+ hAFSCs administration was possibly related to an induction of cellular proliferative activity and a significant decrease in apoptosis. Additional renoprotection might have been obtained by the induction of immunomodulatory effects, characterized by a decrease in proinflammatory cytokines and an increase in anti-inflammatory cytokines, facilitating the control of local immune responses.29
INFUSION OF hAFSCs INTO AN EXPERIMENTAL MODEL OF CHRONIC RENAL DISEASE
We and others have shown the beneficial effects of BMmSCs in experimental models of chronic progressive nephropathy, such as the remnant kidney model.30, 31 We are currently analyzing the potential therapeutic use of hAF-mSCs in an experimental model of chronic kidney disease by analyzing the administration of hAF-mSCs versus BMmSCs to the 5/6 nephrectomy model (Nx).
For this study, AF cells from second-trimester amniocentesis (16–20 weeks of gestation), recommended by advanced maternal age, were obtained at the Clinical Hospital of the University of São Paulo for prenatal diagnosis of fetal genetic mutations. The first sample was discarded because of the risk of contamination with maternal cells. The second aspirated sample consisted of approximately 30 ml of AF and was divided into two samples, one (20 ml) was processed for fetal karyotyping and the other (10 ml) for the study. Only karyotypically normal cases were included in this study. The study protocol was approved by the Local Ethics Committee (0414/2007), and all of the participants signed a written informed consent.
The AF cells, after centrifugation, were placed into a culture flask with α-minimum essential Eagle's medium (Gibco Division, Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (Gibco), 100 U/ml of penicillin, and 100 μg/ml of streptomycin.
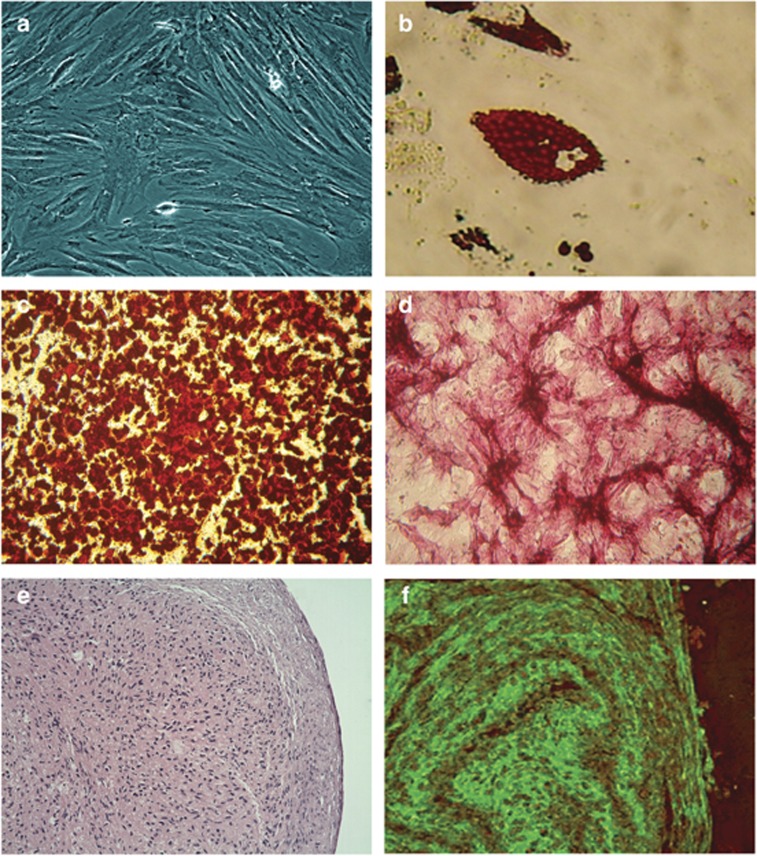
After 72 h, the non-adherent AF cells in the supernatant were removed, and the cells that adhered to the plastic culture flasks were expanded. After reaching 70% confluence, they were detached with trypsin/ethylenediaminetetraacetic acid. The cells exhibited fibroblast-like and spindle-shaped morphologies (Figure 1a).
Figure 1.
The capacity of human amniotic fluid stem cells (hAFSCs) to differentiate into osteogenic, adipogenic, and chondrogenic lineages. (a) In culture, hAFSCs have fibroblast-like morphology. (b) Adipogenic differentiation was evaluated using Oil Red O staining. Cytoplasmic lipid droplets appear red ( × 400). (c, d) Osteogenic differentiation was confirmed by Alizarin red and alkaline phosphatase staining. Calcium deposits and alkaline phosphatase activity appear red ( × 100 and × 400, respectively). (e, f) Chondrogenic differentiation was confirmed by hematoxylin and eosin and immunofluorescence staining using specific monoclonal antibodies against type II collagen ( × 200 and × 400, respectively).
