Abstract
The combination of serum 25-hydroxyvitamin D (25D) and fibroblast growth factor 23 (FGF23) levels predict hard renal outcomes in patients with chronic kidney disease (CKD), independent of classical markers of mineral and bone disorders, including serum phosphorus, parathyroid hormone, 1,25-dihydroxyvitamin D levels, and active vitamin D therapy. In a prospective cohort study of 738 Japanese pre-dialysis outpatients with CKD, we examined potentially non-linear associations between 25D and FGF23 levels and estimated glomerular filtration rate (eGFR) changes in 727 patients with at least a 6-month observation period and no history of admission by acute kidney injury. We used multiple regression analyses with restricted cubic spline functions using annualized eGFR decline as a dependent variable. A significantly non-linear positive relationship between 25D and eGFR changes was observed. The annualized eGFR decline was greater in patients with 25D concentrations <25 and 23 ng/ml in univariate and multivariate analyses, respectively. Above this threshold, the eGFR decline plateaued. FGF23 showed a linear negative association with eGFR changes. After dividing the patients into four groups according to median 25D and FGF23 levels, the annualized eGFR changes in the Low FGF23-Low 25D, High FGF23-High 25D, and High FGF23-Low 25D groups were 0.49 (95% confidence intervals: −2.83 to 3.81), −1.24 (−5.00 to 2.52), −4.77 (−8.85 to −0.69), respectively, relative to the Low FGF23-High 25D group (P for trend, 0.02). Thus, combined use of FGF23 and 25D is useful to predict eGFR change in patients with CKD as well as hard renal outcomes.
Keywords: eGFR slope; FGF23; vitamin D; 25-hydroxyvitamin D; 1,25-dihydroxyvitamin D
Mineral bone disorder (MBD) is prevalent in patients undergoing maintenance dialysis treatment as well as those with chronic kidney disease (CKD) not requiring renal replacement therapy.1 Numerous studies2, 3, 4, 5, 6 have shown that laboratory abnormalities related to CKD-MBD such as elevated serum phosphate levels, when studied separately, predict poor renal outcomes in addition to cardiovascular outcomes. With extensive adjustment of all CKD-MBD-related factors, including serum 1,25-dihdroxyvitamin D (1,25(OH)2D) levels, we previously reported that only 25-hydroxyvitamin D (25D) and fibroblast growth factor 23 (FGF23) levels were predictors of renal outcomes (doubling of serum creatinine level and/or initiation of dialysis) in Japanese patients with CKD.7 Furthermore, two large studies from the United States8, 9 and one from Europe4 have also shown the prognostic value of FGF23 levels for progression to end-stage renal disease in CKD after adjustment for MBD-related factors. Although vitamin D deficiency was reported to be a predictor of adverse hard renal outcomes, studies on its association with changes in GFR are scarce.
The end point of dialysis initiation is critical, as end-stage renal disease is defined as the CKD stage that is dependent on renal replacement therapy, which is often used in epidemiological studies as the gold standard of hard renal outcomes. However, this end point is somewhat subjective in that the timing of dialysis initiation is usually determined by primary physicians. Moreover, it is not necessarily a ‘renal' outcome, as the timing is often dependent not only on renal function but also on the extent of heart function (failure).10 This is very problematic especially when studying the relationship between FGF23 levels and the timing of dialysis initiation, because higher FGF23 levels are associated with cardiovascular events, particularly congestive heart failure.11 From this point of view, changes in the glomerular filtration rate (GFR) represent a truly objective ‘renal' outcome, albeit a soft outcome.
A non-linear relationship between 25D levels and estimated GFR (eGFR) changes was reported in the general population aged 65 years (mean eGFR, 74.5 ml/minper1.73 m2).12 In this study, lower 25D levels were monotonically associated with more rapid eGFR decline, particularly at levels approximately <30 ng/ml. However, no report exists in patients with CKD regarding its association with the eGFR slope. The relationship between FGF23 levels and eGFR changes is also poorly understood in CKD patients, whereas its association with adverse renal outcomes has been demonstrated.8, 9 A recent study reported that C-terminal FGF23 levels predict annual loss of eGFR in patients with immunoglobulin A nephropathy.13 However, it was a relatively small-sized study, and the linearity of this association remains unknown.
In CKD, the associations between MBD-related parameters and changes in eGFR remain unclear. In this study, we assessed the relationships between these two MBD-related biomarkers (25D and FGF23) and annualized eGFR decline in CKD.
