Abstract
Chronic kidney disease-mineral and bone disorder (CKD-MBD) has recently attracted attention in light of its association with clinical outcomes, such as fracture, cardiovascular disease, and mortality. Management of CKD-MBD has therefore come to have a central role in dialysis practice. Cinacalcet, a newly developed drug, has changed prescription patterns in many centers based on different changes in MBD markers than those observed with active vitamin D derivatives. As physicians require real-world evidence to guide their treatment decisions with respect to MBD management, we conducted the Mineral and Bone Disorder Outcomes Study for Japanese CKD Stage 5D Patients (MBD-5D), a 3-year observational study involving prevalent hemodialysis patients with secondary hyperparathyroidism (SHPT). Here, we review the results from the MBD-5D and discuss issues of MBD management in the cinacalcet era. Three years since the introduction of cinacalcet, 40% of hemodialysis patients with SHPT have come to use cinacalcet, enjoying marked improvement in management of circulating MBD markers, such as intact parathyroid hormone (PTH), phosphorus, and calcium. Combination therapy with cinacalcet and a vitamin D receptor activator (VDRA) may allow physicians to choose more suitable prescription patterns based on patient characteristics and therapeutic purposes. We observed an additive association between ‘starting cinacalcet' and ‘increased VDRA dose,' with marked improvement in the control of intact PTH levels. Further, the combination pattern of ‘starting cinacalcet' and ‘decreased VDRA dose' was associated with better achievement of target serum phosphorus and calcium levels. Future studies should examine the effect of different prescription patterns for SHPT treatment on clinical outcomes.
Keywords: cinacalcet, dialysis patients, mineral and bone disorder
Chronic kidney disease-mineral and bone disorder (CKD-MBD) involves disturbances of mineral metabolism, bone disease, and vascular calcification. It has attracted attention in light of its association with fracture, cardiovascular disease, and mortality, underscoring the clinical importance of MBD management. A number of risk factors for mortality among hemodialysis patients have been identified. Circulating MBD markers (phosphorus, calcium, and intact parathyroid hormone (PTH)) are known to be modifiable risk factors, and their management has a central role in dialysis practice. A previous study examined the relative impact of dialysis practices (such as anemia management, MBD management, and adequate dialysis) by calculating population-attributable risk fraction.1 Population-attributable risk fraction of MBD (17.5%) was found to be higher than that of inefficient dialysis (5.1%) or anemia (11.3%), suggesting that MBD management is relatively important for improving clinical outcomes compared with other dialysis practices.
MBD management regimens can change with the introduction of new drugs to the market. Treatment of high intact PTH (iPTH) levels was previously limited to two options: Vitamin D receptor activator (VDRA) and parathyroidectomy. As such, the introduction of cinacalcet, which dramatically reduces iPTH levels, was expected to improve MBD management. In patients with simultaneously high serum levels of iPTH, calcium, and phosphorus, it is often difficult to manage PTH levels with VDRA given the concurrent stimulation of the intestinal absorption of phosphorus and calcium. Because cinacalcet does not increase serum levels of phosphorus or calcium, it may be the ‘silver bullet' for treating patients with simultaneously high levels of iPTH, calcium, and phosphorus.
Randomized controlled trials examining the effect of cinacalcet on MBD markers and cardiovascular events in selected dialysis patient populations have been reported recently.2, 3 However, physicians require real-world evidence to guide their treatment decisions on MBD management. We therefore conducted the Mineral and Bone Disorder Outcomes Study for Japanese CKD Stage 5D Patients (MBD-5D),4 a 3-year case-cohort study involving prevalent hemodialysis patients with secondary hyperparathyroidism (SHPT) based on the whole cohort of patients initially enrolled (8229 patients) and a sub-cohort of patients randomly selected (3276 patients) from the whole cohort. Here, we review the results from the MBD-5D and discuss the issues of MBD management in the cinacalcet era.