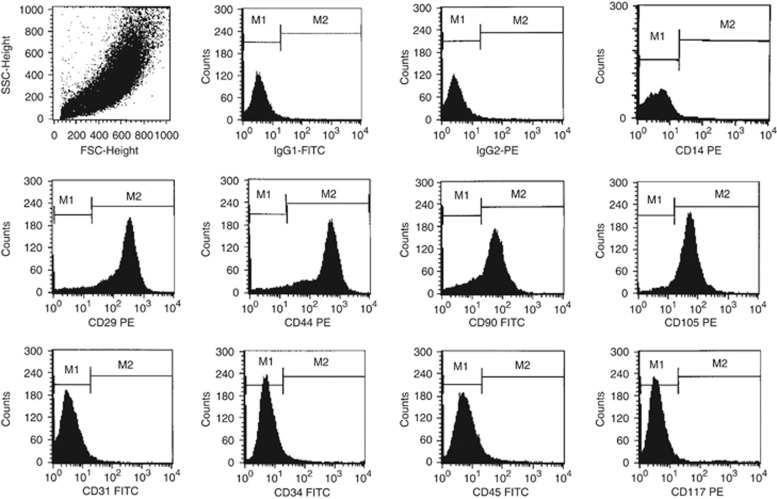
The cells were phenotypically characterized by flow cytometry using fluorescein isothiocyanate- or phycoerythrin-conjugated antibodies. The adherent AF-SCs were positive for the surface markers that are characteristic of mSCs (CD29: 98.0±0.6%, CD44: 96.8±1.0%, CD90: 67.0±3.0%, and CD105: 89.9±4.7%). Only a small percentage of the hAFSCs expressed CD45 (2.1±0.7%, hematopoietic lineage), CD34 (1.3±0.2%, (hematopoietic SC), CD117 (1.5±0.3%, c-kit), and CD31 (1±0.2%, endothelial cells; Figure 1).
The hAFSCs were further characterized on the basis of their multipotent potential for osteogenic, adipogenic, and chondrogenic differentiation, as revealed by positive staining for Alizarin red, alkaline phosphatase, Oil Red O, and hematoxylin, eosin, and immunofluorescence for type II collagen (Figure 2). In addition, RT-PCR experiments demonstrated that these hAFSCs express Oct-4 4/5 and NANOG.
Figure 2.
Surface antigen evaluation by flow cytometry. The human amniotic fluid stem cells (hAFSCs) were positive for CD29, CD44, CD90, and CD105; and negative for CD14, CD31, CD34, CD45, and CD117. IgG1-fluorescein isothiocyanate (FITC) and IgG2-phycoerythrin (PE) antibodies were used as isotype controls.
To investigate the effects of hAF-mSCs on an experimental model of chronic progressive renal disease, hAFSCs were administered to rats subjected to the remnant kidney model. This was compared with an infusion with rat BMmSCs, as previously described.30 Male Wistar rats (n=44) were subjected to a Nx or sham operation. hAF-mSCs (5 × 105 cells) or BMmSCs (5 × 105 cells) at the fourth to seventh cellular passage were inoculated into the kidney subcapsule and the rats were observed by 30 days. The animals were subdivided into six groups: S, sham-operated rats (n=5), S+hAFSCs (n=6), sham rats receiving hAFSCs; S+BMmSCs (n=5), sham rats receiving BMmSCs; Nx (n=9), rats subjected to Nx; Nx+hAFSCs (n=8), Nx rats receiving hAFSCs, and Nx+BMmSCs (n=11), Nx rats receiving BMmSCs. The following parameters were analyzed: blood pressure, urinary protein excretion, serum creatinine, and the glomerulosclerosis index. In addition, kidney infiltration of ED1+cells (macrophages; M), CD43+ cells (lymphocytes), and α-smooth muscle actin (myofibroblasts) was analyzed by immunohistochemistry.
The inoculation of hAFSCs into the kidney subcapsular region of rats subjected to a Nx induced renoprotective effects, characterized by a significant reduction in blood pressure, urinary protein excretion, and the percentage of glomerulosclerosis, similar to the results observed after the administration of BMmSCs (Table 1). However, the marked effect of hAFSCs in reducing the percentage of α-smooth muscle actin, which identifies myofibroblasts, is of relevance, considering that they are the effector cells of renal fibrogenesis and that myofibroblast infiltration correlates with the progression of renal disease (Table 2).