RESULTS
The baseline data of this study are summarized in Table 1. Briefly, about 20% of the cohort had diabetes and 20% had previous CVD at enrollment. Mean eGFR was 35 ml/minper1.73 m2. More than 95% (n=695) of the subjects had complete data without missing values.
Table 1. Characteristics of the study population.
|
Characteristics of the study population (N=727) | |
| Demographic characteristics | |
| Age (years) | 64 (54, 72) |
| Sex (female) | 261 (35.9%) |
| Body mass index (kg/m2) | 23.2±3.47 |
| Diabetes mellitus | 138 (19.0%) |
| Previous CVD | 160 (22.0%) |
| Systolic blood pressure (mm Hg) | 132±18.9 |
| Diastolic blood pressure (mm Hg) | 77.5±10.9 |
| Pulse pressure (mm Hg) | 54.8±15.4 |
| ACE-I/ARB | 532 (73.2%) |
| Active vitamin D | 42 (5.8%) |
| Calcium carbonate | 30 (4.1%) |
| Baseline laboratory test results | |
| Serum creatinine (mg/dl) | 2.05±1.45 |
| eGFR (ml/minper1.73 m2) | 35.3±19.1 |
| CKD stage | |
| CKD stage 1 (eGFR 90) | 3 (0.4%) |
| CKD stage 2 (eGFR 60–89) | 92 (12.7%) |
| CKD stage 3 (eGFR 30–59) | 304 (41.8%) |
| CKD stage 4 (eGFR 15–29) | 219 (30.1%) |
| CKD stage 5 (eGFR<15) | 109 (15.0%) |
| Urinary protein | |
| Urinary protein (−) | 166 (27.3%) |
| Urinary protein (±) | 88 (14.5%) |
| Urinary protein (1+) | 133 (21.8%) |
| Urinary protein (2+) | 136 (22.3%) |
| Urinary protein >(3+) | 86 (14.1%) |
| Corrected calcium (mg/dl) | 9.31±0.57 |
| Phosphate (mg/dl) | 3.49±0.72 |
| Whole PTH (pg/ml) | 19.6 (11.0, 38.2) |
| 25-Hydroxyvitamin D (ng/ml) | 23.5±6.0 |
| 1,25-Dihydroxyvitamin D (pg/ml) | 37.9±18.7 |
| Fibroblast growth factor 23 (pg/ml) | 49 (31.4, 78.8) |
| Albumin (g/dl) | 3.95±0.40 |
| Hemoglobin (g/dl) | 12.4±2.1 |
ACE-I/ARB, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone.
Values are presented as numbers (%), mean±s.d., or median (interquartile range).
During a median follow-up period of 4.4 (IQR, 4.0–4.6) years, 58 (7.9%) patients died, 81 (11.0%) were lost to follow-up, 156 reached a doubling of serum creatinine level, and 146 started dialysis. The mean (s.d,) annualized loss of eGFR was −2.06 (3.12) ml/minper1.73 m2.
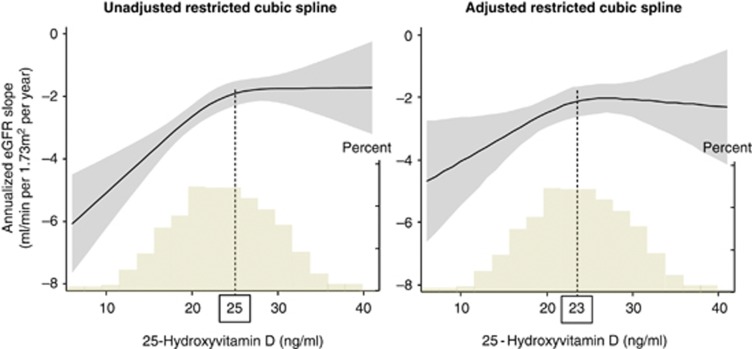
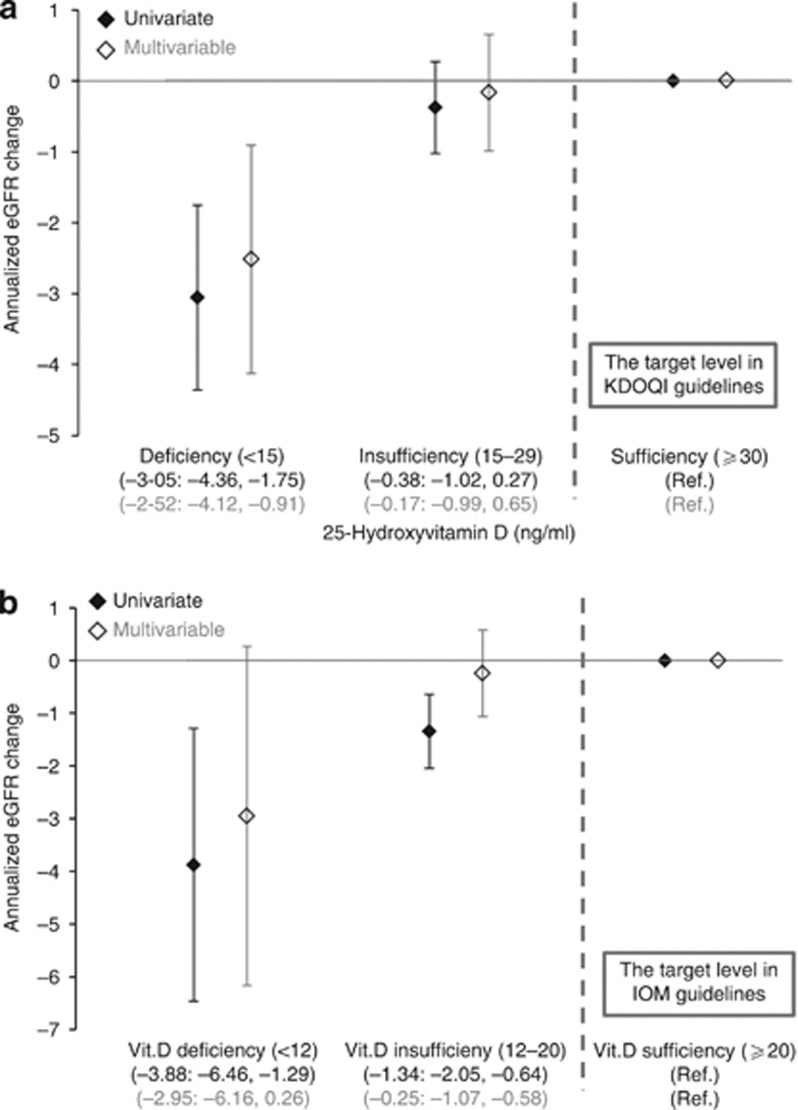
