Abstract
Both the homing of hematopoietic stem cells (HSCs) to the bone marrow and their engraftment in recipients of bone marrow transplants are primarily mediated by the chemokine stromal-derived factor-1 (SDF-1) or CXCL12, which activates CXCR4, its cognate receptor on HSCs. We showed that the recruitment and temporary attachment of CXCR4-expressing cells, such as HSCs and a fraction of mesenchymal stem cells (MSCs), to the kidney, following ischemia/reperfusion acute kidney injury, are similarly mediated by robustly upregulated SDF-1 in the kidney, indicating that such organ injury appears to lead to the transient expression of a facultative stem cell niche. This SDF-1 response of the injured kidney facilitates both the mobilization from the bone marrow and homing of precursor cells, and other CXCR4-expressing cells such as administered MSCs, to the kidney, where they aid in its protection and repair. Similar responses have been observed subsequent to the injury of other solid organs such as the heart, liver, and brain.
Keywords: CXCR4, facultative stem cell niche, stem cell homing, stem cell mobilization
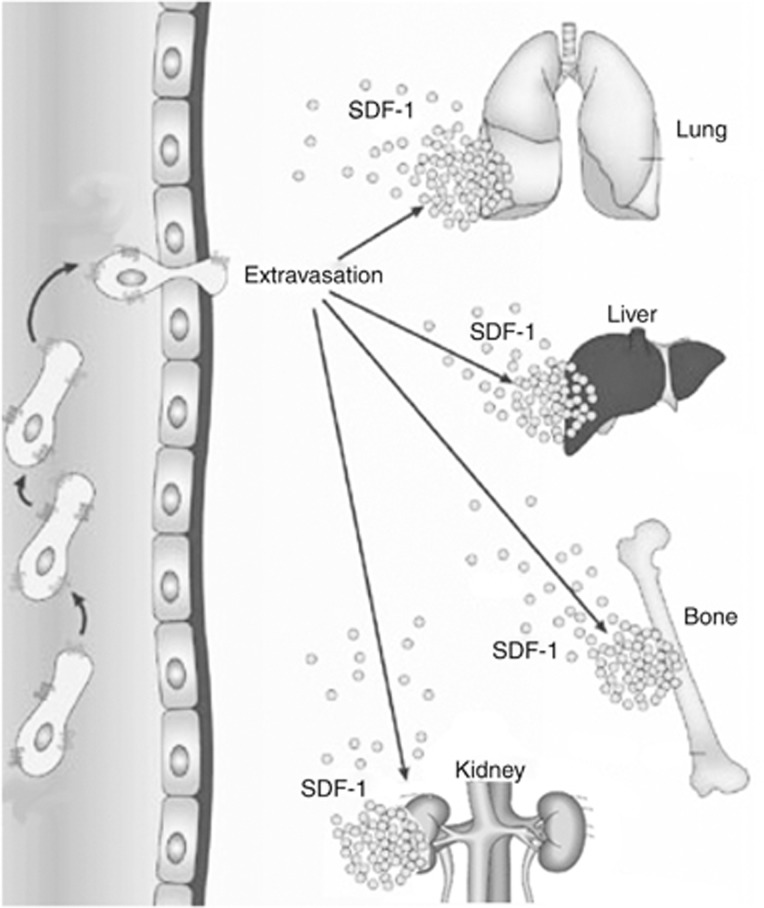
During the last few years, preclinical stem cell research has reached the stage where the feasibility, safety, and efficacy of stem cell therapy for numerous treatment-resistant disorders are being tested in clinical trials. Testing and use of this fundamentally new and promising therapeutic principle not only requires knowledge of the mediator mechanisms whereby these cells function, but also an understanding regarding their trafficking, extravasation, homing, and migration to organs after their administration, and the factors involved in the regulation of these critical processes (Figure 1). The involvement and importance of stromal-derived factor-1 (SDF-1) for homing of hematopoietic stem cells to the bone marrow after hematopoietic stem cell transplantation, the first successful application of stem cell therapy in the clinic, is well established.
Figure 1.
Injury-induced upregulation of stromal-derived factor-1 (SDF-1) in major organs results in mobilization, from the bone marrow, and recruitment of stem cells expressing CXCR4 (SDF-1 receptor).
Acute kidney injury (AKI) is a common and potentially fatal clinical problem, and its therapy is limited to supportive measures. Experimental animal studies have provided data showing that stem cell therapies have the potential to provide a completely new and promising treatment principle for AKI. This following review is intended to provide relevant details regarding the importance of SDF-1 as a stem cell regulatory factor in AKI.