Table 1. Clinical, biochemical, and histological parameters of Nx rats and the controls, which received BMmSCs or hAFSCs.
| Blood pressure (mm Hg) | Urinary protein (mg/24 h) | Serum creatinine (mg/dl) | Glomerulosclerosis index (%) | |
|---|---|---|---|---|
| Sham | 123±3 | 10.9±1 | 0.46±0.03 | 0.3±0.3 |
| Sham+BMmSCs | 132±4 | 7.7±1 | 0.27±0.05 | 1.9±1.1 |
| Sham+hAFSCs | 119±6 | 9.8±2 | 0.54±0.03 | 3.6±2.7 |
| Nx | 185±4a,b,c | 92.9±14a,b,c | 1.05±0.15a,b,c | 26.3±5.5a,b,c |
| Nx+BMmSCs | 162±7a,b,c,d | 31.9±6f | 0.62±0.09d | 5.4±2.4e |
| Nx+hAFSCs | 152±5a,c,f | 39.5±12e | 0.74±0.08c | 5.2±4.5e |
Abbreviations: BMmSCs, bone marrow mesenchymal stem cells; hAFSCs, human amniotic fluid stem cells; Nx, 5/6 nephrectomy model.
Data are presented as mean±s.e.m.
aP<0.01 versus sham.
bP<0.01 versus sham+BMmSCs.
cP<0.05 versus sham+hAFSCs.
dP<0.05 versus Nx.
eP<0.01 versus Nx.
fP<0.001 versus Nx.
Table 2. α-Smooth muscle actin, macrophages, and lymphocytes in kidney specimens from Nx rats and the controls, which received BMmSCs or hAFSCs.
| α-SMA (%) | M (cell/mm2) | Lymphocytes (cell/mm2) | |
|---|---|---|---|
| Sham | 0.3±0.1 | 17±3 | 13±3 |
| Sham+BMmSCs | 0.5±0.2 | 19±3 | 20±5 |
| Sham+hAFSCs | 0.1±0.0 | 9±4 | 67±16a,b |
| Nx | 6.3±0.7a,b,c | 50±8a,b,c | 33±2 |
| Nx+BMmSCs | 2.7±0.4d | 35±2 | 25±2 |
| Nx+hAFSCs | 0.3±0.1d,e | 18±3d | 70±8a,b,e |
Abbreviations: α-SMA, α-smooth muscle actin; BMmSCs, bone marrow mesenchymal stem cells; hAFSCs, human amniotic fluid stem cells; M, macrophages; Nx, 5/6 nephrectomy model.
aP<0.01 versus sham.
bP<0.01 versus sham+BMmSCs.
cP<0.001 versus sham+hAFSCs.
dP<0.001versus Nx.
eP<0.05versus Nx+BMmSCs.
A significant reduction in the number of macrophages was also observed in the Nx animals that received hAFSCs, whereas the BMmSCs inoculation was not associated with this cellular effect in this model. In addition, the administration of hAFSCs induced a significant increase in the number of lymphocytes in the kidney reflecting an ongoing in situ immune response, particularly in the cases transplanted with hAFSCs (Table 2). The characterization of the lymphocyte subsets, cytotoxic mediators, and cytokine production profile will help characterize this pattern of the immune response. Previous studies using a similar model of discordant cellular xenotransplantation reported the presence of an inflammatory infiltrate in the myocardium, suggestive of acute rejection in both immunocompetent rats (with or without immunosuppression) and athymic rats.32
A pertinent issue in the field of cell therapy is defining to what extent the presence of an inflammatory infiltrate can be regarded as definitive evidence of rejection. BMmSCs have previously been shown to have immunosuppressive and immunoregulatory properties. The low expression of class II major histocompatibility complex molecules and the absence of co-stimulatory molecules may contribute to their roles as immunoprivileged cells, allowing them to escape the recognition of alloreactive cells.33, 34 The immunoregulatory effects may also rely on their ability to induce the production of anti-inflammatory cytokines and growth factors35 and generate CD4+ CD25+ regulatory T cells.36 Thus, the presence of an inflammatory infiltrate does not represent evidence for allograft rejection.
In conclusion, these preliminary results suggest that hAFSCs that are successfully isolated and expanded from the AF obtained by routine amniocentesis can promote renoprotection in the Nx model. Considering that the AF cells not used for fetal karyotyping are usually discarded, and that their use does not raise ethical issues, they may represent an alternative source of SCs for cell therapy and regenerative medicine.
All the authors declared no competing interests.