Lower 25D concentrations were associated with more rapid eGFR decline both in the univariate and multivariate analyses (Figure 1). In the univariate analysis, this association proved to be significantly non-linear (P for linearity, 0.017) with a cutoff value below which lower 25D levels were associated with steeper eGFR decline. We estimated the threshold of 25D level at which the slope of this association curve departed significantly from zero by computing the first derivative. The first derivative here means the slope of tangent lines to the cubic spline curves as a function of 25D levels. The flection points were 25 and 23 ng/ml in the univariate and multivariate analyses, respectively. The analyses using the 25D categories based on the KDOQI or IOM guidelines confirmed our findings (Figure 2). For example, patients with 25D levels <15 ng/ml had a significantly steeper eGFR decline compared with patients with vitamin D sufficiency according to the KDOQI criteria (30 ng/ml) (Figure 2a).
Figure 1.
Unadjusted and adjusted associations of 25-hydroxyvitamin D (25D) with annualized estimated glomerular filtration rate (eGFR) decline. Shaded areas represent 95% confidence intervals. Models were performed using restricted cubic splines with four knots. Histograms represent the distribution of 25D in the study population. Annualized eGFR decline was greater in patients with 25D below the concentration of 25 and 23 ng/ml in univariate and multivariate analyses, respectively. In the multivariate analysis, we adjusted for age, sex, diabetes mellitus, previous cardiovascular disease, systolic blood pressure, hemoglobin, albumin, urinary protein, eGFR, corrected calcium, phosphorus, 1,25-dihydroxyvitamin D, whole parathyroid hormone, the seasons of blood sampling, and administration of calcium carbonate, active vitamin D, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Figure 2.
Annualized estimated glomerular filtration rate (eGFR) decline according to 25-hydroxyvitamin D (25D) categories by (a) Kidney Disease Outcomes Quality Initiative (KDOQI) and (b) Institute of Medicine (IOM) guidelines. Patients with vitamin D deficiency presented greater eGFR loss. We adjusted for age, sex, diabetes mellitus, previous cardiovascular disease, systolic blood pressure, hemoglobin, albumin, urinary protein, eGFR, corrected calcium, phosphorus, 1,25-dihydroxyvitamin D, whole parathyroid hormone, the seasons of blood sampling, and administration of calcium carbonate, active vitamin D, and angiotensin converting-enzyme inhibitors or angiotensin receptor blockers.