MBD-5D STUDY DESIGN
The design of the MBD-5D study has been reported in detail previously.4 The target population was hemodialysis patients with SHPT, defined as patients with serum iPTH 180 pg/ml or receiving intravenous VDRA or oral falecalcitriol. Patients with hemodialysis duration 3 years were included in the MBD-5D study. Settings were relatively large dialysis facilities from nine geographically divided regions of Japan (N=86), with the number of facilities chosen in each region proportional to the number of hemodialysis patients in that region. Because MBD markers are tested for periodically and MBD treatment patterns are often changed based on levels of iPTH, calcium and phosphorus, data directly related to MBD markers and treatments were collected every 3 months,5 while other data were collected every 6 months. Patient follow-up started in January 2008 and ended in January 2011. The MBD-5D study was conducted as case-cohort study, including the whole cohort (N=8229) and a randomly selected sub-cohort (N=3276). When we used cardiovascular death or death from any cause as our main outcome, the whole cohort was analyzed as a case-cohort study, because that sample size is required to detect meaningful associations among the Japanese hemodialysis patient population with an infrequent incidence of major outcomes.
Several unique characteristics of the MBD-5D study design warrant mention. First, a case-cohort design was used to facilitate efficient analysis of the whole cohort. This study design is useful when study budget and availability of a clinical research coordinator is insufficient to collect data for all patients thoroughly. As such, in the MBD-5D study, thorough data were prospectively collected for only the subcohort patients, not the whole cohort, thereby saving cost and manpower. For those patients making up the rest of the whole cohort (N=4953), only baseline data and the date and occurrence of main outcomes (i.e., death from any cause or cardiovascular death) were collected initially; detailed data were then collected retrospectively for those non-subcohort patients who developed main outcomes. Second, the MBD-5D is a closed cohort that was started before cinacalcet was marketed. We were therefore able to demonstrate the rate at which cinacalcet prescription spread in daily practice. In addition, when examining the association between cinacalcet prescription and hard clinical outcomes, including only new cinacalcet users who had pretreatment, MBD markers might reduce bias with advanced statistical analysis correcting time-varying cinacalcet prescription and confounders, such as when using marginal structural models.6
CHANGES IN PRESCRIPTION PATTERNS AFTER CINACALCET INTRODUCTION
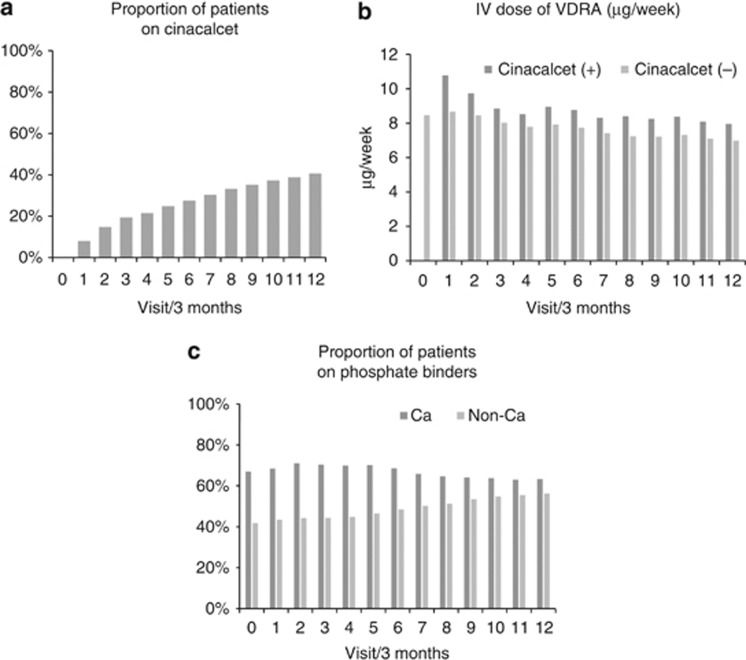
Given that the MBD-5D is an observational study that begun just when cinacalcet first became available in Japan (January 2008), we were able to observe the spread of cinacalcet prescription in daily practice. The proportion of patients receiving cinacalcet increased steadily from 0% to 42% over the 3 years following cinacalcet introduction (Figure 1a). In the years since its release, cinacalcet has come to have a major role in managing MBD. This relatively rapid spread of cinacalcet use suggests hope among members of the medical and pharmaceutical communities that cinacalcet will compensate for the shortcomings of traditional MBD treatment, such as VDRA and phosphate binders.