FUNCTION AND SIGNIFICANCE OF SDF-1
SDF-1 belongs to a complex group of chemokines, which are small molecules involved in the regulation of inflammation and migration of cells.1 There are more than 50 different chemokines and more than 20 receptors that have been cloned. There frequently exists significant promiscuity in the binding of several of these chemokines to multiple receptors, albeit with variable affinity and different responses. SDF-1 is unique in this regard as it only binds to its principal receptor, termed CXCR4, and to a recently identified second receptor CXCR7.2, 3 SDF-1 and CXCR4 are also unique with regard to their importance for embryonic development, with knockout of either one resulting in fetal lethality. The SDF-1/CXCR4 axis regulates trafficking of stem cells during development, in homeostasis, inflammation, and in regeneration. Intracellular signaling of SDF-1 via CXCR4 occurs via G-protein-activated downstream pathways, which includes ras, phosphatidylinositol 3′-kinase, Janus kinase/signal transducers and activators of transcription signaling, activation of transcription factors, as well as linkage to the cytoskeletal system that promotes cell migration.4 The major biological effects of SDF-1 include the induction of motility, chemotactic responses, cell adhesion, and increased adhesion to vascular cellular adhesion molecule-1, intercellular adhesion molecule-1, and fibrinogen, induction of metalloproteinases and angiopoietic factors, as well as proliferation and cell survival. SDF-1 and CXCR4 expressions are regulated by transcription factors including hypoxia-inducible factor-1α and nuclear factor-κB.5 Hypoxia-inducible factor-1α stabilization leads to upregulation of SDF-1 through binding to promoter sites, whereas the von Hippel-Lindau protein negatively regulates SDF-1 expression. SDF-1 has been shown to be upregulated in myocardial infarction, limb ischemia, toxic liver damage, excessive bleeding, total body irradiation, and tissue damage related to chemotherapy.6
SDF-1/CXCR4 AND STEM CELLS
CXCR4 is expressed on hematopoietic stem cells, endothelial progenitor cells, neural stem cells, primordial germ cells, liver oval cells, skeletal muscle satellite cells, embryonic stem cells, and cancer stem cells.4 It is furthermore expressed on subgroups of neutrophiles, monocytes, B cells, and T cells. Accordingly, CXCR4 is one of the major receptors that regulates trafficking of hematopoietic stem cells and progenitor cells. Furthermore, CXCR4 is involved in the regulation of angiogenesis, in part through recruitment of endothelial progenitor cells, and is important in trafficking of tissue stem cells, as well as guiding CXCR4-positive cells during embryogenesis, development, and tissue regeneration. In cancer, CXCR4 regulates locomotion of metastatic cells and is involved in tumor angiogenesis. Knockout of CXCR4 results in death in utero due to hematopoietic and cardiac defects, as well as changes in brain development.7
The SDF-1/CXCR4 axis facilitates tumor cells access to cellular niches such as those in the bone marrow, where it mediates survival and directly stimulates growth of neoplastic cells in a paracrine manner.8 SDF-1 has been shown to be a prognostic marker in acute myelogenous leukemia and in breast cancer, and it is involved in the establishment of the cancer stem-like cell niche.
CXCR4/SDF-1 IN THE KIDNEY
There is limited knowledge regarding the involvement of the SDF-1/CXCR4 axis in the development and homeostasis of the normal kidney, although one report showed SDF-1 expression in the developing kidney.9 Therefore, our group investigated the expression pattern and functions of the SDF-1/CXCR4 system in the normal kidney, as well as after ischemia/reperfusion-induced AKI.10 SDF-1 is expressed in the normal kidney mostly by distal tubular cells in the cortex, as determined by in situ hybridization, immunohistochemistry, and enzyme-linked immunosorbent assay. CXCR4 expression in the kidney is of similar distribution to that of SDF-1. Within 24 h of induction of ischemia/reperfusion AKI, all kidney regions show robust expression of SDF-1 and CXCR4, indicating significant, injury-induced upregulation of both. This was demonstrated using real-time quantitative reverse transcriptase-polymerase chain reaction and enzyme-linked immunosorbent assay. In vitro studies with adenosine 5′ triphosphate depletion of primary, proximal tubular kidney cells in culture showed upregulation of SDF-1 mRNA as early as 90 min after chemically induced ‘hypoxia'. In vitro and in vivo migration and homing assays demonstrated the importance of the SDF-1/CXCR4 axis in cell migration toward injured kidney cells, which was abrogated by neutralizing anti-CXCR4 antibodies. Systemically, we demonstrated a reversal of the physiological bone marrow–plasma SDF-1 gradient, characterized after AKI by plasma levels of SDF-1 that significantly exceeded those present in the bone marrow. This chemokine pattern resulted in the mobilization, from the bone marrow, of CXCR4-expressing progenitor cells into circulation and their recruitment into the injured kidney.
In summary, our data show that renal SDF-1 is an important mediator of homing and migration of CXCR4-positive cells toward the injured kidney. Furthermore, these data indicate that SDF-1 is a major factor involved in kidney repair through recruitment of cells involved in regeneration, including endothelial progenitor cells, as well as administered stem and other progenitor cells.
Acknowledgments
This work was in part supported by funds from the VA Central Office Merit Review Program, the National Kidney Foundation (UT, ID), the American Heart Association (Western Affiliate), the National Institutes of Health, the Western Institute of Biomedical Research, and Allocure.
FET declared no competing interests. CW served as a consultant to Allocure.
Footnotes
TO CITE THIS ARTICLE: Togel FE, Westenfelder C. Role of SDF-1 as a regulatory chemokine in renal regeneration after acute kidney injury. Kidney inter., Suppl. 2011; 1: 87–89.
References
- Segerer S, Nelson PJ, Schlöndorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma E, Kucia M, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. J Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- Mazzinghi B, Ronconi E, Lazzeri E, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–490. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signaling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Yamani MH, Ratliff NB, Cook DJ, et al. Peritransplant ischemic injury is associated with up-regulation of stromal cell-derived factor-1. J Am Coll Cardiol. 2005;46:1029–1035. doi: 10.1016/j.jacc.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Arya M, Ahmed H, Silhi N, et al. Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration. Tumor Biol. 2007;28:123–131. doi: 10.1159/000102979. [DOI] [PubMed] [Google Scholar]
- Gröne HJ, Cohen CD, Gröne E, et al. Spatial and temporally restricted expression of chemokines and chemokine receptors in the developing human kidney. J Am Soc Nephrol. 2002;13:957–967. doi: 10.1681/ASN.V134957. [DOI] [PubMed] [Google Scholar]
- Tögel F, Isaac J, Hu Z, et al. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]