When analyzing the association between 25D levels and eGFR slope, urinary protein levels might be a confounder, as 25D is lost in the urine with D-binding protein and proteinuria per se results in tubulointerstitial injury, leading to the loss of kidney function. Therefore, we adjusted for urinary protein levels. However, representing urinary protein as a categorical variable (by dipstick) might result in residual confounding. Therefore, we restricted the analysis of patients with mild proteinuria (<[2+]) as a sensitivity analysis because of no significant relationship between 25D levels and proteinuria in patients with mild proteinuria (<[2+]) in our study (P=0.42, Kruskal–Wallis).14 Again, in the univariate analysis, we confirmed a similar association between 25D levels and annualized eGFR decline (data not shown).
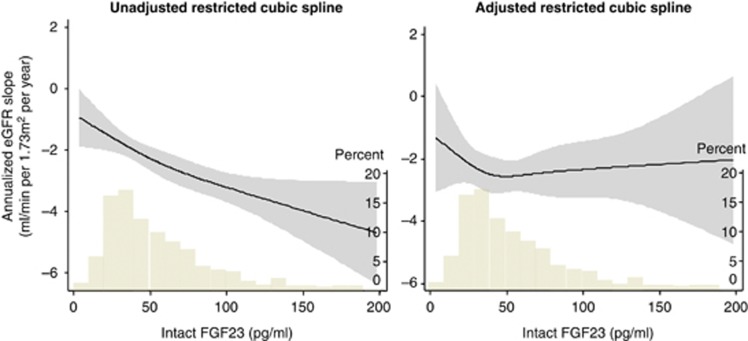
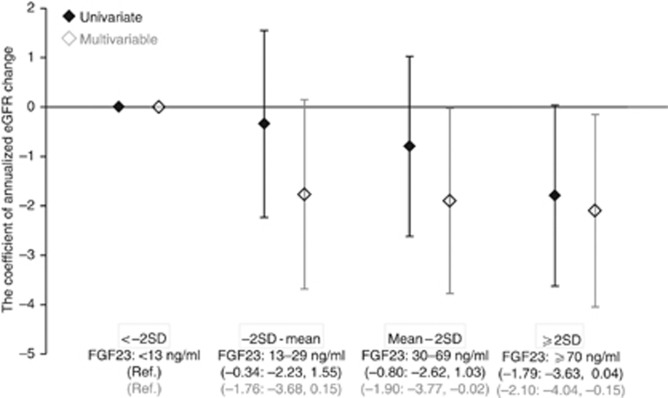
We observed linear negative associations between FGF23 levels and eGFR changes both in the univariate and multivariate analyses (Figure 3, P-value for linearity, 0.427 and 0.463, respectively). Similar data were obtained when using categorical variables of FGF23 (Figure 4).
Figure 3.
Unadjusted and adjusted associations of fibroblast growth factor 23 (FGF23) with annualized estimated glomerular filtration rate (eGFR) decline. Shaded areas represent 95% confidence intervals. Models were performed using restricted cubic splines with three knots. Histograms represent the distribution of FGF23 in the study population. Annualized eGFR decline was greater in patients with higher FGF23 levels. In the multivariate analysis, we adjusted for age, sex, diabetes mellitus, previous cardiovascular disease, systolic blood pressure, hemoglobin, albumin, urinary protein, eGFR, corrected calcium, phosphorus, 1,25-dihydroxyvitamin D, whole parathyroid hormone, the seasons of blood sampling, and administration of calcium carbonate, active vitamin D, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Figure 4.