Figure 1.
Changes in prescription patterns after cinacalcet introduction. Each interval between visits was 3 months. (a) Proportion of patients on cinacalcet: The proportion of patients receiving cinacalcet at each visit. (b) Intravenous dose of vitamin D receptor activator (VDRA; μg/week): mean VDRA dose at each visit by the presence of cinacalcet use. (c) Proportion of patients on phosphate binders: Proportion of patients receiving calcium-based and non-calcium-based phosphate binders at each visit.
MBD-5D findings demonstrated that cinacalcet users were younger, less likely to have comorbid conditions (such as diabetes and cardiovascular disease), and more likely to have high serum levels of calcium, phosphorus, and iPTH than non-users. This observation suggests that cinacalcet was more likely to be prescribed for relatively healthy dialysis patients who were expected to have improved clinical outcomes via better control of MBD markers.
The Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial,2 which is a randomized controlled trial to examine the effect of cinacalcet on cardiovascular events, reported that only 38% (741 patients) of the intervention group (1948 patients who had been randomized to receive cinacalcet) had continued cinacalcet during the follow-up period (median follow-up time of intervention group, 21 months), with an annual continuing rate of only 73%. As such, the proportion of patients receiving cinacalcet continuously in the EVOLVE study was lower than expected.
Regarding the proportion of patients continuing cinacalcet in a real-world, estimated proportion receiving cinacalcet at the end of the follow-up period in the present study (median follow-up time: 33 months) was 70%, with an annual continuing rate of 89%. Several reasons may explain the observation of a higher proportion of patients receiving cinacalcet continuously in the real-world setting in Japan than in the international randomized controlled trial. First, median dose of cinacalcet in the MBD-5D study was 25 mg/day (interquartile range: 25–50 mg/day), which was lower than that in the EVOLVE study (median dose: 55 mg/day). Although differences in race and stature between Japan and the mostly Caucasian and African-American patient population of EVOLVE may have influenced the metabolism of cinacalcet and discrepant clinical outcome findings, the lower cinacalcet dose used in Japan might also have contributed to relatively higher proportion of patients staying on cinacalcet therapy in this country. Second, patients in the MBD-5D study had lower iPTH levels before cinacalcet administration than those in the EVOLVE study (median levels of iPTH, 265 vs. 693 pg/ml). This also may have contributed to the higher proportion of patients receiving cinacalcet continuously.
Regarding changes in administration of other MBD medications, such as VDRA and phosphate binders, after cinacalcet introduction, Figure 1b shows changes in intravenous VDRA doses stratified by cinacalcet use. Notably, intravenous VDRA dosages were greater in patients who received cinacalcet than in those who did not. As such, concomitant therapy with cinacalcet might facilitate successful VDRA administration even in cases in whom a sufficiently high VDRA dose to lower iPTH levels is not well tolerated because of the concomitant maintenance of aggravation of hyperphosphatemia and/or hypercalcemia. Alternatively, physicians using an increased VDRA dosage may be able to concurrently administer cinacalcet to prevent hypocalcemia preemptively. In this way, we can see that introduction of cinacalcet has affected prescription patterns of other MBD medications. Figure 1c shows the proportion of patients receiving calcium-based and non-calcium-based phosphate binders. Although we noted no major changes in the proportion of patients receiving calcium-based phosphate binders (between 63 and 70%), the proportion receiving non-calcium-based phosphate binders increased gradually from 42 to 55% over the 3-year period. Physicians may have tended to favor non-calcium-based phosphate binders in order to avoid calcium overload.