Footnotes
TO CITE THIS ARTICLE: Noronha IL, Cavaglieri RC, Janz FL et al. The potential use of stem cells derived from human amniotic fluid in renal diseases. Kidney inter., Suppl. 2011; 1: 77–82.
References
- Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Priest RE, Marimuthu KM, Priest JH. Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab Invest. 1978;39:106–109. [PubMed] [Google Scholar]
- Gosden CM. Amniotic fluid cell types and culture. Br Med Bull. 1983;39:348–354. doi: 10.1093/oxfordjournals.bmb.a071847. [DOI] [PubMed] [Google Scholar]
- In't Anker PS, Scherjon SA, Kleijburg-van der KC, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- Hoehn H, Salk D. Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods Cell Biol. 1982;26:11–34. doi: 10.1016/s0091-679x(08)61362-x. [DOI] [PubMed] [Google Scholar]
- Brace RA. Amniotic fluid volume and its relationship to fetal fluid balance: review of experimental data. Semin Perinatol. 1986;10:103–112. [PubMed] [Google Scholar]
- Seeds AE. Current concepts of amniotic fluid dynamics. Am J Obstet Gynecol. 1980;138:575–586. doi: 10.1016/0002-9378(80)90289-6. [DOI] [PubMed] [Google Scholar]
- Trounson A. A fluid means of stem cell generation. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt0107-62. [DOI] [PubMed] [Google Scholar]
- Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25:341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- Duenhoelter JH, Pritchard JA. Fetal respiration: quantitative measurements of amnionic fluid inspired near term by human and rhesus fetuses. Am J Obstet Gynecol. 1976;125:306–309. doi: 10.1016/0002-9378(76)90564-0. [DOI] [PubMed] [Google Scholar]
- Minei LJ, Suzuki K. Role of fetal deglutition and micturition in the production and turnover of amniotic fluid in the monkey. Obstet Gynecol. 1976;48:177–181. [PubMed] [Google Scholar]
- Fauza D. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:877–891. doi: 10.1016/j.bpobgyn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Hengstschlager M. Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit. 2002;8:253–257. [PubMed] [Google Scholar]
- Tsai MS, Lee JL, Chang YJ, et al. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- Roubelakis MG, Pappa KI, Bitsika V, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:931–952. doi: 10.1089/scd.2007.0036. [DOI] [PubMed] [Google Scholar]
- Bossolasco P, Montemurro T, Cova L, et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–336. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- Sessarego N, Parodi A, Podesta M, et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93:339–346. doi: 10.3324/haematol.11869. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee Y, Kim H, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, et al. Oct4 expressing cells in human amniotic fluid: a new source for stem cell research. Hum Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- Da Sacco S, Sedrakyan S, Boldrin F, et al. Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. J Urol. 2010;183:1193–1200. doi: 10.1016/j.juro.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coppi P, Callegari A, Chiavegato A, et al. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol. 2007;177:369–376. doi: 10.1016/j.juro.2006.09.103. [DOI] [PubMed] [Google Scholar]
- Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Valli A, Rosner M, Fuchs C, et al. Embryoid body formation of human amniotic fluid stem cells depends on mTOR. Oncogene. 2010;29:966–977. doi: 10.1038/onc.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin L, Giuliani S, Jin D, et al. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40:936–948. doi: 10.1111/j.1365-2184.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel N, Rosner M, Unbekandt M, et al. Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum Mol Genet. 2010;19:3320–3331. doi: 10.1093/hmg/ddq236. [DOI] [PubMed] [Google Scholar]
- Siegel N, Valli A, Fuchs C, et al. Induction of mesenchymal/epithelial marker expression in human amniotic fluid stem cells. Reprod Biomed Online. 2009;19:838–846. doi: 10.1016/j.rbmo.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Perin L, Sedrakyan S, Giuliani S, et al. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One. 2010;5:9357–9373. doi: 10.1371/journal.pone.0009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaglieri RC, Martini D, Sogayar MC, et al. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc. 2009;41:947–951. doi: 10.1016/j.transproceed.2009.01.072. [DOI] [PubMed] [Google Scholar]
- Caldas HC, Fernandes IMM, Gerbi F, et al. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853–855. doi: 10.1016/j.transproceed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Chiavegato A, Bollini S, Pozzobon M, et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol. 2007;42:746–759. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- Tse W, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implication in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Ye Z, Wang Y, Xie HY, et al. Immunosuppressive effects of rat mesenchymal stem cells: involvement of CD4+CD25+ regulatory T cells. Hepatobiliary Pancreat Dis Int. 2008;7:608–614. [PubMed] [Google Scholar]