Annualized estimated glomerular filtration rate (eGFR) decline according to fibroblast growth factor 23 (FGF23) categories. FGF23 concentrations of 13, 30, and 70 are −2 s.d., mean, and 2 s.d. of healthy individuals, respectively. Patients with high FGF23 (2 s.d. of healthy individuals) presented greater eGFR loss. In the multivariate analysis, we adjusted for age, sex, diabetes mellitus, previous cardiovascular disease, systolic blood pressure, hemoglobin, albumin, urinary protein, eGFR, corrected calcium, phosphorus, 1,25-dihydroxyvitamin D, whole parathyroid hormone, the seasons of blood sampling, and administration of calcium carbonate, active vitamin D, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
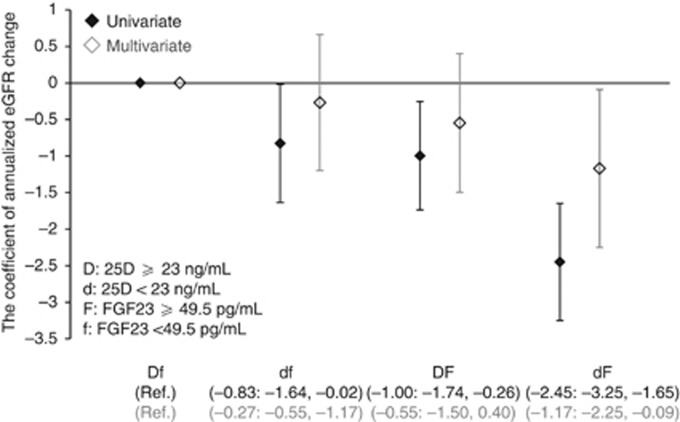
After dividing the patients into four groups based on the median 25D and FGF23 levels, the annualized eGFR changes (95% confidence intervals (CIs)) in the Low FGF23-Low 25D, High FGF23-High 25D, and High FGF23-Low 25D groups were 0.49 (−2.83 to 3.81), −1.24 (−5.00 to 2.52), −4.77 (−8.85 to −0.69), respectively, relative to the Low FGF23-High 25D group (P for trend, 0.02;) (Figure 5).
Figure 5.
Annualized estimated glomerular filtration rate (eGFR) decline and 95% confidence intervals in the patients categorized by fibroblast growth factor 23 (FGF23) and 25-hydroxyvitamin D (25D) median levels. The high FGF23/low 25D group had significantly greater decline in eGFR compared with the low FGF23/high 25D group as a reference. This model was adjusted for age, sex, diabetes mellitus, previous cardiovascular disease, systolic blood pressure, hemoglobin, albumin, urinary protein, eGFR, corrected calcium, phosphorus, 1,25-dihydroxyvitamin D, whole parathyroid hormone, the seasons of blood sampling, and administration of calcium carbonate, active vitamin D, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
As sensitivity analyses, we excluded patients with an observation period of 1 year and then used multiple imputation method for missing values. Similar results were obtained in both analyses (data not shown).
DISCUSSION
In this Japanese cohort study of CKD patients who were not yet receiving dialysis therapy, we observed a non-linear positive association between 25D levels and annualized eGFR changes and a linear negative association between FGF23 levels and eGFR changes. The vitamin D status-related association showed threshold effects, with a detrimental effect observed only within its lower ranges.
Our finding of the positive association between 25D levels and GFR changes was previously reported among community-dwelling older adults12 and renal transplant recipients. Bienaime et al.15 recently reported in a prospective cohort of 634 kidney transplant recipients that a low 25D concentration measured 3 months after transplantation was an independent risk factor for interstitial fibrosis progression confirmed by renal biopsy and was associated with a lower measured GFR 1 year after transplantation. However, in African-American patients with CKD, 25D levels did not predict renal outcome.16 Recently, racial differences were reported to have a role in the association of serum 25D concentration with coronary heart disease events, in that lower serum 25D concentration was associated with an increased risk of incident coronary heart disease events among participants who were white or Chinese but not black or Hispanic.17 Similar phenomena might apply to renal outcome due to genetic variants of vitamin D receptor.18 Our data clearly indicate that 25D levels predict hard and soft renal outcomes in CKD, at least in the Japanese CKD patient population.
There has been much debate as to the optimal 25D concentration. Recent IOM guidelines recommend a concentration of 20 ng/ml,19 as a recent systematic review showed that 30 ng/ml 25D is not consistently associated with improved bone health.20 In contrast to the IOM report, the International Osteoporosis Foundation 2010 position paper on vitamin D recommends a threshold of 75 nmol/l (30 ng/ml) for optimal fall and fracture reduction.21 The recent Kidney Disease Improving Global Outcomes guideline for CKD-MBD does not specify the optimal level of 25D, although the authors recommend nutritional vitamin D administration for patients with intact PTH levels above the upper normal limit of the assay and with vitamin D deficiency.22 It should be noted that this recommendation is based on the control of PTH, not for renal benefit. Regarding renal outcomes, our study sheds light on the optimal level of 25D. The flection point was 25 and 23 ng/ml in the univariate and multivariate analyses, respectively. Therefore, at least 20 ng/ml 25D may be necessary, and 25 ng/ml may be sufficient for improved renal outcomes.