CHANGES IN MBD MARKERS AFTER CINACALCET INTRODUCTION
Along with changes in prescription patterns, circulating MBD markers such as levels of phosphorus, calcium, and iPTH have been found to change after cinacalcet introduction. Table 1 shows changes in MBD markers during the follow-up period of the MBD-5D study. At baseline, the proportion of patients whose values were in the target range for levels of calcium (8.4–10.0 mg/dl) and phosphorus (3.5–6.0 mg/dl) according to the Japanese clinical guidelines were 65.5 and 63.3%, respectively. At the end of follow-up, the proportion of patients whose values fell into the target range for levels of calcium and phosphorus were 73.5 and 67.7%, respectively. Several reasons may explain this small improvement in achieving target levels of calcium and phosphorus. First, the clinical guidelines, published in 2006, were starting to be distributed in daily practice, thereby potentially increasing physicians' awareness of the target range of MBD markers. Second, cinacalcet usage in MBD management has been spreading, as has the usage of non-calcium-based phosphate binder.
Table 1. Changes in circulating MBD marker levels during 3 years of follow-up.
|
Intact PTH |
Phosphorus |
Calcium |
||||
|---|---|---|---|---|---|---|
| Visit number (time period) | Median (pg/ml) | Percentage of achievement of target | Mean (s.d.) (mg/dl) | Percentage of achievement of target | Mean (s.d.) (mg/dl) | Percentage of achievement of target |
| 0 (up to Dec 2007) | 265 | 14.5 | 5.53 (1.37) | 63.3 | 9.45 (0.88) | 65.5 |
| 1 (Jan 2008 to Mar 2008) | 259 | 21.7 | 5.57 (1.39) | 61.9 | 9.45 (0.85) | 68.6 |
| 2 (Apr 2008 to Jun 2008) | 225 | 30.8 | 5.49 (1.35) | 65.2 | 9.49 (0.83) | 69.5 |
| 3 (Jul 2008 to Sep 2008) | 187 | 38.6 | 5.34 (1.37) | 65.7 | 9.58 (0.82) | 67.5 |
| 4 (Oct 2008 to Dec 2008) | 189 | 38.2 | 5.41 (1.38) | 65.6 | 9.55 (0.79) | 69.4 |
| 5 (Jan 2009 to Mar 2009) | 191 | 37.6 | 5.56 (1.41) | 62.1 | 9.46 (0.80) | 72.1 |
| 6 (Apr 2009 to Jun 2009) | 172 | 42.3 | 5.44 (1.36) | 64.4 | 9.51 (0.78) | 70.8 |
| 7 (Jul 2009 to Sep 2009) | 160 | 45.0 | 5.27 (1.37) | 67.1 | 9.56 (0.81) | 68.5 |
| 8 (Oct 2009 to Dec 2009) | 172 | 40.9 | 5.39 (1.39) | 65.0 | 9.51 (0.77) | 71.4 |
| 9 (Jan 2010 to Mar 2010) | 173 | 41.6 | 5.52 (1.37) | 63.7 | 9.47 (0.75) | 73.6 |
| 1 0 (Apr 2010 to Jun 2010) | 165 | 42.5 | 5.47 (1.36) | 65.3 | 9.50 (0.76) | 73.1 |
| 11 (Jul 2010 to Sep 2010) | 150 | 46.1 | 5.24 (1.34) | 69.1 | 9.58 (0.77) | 71.0 |
| 12 (Oct 2010 to Dec 2010) | 160 | 44.1 | 5.32 (1.36) | 67.7 | 9.52 (0.74) | 73.5 |
Abbreviations: MBD, mineral and bone disorder; PTH, parathyroid hormone.
Target levels of serum calcium, phosphorus, and intact PTH were defined as 8.4–10.0 mg/dl, 3.5–6.0 mg/dl, and 60–180 pg/ml, respectively.
Median values for iPTH levels decreased from 265 to 160 pg/ml during the follow-up period, and the proportion of patients whose values fell into the target range for iPTH according to the Japanese clinical guidelines improved from 14.5% at baseline to 44.1% at the end of follow-up. In contrast, however, the proportion of patients with iPTH levels <60 pg/ml increased from 2.8 to 12.4% over follow-up. Given that findings from previous studies suggest that low iPTH levels are associated with increased mortality, physicians may need to avoid oversuppression of iPTH levels. However, the associations observed between low iPTH levels and mortality are based solely on the results of observational studies. It remains to be determined in future studies whether oversuppressing iPTH levels in the course of MBD treatment with compounds such as cinacalcet and VDRA does increase mortality or not.
PRESCRIPTION PATTERNS AND MBD MARKERS IN THE CINACALCET ERA
Prescription patterns for MBD have shown extensive variation since cinacalcet introduction, granting physicians the freedom to choose suitable prescription modalities according to patient characteristics and therapeutic purposes. Here, we tried to clarify the associations between prescription patterns and subsequent levels of MBD markers among hemodialysis patients with SHPT who did not receive cinacalcet at baseline.7 Prescription patterns were defined by two factors: cinacalcet administration and change of VDRA dose. Changes in the proportion of VDRA dose between two consecutive visits (3-month interval between visits) were defined as either ‘increased VDRA' (>25%), ‘decreased VDRA' (< −25%), or ‘stable VDRA' (from –25 to 25%). Cinacalcet administration was defined by new use of this agent as either ‘starting cinacalcet' or ‘not starting cinacalcet'. The primary outcome was improvement in iPTH level by at least one category (<180, 180–299, 300–499, and ⩾500 pg/ml). Secondary outcomes were achievements of guideline-targeted levels for calcium (8.4–10.0 mg/dl) and phosphorus (3.5–6.0 mg/dl), respectively.
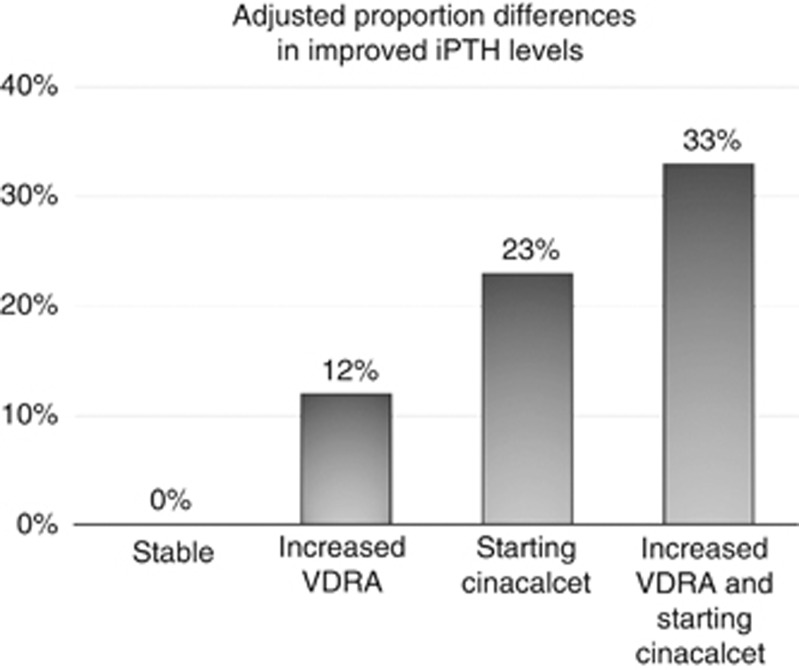
Table 2 shows adjusted associations between prescription patterns and changes in circulating MBD markers. Compared with stable treatment, patterns of ‘starting cinacalcet' and ‘increased VDRA dose' were separately associated with 25 and 13% increases in improved iPTH levels, respectively. Combination of ‘starting cinacalcet' and ‘increased VDRA dose' was associated with the highest proportion difference (34%) for improved iPTH levels. Figure 2 shows the separate and combined association of ‘starting cinacalcet' and ‘increased VDRA dose' with improvement in iPTH level. We found an additive association of their combination patterns (33%) in iPTH improvement, nearly equal to the sum of the associations of the two factors alone (12 and 23%). As to phosphorus and calcium, the combination pattern of ‘starting cinacalcet' and ‘decreased VDRA dose' was associated with achieving target levels of phosphorus (proportion difference: 0.12, 95% CI: 0.04–0.20) and calcium (proportion difference: 0.09, 95% CI: 0.01–0.17).