Our results suggest that vitamin D supplementation and any measures that decrease FGF23 levels have potential renal function benefits. However, they should be interpreted with caution, because this investigation was only an observational study. As observational studies cannot confer a causal relationship, intervention studies are required to clearly show the benefit of nutritional vitamin D supplementation or phosphate binder use. A recent small, uncontrolled study showed that oral cholecalciferol administration decreases albuminuria and urinary transforming growth factor β levels in patients with type 2 diabetic nephropathy taking renin–angiotensin–aldosterone (RAAS) system inhibitors.23 In the field of transplantation, we have launched a randomized, controlled study of nutritional vitamin D and continuous erythropoietin receptor activator (CERA) (CANDLE-KIT trial) in anemic recipients, which will address this issue (http://clinicaltrials.gov/ct2/ show/NCT01817699?term=NCT01817 699&rank=1).
Intra-renal RAAS activation, which has been shown to reduce the expression of FGF23 co-receptor Klotho,24 might explain the observed linear negative relationship between FGF23 levels and annualized eGFR changes, as the reduction of Klotho presumably leads to increased FGF23 expression via increased FGF23 resistance on the proximal tubules. Alternatively, increased FGF23 levels might be a surrogate biomarker of tubulointerstitial injury that predicts CKD progression,25 given that high serum levels of FGF23 are detected in patients with autosomal-dominant polycystic kidney disease, where cyst-induced tubular damage is observed.26, 27
No relationship between 25D levels and eGFR change in patients with 25D >25 and >23 ng/ml in univariate and multivariate analyses, respectively, implies no clinical usefulness of 25D for prediction of this soft renal outcome in this sufficient range of 25D. However, even in this range of 25D, the addition of FGF23 improved prediction of eGFR. Figure 5 shows that eGFR change in the Group DF was significantly lower than that in the Group Df by about 1 ml/min/1.73 m2 in univariate analysis, which is a clinically relevant difference. This is also true in patients with 25D <23 ng/ml, as the difference in eGFR change between the Groups dF and df exceeded 1.5 ml/min/1.73 m2. Thus, the combined use of 25D and FGF23 is more useful than the use of either alone to predict eGFR decline.
Our study has several limitations. First, we did not perform direct measurements of GFR. Second, our ability to ascertain the impact of 25(OH)D and FGF23 concentration on eGFR changes may have been limited by the use of only one follow-up measurement of eGFR. Third, as with all studies of changes in GFR, our study is subject to potential survival bias. However, only 8% of the enrolled patients died during the entire observation period. The strengths of this study include its large population size and reduction of potential confounds by adjustment for well-defined potential confounding variables, particularly for MBD markers such as serum phosphorus levels, 1,25(OH)2D levels, and active vitamin D therapy. Therefore, the effect of vitamin D status on the eGFR slope was independent of serum 1,25(OH)2D levels and active vitamin D therapy. Another strength of our study is the fact that unlike in North America, nutritional vitamin D is not widely supplemented in Japan, and physicians cannot prescribe nutritional vitamin D. Indeed, a recent study reported that the prevalence of dietary vitamin supplement use, including vitamin D, was only 3.9% in Japan.28 There is no fortified food in Japan. Moreover, no combination of bisphosphonate and nutritional vitamin D drug has been approved in Japan. In contrast, in North America, this type of study with only baseline data might not reveal the true effect of vitamin D status, as a concurrent increase of 25(OH)D levels and decrease of PTH levels over 10 years was observed in Canada,29 and a similar phenomenon was reported in other cohorts such as the Chronic Renal Insufficiency Cohort (CRIC) owing to the increasing number of patients receiving nutritional vitamin D (personal communication).
In conclusion, high FGF23 and low 25D levels predicted an annualized eGFR decline. There was a cutoff level below which lower 25D levels were associated with a steeper eGFR decline. A future study is needed to test whether renal disease progression is prevented by vitamin D supplementation in patients with vitamin D deficiency or an intervention to decrease FGF23 levels, such as treatment with a phosphate binder.
MATERIALS AND METHODS
Study population
This study (the Osaka Vitamin D Study in CKD; OVIDS-CKD) was a prospective observational cohort study conducted in Osaka, Japan as described previously.7 Briefly, we recruited 738 pre-dialysis CKD outpatients in two major hospitals in Osaka, Japan who agreed to be enrolled from May 2005 through July 2007. The Ethics Committee of Osaka University Hospital approved this study (approval number 07142). We restricted the analyses to 727 pre-dialysis CKD outpatients with an observation period for >6 months and having no history of admission by acute kidney injury, because the eGFR slope in patients with a shorter observation period was very inaccurate and because the purpose of our study was to examine possible associations of 25D and FGF23 with the progression of CKD.