Table 2. Association between prescription patterns and changes in circulating MBD markers.
|
Prescription patterns |
Improvement in intact PTH |
Achievement of target phosphorus |
Achievement of target calcium |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cinacalcet | VDRA | Absolute change (pg/ml) | PDa | 95% CI | Absolute change (mg/dl) | PDb | 95% CI | Absolute change (mg/dl) | PDc | 95% CI |
| Not | Decreased | −67.8 | 0.01 | −0.03 to 0.06 | −0.29 | 0.03 | −0.02 to 0.07 | −0.14 | 0.01 | −0.03 to 0.05 |
| Not | Stable | −56.7 | reference | −0.12 | reference | 0.13 | reference | |||
| Not | Increased | −127.6 | 0.13 | 0.09 to 0.17 | −0.01 | 0.00 | −0.03 to 0.04 | 0.32 | −0.02 | −0.06 to 0.01 |
| Starting | Decreased | −141.8 | 0.19 | 0.10 to 0.28 | −0.82 | 0.12 | 0.04 to 0.20 | −0.69 | 0.09 | 0.01 to 0.17 |
| Starting | Stable | −205.1 | 0.25 | 0.19 to 0.31 | −0.49 | 0.03 | −0.02 to 0.09 | −0.35 | 0.08 | 0.02 to 0.14 |
| Starting | Increased | −266.8 | 0.34 | 0.25 to 0.42 | −0.35 | 0.07 | 0.00 to 0.15 | −0.16 | 0.03 | −0.05 to 0.11 |
Abbreviations: CI, confidence interval; MBD, mineral and bone disorder; PD, proportion difference; PTH, parathyroid hormone; VDRA, vitamin D receptor activator.
PD for improvement in intact PTH levels (decrease at least one category) adjusting for age, gender, dialysis duration, baseline intact PTH levels, phosphorus levels, calcium levels, use of calcium- or non-calcium-based phosphate binders, single Kt/V, and dialysis calcium.
PD for achievement of target phosphorus levels (3.5–6.0 mg/dl) adjusting for potential confounders.
PD for achievement of target calcium levels (8.4–10.0 mg/dl) adjusting for potential confounders.
Permission obtained from the American Society of Nephrology.7
Figure 2.
Additive association of starting cinacalcet and increased vitamin D receptor activator (VDRA) dose with improvement in intact parathyroid hormone (PTH) levels. Figure shows separate and combined associations of ‘starting cinacalcet' and ‘increased VDRA dose' with improvement in intact PTH (iPTH) level. We estimated proportion differences for improvement in iPTH levels compared with the reference group, using generalized estimating equations, after adjusting for age, gender, dialysis duration, baseline iPTH levels, phosphorus levels, calcium levels, use of calcium- or non-calcium-based phosphate binders, single Kt/V, and dialysis calcium. Permission obtained from the American Society of Nephrology.7
Thus the present study provides dialysis physicians with real-world evidence about the types of prescription patterns of cinacalcet and VDRA, which are associated with improved control of certain MBD markers. First, the study suggests that ‘starting cinacalcet' and ‘increased VDRA dose' allows better control of iPTH levels than other patterns, and their combination may exert additive effects. Before cinacalcet was marketed in Japan, >80% of dialysis patients with SHPT had iPTH levels exceeding the guideline-defined upper limit set at <180 pg/ml, with 77% of these patients already prescribed some form of VDRA.8 Combination of cinacalcet and VDRA therapy therefore appears to allow better control of iPTH levels. VDRA binds to vitamin D receptor and inhibits PTH synthesis in the parathyroid gland but does not inhibit PTH secretion. Cinacalcet, in contrast, modulates calcium-sensing receptor and inhibits PTH secretion. These differences in mechanisms of action may provide additive clinical benefit in the management of SHPT. However, further study is required to determine whether or not combination therapy allows a similarly effective control of iPTH levels in refractory SHPT patients with hyperplastic nodules.
Second, ‘starting cinacalcet' and ‘decreased VDRA dose' was found to be associated with improved achievement of guideline-defined serum calcium and serum phosphorus levels. In the MBD-5D sub-cohort, 49.6% had hypercalcemia or hyperphosphatemia,8 both of which have been shown to be associated with reduced rates of survival.9 Among SHPT patients with hypercalcemia or hyperphosphatemia, VDRA treatment is unlikely to be increased and likely to be decreased,10 as increased dosage alone may aggravate hypercalcemia and hyperphosphatemia. As such, the combination of ‘starting cinacalcet' and ‘decreased VDRA dose' appears to be a reasonable alternative for achieving control of both serum calcium and serum phosphorus without reducing the likelihood to control iPTH levels. In addition, ‘starting cinacalcet' and ‘increased VDRA dose' was not associated with less frequent in achievement of guideline-defined serum calcium or serum phosphorus levels, supporting the use of this treatment pattern for the management of patients with severe SHPT as well. Several potential mechanisms may be behind the observed decrease in serum calcium under the ‘starting cinacalcet' and ‘decreased VDRA dose' regimen. Calcium-sensing receptor is expressed not only in the parathyroid gland but also in the intestine, bone, and thyroid gland. Activation of calcium-sensing receptor may therefore result in reduced calcium uptake in the intestine, decreased turnover of the bone, and increased calcitonin secretion from C cells in the thyroid gland, all of which should contribute to decreasing serum calcium levels. A decrease in VDRA dose should also lead to a reduction in intestinal calcium absorption. Although the mechanism underlying the decrease in serum phosphorus observed with ‘starting cinacalcet' and ‘decreased VDRA dose' is unclear, decreased bone turnover may be involved.
Combination of cinacalcet and VDRA may therefore prove beneficial in effectively managing circulating MBD markers. However, potential health benefits of a better control of MBD markers with such combination therapy were not examined in the present study, and defining optimal treatment regimens for the best possible control of iPTH, phosphorus, and calcium levels by combining cinacalcet and VDRA will be the subject of future studies.
SUMMARY
Following the introduction of cinacalcet, prescription patterns for MBD in dialysis patients have changed and the management of circulating MBD markers have improved. The combination of ‘starting cinacalcet' and ‘increased VDRA dose' has been found to exert an additive effect in improving serum iPTH levels, while the pattern of ‘starting cinacalcet' and ‘decreased VDRA dose' was associated with better achievement of target phosphorus and calcium levels. The concomitant use of cinacalcet and VDRA may improve management of MBD markers. Future studies are needed to investigate the effect of various combinations of cinacalcet and VDRA prescription patterns on clinical outcomes.