Baseline investigation and laboratory measurements
Data regarding baseline characteristics, including previous CVD history and medication use, were collected from patient records. Previous cardiovascular disease (CVD) was defined as a history of stroke, coronary artery disease, heart failure, aortic disease, valvular disease, or peripheral arterial disease. Patients underwent blood pressure measurement while seated in an office. Blood and urine samples were obtained at enrollment. After a 30-min incubation time, blood samples were centrifuged for serum separation and the sera were frozen and stored at −80 °C until analyses. Blood chemistry parameters (creatinine, albumin (Alb), calcium (Ca), and inorganic phosphorus) were measured using standard automated techniques. Full-length 1–84 parathyroid hormone (PTH) was measured using a third-generation assay (Whole PTH, Scantibodies, Santee, CA). The biologically active form of FGF23 (intact FGF23) was measured using a sandwich enzyme-linked immunosorbent assay system (Kainos Laboratories, Tokyo, Japan). Normal ranges of whole PTH and intact FGF23 are 9–39 and 10–50 pg/ml, respectively. We used this assay, because C-terminal FGF23 fragments were reported to be present as circulating FGF23 in serum from patients with end-stage renal disease.30 Moreover, unlike in hemodialysis patients, the correlation between the two assays is poor in pre-dialysis patients.31, 32 Serum Ca levels were corrected for Alb using the following formula (corrected Ca=total Ca+(4.0−Alb) × 0.8, if Alb<4.0 g/dl). Urinary protein levels were measured semiquantitatively with a dipstick test.
Exposures of interest
The primary exposures were serum 25D and FGF23 concentrations. For vitamin D status categories, we used the Institute of Medicine (IOM) definition (deficiency, <12 ng/ml; inadequacy, 12 and <20 ng/ml; sufficiency, 20 ng/ml)19 and the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (deficiency, <15 ng/ml; inadequacy, 15 and <30 ng/ml; sufficiency, 30 ng/ml).33 We also created four groups using the s.d. of the log (FGF23) according to its distribution in healthy subjects.34 To examine the combined effect of vitamin D status and FGF23 levels, we then categorized the patients into four groups according to the median of 25D and FGF23 concentrations (23.0 ng/ml and 49.5 pg/ml, respectively).
Study outcomes
Patients were seen regularly at a frequency dependent on their conditions and renal function, which was usually every 1–3 months, and followed prospectively until the study end date (1 July 2010), death, or lost to follow-up. We defined the study outcome as annualized eGFR decline; (final eGFR−baseline eGFR)/follow-up duration (years). The final eGFR was the eGFR at the time when the serum creatinine level doubled. If the patients achieved the renal event of initiation of dialysis or died before serum creatinine levels doubled, the eGFR at the visit just before the event was used as the ‘final eGFR.' The estimated GFR was calculated according to a Japanese standard formula based on inulin clearance (194 × creatinine−1.094 × age−0.287 (if female × 0.739)).35 Originally, annualized eGFR decline was a secondary outcome of this cohort study.
Statistical analyses
Data are presented as the mean±s.d. or the median and interquartile range (IQR), as appropriate. We created multiple dichotomous variables of proteinuria and the season of blood sampling with urinary protein (−) by the dipstick test and winter as references, respectively. Herein, spring denotes March to May, summer June to August, autumn September to November, and winter December to February. In the multivariable analysis, we adjusted for important clinical variables associated with CKD progression or MBD. We used binary variables for administration of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, calcium carbonate, and active vitamin D (alfacalcidol and calcitriol). Calcium carbonate was the only phosphate binder available for pre-dialysis CKD patients in Japan. As the associations between 25D and FGF23 levels and the eGFR slope might be non-linear, we also applied restricted cubic spline models to the serum 25D and FGF23 concentrations. Linearity was checked using the quadratic term of each continuous variable. As sensitivity analyses, we also compared the results of the complete case analysis with those obtained by imputing missing data. We generated five multiple imputation data sets using the normal multivariate model. We did not impute missing values of urinary protein because they were missing randomly (i.e., they were missing in patients with low or high urinary protein levels). The statistical test was two-tailed, and P<0.05 was considered statistically significant. All statistical analyses were performed using Stata/SE 11.1 (Stata Corp., College Station, TX).