Acknowledgments
We would like to thank the MBD-5D steering committee members and the MBD-5D study advisory investigators: Masashi Suzuki (Shinrakuen Hospital), Yoshindo Kawaguchi (Shiomidai Hospital), Akira Saito (Yokohama Daiichi Hospital), Yoshiki Nishizawa (Osaka City University Graduate School of Medicine), Yusuke Tsukamoto (Shuwa General Hospital), Satoshi Kurihara (Tsukinomori Clinic), Takashi Akiba (Tokyo Women's Medical University), Eriko Kinugasa (Showa University Northern Yokohama Hospital), Yuzo Watanabe (Kasugai Municipal Hospital), Yoshihiro Tominaga (Nagoya Daini Red Cross Hospital), Takashi Shigematsu (Wakayama Medical University), Masaaki Inaba (Osaka City University Graduate School of Medicine), Jun Minakuchi (Kawashima Hospital), Hideki Hirakata (Fukuoka Red Cross Hospital), Keitaro Yokoyama (Jikei University School of Medicine), Naoki Kimata (Tokyo Women's Medical University), Fumihiko Koiwa (Showa University Fujigaoka Hospital), Ryoichi Ando (Musashino Red Cross Hospital), Junichiro J. Kazama (Niigata University), Takatoshi Kakuta (Tokai University School of Medicine), Hirotaka Komaba (Tokai University School of Medicine), Daijo Inaguma (Nagoya Daini Red Cross Hospital), Eiji Ishimura (Osaka City University Graduate School of Medicine), Hideki Tahara(Osaka City University Graduate School of Medicine), Kazuhiko Tsuruya (Kyushu University), and Akira Fujimori (Konan Hospital). This supplement was supported by a grant from the 58th Annual Meeting of the Japanese Society for Dialysis Therapy.
Funding for the MBD-5D was provided by Kyowa Hakko Kirin (manufacturer of intravenous calcitriol, cinacalcet hydrochloride, and sevelamer hydrochloride). MF has acted as a consultant for Kyowa Hakko Kirin, Chugai, Bayer Japan, Novartis, JT, Abbvie, and Astellas. MF has also received honoraria from Kyowa Hakko Kirin, Chugai, Bayer Japan, and Astellas and has received grants (research support) from Kyowa Hakko Kirin. TA has acted as a consultant for Kyowa Hakko Kirin, REATA and Abbvie, has received grants (research support) from Baxter, Bayer, Chugai, Daiichi-Sankyo, Kyowa Hakko Kirin, and is a member of speakers' bureau of Kyowa Hakko Kirin. SF has acted as a scientific advisor for Kyowa Hakko Kirin and has received grants (research support) from Kyowa Hakko Kirin. The other authors declared no competing interests.
References
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Investigators ET, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Akizawa T, Fukagawa M, et al. Mineral and bone disorders outcomes study for Japanese chronic kidney disease stage 5D patients: rationale and study design. Ther Apher Dial. 2011;15:169–175. doi: 10.1111/j.1744-9987.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Fukuhara S, Fukagawa M, et al. Results of the survey on practice patterns including MBD management at dialysis facilities: as part of the MBD-5D J Jpn Soc Dial Ther 201144557–566.(Japanese). [Google Scholar]
- Bradbury BD, Brookhart MA, Winkelmayer WC, et al. Evolving statistical methods to facilitate evaluation of the causal association between erythropoiesis-stimulating agent dose and mortality in nonexperimental research: strengths and limitations. Am J Kidney Dis. 2009;54:554–560. doi: 10.1053/j.ajkd.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Fukagawa M, Fukuma S, Onishi Y, et al. Prescription patterns and mineral metabolism abnormalities in the cinacalcet era: results from the MBD-5D study. Clin J Am Soc Nephrol. 2012;7:1473–1480. doi: 10.2215/CJN.13081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa M, Komaba H, Onishi Y, et al. Mineral metabolism management in hemodialysis patients with secondary hyperparathyroidism in Japan: baseline data from the MBD-5D. Am J Nephrol. 2011;33:427–437. doi: 10.1159/000327654. [DOI] [PubMed] [Google Scholar]
- Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Tentori F, Albert JM, Young EW, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2009;24:963–972. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]