Acknowledgments
This supplement was supported by a grant from the 58th Annual Meeting of the Japanese Society for Dialysis Therapy.
TH has received consulting and lecture fees from Kyowa-Medex Company. TH has also received lecture fees and grant support from Chugai-Pharmaceutical Company. NF has received grant support from The Kidney Foundation, Japan and Kyowa Hakko Kirin. IM and YT have received grant support from Chugai-Pharmaceutical Company. YT has also received lecture fees from Chugai-Pharmaceutical Company, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, Torii Pharma, and Kissei Pharma. The other authors declared no competing interests.
References
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17:2928–2936. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Trivedi BK, Kalantar-Zadeh K, et al. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:825–831. doi: 10.2215/CJN.02101205. [DOI] [PubMed] [Google Scholar]
- Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- Nakano C, Hamano T, Fujii N, et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7:810–819. doi: 10.2215/CJN.08680811. [DOI] [PubMed] [Google Scholar]
- Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Kimura T, Sasaki K, et al. Plasma B-type natriuretic peptide level predicts kidney prognosis in patients with predialysis chronic kidney disease. Nephrol Dial Transplant. 2012;27:3885–3891. doi: 10.1093/ndt/gfs365. [DOI] [PubMed] [Google Scholar]
- Scialla JJ, Xie H, Rahman M, for the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators et al. Fibroblast growth factor 23 and cardiovascular events in chronic kidney disease J Am Soc Nephrol 2013(in press).
- de Boer IH, Katz R, Chonchol M, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6:2141–2149. doi: 10.2215/CJN.02640311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg S, Qureshi AR, Olivecrona S, et al. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol. 2012;7:727–734. doi: 10.2215/CJN.10331011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano T, Fujii N, Matsui I, et al. Guideline-practice gap in the management of predialysis chronic kidney disease mineral bone disorder in Japan. Ther Apher Dial. 2011;15 (Suppl 1:2–8. doi: 10.1111/j.1744-9987.2011.00918.x. [DOI] [PubMed] [Google Scholar]
- Bienaime F, Girard D, Anglicheau D, et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol. 2013;24:831–841. doi: 10.1681/ASN.2012060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialla JJ, Astor BC, Isakova T, et al. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. 2013;24:125–135. doi: 10.1681/ASN.2012070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310:179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308:1898–1905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press: Washington, DC, USA; 2011. [PubMed] [Google Scholar]
- Ross AC, Taylor CL, Yaktine AL, et al. Dietary Reference Intakes for Calcium and Vitamin D. National Academy Press: Washington, DC, USA; 2011. [PubMed] [Google Scholar]
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009. pp. S1–S130. [DOI] [PubMed]
- Kim MJ, Frankel AH, Donaldson M, et al. Oral cholecalciferol decreases albuminuria and urinary TGF-beta1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney Int. 2011;80:851–860. doi: 10.1038/ki.2011.224. [DOI] [PubMed] [Google Scholar]
- Yoon HE, Ghee JY, Piao S, et al. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011;26:800–813. doi: 10.1093/ndt/gfq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohle A, Mackensen-Haen S, von Gise H, et al. The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract. 1990;186:135–144. doi: 10.1016/S0344-0338(11)81021-6. [DOI] [PubMed] [Google Scholar]
- Pavik I, Jaeger P, Ebner L, et al. Soluble klotho and autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:248–257. doi: 10.2215/CJN.09020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavik I, Jaeger P, Kistler AD, et al. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 2011;79:234–240. doi: 10.1038/ki.2010.375. [DOI] [PubMed] [Google Scholar]
- Nanri A, Foo LH, Nakamura K, et al. Serum 25-hydroxyvitamin d concentrations and season-specific correlates in Japanese adults. J Epidemiol. 2011;21:346–353. doi: 10.2188/jea.JE20100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Greene-Finestone LS, Langsetmo L, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. J Bone Miner Res. 2012;27:1381–1389. doi: 10.1002/jbmr.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber TJ, Liu S, Indridason OS, et al. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- Bacchetta J, Dubourg L, Harambat J, et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- Smith ER, Ford ML, Tomlinson LA, et al. Instability of fibroblast growth factor-23 (FGF-23): implications for clinical studies. Clin Chim Acta. 2011;412:1008–1011. doi: 10.1016/j.cca.2011.02.009. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- Imel EA, Peacock M, Pitukcheewanont P, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–2061. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]